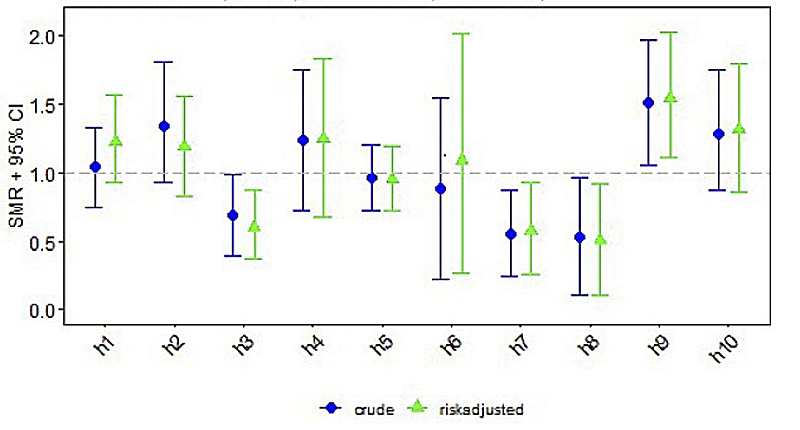
Figure 1 Standardised mortality/morbidity ratio (SMR) chart: seizures (AUC = 0.631).
DOI: https://doi.org/10.4414/smw.2021.20489
hypoxic-ischaemic encephalopathy
neonatal intensive care unit
paediatric intensive care unit
(amplitude-integrated) electroencephalogram
confidence interval
In Switzerland, neonates with hypoxic-ischaemic encephalopathy (HIE) have been treated according to the national cooling protocol and are registered in the Swiss National Asphyxia and Cooling Register since 2011 [1, 2]. Each year, data from approximately 70–80 cases of HIE treated in 10 centres across Switzerland are entered into the register.
A previous study reported streamlined processes and improved management of neonatal care in HIE after the implementation of the Swiss National Asphyxia and Cooling Register [2]. However, additional complications, which might require specific medical treatment, may occur because of perinatal asphyxia or secondary effects of the intensive care treatment, and such as seizures, arterial hypotension and infections [3]. These short-term outcomes are known in neonatal care to increase overall morbidity and mortality and worsen the neurodevelopmental outcome, with specific evidence in neonates with HIE [4–6].
Therapeutic hypothermia for moderate to severe HIE has become standard of care in industrialised countries across the world [7]. However, short-term outcomes and complications during therapeutic hypothermia have mainly been reported in the context of the initial randomised controlled trials evaluating its safety and efficacy [7–9]. The cooling process itself and short-term outcomes may differ between countries and even between centres [10, 11].
Investigating outcome quality and benchmarking is essential to assess quality of care and identify centre-specific areas for quality improvement [3, 10–12]. This study investigated centre-specific differences in short-term outcome indicators in neonates with HIE receiving therapeutic hypothermia registered in the Swiss National Asphyxia and Cooling Register between 2011 and 2018.
Data collection, evaluation and publication for this study were approved by the Swiss Association of Research Ethics Committees and the Swiss Federal Commission for Privacy Protection in Medical Research (KEK-ZH-Nr2014-0551 and KEK-ZH-Nr2014-0552).
In 2011, the centres participating in SwissNeoNet agreed on a protocol for standardised treatment of HIE and subsequently neonates with HIE are registered in the Swiss National Asphyxia and Cooling Register (appendix 2). This is a population-based retrospective cohort study including Swiss term and near-term neonates (≥35 weeks gestational age) with HIE, born between 1 January 2011 and 31 December 2018 and receiving therapeutic hypothermia in 11 centres (9 neonatal intensive care units [NICUs] and 2 paediatric intensive care units [PICUs] for internal logistic reasons). Neonates receiving off-protocol cooling on the basis of the clinical judgement of the attending neonatologist were included in the analysis. Exclusion criteria for therapeutic hypothermia are depicted in the national cooling protocol (appendix 2).
Demographical maternal, pregnancy, delivery and neonatal data were extracted from the national register. In order to compare severity of encephalopathy on admission between centres, Thompson scores [13] (used by one centre) were converted to Sarnat scores [14] (used by all other centres) according to Thompson et al. (Thompson score 1–6 = Sarnat score 1; Thompson score 7–12 = Sarnat score 2; Thompson score 13–22 = Sarnat score 3) [15].
