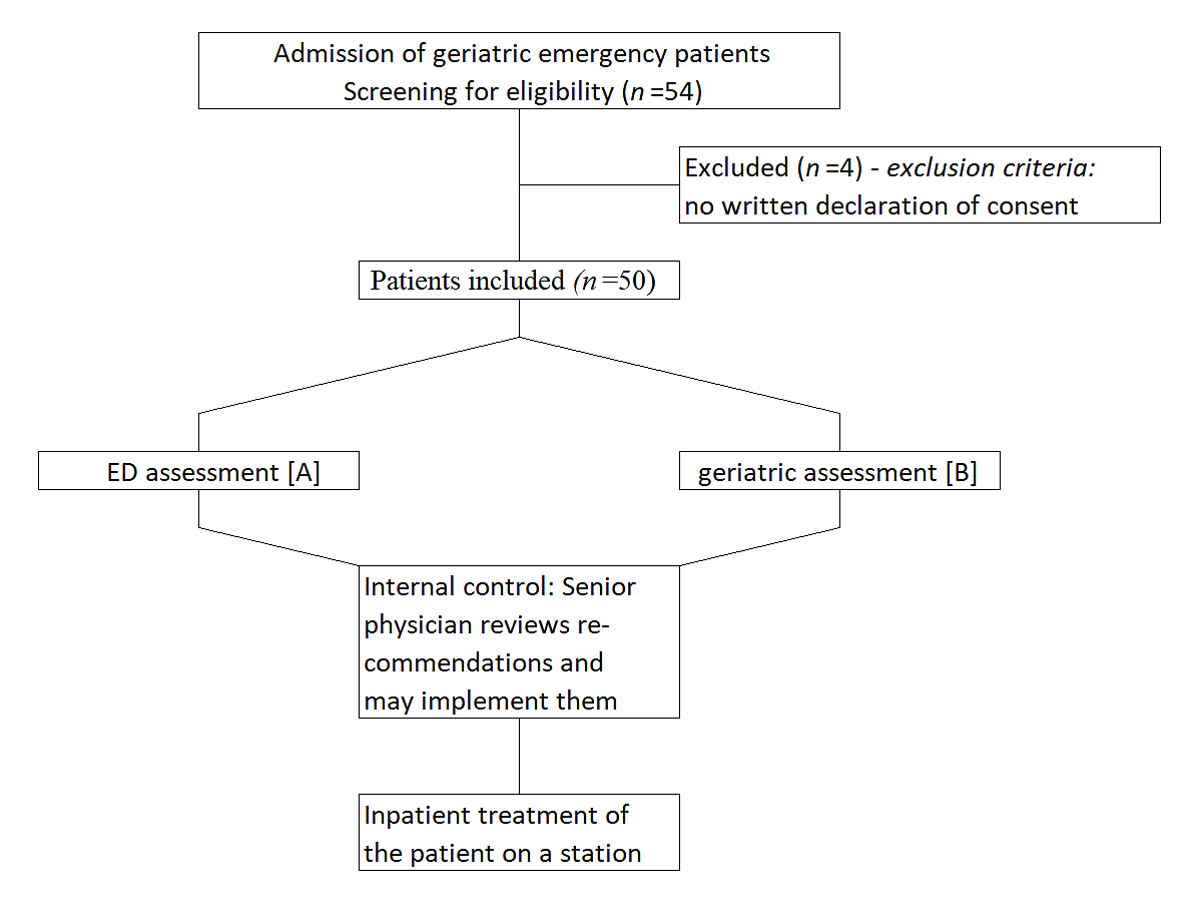
Figure 1 Flowchart of the study.
DOI: https://doi.org/10.4414/smw.2021.20500
Due to increasing life expectancy within an ageing society, treatment of geriatric patients in a hospital setting often has to address specific clinical challenges, such as drug interactions during poly-medication, increasing frailty, cognitive decline and multiple diseases. This applies especially to the emergency department (ED) [1], where fast and efficient medicine adapted to patients needs is crucial. In the field of emergency medicine, sustained success of inpatient treatment depends on adequate diagnosis and initiation of age-appropriate therapy. An important aspect of drug therapy management is that, as a result of changed pharmacokinetics and pharmacodynamics in older patients, many therapeutic agents are frequently associated with adverse drug effects (ADEs). This is, among others, a risk factor for longer hospitalisation, delirium and falls [2–5]. This is of particular relevance as geriatric patients are experience an increased rate of complications during inpatient hospital treatment [6, 7]. In order to reduce drug-related complications, several screening tools have been developed in recent years to detect potentially inadequate drugs (PIMs) for older adults [8, 9]. PIMs are defined as drugs that should be avoided in geriatric patients owing to an increased risk of adverse effects [10]. In addition to the Beer criteria for the American drug market, the FORTA (“Fit for the Aged”) classification is available in Germany.
