Single embryo transfer in all infertile couples treated with assisted reproduction produces excellent results and avoids multiple births
DOI: https://doi.org/10.4414/smw.2021.20499
Reproductive Medicine and Gynaecological Endocrinology (RME), University Hospital, University of Basel, Switzerland
Summary
Assisted reproductive technology (ART) is an efficacious and frequently used treatment of infertility. Multiple births resulting from the widespread practice of transfer of more than one embryo per treatment trial have reached epidemic proportions. Since the revision of the Swiss law on ART in September 2017, up to 12 embryos per couple may now be developed in vitro and cryopreserved in Switzerland. This potentially allows for the selection and transfer of only one embryo to avoid multiple birth with ART. We decided to offer transfer of one embryo to all infertile patients undergoing ART in our institution. In this retrospective cohort study the cumulative pregnancy and live-birth rates after universal transfer of one embryo from January 2018 to December 2019 were analysed. The cumulative pregnancy rate per oocyte collection and up to five transfer cycles was as high as 48.9%, whereas the cumulative live-birth rate reached 33.4% and none were multiple births. These results were age-dependent, with best outcome in patients aged 37 years or younger. When still remaining cryopreserved embryos were taken into account, the cumulative birth-rates could exceed 60% per oocyte collection in all age groups. The consequent adoption of a single embryo transfer significantly reduced the incidence of multiple births in the department of obstetrics and the number of prematurely born infants resulting from multiple pregnancies in the department of neonatology. Universal elective single embryo transfer is feasible in Switzerland, benefits infertile couples treated with ART and reduces the number of multiple births in obstetrics and of newborn children hospitalised into neonatal care.
Introduction
According to the World Health Organization (WHO), infertility is a disease of the reproductive system defined by the failure to achieve pregnancy after 12 months or more of regular unprotected sexual intercourse [1, 2]. The incidence of this condition is frequent in all populations and may affect between 7% and 15% of all couples during some time of their reproductive life. Assisted reproductive technology (ART) provides the most effective medical treatment to overcome infertility. ART usually consists of a hormonal treatment, during which the ovaries are stimulated to produce multiple ovarian follicles, from which oocytes can be retrieved. This hormonal treatment mostly consists of gonadotropin preparations combined with a gonadotropin releasing hormone (GnRH) agonist or a GnRH antagonist to prevent premature ovulation. Subsequently, fertilisation of all harvested oocytes takes places in a specialised laboratory. After fertilisation, several embryos may develop. Conventionally, several embryos are transferred into the uterus of the patient, and remaining embryos are cryopreserved and stored for later use. Current efficacy of ART stems from the possibility to select embryos from a cohort of oocytes that were fertilised through conventional in vitro fertilisation (IVF) or with intracytoplasmic injection of sperm (ICSI). The identification of the early processes of fertilisation and early embryonic growth up to the blastocyst stage, which is reached 5 days after fertilisation, also adds to current efficacy of ART. However, this widely used therapeutic strategy is not without risks. The most common complication of the transfer of more than one embryo per cycle consists of multiple pregnancy, which frequently results in premature delivery with all its inherent risks for both the mother and the infants [3–5]. Another frequent adverse event is the ovarian hyperstimulation syndrome (OHSS), which in its severe form occurs in approximately 1.7 to 8.5% of all ART treatments [6, 7].
New methods and technologies have been introduced to avoid these complications. In comparison with the older GnRH-agonist based protocol, the risk of OHSS is significantly reduced by using the GnRH antagonist protocol instead of a GnRH agonist [6]. Human chorionic gonadotropin (hCG) has been used to induce the final stages of follicular maturation and to resume meiosis. However, hCG is also implicated in promoting OHSS [8]. In modern ovarian hyperstimulation protocols, GnRH antagonists are now added to ovarian stimulation with gonadotropins to prevent premature ovulation and, once the follicles reach the appropriate size, a GnRH agonist replaces hCG to trigger the endogenous luteinising hormone (LH) surge [9]. In addition to novel ovarian stimulation protocols, ultrarapid vitrification has improved cryopreservation of embryos such that, instead of transferring embryos immediately after oocyte collection, all embryos may now be stored frozen with virtually no risk of damage for later thawing and transfer. This treatment strategy, commonly termed “freeze all”, has significantly reduced the incidence of OHSS, as recently demonstrated in a prospective randomised clinical trial [10]. Furthermore, the improvement of in vitro culture techniques of oocytes and embryos has paved the way to reducing the number of embryos needed for successful pregnancy. The decision to transfer only one embryo per treatment trial opened the gate for the concept of elective single embryo transfer (eSET) [11]. The use of eSET has considerably reduced the risk of multiple delivery after ART in a number of European countries, such as Belgium, Sweden and Finland [12–14]. Although the practice of eSET is being firmly established in those countries and elsewhere [15], it is still not being applied universally to all infertile couples treated with ART.
As a result of the prevailing legal restrictions, Swiss reproductive physicians have long been unable to adopt most of these improvements in ART. In comparison with international standards, Swiss outcome results remained moderate and the incidence of multiple deliveries high [16–18]. After long and controversial debates, the new law dealing with ART was finally introduced [19]. Since September 2017, the cryopreservation of up to 12 embryos per couple is allowed and the principle of eSET can now be applied potentially to all infertile couples. However, misbelieve about the feasibility and benefits of eSET still prevails among patients, physicians and embryologists alike.
We decided to apply the principle of eSET to all infertile couples treated with ART in our institution. The present communication describes the results, including the effects of this policy on the outcome results of ART and on the incidence of multiple births in the obstetrics and neonatology intensive care departments.
Material and methods
As part of a research project funded by the Swiss National Fund (SNF) dealing with the identification and characterisation of epigenetic variation in human spermatozoa and its impact on embryonic development (No. 320030_189264), we systematically analysed the data of all new patients treated with ART in 2018 and 2019 (EKNZ 2020-00330). We here describe the results of this retrospective single-centre cohort study and also include the number of multiple delivery births that occurred in the department of obstetrics of the University Hospital and the number of premature neonates hospitalised in the intensive care unit of the neonatology department of the University Children’s Hospital of Basel from 2016 to 2019.
Data extraction
In the Reproductive Medicine and Gynaecological Endocrinology (RME) department, University Hospital Basel, all data of infertile couples are systematically registered in an online database (FertiMed®, Philippe Belloni, Reinach BL, Switzerland). Prior to ART the patients are informed about the legal requirements concerning data collection, including data reporting to the authorities, and all couples signed a consent form prior to treatment. For the purpose of this retrospective analysis, data of all infertile couples treated with ART in 2018 and 2019 were extracted and processed. We excluded all infertile couples who underwent thawing cycles with oocytes in the pronuclear stage or embryos that were cryopreserved before 2018. We also excluded all treatments with preimplantation genetic testing (PGT) and fertility preservation. The outcome of all pregnancies and deliveries was systematically obtained by written letters to the respective obstetricians.