The members of the quality improvement committee of the Swiss National Asphyxia and Cooling Register developed short-term outcomes as per Donabedian [16] and following the guidelines of the Swiss Academy of Medical Sciences. The short-term outcomes investigated were: seizures, arterial hypotension, infections and mortality. Outcome definitions from the cooling protocol forms/definitions (see appendix 1) were applied. Seizures were defined as “clinical or subclinical seizures identified on (amplitude-integrated) electroencephalogram (aEEG/EEG)” during therapeutic hypothermia and rewarming. Arterial hypotension was defined as “hypotension requiring treatment as defined by unit policies” during days 1–4. Infections requiring antibiotic therapy consisted of pneumonia (consistent chest X-ray findings), culture-proven sepsis (early onset and late onset) and necrotising enterocolitis (Bell stage 2–3 [17]). Death included all mortality until discharge from NICU/PICU.
Dichotomous short-term outcomes were defined to generate standardised comparisons. For each outcome, the predefined standard included data completeness of 90% of valid patient responses per centre. Missing data (based on plausibility) was imputed as follows: 16 neonates with unknown survival status were determined to be survivors by analysis of discharge data; 1 neonate with unknown birth location was determined to be outborn based on place of birth.
Descriptive analyses of the de-identified centre to centre analysis were provided. A chi-square test was used to test for outcome differences between centres and standardised observed-to-expected values for each centre compared with the entire network using indirectly standardised mortality/morbidity ratio charts were calculated. The area under the curve was calculated for each short-term outcome. Risk adjustment was performed for all outcomes based on: male sex, small for gestational age, Sarnat score on admission, composite pregnancy complication (maternal diabetes, maternal fever or pre-eclampsia), and composite delivery sentinel events (placental abruption, ruptured uterus, shoulder dystocia, cord mishaps, or head entrapment). All analyses were performed in R version 3.6.1 [18].
A total of 680,664 live births were registered in Switzerland between 1 January 2011 and 31 December 2018 and 578 neonates with HIE receiving therapeutic hypothermia were registered in the Swiss National Asphyxia and Cooling Register during the study period. Therefore, the estimated incidence of HIE requiring therapeutic hypothermia was 0.85 per 1000 livebirths in Switzerland.
For the following evaluations, the datasets from one centre were removed as data did not fulfil the criteria for evaluation (n = 8; 8/578; 1.4%). Therefore, 10 (out of 11) centres performing therapeutic hypothermia participated in this study and 570 cooled neonates were included in the analysis (570/578; 98.6%).
Centre-specific baseline pregnancy, maternal, delivery and neonatal characteristics are presented in table 1. The rates of outborns varied between 50% and 100% (no labour and delivery ward in this centre). A total of 490 (490/570; 86%) cooled neonates were discharged alive from NICU/PICU.
Table 1 Baseline descriptive statistics.