Another risk factor for elderly patients, in addition to PIMs, is polypharmacy (usually defined as taking more than five drugs [11]). Because of the complex situation concerning older patients, guidelines such as the FORTA classification aid healthcare professionals with drug management but cannot replace assessment by a geriatrician
A common problem in most hospitals is the lack of instant availability of specific geriatric competence. Furthermore, new pandemic scenarios (COVID-19) call for reductions of face-to-face contact with patients at risk.
With increasing technical advances, the introduction of a telemedical geriatric consultation in the ED might constitute a solution to these challenges. Telemedical procedures have now been implemented and successfully applied in many areas of medicine [12], but evaluations of this method in real-life settings are still few and far between.
Therefore, the aim of this prospective clinical pilot study was to determine if an early tele-geriatric co-evaluation might contribute to improved geriatric treatment of elderly patients in an ED setting [13].
For this purpose an instant telemedical consultation was carried out by a geriatrician independently of the standard assessment by the ED medical staff. The therapeutic recommendations regarding drug management resulting from the two assessments were compared with each other. A special focus was on the use of PIMs for geriatric patients, according to the FORTA classification [9].
The patients of this prospective clinical pilot study were randomly selected from a population of geriatric patients aged ≥70 years who were admitted to the interdisciplinary ED of a single tertiary care medical centre (University Hospital RWTH Aachen, Germany). Another inclusion criterion was an “Identification of Seniors at Risk” score (ISAR score) of ≥2 [14]. The study enrolment was independent of the admission diagnosis. However, the study population mainly consisted of patients with internal medical diseases. Exclusion criteria were:
The study was conducted from November 2017 to February 2018. Informed consent was obtained from all participants in the study. The study was approved by the Ethics Committee of the University Hospital RWTH Aachen (EK 243/17) and performed according to the guidelines of the Declaration of Helsinki. The study was retrospectively registered at ClinicalTrials.gov (NCT04148027).
After admission to the ED, patients received standard treatment by a medical team from internal medicine, neurology and general surgery headed by a board-certified senior physician (see fig. 1 below).
Independently of this, a board-certified geriatrician was remotely linked for co-evaluation by video transmission (AVIZIA CA750, IVCi, New York, USA). The geriatrician was located in another hospital (Franziskus Hospital Aachen), to which the department for geriatrics of the University Hospital RWTH Aachen is outsourced.
Workflow of the telemedical assessment was aided by a research assistant in the ED as follows. The geriatrician was first given a short summary of the medical history and current reason for presentation in the ED, as well as the results of newly performed examinations such as laboratory tests, ECGs or X-rays. The geriatrician then had the choice of performing an independent interview with the patient or performing a telemedical physical assessment. The telemedical assessment was made with the help of the research assistant in the ED, who carried out a focused physical examination of the patient on behalf of the geriatrician. Depending on this telemedical assessment, the geriatrician provided a diagnosis and recommendations for further treatment.
In 22% of the cases, presentation of the patient’s history to the geriatrician was via telephone, since the video transmission device in Franziskus Hospital was also used by other departments.
Following the telemedical assessment, therapeutic recommendations provided by the geriatrician were evaluated by the responsible senior physician from the ED, who then made a final decision on the implementation of these recommendations before the patient was admitted to hospital for inpatient treatment.
As the board-certified geriatricians of the participating department were not on continuous round-the-clock duty, patients were enrolled in the study only on weekdays between 08:00 and 17:00. During this period, a telemedical assessment was made once a day at a previously agreed time. If there was only one possible patient who met the inclusion criteria, this patient was included. If there were several potentially eligible patients, inclusion was randomised by lot.
For bias prevention, ED physicians and the geriatrician were blinded on the endpoints and evaluation criteria of the study.
This was followed by an analysis of the two study arms with regard to the drug therapy recommendations. A distinction was made between recommendations for immediate drug interventions such as the administration of pro re nata (PRN) medications and recommendations on preexisting medication. In addition, we examined the different treatment recommendations to determine whether the interventions could reduce the use of PIMs for geriatric patients. To this end, we evaluated the treatment recommendations following the FORTA classification [9].