Treatment procedures
Ovarian hyperstimulation invariably consisted of daily subcutaneous injections of either urinary or recombinant gonadotropin preparations in a GnRH antagonist protocol, as previously described [9]. Ovarian hyperstimulation was monitored both with vaginal ultrasound and with repeated measurements of the serum oestradiol and progesterone concentrations. Except in patients diagnosed with hypogonadotropic hypogonadism (WHO I) the final maturation of the ovarian follicles was always triggered with a GnRH agonist (Decapeptyl® 0.2 mg, Ferring, Saint-Prex, Switzerland). Triggering was done in the presence of 1000 pmol oestradiol concentration per mature follicle and in the presence of progesterone levels rising above the basal serum level. Exactly 36 hours after trigger with the GnRH agonist or 37 hours after ovulation induction with hCG, oocytes were harvested through ultrasound-guided transvaginal aspiration of all visible ovarian follicles. Up to 12 oocytes in the pronuclear stage were cultured to the blastocyst stage in a time lapse incubator (ESCO Medical Miri, Arhus, Denmark). If more than 12 oocytes became fertilised, the remainder were stored frozen in the zygote stage.
In all cases, only one embryo was transferred per treatment trial. Embryo transfer was always carried out under transabdominal ultrasound guidance. The remaining embryos were vitrified in the blastocyst stage, either 5 or 6 days after fertilisation. Luteal phase support was initiated immediately after oocyte collection, using vaginal progesterone supplementation (600 mg daily, Utrogestan®, Vifor, Villars-sur-Glâne, Switzerland) supplemented with low dose hCG (Choriomon®, IBSA, Lugano, two or three times 2000 IU, every third day).
Freezing of all embryos was carried out in the following situations:
- In the presence of high circulating oestradiol levels at ovulation induction (15,000 pmol/l or higher).
- In the presence of high circulating progesterone levels at ovulation induction (6.2 nmol/l or higher).
- When 15 or more oocytes were harvested.
- In the presence of any abnormality of the endometrium, as visualised with ultrasound, such as serometra, polyps or adhesions.
If all embryos were stored frozen, a thawing cycle was initiated at the earliest in the subsequent treatment cycle [20] or later.
In thawing cycles, proliferation of the endometrium was induced with oestradiol valerate (6 mg daily, Progynova®, Bayer, Zürich, Switzerland). After proliferation of the endometrium, as verified by ultrasound, the secretory phase was initiated by supplementing vaginal progesterone (600 mg daily, Utrogestan®) to oestradiol valerate. One embryo was thawed and transferred after 5 days.
Pregnancy was defined by the presence of at least 100 IU/l hCG in the serum 14 days after oocyte collection or 9 days after thawing and transfer of one single blastocyst embryo. All pregnancies were followed sequentially until week 13 of pregnancy. After that, the pregnant patients were referred back to their obstetrician.
All in house diagnostic and therapeutic procedures are described in written protocols. Quality management is both certified (DIN EN ISO 9001:2015) and accredited (ISO/IEC 17025:2017). At all stages gametes and embryos are systematically identified through a matching system, IMT Matcher, provided by IMT International, Gynemed GmbH, Lensahn, Germany. Semen analysis is consistently performed according to the WHO guidelines (2010) [21] and selected samples are quality controlled both internally (monthly means) and externally (QuaDeGa). RME participates in the FIVNAT-CH data collection.
Data analysis