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 107 | 53 | 72 | 30 | 128 | 14 | 50 | 29 | 41 | 46 | 570 |
| Gestational age, median (IQR) | 39.7 (38.1–40.6) | 39.1 (38.0–40.4) | 40.0 (39.1–41.0) | 38.9 (37.6–40.2) | 39.8 (38.7–40.7) | 40.1 (38.9–40.9) | 39.9 (37.9–40.6) | 39.4 (37.9–41.4) | 39.9 (38.0–41.0) | 39.9 (39.0–41.0) | 39.8 (38.3–40.7) |
| Birthweight <P10, n (%) | 27 (25.2%) | 5 (9.4%) | 14 (19.4%) | 1 (3.3%) | 23 (18.0%) | 5 (35.7%) | 7 (14.0%) | 5 (17.2%) | 4 (9.8%) | 8 (17.4%) | 99 (17.4%) |
| Male sex, n (%) | 69 (64.5%) | 29 (54.7%) | 38 (52.8%) | 16 (53.3%) | 72 (56.2%) | 10 (71.4%) | 22 (44.0%) | 16 (55.2%) | 25 (61.0%) | 24 (52.2%) | 321 (56.3%) |
| Sarnat Score on admission, median (IQR) | 2 (1–2) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (1–2) | 2 (2–2) | 2 (2–3) | 2 (2–2) | 2 (2–2) | 2 (2–2) |
| Composite pregnancy complication*, n (%) | 12 (11.2%) | 4 (7.5%) | 11 (15.3%) | 3 (10.0%) | 15 (11.7%) | 3 (21.4%) | 9 (18.0%) | 3 (10.3%) | 3 (7.3%) | 4 (8.7%) | 67 (11.8%) |
| Composite delivery sentinel events†, n (%) | 29 (27.1%) | 20 (37.7%) | 21 (29.2%) | 13 (43.3%) | 49 (38.3%) | 8 (57.1%) | 18 (36.0%) | 10 (34.5%) | 21 (51.2%) | 16 (34.8%) | 205 (36.0%) |
| Pathological CTG, n (%) | 71 (66.4%) | 26 (49.1%) | 26 (36.1%) | 12 (40.0%) | 46 (35.9%) | 5 (35.7%) | 20 (40.0%) | 10 (34.5%) | 18 (43.9%) | 28 (60.9%) | 262 (46.0%) |
| Emergency C-section, n (%) | 57 (53.3%) | 29 (54.7%) | 24 (33.3%) | 12 (40.0%) | 44 (34.4%) | 5 (35.7%) | 23 (46.9%) | 12 (41.4%) | 19 (46.3%) | 5 (10.9%) | 230 (40.4%) |
| APGAR 1, median (IQR) | 1 (0–2) | 1 (0–2) | 2 (1–4) | 1 (0–2) | 1 (0–2) | 2 (1–3) | 1 (0–2) | 1 (1–3) | 2 (0–3) | 1 (0–3) | 1 (0–2) |
| APGAR 5 min, median (IQR) | 3 (2–5) | 2 (1–4) | 4 (2–5) | 3 (1–4) | 3 (2–5) | 3 (2–4) | 3 (2–4) | 3 (3–5) | 4 (2–5) | 3 (1–5) | 3 (2–5) |
| APGAR 10 min, median (IQR) | 5 (3–7) | 4 (2–6) | 4 (2–6) | 3 (2–5) | 5 (3–7) | 5 (4–6) | 5 (4–6) | 5 (4–7) | 4 (3–6) | 4 (2–6) | 4 (3–6) |
| Resuscitation >10 min, n (%) | 63 (58.9%) | 39 (73.6%) | 44 (61.1%) | 25 (83.3%) | 54 (42.2%) | 8 (57.1%) | 25 (50.0%) | 16 (55.2%) | 23 (56.1%) | 24 (52.2%) | 321 (56.3%) |
| Umbilical artery pH ≤7.0, n (%) | 64 (68.8%) | 28 (68.3%) | 29 (50.0%) | 17 (63.0%) | 65 (60.2%) | 7 (58.3%) | 33 (71.7%) | 14 (50.0%) | 16 (44.4%) | 17 (38.6%) | 290 (58.85) |
| Base deficit ≥16 mmol/l, n (%) | 37 (46.8%) | 16 (34.8%) | 44 (71.0%) | 7 (29.2%) | 5 (4.5%) | 4 (28.6%) | 1 (2.6%) | 2 (8.3%) | 15 (41.7%) | 6 (15.8%) | 137 (29.0%) |
H = hospital; IQR = interquartile range; P10 = 10th percentile * Composite pregnancy complication includes: maternal diabetes, maternal fever or pre-eclampsia; † composite delivery sentinel events includes: placental abruption, ruptured uterus, shoulder dystocia, cord mishaps or head entrapment.
Centre-specific data on short-term outcomes are depicted in table 2. Clinical or subclinical seizures were reported in a median of 32% (range 17–49%). Arterial hypotension occurred in a median of 62% (range 30–90%). Median infection rate was 10% (range 0–31%; medians for type of infection were: culture positive sepsis 5%, pneumonia 3%, necrotising enterocolitis 2%). Median mortality rate until discharge across all included Swiss centres was 14% (range 0–25%). The differences between centres for all four short-term outcomes were statistically significant, with p-values ranging between <0.001 and 0.01.
Table 2 Centre specific short-term outcomes.