The “Identification of Seniors at Risk” score (ISAR score) [14] was applied to identify elderly patients at risk for adverse outcomes such as prolonged hospitalisation, functional decline and death during the first 6 months after an ED visit. It is comprised of six items, all answered with yes or no (need for support, acute changes in need for support, hospitalisation, sensory impairment, cognitive decline, multimorbidity) by the medical staff together with the affected patient, with possible support by a relative. An ISAR score of ≥2 is considered positive and indicates an increased risk of a poor health outcome.
The “Fit for the Aged” (FORTA) classification is a guide to the pharmacological treatment of elderly patients [9]. A total of 225 drugs are listed in the FORTA classification. These are assigned to 25 main clinical indications. The drugs are also classified into categories A, B, C and D, with positive and negative recommendations. Category A includes drugs whose benefits to older patients are considered to be clear. Category B includes drugs that, although useful, have some limitations in terms of safety and efficacy. Drugs in category C are expected to have an unfavourable benefit-risk relation for geriatric patients. Category D drugs should always be avoided.
First, we carried out a descriptive analysis of the patient population for age, sex, ISAR score and the number of preexisting medications, which were assigned for each patient to match main pharmacological subgroups according to a register for pharmaceutical drugs in Germany (“Rote Liste”) [15].
The primary endpoint was the number of pharmacological recommendations given by standard ED assessment and by telemedical geriatric assessment. A distinction was made between recommendations for immediate drug interventions, such as PRN medication or antibiotics, and recommendations on preexisting medication.
Another endpoint was the difference between the two study arms in the total number of preexisting medications taken by the patient before and after assessment.
In addition, we analysed the number of pharmacological recommendations per patient for (1) discontinuation of a preexisting drug, (2) start of a new drug, and/or (3) dose change of a preexisting drug.
Preexisting medications with regard to FORTA categories C and D were analysed. The endpoint was the number of successfully discontinued PIMs in the two study arms. As stated above, we used the FORTA classification to categorise each drug into A, B, C and D categories.
Statistical analysis was performed using Statistics Package for Social Sciences - SPSS 24 (SPSS Inc. Chicago, IL, USA).
We used the McNemar test for the comparison of pharmacological interventions undertaken during presentation in the ED. Student’s one sample t-test was used to compare the total number of drugs before and after intervention. Results were reported using mean and standard deviation and as significant at a p-level of <0.05.
During the screening process, 54 patients were randomly selected for the study. Four patients did not agree to study participation, therefore a total of 50 patients were included (fig. 1).

Figure 1 Flowchart of the study.
Among the 50 patients (age [mean ± standard deviation] 82.22 ± 6.03 years; 30 male, 20 female; ISAR score 3.8 ± 1.1 points), the mean number of preexisting medications was 8.76 ± 3.97. In total, 45/50 patients had more than five preexisting medications and thus met the criterion for polypharmacy [11].
According to the “Rote Liste” classification [14], 78% (n = 39) patients took β-receptor blockers, calcium channel blockers and/or inhibitors of the renin-angiotensin system (β-R/CA/RAAS), 64% (n = 32) took platelet aggregation inhibitors, 66% (n = 33) diuretics, 58% (n = 29) lipid-lowering drugs, 44% (n = 22) proton pump inhibitors, 38% (n = 19) anticoagulants, 38% ( n= 19) analgesics, 30% (n = 15) antidiabetics, 28% (n = 14) thyroid hormone, 26% (n = 13) psychotropic drugs, 26% (n = 13) urological/hyperuricaemia agents, 14% (n = 7) antianginals, 14% (n = 7) broncholytics, 12% (n = 6) other antihypertensives (such as moxonidine or doxazosin), 10% (n = 5) Parkinson’s medication, 8% (n = 4) antiepileptic drugs, 8% (n = 4) antiarrhythmics, 6% (n = 3) antiemetics, 6% (n = 3) corticoids, 6% (n = 3) hypnotics and 58% (n = 29) nonspecific drugs such as memantine or calcitriol.
There was a highly significant difference between the geriatrician and ED physicians in the frequency of recommendations regarding medication (p <0.001, n = 50; table 1). For the 50 patients, a total of 164 drug recommendations (3.28 ± 2.22 per patient) were made by the geriatrician, whereas the ED physicians made only 64 recommendations (1.24 ± 1.71 per patient). The geriatrician altered the preexisting medication in 96% of cases, i.e., 48 patients, whereas the ED physicians changed the preexisting medication in only 46% of cases, i.e., 23 patients. Table 2 shows the distribution of the most common drug groups (at least three therapy changes in one of the interventions) for which a change was recommended after geriatric and ED assessment.
