Impact of the new TNM Staging System (8th edition) on oral tongue cancers
DOI: https://doi.org/10.4414/smw.2021.20493
Yanic
Ammannab, Ulrich
Beutnerb, Domenic G.
Vitalc, Grégoire
Morandc, Martina A.
Broglie Daeppenc, Diana
Bornd, Sandro J.
Stoecklia, Gerhard F.
Hubera
a Klinik für Hals-Nasen-Ohrenheilkunde, Kantonsspital St. Gallen, Switzerland
b Klinik für Chirurgie, Kantonsspital St. Gallen, Switzerland
c Klinik für Ohren-, Nasen und Halschirurgie, Universitätsspital Zürich, Switzerland
d Klinik für Pathologie, Kantonsspital St. Gallen, Switzerland
Summary
AIM OF THE STUDY
For tumours of the oral tongue, the most recent 8th edition of the AJCC/UICC staging system has introduced depth of infiltration (DOI) as a novel parameter. With this study we wanted to investigate its impact regarding this risk stratification compared with the preceding 7th edition.
METHODS
Between 2008 and 2017, 161 patients of two tertiary referral centres in Switzerland (Kantonsspital St. Gallen and University Hospital Zurich) with T1 N0 or T2 N0 tongue cancers were enrolled in this study. The primary tumours were restaged according to the 8th edition of the TNM classification. Kaplan-Meier curves for overall and disease-specific survival were calculated.
RESULTS
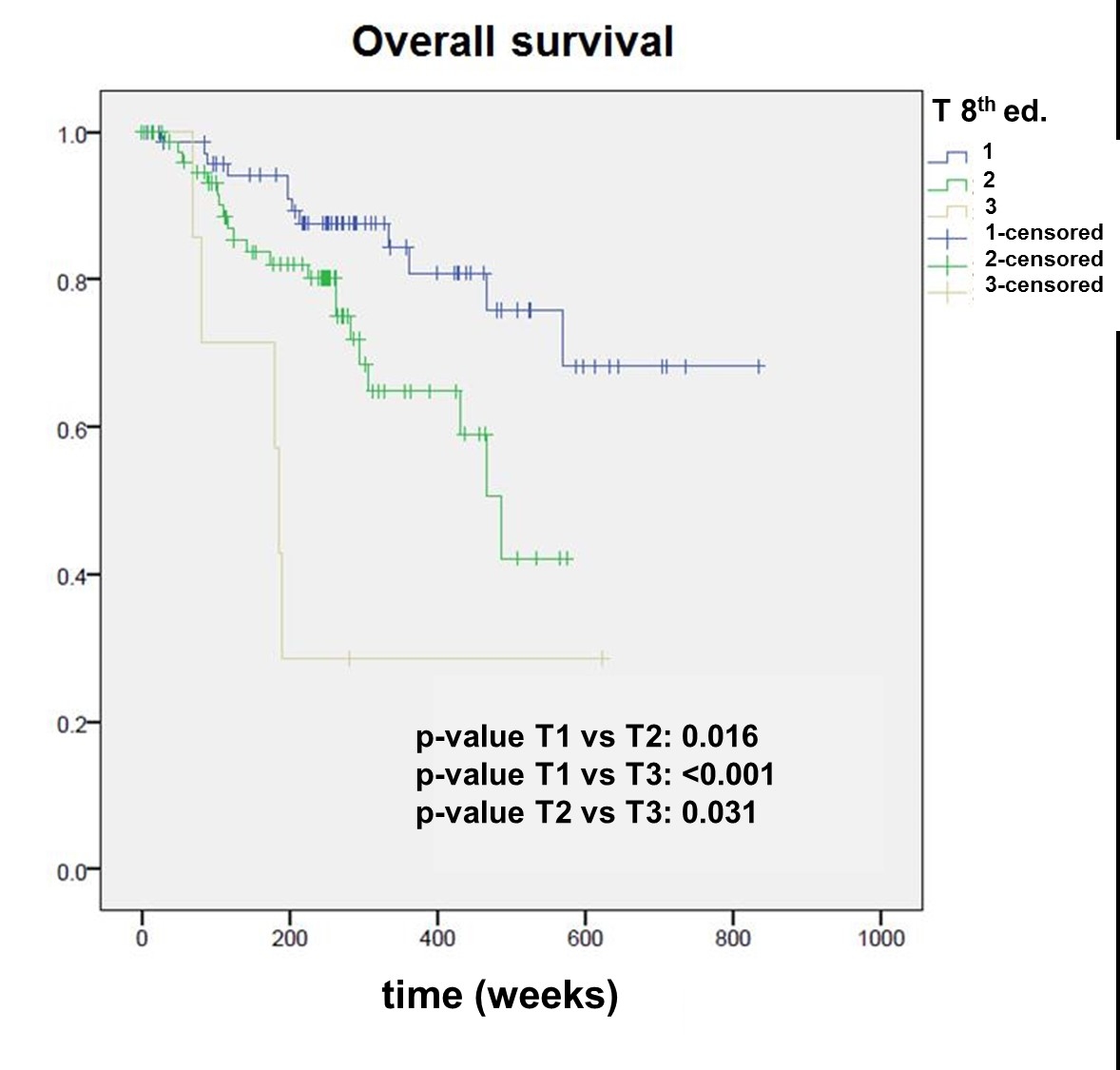
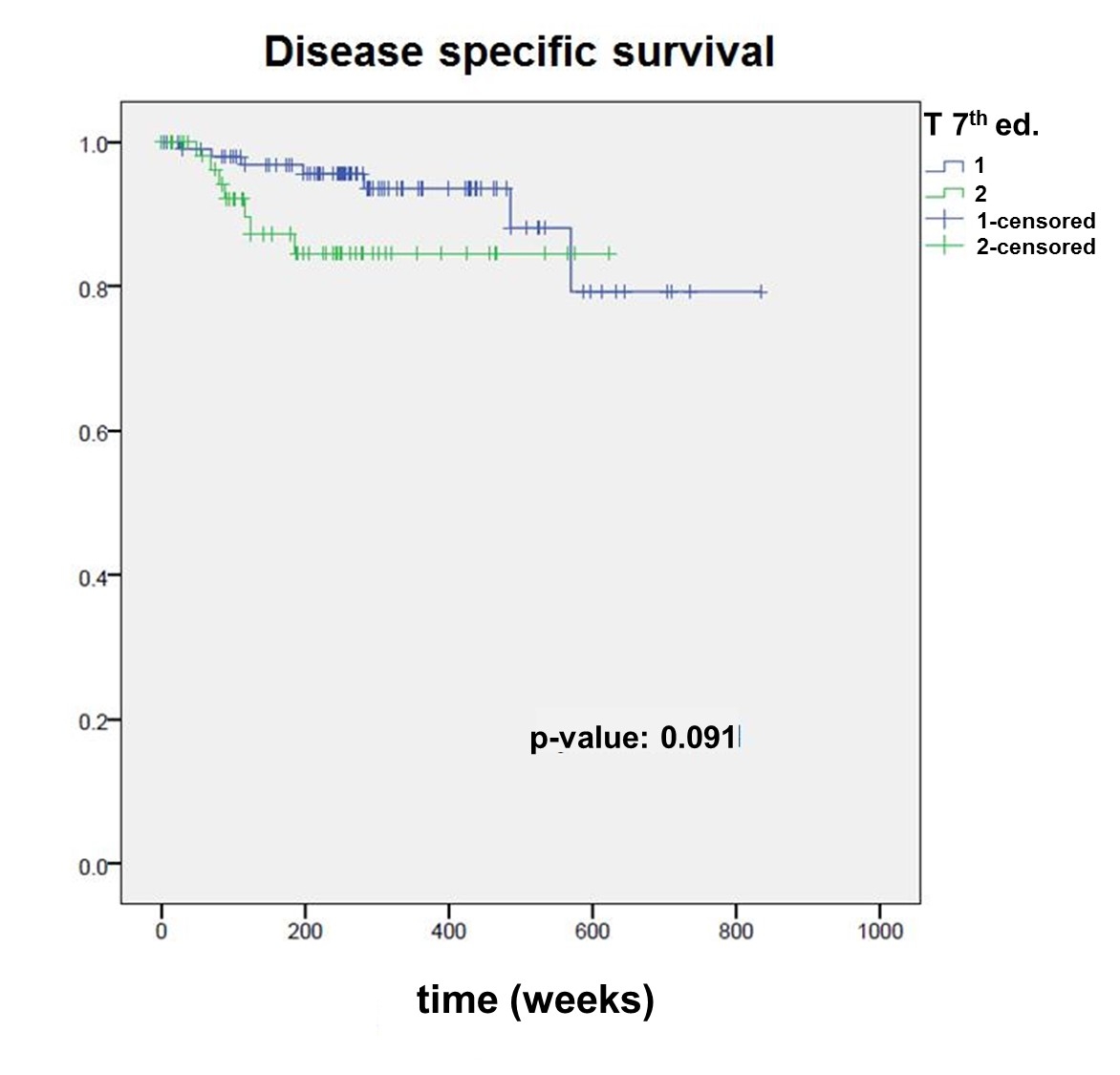
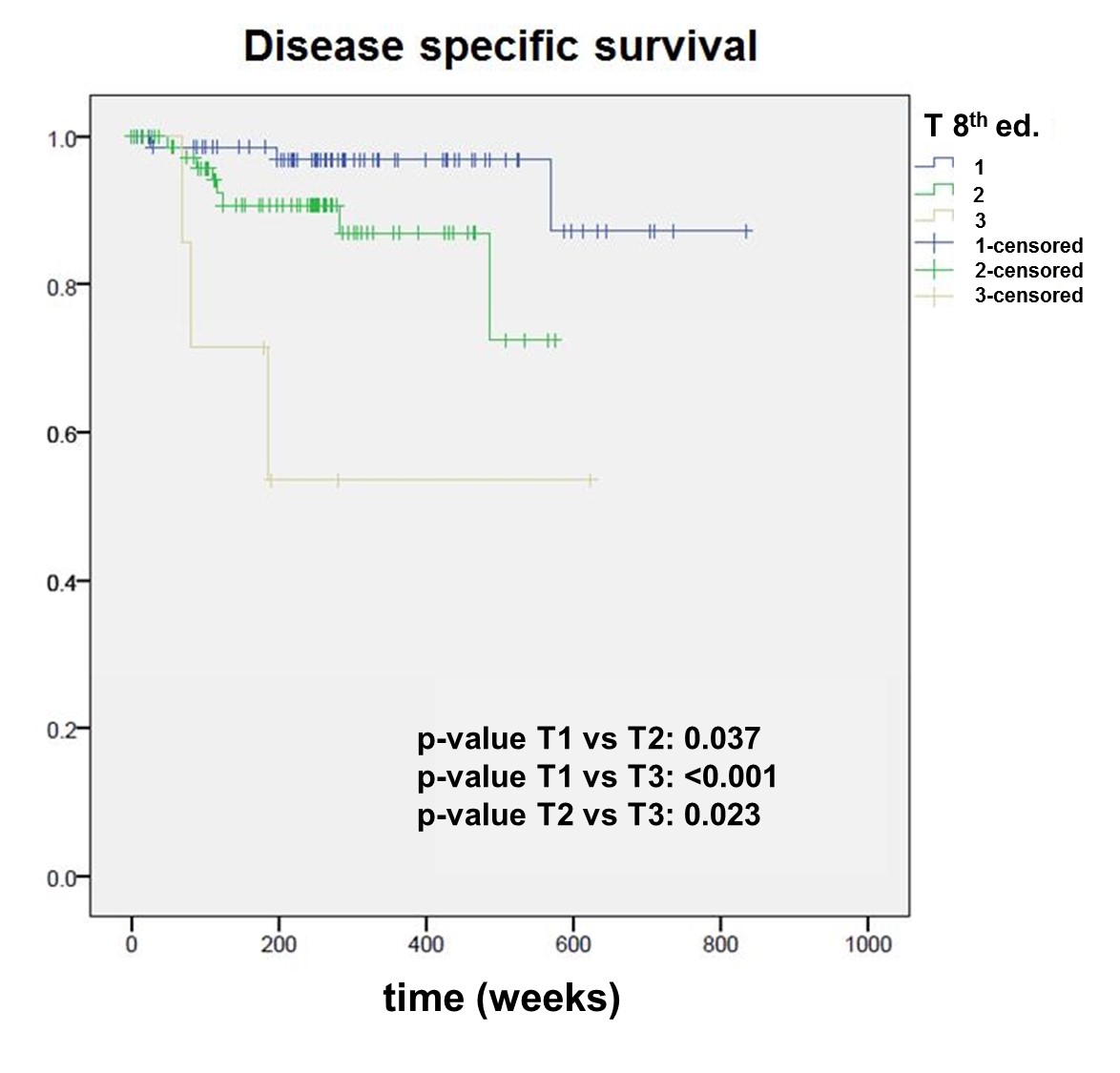
According to the 7th edition, of the 161 patients, 102 were staged after surgery as pT1 (stage I) and 59 as pT2 (stage II). According to the 8th edition, 36 patients (22.4%) were re-staged to a higher stage. Of these 36 patients, 8 (22.2%) experienced a recurrence, and 9 (25%) died. In the remaining, not re-staged group, 20 patients (16.0%) experienced a recurrence (p = 0.55) and 14 (11.2%) died (p = 0.025*). The 7th edition showed a statistically significant difference between pT1 and pT2 tumours for overall survival (p = 0.025), but not for disease-specific survival (p = 