The cumulative pregnancy and live-birth rates were analysed per ovarian hyperstimulation followed by oocyte collection, and calculated based on the sequential transfer of single embryos in the stimulated cycle and in subsequent thawing cycles during the observation period from 1 January 2018 to 31 December 2019. The pregnancy and live-birth outcome rates were calculated using the method given by Malizia and coworkers [22] with some modifications. The “optimistic” cumulative live-birth rate scenario assumes that all patients who did not return for subsequent treatment cycles had the same likelihood of a pregnancy and live-birth. The “conservative” method was not applied in this set of patients. Instead, potential additional live births beyond the period of observation were calculated from the number of still cryopreserved blastocysts. For each of the four age groups, the implantation and live-birth probability per blastocyst was calculated. By multiplying the child/blastocyst ratio with the number of blastocysts still available, we calculated the “maximum” live-birth rate, if all remaining embryos would be thawed one by one during sequential thawing cycles.
Differences between groups were analysed with the chi-square test.
Results
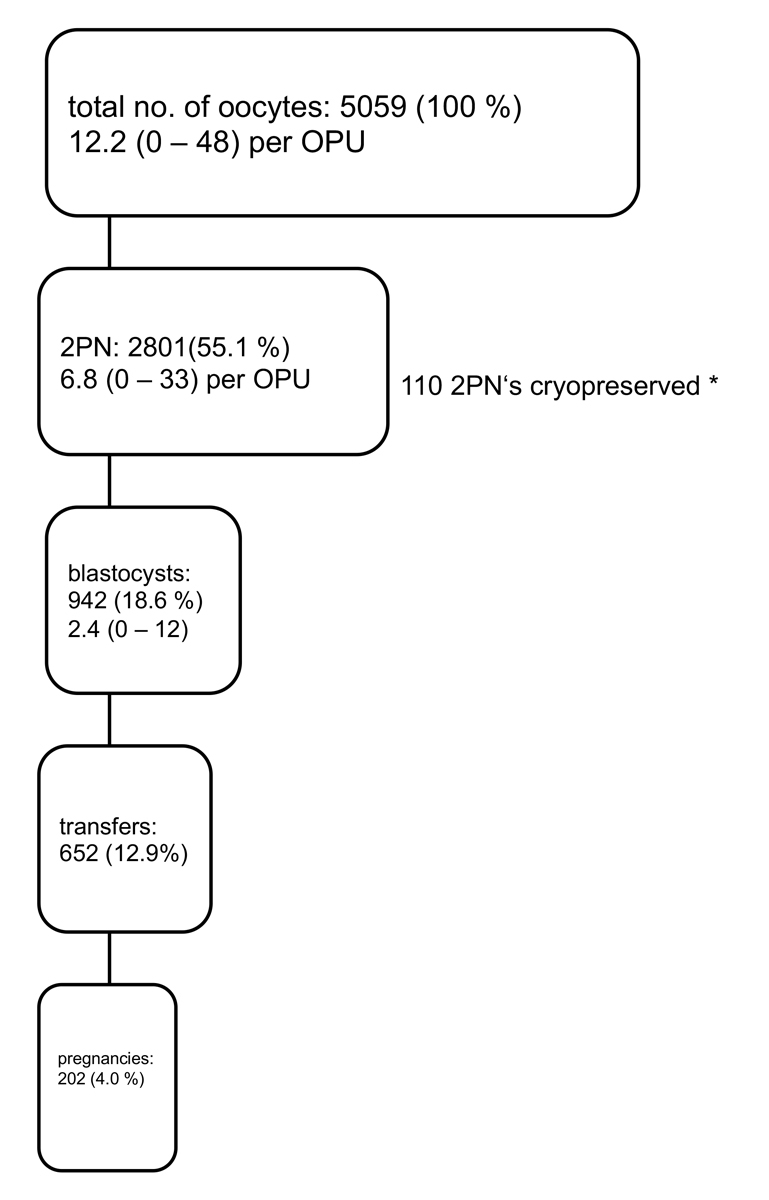
The characteristics of the 381 infertile couples treated with ART and having undergone oocyte collection for IVF and ICSI in 2018 and 2019 (without cancelled cycles, without PGT and leaving out fertility protection treatments) are summarised in table 1. After ovarian hyperstimulation and ovulation induction, 413 oocyte collections were performed in 381 infertile women and 5059 oocytes were harvested (mean number of oocytes collected per aspiration: 12.2, fig. 1). In this cohort there were no treatments in which no oocyte was harvested. In five treatment trials, only one oocyte was collected (1.2%). IVF was performed in 114 treatments (27.6%), ICSI in 299 treatments (72.4%). In 16 treatment cycles, fertilisation failed to occur (3.9%). Two pronuclei were identified in 2801 oocytes (fertilisation rate: 55.4%). In 22 treatments, none of the fertilised oocytes developed to the blastocyst stage (6.8%). In cases with more than 12 fertilised oocytes, a total of 119 zygotes were stored frozen. A total of 942 embryos developed to the blastocyst stage (33.6%). In 415 treatment cycles, all embryos were stored frozen without transfer to the uterus (freeze all: 35.1%), mostly because more than 14 oocytes were harvested. During the observation period, four cases with OHSS were observed (0.97%), all after transfer of an embryo in the stimulated treatment cycle supplemented with hCG. Two severe cases needed to be hospitalised, but recovered without late complications.
Table 1 Characteristics of the 413 couples during the cycle for oocyte harvest.
|
Characteristic
|
|
|
Mean value (SD)
|
Range
|
| Female age (years) |
35.8 (3.9) |
25–45 |
|
<35 years, n (%) |
164 (39.7%) |
|
|
| 35–37 years, n (%) |
102 (24.7%) |
|
|
| 38– 39years, n (%) |
63 (15.2%) |
|
|
| ≥40 years, n (%) |
30 (20.3%) |
|
|
| Female BMI (kg/m2) |
23.9 (4.5) |
17.1–42.8 |
| Antral follicle count (n) |
18.3 (10.6) |
2–73 |
| AMH (pmol/l) |
18.6 (16.8) |
0.3.–181 |
| Basal FSH (IU/l) |
7.9 (2.5) |
3.1–28.2 |
| Male age (years) |
38.6 (6.2) |
24–67 |
| Male BMI (kg/m2) |
26.2 (4.0) |
14.0–50.2 |
| FSH (IU/l) |
6.7 (6.4) |
0.2–53.5 |
| Testosterone (nmol/l) |
16.1 (5.7) |
3.9–50.3 |
|
Normozoospermia, n (%) |
183 (44.3%) |
|
|
| Abnormal semen quality, n (%) |
187 (45.3%) |
|
|
| Azoospermia, n (%) |
28 (6.8%) |
|
|
| Cryptozoospermia, n (%) |
15 (3.6%) |
|
|
| Duration of infertility (months) |
48.5 (38.0) |
3–331 |
| AMH at stimulation start (pmol/l) |
14.5 (12.5) |
0.8–98.1 |
| FSH administered (IU) |
3776 (1527) |
1025–7800 |
| Oestradiol level at trigger (pmol/l) |
8453 (4453) |
1168–37532 |
| Progesterone level at trigger (nmol/l) |
2.7 (1.3) |
0.2–8.2 |
| Oocytes harvested per case (no.) |
12.2 (7.6) |
1–48 |
| Oocytes fertilised per case (no.) |
6.8 (4.9) |
0–33 |
| Blastocysts (n) |
3.1 (2.2) |
0–11 |
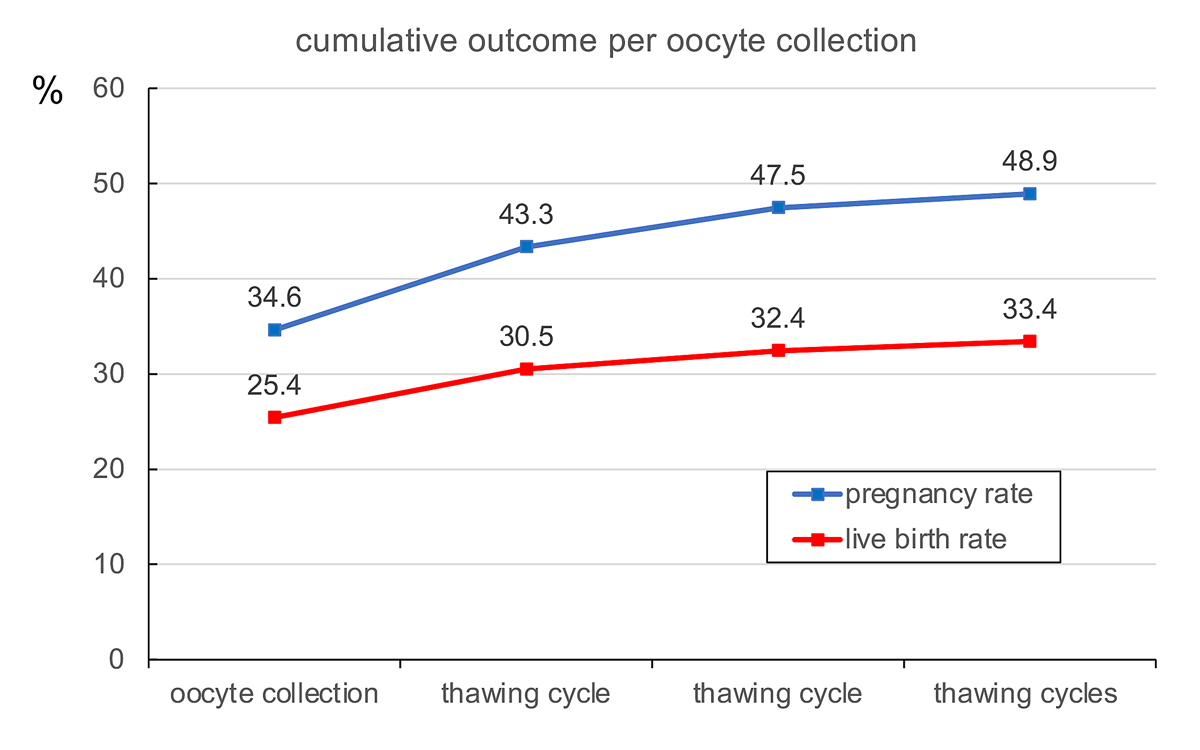
A total of 202 pregnancies resulted from the transfer of 652 single embryos (cumulative pregnancy rate per oocyte collection: 48.7%, fig. 2). Sixty pregnancies ended in miscarriage (29.7%), four were ectopic pregnancies (2.0%); 138 pregnancies remained intact (68.3%). All were singleton pregnancies. Three children died postnatally (one trisomy 18, one owing to a hypertrophic cardiomyopathy of unknown origin and one owing to multiple organ malformations, including congenital absence of the biliary ducts). Exactly 50% of the children were boys and 50% girls.