Table 1 Comparison of the different forms of pharmacological recommendations between geriatric and ED assessment. The table shows (1) the total number of drug recommendations, (2) the number of recommendations per patient, and (3) the number of affected patients for each form of therapy recommendation. With regard to the FORTA C/D drugs, only the number of recommendations in relation to the total number of FORTA C/D drugs is shown.
| Number of therapy recommendations | Different forms of medical intervention | A vs B | |
|---|---|---|---|
| ED assessment (A) | Geriatric assessment (B) | p-value | |
| Total number for immediate drug therapy | 55 | 30 | 0.039 |
| Number per patient (mean ± SD) | 1.10 ± 1.09 | 0.6 ± 0.81 | |
| In % of all patients | 62% (31/50) | 46% (23/50) | |
| Total number for preexisting medication | 62 | 164 | <0.001 |
| Number per patient (mean ± SD) | 1.24 ± 1.71 | 3.28 ± 2.22 | |
| In % of all patients | 46% (23/50) | 96% (48/50) | |
| Different forms of drug recommendations for the preexisting medication | |||
| Total number for discontinuing a drug | 30 | 87 | < 0.001 |
| Number per patient (mean ± SD) | 0.60 ± 1.25 | 1.74 ± 1.32 | |
| In % of all patients | 30% (15/50) | 82% (41/50) | |
| Total number for starting a new drug | 25 | 43 | 0.004 |
| Number per patient (mean ± SD) | 0.50 ± 0.93 | 0.86 ± 0.93 | |
| In % of all patients | 34% (17/50) | 56% (28/50) | |
| Total number for changing the dose of a drug | 7 | 34 | 0.001 |
| Number per patient (mean ± SD) | 0.14 ± 0.35 | 0.68 ± 0.82 | |
| In % of all patients | 14% (7/50) | 50% (25/50) | |
| Total number for drugs of FORTA classifications C/D (%) | 12.3% (8/65) | 53.9 (35/65) | <0.001 |
ED = emergency department; SD = standard deviation
Table 2 Distribution of the most common drug groups in the preexisting medication (at least three therapy changes in one of the interventions) for which a therapy change was recommended after geriatric and emergency department (ED) assessment.
| Number of therapy recommendations on preexisting medication | Antianginals | Anticoagulants | Diuretics | Antidiabetics | Thyroid hormone | β-R/CA/RAAS* | Analgesics | Hypnotics |
Platelet aggregation
inhibitors |
|---|---|---|---|---|---|---|---|---|---|
| Total number for discontinuing a drug | |||||||||
| ED assessment | 1/30 (3.3%) | 4/30 (13.3%) | 9/30 (30%) | 4/30 (13.3%) | 0 | 6/30 (20%) | 0 | 0 | 1/30 (3.3%) |
| Geriatric assessment | 4/87 (4.6%) | 2/87 (2.3%) | 21/87 (24.1%) | 9/87 (10.3%) | 0 | 20/87 (22.9%) | 8/87 (9.2%) | 4/87 (4.6%) | 1/87(1.1%) |
| Total number for starting a new drug | |||||||||
| ED assessment | 0 | 4/25 (16%) | 4/25 (16%) | 0 | 1/25 (4%) | 3/25 (12%) | 3/25 (12%) | 2/25 (8%) | 3/25 (12%) |
| Geriatric assessment | 0 | 4/43 (9.3%) | 7/43 (16.3%) | 5/43 (11.2%) | 1/43 (2.3%) | 8/43 (18.6%) | 5/43 (11.6%) | 0 | 2/43 (4.7%) |
| Total number for changing the dose of a drug | |||||||||
| ED assessment | 0 | 0 | 1/7 (14.3%) | 1/7 (14.3%) | 1/7 (14.3%) | 3/7 (42.9%) | 0 | 1/7 (14.3%) | 0 |
| Geriatric assessment | 0 | 0 | 3/34 (8.8%) | 5/34 (14.7%) | 5/34 (14.7%) | 10/34 (29.4%) | 1/34 (2.9%) | 3/34 (8.8%) | 0 |
* β-receptor blockers, calcium-channel blockers and/or inhibitors of the renin-angiotensin system
Following the pharmacological therapy recommendations made by the geriatric specialist, the number of prescribed drugs was significantly reduced (t(49) = 4.165, p <0.001, n = 50; number of drugs before evaluation 8.76 ± 3.97 vs 7.82 ± 3.77 after evaluation). Interventions by the ED physicians had no statistically significant effect on the number of prescribed drugs (t(49) = 0.622, p = 0.537; n = 50; drugs before evaluation 8.76 ± 3.97 vs 8.62 ± 3.8 after evaluation).