0.091), whereas the 8th edition was able to well discriminate between pT1, pT2 and pT3 for both overall (pT1 vs pT2, p = 0.016*; pT2 vs pT3, p = 0.031*) and disease-specific survival (pT1 vs pT2, p = 0.037*; pT2 vs pT3, p = 0.023*).
CONCLUSION
The recent TNM 8th edition provides a more accurate prediction of overall and disease-specific survival for this subgroup of patients. Hence, a more aggressive treatment should be considered for patients re-staged to pT3 due to depth of infiltration.
Introduction
The Union for International Cancer Control (UICC) / American Joint Committee on Cancer (AJCC) TNM classification of malignant tumours is a globally recognised standard categorising tumours with respect to local extension (T), regional (N) and distant metastases (M) [1]. The aim is to stratify tumours of a distinct location into cohorts with comparable prognosis, potentially allowing tailored treatment. With regard to tumours of the oral tongue, the most recent 8th edition has introduced depth of infiltration (DOI) as a novel parameter in local extension [2]. In the preceding editions only superficial extension of the primary tumour and infiltration of surrounding structures were considered for pT classification. Nevertheless, it has been known for years that DOI was a strong prognosticator for the risk of lymph node metastasis, locoregional recurrence and survival [3]. However, this had not been implemented until recently in the TNM system, most likely due to the different thresholds of DOI reported in different studies [3–5]. The goal of including DOI was to better discriminate prognostic groups with regard to risk of recurrence and survival, in particular in early oral cancer.
The relevant differences between the 7th and 8th edition of the UICC/AJCC staging system, the meaning of true DOI and the enhanced discrimination of T-stage in the survival of patients with tongue tumours with use of the recent 8th edition are summarised in figure 1.