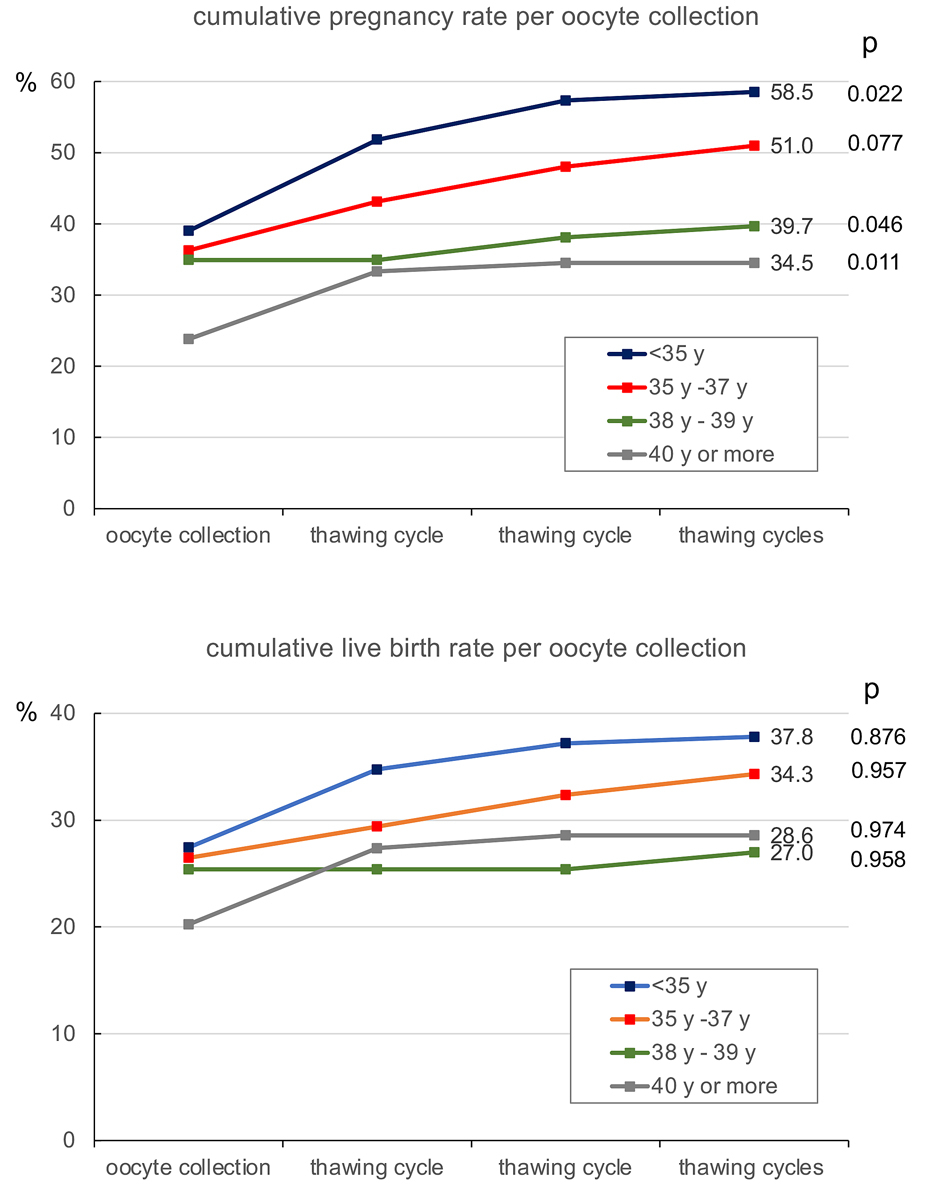
The cumulative pregnancy and live-birth rates per oocyte collection are depicted in fig. 1. During the observation period (2018 and 2019) both the pregnancy and the live-birth rates continued to rise with each subsequent transfer of one embryo per treatment. As female age is the most significant factor in determining the outcome of ART, both the cumulative pregnancy and live-birth rates were calculated according to the age of the treated patients at the moment of oocyte collection (fig. 3). The age categories were taken from Malizia and coworkers [22]. Both the cumulative pregnancy and live-birth rates correlated proportionally with the age of the patients.