For the individual forms of drug recommendation (discontinuation of a preexisting drug, start of a new drug, dose change of a preexisting drug), there was also a highly significant difference between geriatric and ED assessment.
Significantly more drugs were discontinued following the geriatrician’s recommendations (p <0.00, n = 50). The geriatrician discontinued one or more medications in 41 (82%) of all telemedically examined patients, whereas ED physicians withdrew preexisting medications in only 15 (30%) patients. The total number of medications discontinued by the geriatrician was 87 (1.74 ± 1.32 per patient) and by the ED physicians 30 (0.60 ± 1.25 per patient).
For the rates of new drug prescriptions, the values for the ED were significantly lower than for the geriatric assessment (p = 0.004, n = 50). Forty-three new drugs were added in 28 patients (56%) by the geriatrician (0.86 ± 0.93 per patient). Twenty-five new drugs were added by the ED physicians (0.50 ± 0.93 per patient). This change affected 17 patients (34%).
The geriatrician recommended dose changes significantly more frequently than the ED physicians (p = 0.001, n = 50). The geriatrician suggested a dose change 34 times (0.68 ± 0.82 per patient). The ED physicians recommended dose changes only 7 times (0.14 ± 0.35 per patient). Geriatrics specialists recommended a dose modification of preexisting medications in 50% (25/50) of the patients. ED physicians prescribed alterations in dosage in only 14% (7/50) of the patients.
There was a significant difference between the geriatrician and ED physicians in the frequency of recommendations for immediate drug therapy (p = 0.039, n = 50; table 1). The total number of pharmacological recommendations was 30 for the geriatrician (0.6 ± 0.81 per patient) and 55 for the ED physicians (1.10 ± 1.09 per patient). For immediate recommendations in the ED, acute pharmacological treatment was undertaken in 31 patients (62%), whereas the geriatrician initiated acute pharmacological treatment in only 23 patients (46%).
Thirty-nine patients (78%) had one or more preexisting medications in categories C and D of the FORTA classification, which should be avoided in geriatric patients. In total, 42 drugs were categorised as class C and 23 as class D.
Among these, 16.9% were analgesics, 15.4% psychotropics, 12.3% hypnotics/sedatives, 10.8% antihypertensives (including moxonidine), 9.2% diuretics, 6.2% amiodarone, 6.2% antidiabetics, 4.6% antianginals (including molsidomine), 4.6% β-receptor blockers, calcium-channel blockers and / or inhibitors of the renin-angiotensin system, 4.6% antiepileptics, 3.1% urologicals, 3.1% antiemetics, 1.5% gastrointestinal drugs and 1.5% nonspecific.
Comparison between the geriatric and ED assessment of recommendations for drugs meeting FORTA classes C and D revealed a highly significant difference (p <0.001, number of FORTA C/D drugs = 65). The geriatrician changed 53.9% of the drugs (35/65) whereas the ED physicians only changed 12.3% (8/65).
The results of our present study show that an early geriatric assessment had a significant influence on drug therapy as compared with ED standard treatment. In particular, there was a significant effect regarding recommendations on preexisting medication. As a result of the geriatric examination, significantly more drugs were newly prescribed, discontinued or had their dosage changed. Overall, this led to the total number of prescribed drugs being significantly reduced. Conversely, ED physicians were significantly more likely to recommend an immediate pharmacological treatment such as PRN medication. One reason for this is that the telemedical geriatric consultation was not carried out immediately. PRN medication against pain or nausea had already been prescribed before the telemedical consultation. A therapeutic recommendation by the geriatrician was therefore no longer necessary. A comparison of the two study arms in regard to this is therefore difficult.
Another very important aspect of the study was that the geriatric assessment resulted in significantly more PIMs being recognised and the medication adjusted. There was no relevant difference between two study arms with regard to the type of drugs for which therapy changes were most frequently recommended (as shown in table 2).
The most frequent therapeutic recommendations concerned diuretics and substances of the β-R/CA/RAAS group. After geriatric consultation, there were significantly more cases in which the therapy with FORTA drugs of category C or D was changed compared with the ED physicians (54% vs 12%). However, owing to the recommendations of the geriatricians, there were significantly more cases in which the therapy was changed for FORTA drugs of category C or D compared with the ED doctors. Whether this also had an effect on the aspects that are relevant for the patient, such as reduced risk of delirium and falls, shortened hospital stay and reduced mortality, is certainly the most important issue. Because of the small cohort of this pilot study, this question could not be answered. Further studies will be needed to evaluate this.