Figure 1 The relevant differences between the 7th and 8th editions of the UICC/AJCC staging system, the meaning of true depth of infiltration (DOI) and the enhanced discrimination of T-stage in the survival of tongue tumours by the new edition are visually summarised.
The aim of this study was to compare the prognostic accuracy of the 8th and the 7th editions of the TNM classification in a highly homogeneous cohort of patients treated for early cancer of the oral tongue.
Material and methods
Between 2008 and 2017, a total of 161 patients were prospectively enrolled in the sentinel node biopsy protocol at two tertiary referral centres in Switzerland (Kantonsspital St. Gallen and University Hospital Zurich). For the present study the tumours were retrospectively restaged according to the 8th edition of the TNM classification. The study was approved by the local ethics Board (KEK-ZH: 2016-01799). The inclusion criteria were histologically confirmed squamous cell carcinoma of the oral tongue staged cT1 or T2 (7th edition of the TNM system) without clinical or imaging (ultrasound, computed tomography or magnetic resonance imaging) evidence of metastatic disease to the neck (cN0). The primary treatment consisted of panendoscopy, transoral partial glossectomy and sentinel node biopsy, according to a previously published protocol [6]. In the case of occult metastatic disease detected by sentinel node biopsy, comprehensive neck dissection was performed as a staged procedure. Radio-chemotherapy was not given. Patients with second primary tumours or previous treatment for a head and neck cancer were excluded from the study. Patients were routinely followed up for 5 years and their disease status documented. The minimum follow-up period was 2 years.
All patients were prospectively staged according to the 7th edition of the TNM system and retrospectively re-staged according to the 8th edition. Kaplan-Meier curves for overall survival and disease-specific survival were calculated. Stratification into T-categories was compared between the two editions by the means of a log-rank test. For statistical analysis SPSS was used. The statistical analysis was performed by a statistician (UB).
Results
The study cohort consisted of 103 males and 58 females with a median age at diagnosis of 59 years (range 28–90).
According to the 7th edition, out of the 161 patients, 102 were staged after surgery as pT1 (stage I) and 59 as pT2 (stage II). According to the 8th edition, 36 patients (22.4%) were assigned a higher stage, 29 patients (18.1%) from pT1 to pT2 and 7 (4.3%) from pT2 to pT3. In this group, eight patients (22.2%) experienced a recurrence and nine (25%) died. In the remaining, not re-staged group of 125 patients (77.6%), 20 (16.0%) experienced a recurrence (p = 0.55) and 14 (11.2%) died (p = 0.025).
The corresponding numbers of patients (regarding sentinel node status, overall and disease-specific survival) staged according to either the 7th or the 8th edition of the TNM system are shown in table 1.
Table 1 Numbers of patients (regarding sentinel node status, overall and disease specific survival) staged according to either the 7th or the 8th edition of the TNM system.
|
pT stage
|
SNB+
n (%)
|
OS
|
DSS
|
| 7th edition |
T1 (n = 102) |
29 (28.4%) |
79.4% |
93.1% |
| T2 (n = 59) |
22 (37.3%) |
71.2% |
88.1% |
| 8th edition |
T1 (n = 73) |
18 (24.7%) |
83.6% |
95.9% |
| T2 (n = 81) |
29 (35.8%) |
74.1% |
90.1% |
| T3 (n = 7) |
4 (57.1%) |
28.6% |
57.1% |
The Kaplan Meier survival curves stratified by pT category according to both editions are shown in figures 2–5
. The 7th edition reveals a statistically significant difference between pT1 and pT2 tumours for overall survival (p = 0.025), but not for disease-specific survival (p = 0.091). The 8th edition was able to well discriminate between pT1, pT2 and pT3 for both overall (pT1 vs pT2, p = 0.016; pT2 vs pT3, p = 0.031) and disease-specific survival (pT1 vs pT2, p = 0.037; pT2 vs pT3, p = 0.023).