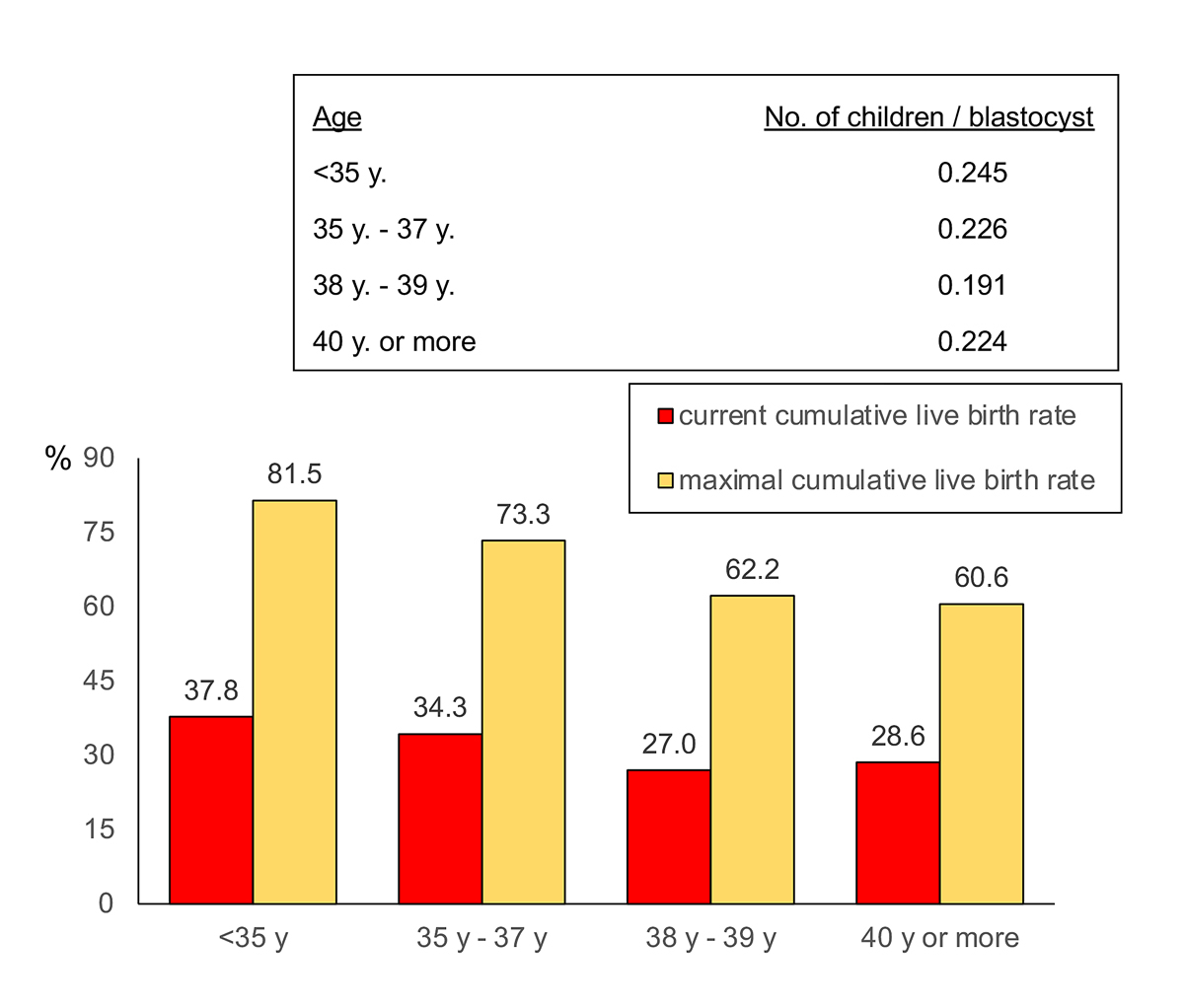
The maximum expected cumulative live-birth rate resulting from the thawing and transfer of all still remaining cryopreserved blastocysts is given in fig. 4 and exceeded 60% in all age groups.
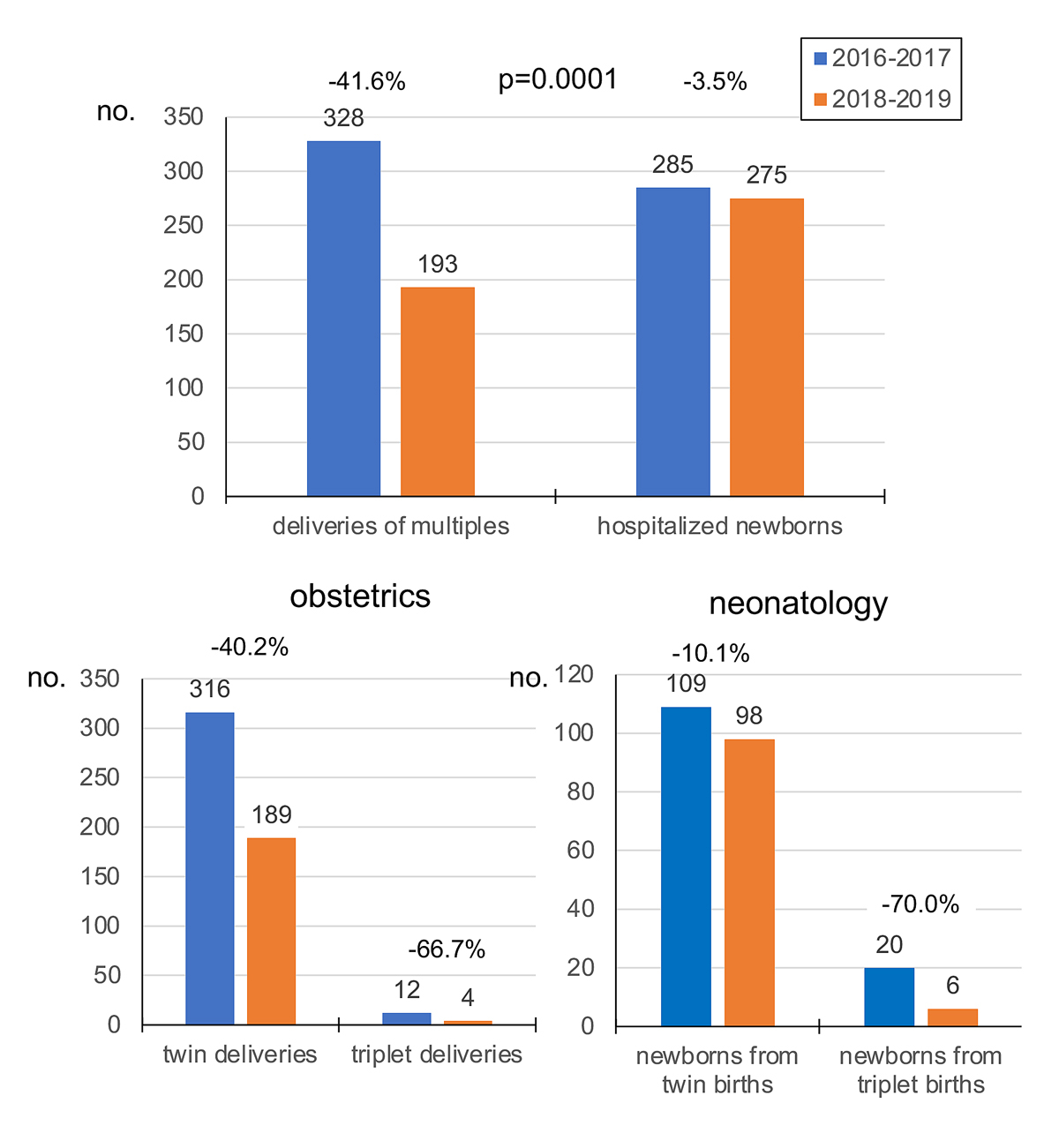
The impact of universal single embryo transfer in all couples in our institution on the number of multiple deliveries in the department of obstetrics and on the intensive care unit of the department of neonatology of the University Children’s Hospital (UKBB) was examined as well (fig. 5). The number of twin deliveries and the number of newborn children from multiple births who had to be hospitalised in the department of neonatology decreased significantly between 2016–2017 and 2018–2019 (p = 0.0001). In detail, the overall number of deliveries of twins and triplets, respectively, decreased from 316 in 2016–2017 to 189 in 2018–2019 (−40.2%) and from 12 to 4 (−66.7%) (p = 0.311). The number of newborns from twin pregnancies decreased from 109 in 2016–2017 to 98 in 2018–2019 (−10.1%) and the number of newborns from triplet pregnancies dropped from 20 in 2016–2017 to 6 in 2018–2019 (−70.0%).