However, several studies have shown that polypharmacy and the use of PIMs for elderly patients are associated with increased morbidity and mortality [11, 16, 17]. Reasons for this are altered pharmacokinetics and pharmacodynamics as a result of the metabolic changes that occur with aging [3]. This results in drug-associated complications such as drug interactions, drug-disease interactions and adverse drug reactions.
Examples of PIMs are certain antidepressants or anticholinergics that have deliriogenic potential, or benzodiazepines, which are also associated with an increased risk of falling [5]. Screening tools such as the Beer criteria [8] or the FORTA classification [9] can be used to detect possible PIMs, but do not replace geriatric expertise. This again is of particular relevance for the ED, as many drugs that are frequently used there increase the risk of ADEs. These include nonsteroidal inflammatory drugs, opioids and benzodiazepines. These are associated, among other things, with acute kidney failure, increased falls and peptic ulcers [18]. In addition, ADEs are a common reason for admission to the ED in older patients in the first place [19]. A review by Alhawassi et al. came to the conclusion that, conservatively estimated, in 1 in 10 elderly patients an ADE is the reason for admission to the hospital or occurs during inpatient treatment [20].
The patients in the present study also had a relevant risk constellation. In 90% of the patients the criteria for polypharmacy were met. In addition, 78% of the patients took one or more drugs that were included in the FORTA classification categories C or D. The most frequently inappropriately used drugs were analgesics (16.9%), psychotropics (15.4%) and sedatives (12.3%). Here the influence of a geriatric co-evaluation was shown to be most helpful. Whereas ED physicians intervened only sometimes, the geriatricians recommended therapy adjustment for over half of the critical medications.
In addition to medical therapy, a variety of concepts have been developed to improve the care of geriatric patients in the ED [21, 22]. These approaches include changes to staffing, physical infrastructure, care delivery, case management or discharge planning [23–27]. These efforts also resulted in the publication of the Geriatric Emergency Department Guidelines in 2014 [28], which include recommendations for improved care of geriatric emergency patients. Whether these approaches will lead to an improvement in patient outcomes is unclear, as there are only a small number of studies. The heterogeneity of the possible interventions also makes the evaluation of outcomes difficult. A systematic review by Hughes et al. [29] analysed 15 studies with different intervention approaches. These mainly included measures in the areas of case management, discharge planning and medication management. Overall, there was a small positive effect on functional status. However, impact on other domains such as quality of life, patient experience, hospitalisation after the intervention or rate of hospital readmission is still unclear. Only a few studies utilising two or more of these interventions showed a small positive effect in terms of lower hospitalisation and readmission rates.
Despite the previously insufficient evidence in favour of age-adapted emergency treatment, the implementation of geriatric-specific interventions in the ED seems advantageous. An early, age-adapted adjustment of medication, as in the present study, could be beneficial, especially in combination with other non-drug-related interventions.
Another important aspect of our study was that the geriatric assessment was carried out in a telemedical setting. Telemedicine has already become established in many medical fields and has been proven to have positive effects [30]. Especially for elderly patients, its significance is increasing owing to limited resources and elevated susceptibility to contagious diseases, as currently demonstrated by the COVID-19 pandemic. The telemedical approach is a possible solution to the limited availability of medical staff with special geriatric expertise.
With regard to the use of telemedicine in geriatric patients, its feasibility and effectiveness have already been shown in several studies [31] in different settings such as hospitals, communities for the elderly or nursing homes. Various telemedical communication forms, such as video consultation, consultation by telephone or transmission of vital signs via computer, have been used [32]. The special use of a telegeriatric intervention immediately after admission in the ED has not been further investigated so far, so this pilot study evaluated its feasibility and effectiveness for the first time and was therefore innovative in this field.
A possible limitation of our study is the monocentric study design with a limited number of patients included. Further multicentre evaluation with larger samples is needed, combined with evaluation of longitudinal outcomes, such as duration of hospitalisation, readmission rates, complications and mortality.
Another limitation of the study is that, in some cases (22%), for organisational reasons, presentation of patients had to be solely by telephone and not by video transmission. In addition, the geriatricians were not continuously available, so that patients were only enrolled in the study on weekdays between 08:00 and 17:00. Another bias can be assumed with regard to the recommendations on acute pharmacological treatment, since several patients needed immediate pharmacological treatment from the ED physicians after admission to the ED. In some cases, this had to be reported to the geriatricians, which probably resulted in a lower number of recommendations.