Discussion
Former UICC/AJCC TNM staging systems (e.g., 7th edition) for oral tongue carcinomas were based on the maximum tumour extent and the involvement of the nearby anatomical structures. In the 8th edition, DOI was incorporated into the staging system (and extrinsic muscle involvement excluded as a criterion for staging a tumour pT4).
DOI has been known to be of prognostic value for several years [4]. As a matter of fact, independent of the TNM staging, DOI was included in the treatment decisions by tumour boards all over the world, being known as a predictive factor for occult metastatic disease, higher loco-regional recurrence and worse outcome [7–13]. Therefore most centres decided to treat patients with a “considerable” DOI more aggressively (e.g., elective neck dissection vs sentinel node biopsy, lower threshold for giving adjuvant radiotherapy) – although the threshold varied between different institutions since different studies came up with different conclusions regarding risk profile.
We observed statistically significant differences in the overall and disease-specific survival between pT1 and pT2, and pT2 and pT3 in patients staged according to the 8th edition (figs 2–5
). The importance of DOI as a predictor for nodal metastasis [14] and recurrences [15] is well described in the literature. However, as mentioned before, different cut‐off values have been used to identify the high‐risk groups. In our cohort, the rate of sentinel node biopsy positivity in tumours staged according to UICC/AJCC 8th edition was 24.7%, 35.8% and 57.1% for pT1, pT2 and pT3 tumours, respectively, demonstrating a disproportionately high number in the re-staged pT3 group. Our findings are in line with Mascitti et al. [16] who also found a higher risk for death (25% vs 11.2%, p = 0.04) for individuals assigned a higher grade.
In summary, the 8th edition of the UICC/AJCC criteria seems to allow for improved stratification of patients with tongue cancer, justifying more intense treatment for those patients with higher DOI, especially those being re-staged pT3 because of their DOI.
Conclusion
The inclusion of DOI to the UICC/AJCC 8th staging system assigned a higher stage to a significant number of early oral tongue cancer patients. The newer staging system provides a more accurate prediction of overall and disease-specific survival in this subgroup of patients. A more aggressive treatment, such as elective neck dissection or adjuvant radio-chemotherapy should be considered for patients re-staged as pT3 due to DOI.
References
1
Asare
EA
,
Grubbs
EG
,
Gershenwald
JE
,
Greene
FL
,
Aloia
TA
. Setting the “stage” for Surgical Oncology fellows: Pierre Denoix and TNM staging. J Surg Oncol. 2019;119(7):823–823. doi:.https://doi.org/10.1002/jso.25404
2de Franceschi L, Santo JMMM, de Abreu AM, Kulcsar MAV, Cernea CR, Garcia MRT, et al. Staging of oral cavity cancer in the 8th edition of the TNM classification: the role of computed tomography in the assessment of depth of invasion and extranodal extension. Arch Head Neck Surg [Internet]. 2018;47(1):e0869. [Cited 2020 Oct 23]. Available from: http://archivesheadnecksurgery.com/article/doi/10.4322/ahns.2018.0869
3
Tam
S
,
Amit
M
,
Zafereo
M
,
Bell
D
,
Weber
RS
. Depth of invasion as a predictor of nodal disease and survival in patients with oral tongue squamous cell carcinoma. Head Neck. 2019;41(1):177–84. doi:.https://doi.org/10.1002/hed.25506
4
Shinn
JR
,
Wood
CB
,
Colazo
JM
,
Harrell
FE, Jr
,
Rohde
SL
,
Mannion
K
. Cumulative incidence of neck recurrence with increasing depth of invasion. Oral Oncol. 2018;87:36–42. doi:.https://doi.org/10.1016/j.oraloncology.2018.10.015
5
Morand
GB
,
Ikenberg
K
,
Vital
DG
,
Cardona
I
,
Moch
H
,
Stoeckli
SJ
, et al.