Discussion
ART has become a globally used treatment of infertility [23]. Up to 2016 at least 1,816,760 children were born after ART in Europe [24]. The current efficacy of ART largely depends on access to a set of multiple oocytes, which can be subjected to fertilisation and from which a number of embryos may develop (fig. 1). Retrospective analysis of large-scale databases has demonstrated that, ideally, between 10 and 15 oocytes are needed to achieve the best success rate with few complications [18, 25]. In addition, the low implantation rate per embryo has traditionally been compensated by the transfer of more than one embryo per treatment trial, especially in older patients. This practice has resulted in an epidemic rise in the number of multiple deliveries [26]. Multiple deliveries are burdened with complications such as prematurity, discordant fetal birth weights and placentation abnormalities [27, 28]. The risk of these complications and their frequent long-lasting sequelae in both the mother and her infants are often underestimated or not taken into consideration. For financial reasons, for reasons of public awareness and to reduce workload both the infertile patients and the involved physicians and embryologists perceive the pressure to achieve pregnancy as fast as possible. The professional dissociation between the short-term outcome results in reproductive medicine (pregnancy rate, live birth rate) on the one hand, and much later obstetric and neonatal outcomes (multiple delivery, long-term health of the child) on the other, renders it difficult to convince all stakeholders to limit the number of embryos transferred per treatment cycle. Although the revised Swiss law on assisted reproductive technology now allows the cryopreservation of up to 12 embryos per treatment cycle, the principle of transfer of only one embryo per treatment trial in all infertile couples (regardless their age) is still far from being accepted by all stakeholders.
Numerous studies have demonstrated the beneficial effect of the transfer of only one embryo on the likelihood of pregnancy and live birth, and its impact on nation-wide recorded incidences of multiple delivery [11–13]. The feasibility of this approach in older patients has also been demonstrated repeatedly [29, 30]. The analysis of register data on ART of the United States, of Australia and New Zealand and of Europe demonstrate the increased use of single embryo transfer and the concomitant decrease in the number of multiple deliveries, including twins [15]. After the instalment of the revised law on ART in Switzerland in September 2017, we decided to embark on universal eSET in all patients treated with ART in our institution. This decision was motivated by the urge to avoid all complications in ART at the benefit not only of the infertile couples themselves, but also of the children. This decision was heavily discussed among the various professional groups within the team. Embryologists have the tendency to overestimate morphological assessment of embryos and both clinicians and nurses are inclined to fulfil all wishes of their patients, but finally the principle of eSET was accepted by all. Controversial discussions are occasionally ongoing with some infertile couples, who wish to maximise their chances through the transfer of more than one embryo. Concerns about the future wellbeing of the children resulting from eSET is accepted by all informed couples. During the observation period 2018–2019, all patients were universally treated with eSET and no multiple pregnancies and births occurred.
In addition to universal eSET in all couples, the “freeze all” strategy was applied to 35.1% of all treatments, mainly to avoid OHSS. Recent data from a multicentre study demonstrated that the pregnancy and live-birth rates are not reduced by this option [31]. Despite this precaution, four cases with OHSS were observed after embryo transfer in the stimulated cycle as a result of luteal phase supplementation with low doses of hCG.
During the observation interval 2018 to 2019, a cumulative pregnancy rate of 48.9% and a live birth rate of 33.4% per oocyte collection were achieved (fig. 1). To our knowledge, only one other study has provided cumulative live-birth rates after a single complete ART cycle (including transfer of all blastocysts) [32]: in this study the live-birth rate after one complete cycle varied between 63.8% in women under 31 years to 4.7% in women over 40 years of age. We calculated that, if all remaining blastocysts are thawed and transferred, the maximum cumulative live birth rate of one complete treatment would exceed 60% in all age groups (fig. 3). This hypothetical outcome implies, however, that the developmental potential of all blastocysts, including those already thawed and transferred, is similar. The cumulative likelihood of successful treatment of up to three complete treatment cycles with ART has demonstrated live birth rates as high as 80% [33].
The mean age of the treated patients in this cohort (35.8 years) may contribute to high miscarriage rate in the present cohort (29.7%), together with ovarian insufficiency [34], which was diagnosed in many infertile patients in this cohort (table 1). Two prospective randomised clinical trials have demonstrated that preimplantation genetic testing of the chromosome complement (PGT-A) of the trophectoderm of blastocyst embryos has the potential to identify embryos with a higher implantation potential and to avoid miscarriages caused by aneuploidy [35, 36]. These findings, however, have not been confirmed by all [37]. The option of PGT-A was not included in this retrospective cohort analysis.
ART is a costly treatment and the infertile couples have to carry the entire financial burden of the treatment. Financial restrictions push many patients to wait a long time until they embark on their first ART trial. Along with Albania, Armenia and Georgia, Switzerland offers no financial support whatsoever to the infertile couples undergoing ART [38]. The decision of the Federal Office of Public Health (BAG, 1 July 2019) to fund fertility protection in individuals diagnosed with malignant disease provides some hope for relief. Funding of ART will not only allow more infertile couples to opt for treatment, but should be linked to universal single embryo transfer in all couples and to a more stringent “freeze all” to avoid OHSS. Previous health economic studies have clearly demonstrated that through prevention of multiple births the medical costs of ART and its complications can be reduced to such an extent that ART becomes money sparing [39–41], especially if more than one child is to be born at different time points after one ART attempt [42]. In addition, overuse of ART can be avoided through the introduction of stringent entrance criteria [43, 44], which may be controlled systematically through the obligatory cantonal physician supervision visits already in place.