An early assessment by a geriatrician of elderly patients admitted to the ED has a significant impact on the number of drug interventions. As shown in our study, the evaluation by a geriatric physician significantly reduced the total number of prescribed medications. In addition, the number of PIMs for elderly patients was significantly lowered. Another important aspect was that the geriatric consultation was carried out using telemedical procedures. In view of limited medical resources and pandemic situations, these are becoming increasingly important, especially for elderly patients at risk. The results lead to the conclusion that an early geriatric intervention in the ED, possibly in the form of telemedical consultation, can be a very effective approach to addressing these problems. For further evaluation, however, a prospective study with larger case numbers is required.
RR and JCB are cofounders and shareholders of “Docs in clouds”, a telemedicine company. No products of the company were used in the study.
1 Martin-Khan M Burkett E Schnitker L Jones RN Gray LC . Methodology for developing quality indicators for the care of older people in the Emergency Department. BMC Emerg Med. 2013;13(1):23. doi:.https://doi.org/10.1186/1471-227X-13-23
2 Rowe JW Andres R Tobin JD Norris AH Shock NW . The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31(2):155–63. doi:.https://doi.org/10.1093/geronj/31.2.155
3 Klotz U . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. doi:.https://doi.org/10.1080/03602530902722679
4 Hübscher A Isenmann S . Delir: Konzepte, Ätiologie und klinisches Management [Delirium: Concepts, Etiology, and Clinical Management]. Fortschr Neurol Psychiatr. 2016;84(4):233–44. Article in German. doi:https://dio.org/10.1055/s-0042-104502.
5 Burkhardt H Wehling M Gladisch R . Pharmakotherapie älterer Patienten [Pharmacotherapy of elderly patients]. Internist (Berl). 2007;48(11):1220–31, 1222–4, 1226–31. Article in German. doi:.https://doi.org/10.1007/s00108-007-1947-1
6 Schnitker LM Martin-Khan M Burkett E Brand CA Beattie ER Jones RN Research Collaboration for Quality Care of Older Persons: Emergency Care Panel . Structural quality indicators to support quality of care for older people with cognitive impairment in emergency departments. Acad Emerg Med. 2015;22(3):273–84. doi:.https://doi.org/10.1111/acem.12617
7 Long SJ Brown KF Ames D Vincent C . What is known about adverse events in older medical hospital inpatients? A systematic review of the literature. Int J Qual Health Care. 2013;25(5):542–54. doi:.https://doi.org/10.1093/intqhc/mzt056
8 Beers MH Ouslander JG Rollingher I Reuben DB Brooks J Beck JC UCLA Division of Geriatric Medicine . Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151(9):1825–32. doi:.https://doi.org/10.1001/archinte.1991.00400090107019
9 Werner H . Arzneimitteltherapie im Alter: zu viel und zu wenig, was tun? – Ein neues Bewertungssystem: fit for the aged (FORTA) [Drug therapy in the aged: too much and too little, what to do? A new evaluation system: fit for the aged (FORTA)]. Dtsch Med Wochenschr. 2009;134(3):95–6, author reply 96. Article in German. doi:.https://doi.org/10.1055/s-0028-1105898
10 Galli TB Reis WC Andrzejevski VM . Potentially inappropriate prescribing and the risk of adverse drug reactions in critically ill older adults. Pharm Pract (Granada). 2016;14(4):818. doi:.https://doi.org/10.18549/PharmPract.2016.04.818
11 Masnoon N Shakib S Kalisch-Ellett L Caughey GE . What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi:.https://doi.org/10.1186/s12877-017-0621-2
12 Marx G Beckers R Brokmann JC Deisz R Pape HC . Telekooperation für die innovative Versorgung am Beispiel des Universitätsklinikums Aachen : Telematik in der Intensivmedizin (TIM), Telenotarzt und Telemedizinische intersektorale Rehabilitationsplanung in der Alterstraumatologie (TIRA) [Tele-cooperation for innovative care using the example of the University Hospital Aachen. Telematics in intensive care medicine, emergency medicine, and telemedical intersectoral rehabilitation planning in geriatric trauma]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(10):1056–61. Article in German. doi:.https://doi.org/10.1007/s00103-015-2224-4
13Lecce C. Einsatz von Telemedizin bei geriatrischen Notfallpatienten [dissertation]. Aachen (Germany): RWTH Aachen University; 2021.
14 Yao JL Fang J Lou QQ Anderson RM . A systematic review of the identification of seniors at risk (ISAR) tool for the prediction of adverse outcome in elderly patients seen in the emergency department. Int J Clin Exp Med. 2015;8(4):4778–86.
15Rote Liste. Rote Liste - Arzneimittelinformationen für Deutschland. https://www.rote-liste.de/, 2018.