Preoperative assessment of CD44-mediated depth of invasion as predictor of occult metastases in early oral squamous cell carcinoma. Head Neck. 2019;41(4):950–8. doi:.https://doi.org/10.1002/hed.25532
6
Schilling
C
,
Stoeckli
SJ
,
Vigili
MG
,
de Bree
R
,
Lai
SY
,
Alvarez
J
, et al.
Surgical consensus guidelines on sentinel node biopsy (SNB) in patients with oral cancer. Head Neck. 2019;41(8):2655–64. doi:.https://doi.org/10.1002/hed.25739
7
Kano
S
,
Sakashita
T
,
Tsushima
N
,
Mizumachi
T
,
Nakazono
A
,
Suzuki
T
, et al.
Validation of the 8th edition of the AJCC/UICC TNM staging system for tongue squamous cell carcinoma. Int J Clin Oncol. 2018;23(5):844–50. doi:.https://doi.org/10.1007/s10147-018-1276-5
8
Chandler
K
,
Vance
C
,
Budnick
S
,
Muller
S
. Muscle invasion in oral tongue squamous cell carcinoma as a predictor of nodal status and local recurrence: just as effective as depth of invasion?
Head Neck Pathol. 2011;5(4):359–63. doi:.https://doi.org/10.1007/s12105-011-0296-5
9
Shim
SJ
,
Cha
J
,
Koom
WS
,
Kim
GE
,
Lee
CG
,
Choi
EC
, et al.
Clinical outcomes for T1-2N0-1 oral tongue cancer patients underwent surgery with and without postoperative radiotherapy. Radiat Oncol. 2010;5(1):43. doi:.https://doi.org/10.1186/1748-717X-5-43
10
Bonnardot
L
,
Bardet
E
,
Steichen
O
,
Cassagnau
E
,
Piot
B
,
Salam
AP
, et al.
Prognostic factors for T1-T2 squamous cell carcinomas of the mobile tongue: A retrospective cohort study. Head Neck. 2011;33(7):928–34. doi:.https://doi.org/10.1002/hed.21567
11
Almangush
A
,
Bello
IO
,
Keski-Säntti
H
,
Mäkinen
LK
,
Kauppila
JH
,
Pukkila
M
, et al.
Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36(6):811–8. doi:.https://doi.org/10.1002/hed.23380
12
Al-Rajhi
N
,
Khafaga
Y
,
El-Husseiny
J
,
Saleem
M
,
Mourad
W
,
Al-Otieschan
A
, et al.
Early stage carcinoma of oral tongue: prognostic factors for local control and survival. Oral Oncol. 2000;36(6):508–14. doi:.https://doi.org/10.1016/S1368-8375(00)00042-7
13
Ganly
I
,
Patel
S
,
Shah
J
. Early stage squamous cell cancer of the oral tongue--clinicopathologic features affecting outcome. Cancer. 2012;118(1):101–11. doi:.https://doi.org/10.1002/cncr.26229
14
Kane
SV
,
Gupta
M
,
Kakade
AC
,
D’ Cruz
A
. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32(7):795–803. doi:.https://doi.org/10.1016/j.ejso.2006.05.004
15
Keski-Säntti
H
,
Atula
T
,
Tikka
J
,
Hollmén
J
,
Mäkitie
AA
,
Leivo
I
. Predictive value of histopathologic parameters in early squamous cell carcinoma of oral tongue. Oral Oncol. 2007;43(10):1007–13. doi:.https://doi.org/10.1016/j.oraloncology.2006.11.015
16
Mascitti
M
,
Rubini
C
,
De Michele
F
,
Balercia
P
,
Girotto
R
,
Troiano
G
, et al.
American Joint Committee on Cancer staging system 7th edition versus 8th edition: any improvement for patients with squamous cell carcinoma of the tongue?
Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126(5):415–23. doi:.https://doi.org/10.1016/j.oooo.2018.07.052