The present data demonstrate that iatrogenic multiple deliveries can be avoided almost completely through the adoption of eSET in all couples undergoing ART. This can only be achieved through a collaborative and convincing effort of all team members and should be facilitated through reimbursement of treatment costs in the near future.
Acknowledgements
The support and acceptance of universal eSET by all members of the staff of RME is highly acknowledged (M. Fischer, S. Dinkel, S. Stallkamp). I am grateful to Prof. Irene Hoesli for providing me the multiple delivery rates in the Department of Obstetrics of the Women’s Hospital of the University and to Prof. Sven Schulzke for providing the data on neonate care resulting from multiple births in the Children’s Hospital of the University.
References
1
Zegers-Hochschild
F
Adamson
GD
de Mouzon
J
Ishihara
O
Mansour
R
Nygren
K
International Committee for Monitoring Assisted Reproductive Technology
World Health Organization
. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod. 2009;24(11):2683–7. doi:.https://doi.org/10.1093/humrep/dep343
2
Zegers-Hochschild
F
Adamson
GD
de Mouzon
J
Ishihara
O
Mansour
R
Nygren
K
International Committee for Monitoring Assisted Reproductive Technology
World Health Organization
. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–4. doi:.https://doi.org/10.1016/j.fertnstert.2009.09.009
3
Pinborg
A
Loft
A
Rasmussen
S
Nyboe Andersen
A
. Hospital care utilization of IVF/ICSI twins followed until 2-7 years of age: a controlled Danish national cohort study. Hum Reprod. 2004;19(11):2529–36. doi:.https://doi.org/10.1093/humrep/deh474
4
Levin
S
Sheiner
E
Wainstock
T
Walfisch
A
Segal
I
Landau
D
Infertility Treatments and Long-Term Neurologic Morbidity of the Offspring. Am J Perinatol. 2019;36(9):949–54. doi:.https://doi.org/10.1055/s-0038-1675159
5
Rodriguez-Wallberg
KA
Lundberg
FE
Ekberg
S
Johansson
ALV
Ludvigsson
JF
Almqvist
C
Mortality from infancy to adolescence in singleton children conceived from assisted reproductive techniques versus naturally conceived singletons in Sweden. Fertil Steril. 2020;113(3):524–32. doi:.https://doi.org/10.1016/j.fertnstert.2019.10.018
6
Toftager
M
Bogstad
J
Bryndorf
T
Løssl
K
Roskær
J
Holland
T
Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31(6):1253–64. doi:.https://doi.org/10.1093/humrep/dew051
7
Malchau
SS
Henningsen
AA
Forman
J
Loft
A
Nyboe Andersen
A
Pinborg
A
. Cumulative live birth rate prognosis based on the number of aspirated oocytes in previous ART cycles. Hum Reprod. 2019;34(1):171–80. doi:.https://doi.org/10.1093/humrep/dey341
8
Pfeifer
S
Butts
S
Dumesic
D
Fossum
G
Gracia
C
La Barbera
A
Practice Committee of the American Society for Reproductive Medicine
. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–47. doi:.https://doi.org/10.1016/j.fertnstert.2016.08.048
9
Humaidan
P
Polyzos
NP
Alsbjerg
B
Erb
K
Mikkelsen
AL
Elbaek
HO
GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod. 2013;28(9):2511–21. doi:.https://doi.org/10.1093/humrep/det249
10
Stormlund
S
Sopa
N
Zedeler
A
Bogstad
J
Prætorius
L
Nielsen
HS
Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ. 2020;370:m2519. doi:.https://doi.org/10.1136/bmj.m2519
11
Tiitinen
A
Halttunen
M
Härkki
P
Vuoristo
P
Hyden-Granskog
C
. Elective single embryo transfer: the value of cryopreservation. Hum Reprod. 2001;16(6):1140–4. doi:.https://doi.org/10.1093/humrep/16.6.1140
12
Gerris
J
De Neubourg
D
Mangelschots
K
Van Royen
E
Vercruyssen
M
Barudy-Vasquez
J
Elective single day 3 embryo transfer halves the twinning rate without decrease in the ongoing pregnancy rate of an IVF/ICSI programme. Hum Reprod. 2002;17(10):2626–31. doi:.https://doi.org/10.1093/humrep/17.10.2626
13
Karlström
PO
Bergh
C
. Reducing the number of embryos transferred in Sweden-impact on delivery and multiple birth rates. Hum Reprod. 2007;22(8):2202–7. doi:.https://doi.org/10.1093/humrep/dem120
14
Tiitinen
A
Unkila-Kallio
L
Halttunen
M
Hyden-Granskog
C
. Impact of elective single embryo transfer on the twin pregnancy rate. Hum Reprod. 2003;18(7):1449–53. doi:.https://doi.org/10.1093/humrep/deg301
15
De Geyter
Ch
Wyns
C
Calhaz-Jorge
C
de Mouzon
J
Ferraretti
AP
Kupka
M
20 years of the European IVF monitoring (EIM) registry: what have we learned? A comparison with registries from two other regions. Hum Reprod. 2020;35(12):2832–49. doi:.https://doi.org/10.1093/humrep/deaa250
16
Van den Bergh
M
Hohl
MK
De Geyter
Ch
Stalberg
AM
Limoni
C
. Ten years of Swiss National IVF Register FIVNAT-CH. Are we making progress?
Reprod Biomed Online. 2005;11(5):632–40. doi:.https://doi.org/10.1016/S1472-6483(10)61173-X
17
Fehr
P
Nygren
KG
De Geyter
C
. Effekt unterschiedlicher Strategien beim Embryotransfer auf die Ergebnisse der assistierten Reproduktion
[Effect of different embryo transfer strategies on the outcome of assisted reproduction].
Ther Umsch. 2009;66(12):825–9. doi:.https://doi.org/10.1024/0040-5930.66.12.825
18
De Geyter
C
Fehr
P
Moffat
R
Gruber
IM
von Wolff
M
. Twenty years’ experience with the Swiss data registry for assisted reproductive medicine: outcomes, key trends and recommendations for improved practice. Swiss Med Wkly. 2015;145:w14087.
19Bundesgesetz über die medizinisch unterstützte Fortpflanzung (Fortpflanzungsmedizingesetz, FMedG).
20
Santos-Ribeiro
S
Siffain
J
Polyzos
NP
van de Vijver
A
van Landuyt
L
Stoop
D
To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt?