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | Median[N; %] | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (total = 570) | 107 | 53 | 72 | 30 | 128 | 14 | 50 | 29 | 41 | 46 | ||
| Seizures, n (%) | 36 (33.6%) | 23 (43.4%) | 16 (22.2%) | 12 (40.0%) | 40 (31.2%) | 4 (28.6%) | 9 (18.0%) | 5 (17.2%) | 20 (48.8%) | 19 (41.3%) | 184 (32.3%) | 0.009 |
| Arterial hypotension, n (%) | 50 (46.7%) | 28 (52.8%) | 65 (90.3%) | 18 (60.0%) | 96 (75.0%) | 5 (35.7%) | 28 (56.0%) | 15 (51.7%) | 35 (85.4%) | 14 (30.4%) | 354 (62.1%) | <0.001 |
| Infection, n (%) | 6 (5.6%) | 3 (5.7%) | 2 (2.8%) | 5 (16.7%) | 18 (14.1%) | 0 | 3 (6.0%) | 9 (31.0%) | 3 (7.3%) | 5 (10.9%) | 54 (9.5%) | <0.001 |
| Mortality, n (%) | 12 (11.2%) | 12 (22.6%) | 18 (25.0%) | 7 (23.3%) | 14 (10.9%) | 0 | 3 (6.0%) | 4 (13.8%) | 6 (14.6%) | 4 (8.7%) | 80 (14.0%) | 0.010 |
H = hospital; H3 and H5 paediatric intensive care units, all other hospitals neonatal intensive care units.
Standardised mortality/morbidity rates are given for each short-term outcome per centre, consisting of crude and risk adjusted ratios with 95% confidence intervals (CIs) (figs 1–4 ). Persistent seizures at hospital discharge were recorded in a median of 13% (74/570; data not shown). The incidence of seizures, arterial hypotension, and infections did not differ significantly between the whole cohort of cooled neonates (figs 1–4 ) and survivors (data not shown).

Figure 1 Standardised mortality/morbidity ratio (SMR) chart: seizures (AUC = 0.631).
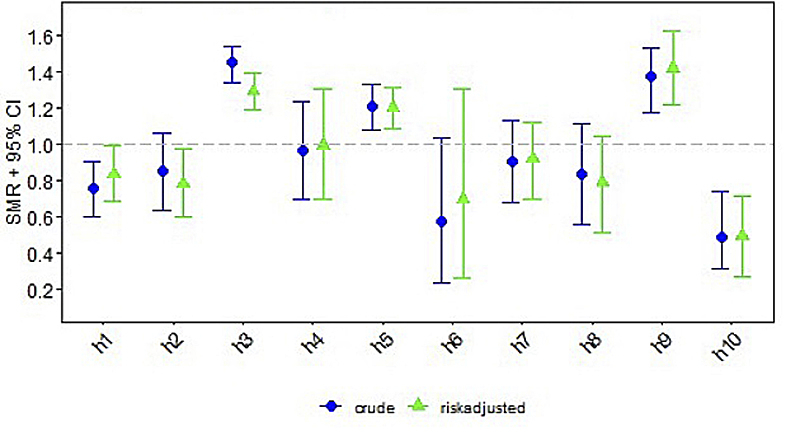
Figure 2 Standardised mortality/morbidity ratio (SMR) chart: arterial hypotension (AUC = 0.658).
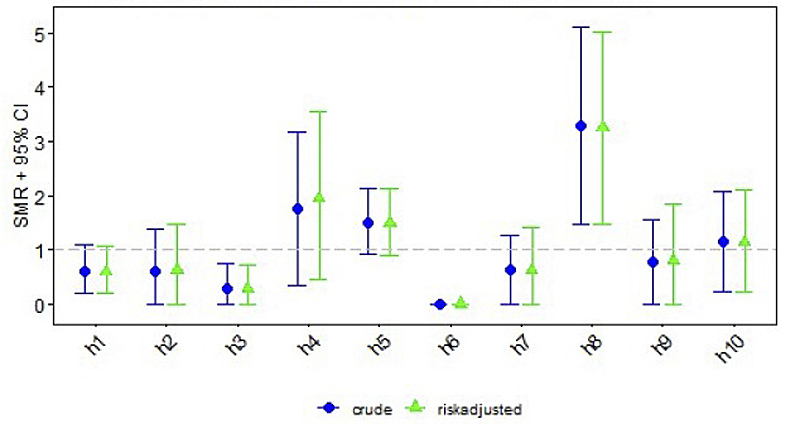
Figure 3 Standardised mortality/morbidity ratio (SMR) chart: infections (AUC = 0.569).