16 Milton JC Hill-Smith I Jackson SHD . Prescribing for older people. BMJ. 2008;336(7644):606–9. doi:.https://doi.org/10.1136/bmj.39503.424653.80
17 Muhlack DC Hoppe LK Weberpals J Brenner H Schöttker B . The Association of Potentially Inappropriate Medication at Older Age With Cardiovascular Events and Overall Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. J Am Med Dir Assoc. 2017;18(3):211–20. doi:.https://doi.org/10.1016/j.jamda.2016.11.025
18 Kim M Mitchell SH Gatewood M Bennett KA Sutton PR Crawford CA Older adults and high-risk medication administration in the emergency department. Drug Healthc Patient Saf. 2017;9:105–12. doi:.https://doi.org/10.2147/DHPS.S143341
19 Laatikainen O Sneck S Bloigu R Lahtinen M Lauri T Turpeinen M . Hospitalizations Due to Adverse Drug Events in the Elderly-A Retrospective Register Study. Front Pharmacol. 2016;7:358. doi:.https://doi.org/10.3389/fphar.2016.00358
20 Alhawassi TM Krass I Bajorek BV Pont LG . A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–86 .
21 Sinha SK Bessman ES Flomenbaum N Leff B . A systematic review and qualitative analysis to inform the development of a new emergency department-based geriatric case management model. Ann Emerg Med. 2011;57(6):672–82. doi:.https://doi.org/10.1016/j.annemergmed.2011.01.021
22 Aminzadeh F Dalziel WB . Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39(3):238–47. doi:.https://doi.org/10.1067/mem.2002.121523
23Preston LR, Chambers D, Campbell F, Cantrell A, Turner J, Goyder E. What evidence is there for the identification and management of frail older people in the emergency department? A systematic mapping review. National Institute for Health Research. Southampton, UK: Queen’s Printer and Controller of HMSO; 2018.
24 Conroy SP Stevens T Parker SG Gladman JR . A systematic review of comprehensive geriatric assessment to improve outcomes for frail older people being rapidly discharged from acute hospital: ‘interface geriatrics’. Age Ageing. 2011;40(4):436–43. doi:.https://doi.org/10.1093/ageing/afr060
25 Fealy G McCarron M O’Neill D McCallion P Clarke M Small V Effectiveness of gerontologically informed nursing assessment and referral interventions for older persons attending the emergency department: systematic review. J Adv Nurs. 2009;65(5):934–45. doi:.https://doi.org/10.1111/j.1365-2648.2009.04961.x
26 Jay S Whittaker P Mcintosh J Hadden N . Can consultant geriatrician led comprehensive geriatric assessment in the emergency department reduce hospital admission rates? A systematic review. Age Ageing. 2017;46(3):366–72. doi:.https://doi.org/10.1093/ageing/afw231
27 Lowthian JA McGinnes RA Brand CA Barker AL Cameron PA . Discharging older patients from the emergency department effectively: a systematic review and meta-analysis. Age Ageing. 2015;44(5):761–70. doi:.https://doi.org/10.1093/ageing/afv102
28 American College of Emergency PhysiciansAmerican Geriatrics SocietyEmergency Nurses AssociationSociety for Academic Emergency MedicineGeriatric Emergency Department Guidelines Task Force . Geriatric emergency department guidelines. Ann Emerg Med. 2014;63(5):e7–25. doi:.https://doi.org/10.1016/j.annemergmed.2014.02.008
29 Hughes JM Freiermuth CE Shepherd-Banigan M Ragsdale L Eucker SA Goldstein K Emergency Department Interventions for Older Adults: A Systematic Review. J Am Geriatr Soc. 2019;67(7):1516–25. doi:.https://doi.org/10.1111/jgs.15854
30 Hilty DM Ferrer DC Parish MB Johnston B Callahan EJ Yellowlees PM . The effectiveness of telemental health: a 2013 review. Telemed J E Health. 2013;19(6):444–54. doi:.https://doi.org/10.1089/tmj.2013.0075
31 Narasimha S Madathil KC Agnisarman S Rogers H Welch B Ashok A Designing Telemedicine Systems for Geriatric Patients: A Review of the Usability Studies. Telemed J E Health. 2017;23(6):459–72. doi:.https://doi.org/10.1089/tmj.2016.0178
32 Brignell M Wootton R Gray L . The application of telemedicine to geriatric medicine. Age Ageing. 2007;36(4):369–74. doi:.https://doi.org/10.1093/ageing/afm045
Contributed equally to this paper
RR and JCB are cofounders and shareholders of “Docs in clouds”, a telemedicine company. No products of the company were used in the study.