Fertil Steril. 2016;105(5):1202–1207.e1. doi:.https://doi.org/10.1016/j.fertnstert.2015.12.140
21
Cooper
TG
Noonan
E
von Eckardstein
S
Auger
J
Baker
HW
Behre
HM
World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45. doi:.https://doi.org/10.1093/humupd/dmp048
22
Malizia
BA
Hacker
MR
Penzias
AS
. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236–43. doi:.https://doi.org/10.1056/NEJMoa0803072
23
de Mouzon
J
Chambers
GM
Zegers-Hochschild
F
Mansour
R
Ishihara
O
Banker
M
International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2012†. Hum Reprod. 2020;35(8):1900–13. doi:.https://doi.org/10.1093/humrep/deaa090
24
Wyns
C
Bergh
C
Calhaz-Jorge
C
De Geyter
C
Kupka
MS
Motrenko
T
European IVF-monitoring Consortium (EIM)‡ for the European Society of Human Reproduction and Embryology (ESHRE)
. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open. 2020;2020(3):hoaa032. doi:.https://doi.org/10.1093/hropen/hoaa032
25
Sunkara
SK
Rittenberg
V
Raine-Fenning
N
Bhattacharya
S
Zamora
J
Coomarasamy
A
. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–74. doi:.https://doi.org/10.1093/humrep/der106
26
Arlettaz Mieth
R
Ersfeld
S
Douchet
N
Wellmann
S
Bucher
HU
. Higher multiple births in Switzerland: neonatal outcome and evolution over the last 20 years. Swiss Med Wkly. 2011;141:w13308. doi:.https://doi.org/10.4414/smw.2011.13308
27
Källén
B
. Maternal morbidity and mortality in in-vitro fertilization. Best Pract Res Clin Obstet Gynaecol. 2008;22(3):549–58. doi:.https://doi.org/10.1016/j.bpobgyn.2008.02.001
28
Magnusson
Å
Wennerholm
UB
Källén
K
Petzold
M
Thurin-Kjellberg
A
Bergh
C
. The association between the number of oocytes retrieved for IVF, perinatal outcome and obstetric complications. Hum Reprod. 2018;33(10):1939–47. doi:.https://doi.org/10.1093/humrep/dey266
29
Veleva
Z
Vilska
S
Hydén-Granskog
C
Tiitinen
A
Tapanainen
JS
Martikainen
H
. Elective single embryo transfer in women aged 36-39 years. Hum Reprod. 2006;21(8):2098–102. doi:.https://doi.org/10.1093/humrep/del137
30
Niinimäki
M
Suikkari
AM
Mäkinen
S
Söderström-Anttila
V
Martikainen
H
. Elective single-embryo transfer in women aged 40-44 years. Hum Reprod. 2013;28(2):331–5. doi:.https://doi.org/10.1093/humrep/des399
31
Stormlund
S
Sopa
N
Zedeler
A
Bogstad
J
Prætorius
L
Nielsen
HS
Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ. 2020;370:m2519. doi:.https://doi.org/10.1136/bmj.m2519
32
Zhu
Q
Chen
Q
Wang
L
Lu
X
Lyu
Q
Wang
Y
Live birth rates in the first complete IVF cycle among 20 687 women using a freeze-all strategy. Hum Reprod. 2018;33(5):924–9. doi:.https://doi.org/10.1093/humrep/dey044
33
Leijdekkers
JA
Eijkemans
MJC
van Tilborg
TC
Oudshoorn
SC
McLernon
DJ
Bhattacharya
S
OPTIMIST group
. Predicting the cumulative chance of live birth over multiple complete cycles of in vitro fertilization: an external validation study. Hum Reprod. 2018;33(9):1684–95. doi:.https://doi.org/10.1093/humrep/dey263
34
Sunkara
SK
Khalaf
Y
Maheshwari
A
Seed
P
Coomarasamy
A
. Association between response to ovarian stimulation and miscarriage following IVF: an analysis of 124 351 IVF pregnancies. Hum Reprod. 2014;29(6):1218–24. doi:.https://doi.org/10.1093/humrep/deu053
35
Scott
RT
Jr
Upham
KM
Forman
EJ
Hong
KH
Scott
KL
Taylor
D
Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. doi:.https://doi.org/10.1016/j.fertnstert.2013.04.035
36
Tiegs
AW
Tao
X
Zhan
Y
Whitehead
C
Kim
J
Hanson
B
A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115(3):627–37. doi:.https://doi.org/10.1016/j.fertnstert.2020.07.052
37
Munné
S
Kaplan
B
Frattarelli
JL
Child
T
Nakhuda
G
Shamma
FN
STAR Study Group
. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–1079.e7. doi:.https://doi.org/10.1016/j.fertnstert.2019.07.1346
38
Calhaz-Jorge
C
De Geyter
CH
Kupka
MS
Wyns
C
Mocanu
E
Motrenko
T
Survey on ART and IUI: legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod Open. 2020;2020(1):hoz044. doi:.https://doi.org/10.1093/hropen/hoz044
39
Fiddelers
AA
van Montfoort
AP
Dirksen
CD
Dumoulin
JC
Land
JA
Dunselman
GA
Single versus double embryo transfer: cost-effectiveness analysis alongside a randomized clinical trial. Hum Reprod. 2006;21(8):2090–7. doi:.https://doi.org/10.1093/humrep/del112
40
Dixon
S
Faghih Nasiri
F
Ledger
WL
Lenton
EA
Duenas
A
Sutcliffe
P
Cost-effectiveness analysis of different embryo transfer strategies in England. BJOG. 2008;115(6):758–66. doi:.https://doi.org/10.1111/j.1471-0528.2008.01667.x
41
Connolly
MP
Ledger
W
Postma
MJ
. Economics of assisted reproduction: access to fertility treatments and valuing live births in economic terms. Hum Fertil (Camb). 2010;13(1):13–8. doi:.https://doi.org/10.3109/14647270903401747
42
van Heesch
MM
van Asselt
AD
Evers
JL
van der Hoeven
MA
Dumoulin
JC
van Beijsterveldt
CE
Cost-effectiveness of embryo transfer strategies: a decision analytic model using long-term costs and consequences of singletons and multiples born as a consequence of IVF. Hum Reprod. 2016;31(11):2527–40. doi:.https://doi.org/10.1093/humrep/dew229
43
Hunault
CC
Habbema
JD
Eijkemans
MJ
Collins
JA
Evers
JL
te Velde
ER
. Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Hum Reprod. 2004;19(9):2019–26. doi:.https://doi.org/10.1093/humrep/deh365
44
Bensdorp
AJ
van der Steeg
JW
Steures
P
Habbema
JDF
Hompes
PGA
Bossuyt
PMM
CECERM study group
. A revised prediction model for natural conception. Reprod Biomed Online. 2017;34(6):619–26. doi:.https://doi.org/10.1016/j.rbmo.2017.03.014