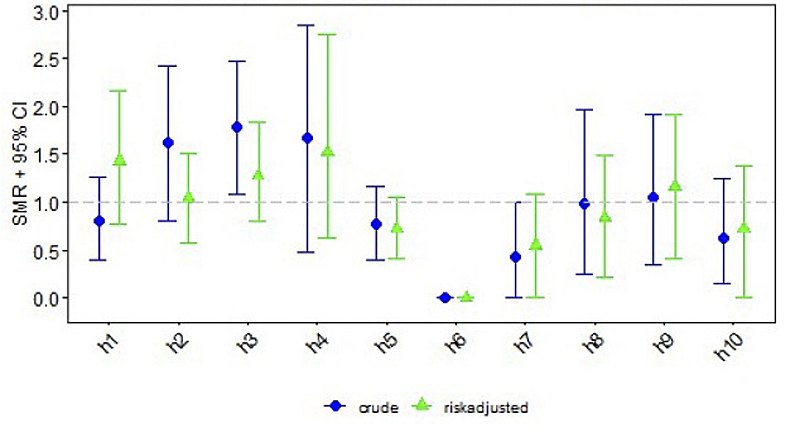
Figure 4 Standardised mortality/morbidity ratio (SMR) chart: mortality (AUC = 0.784).
This study assessed centre-specific short-term outcomes of neonates with HIE receiving therapeutic hypothermia between 2011 and 2018 in Switzerland. Short-term outcomes such as seizures, arterial hypotension, infections and mortality showed significant differences between the different centres after adjustment for clinical confounders.
The reported short-term outcomes of seizures, arterial hypotension and infection have not been associated with the therapeutic hypothermia treatment itself [7]. In contrast, thrombocytopenia and sinus bradycardia are aggravated by therapeutic hypothermia [7]. They thus can be considered as adverse events rather than short-term outcomes and were not interpreted as centre-specific short-term outcomes.
Seizures can serve as a surrogate marker for expected neurodevelopmental outcome [19]. Documented clinical or subclinical seizure activity during therapeutic hypothermia and rewarming varied between the centres from 17% to 49%; seizures persisted at hospital discharge in a median of 13%. Occurrence of seizures was not adjusted for sedation and analgesia management, but was adjusted for severity of encephalopathy amongst other confounders. Hence severity of encephalopathy cannot be an explanation of the variability in the frequency of seizures between centres. Some of the centres routinely use continuous infusions of midazolam for sedation, which is known to have an antiepileptic effect, and might therefore have fewer seizures in their population. Another reason for the centre-specific difference in seizure frequency could be the detection of seizures. With the implementation of therapeutic hypothermia and introduction of the Swiss National Asphyxia and Cooling Register in 2011, aEEG monitoring has become standard of care in therapeutic hypothermia in all Swiss centres and is continued throughout rewarming [2]. Detection of seizures on aEEG is strongly dependent on the training and education of the bedside medical team members [20]. Furthermore, seizure detection is not straightforward and requires specific expertise in neonates with HIE receiving therapeutic hypothermia [21]. Over- and underdetection of neonatal seizures has been reported to be a common problem in neonates with HIE [22]. For this reason, neonatal neuro-critical care teams have been established in high volume centres for HIE [21, 22], providing advice on tailored seizure management. To improve seizure management, it is mandatory to foster education in aEEG seizure recognition by bedside staff and clarify responsibilities regarding aEEG interpretation. Furthermore, there is a need for comparison of local sedation/analgesia protocols, which obviously impact the incidence of seizures.
Some differences in the reported incidence of arterial hypotension might be explained by differing unit policies because of vague outcome definitions provided by the Swiss National Asphyxia and Cooling Register. Arterial hypotension is defined as “hypotension requiring treatment defined by unit policies”. Arterial hypotension can be attributed to myocardial hypoxia, bradycardia with reduced cardiac output and sedation. Attention to arterial hypotension is clinically relevant as arterial hypotension in neonates without intact autoregulation induces reduced cerebral blood flow, thereby might further aggravate neuronal damage, and needs to be avoided whenever possible [4]. In the centres, blood pressure measurement is not unified. Some centres use continuous invasive blood pressure monitoring and others use intermittent cuff blood pressure measurements. Furthermore, the definition of arterial hypotension varied between the centres, as did thresholds for treating arterial hypotension. Interestingly, arterial hypotension requiring treatment occurred more frequently on PICUs than NICUs. This might imply that paediatric intensive care specialists may use more sedative medications leading to arterial hypotension, or one might speculate that paediatric intensive care specialists may treat arterial hypotension more aggressively than neonatal intensive care specialists. According to the local representatives, the majority reported arterial hypotension as long as specific treatment was needed to maintain the mean arterial blood pressure in the range of the respective gestational age. Some units, however, no longer consider a cooled neonate hypotensive if age-appropriate blood pressures were maintained with fluid boluses or inotropic support, which might explain the rates of arterial hypotension ranging between the centres from 30% to 90%. One conclusion from these data is the need to clarify the definition of arterial hypotension in the register and analyse the use of inotropes and sedation in this population.
Increased risk for infections during therapeutic hypothermia has been described previously [6]. In the literature, the association of (maternal/placental/neonatal) infection/inflammation in neonates with HIE and unfavourable outcome has been emphasised [6, 23, 24]. The short-term outcome of infection (defined as culture proven sepsis, pneumonia, necrotising enterocolitis Bell stage 2–3) varied between centres. One reason could be that some units treat neonates with HIE receiving therapeutic hypothermia with antibiotics empirically because of limited clinical assessment during therapeutic hypothermia. The next step is to investigate the site protocols for diagnosing infection (laboratory tests, e.g., C-reactive protein vs interleukin-6) and treatment thresholds for antibiotic therapy. In addition, there is a need to compare the local feeding protocols during therapeutic hypothermia regarding the incidence of necrotising enterocolitis (e.g., parenteral nutrition, protocols for increasing enteral feeds).
Higher rates of mortality of up to 25% were seen in centres treating neonates with more severe encephalopathy, reflected in a higher Sarnat score on admission to the NICU/PICU. Mortality during NICU/PICU stay of neonates with HIE is often related to withdrawal of care in cases with futile neurodevelopmental prognosis and with parental consent. Counselling parents to redirect care might also differ between the NICUs/PICUs. Reasons for mortality are currently not registered, amending the data entry is being considered (mortality due to redirection of care versus death despite full intensive care).
One centre reported no deaths, which was most likely an effect of the low case number.
The aetiology of HIE is important since the timing of hypoxic-ischaemic injury is crucial regarding the neuroprotective effect of therapeutic hypothermia. Routine investigation of placenta pathology might help to determine the onset of hypoxia/ischemia and explain the aetiology of HIE. One hypothesis is that the worse outcomes in centres H2 and H3 in absence of perinatal sentinel events might be due to prolonged intrauterine hypoxia.
We compared the short-term outcomes in our study with other national cohorts, namely the UK TOBY Cooling Register cohort and a Dutch cohort [10, 11]. Inclusion criteria for therapeutic hypothermia and baseline demographics (including severity of encephalopathy on admission) were similar to the data of the Swiss cohort. Data from the UK TOBY Register [10] included 1384 returned data forms on cooled neonates with HIE registered between 2006 and 2011. The Dutch study [11] is a retrospective single centre study including 168 neonates receiving therapeutic hypothermia for HIE between 2008 and 2016.
The incidence of seizures was not reported in the Dutch study and was 44% in the UK TOBY cohort in 2011, similar to Swiss data with an incidence of 32%. Arterial hypotension, defined as a (persistent) mean arterial blood pressure of less than 40 mm Hg, occurred in 81% of the Dutch neonates with HIE, 79% of these requiring inotropic support, and in 40% of the cases in the UK TOBY Cooling Register. The mean incidence in the Swiss cohort was 62%. The differences in incidence of arterial hypotension strongly relate to the definition of hypotension itself and to sedation management. Moreover, it was not reported throughout whether neonates were mechanically ventilated during therapeutic hypothermia and thus required some sedation.
The Dutch cohort reported an incidence of 17% for sepsis (culture positive sepsis and culture negative sepsis) and of 2% for necrotising enterocolitis. Similarly, culture proven sepsis was noted in 19%, pneumonia in 1% and necrotising enterocolitis in 0.7% in the UK TOBY cohort. When similar definitions for infection were applied, our cohort had a comparatively low infection rate of 10.2% (culture positive sepsis 5.3%, pneumonia 2.6%, necrotising enterocolitis 2.3%).
Mortality was 32% in the Dutch study and 20% in the UK TOBY cohort. The Swiss mortality rate was 14%. The mortality rate decreased from 22% in 2007 to 15% in 2011 in the UK TOBY cohort [10] and the Swiss cohort included cooled neonates with HIE born more recently than neonates in the UK TOBY cohort.
One strength is the harmonised national inclusion criteria for therapeutic hypothermia and the standardised cooling protocol. Despite some local differences, this led to a comparatively large cohort of neonates with HIE receiving therapeutic hypothermia according to the same cooling protocol. Datasets were characterised by completeness of data. Data monitoring was performed.
This study has several limitations due to the retrospective design. The data were collected prospectively with single data entry. Placenta pathology was not routinely reported. Definitions of the short-term outcomes were applied according to the Swiss National Asphyxia and Cooling Register, but some definitions were vague, leaving room for interpretation when coding outcomes and entering data into the register. Individual care protocols for seizures, arterial hypotension and infection differed between the centres and warrant further investigation. Except for mortality, risk adjustment was of low predictive validity.
Investigating outcome quality using routinely collected clinical data is well established [12, 25]. Outcome quality is closely linked to (infra)structural quality and process quality [26]. Short-term outcomes serve as hints about the quality in the specific centre, provided that centres with a similar level of care and similar local infrastructure are compared [27]. Benchmarking and peer review leads to quality improvement and transparency in health care [12]. Benchmarking between different NICUs/PICUs is needed to continuously improve easily modifiable short-term outcomes in neonates. Centres with low outcome quality will be supported in quality improvement strategies and centres with high outcome quality will be affirmed in their high quality work. The iterative process of discussing differences during peer review will lead to continuous learning and to ultimately establishing best practice. Comparing outcomes automatically triggers evaluation of underlying processes, which will be published separately. Similarly, longitudinal evolution of the Swiss National Asphyxia and Cooling Register will be assessed separately. This paper focuses on short-term outcomes until discharge from NICU/PICU and does not delineate changes in short-term outcomes over time since implementation of the register. Further research will investigate centre-specific differences in neurodevelopmental outcomes at 2 years and 5 years of age, which is a surrogate marker for the impact of short-term outcomes.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
The Swiss National Asphyxia and Cooling Register: Aarau: Cantonal Hospital Aarau, Children's Clinic, Department of Neonatology (P. Meyer, G. Zeilinger, G. Konetzny); Basel: University Children's Hospital Basel (UKBB), Department of Neonatology (S.M. Schulzke, S. Wellmann, M. Hug); Berne: University Hospital Berne, Department of Pediatric Intensive Care (T. Humpl, B. Wagner, K. Daetwyler, S. Pilgrim, M. Hug); Chur: Children's Hospital Chur, Department of Neonatology (T. Riedel, W. Bär, B. Scharrer, N. Binz); Lausanne: University Hospital (CHUV), Department of Neonatology (A. Truttmann, J.-F. Tolsa, A. Torregossa, J. Schneider); Lucerne: Children's Hospital of Lucerne, Neonatal and Pediatric Intensive Care Unit (M. Stocker, T. M. Berger, M. Fontana, K. Schwendener); St.Gallen: Children's Hospital St. Gallen, Neonatal and Pediatric Intensive Care Unit (B. Rogdo, J. P. Micallef, I. Hoigné, A. Birkenmaier); Winterthur: Clinic of Neonatology, Cantonal Hospital Winterthur (L. Hegi, M. Kleber); Zurich: University Hospital Zurich (3), Department of Neonatology (D. Bassler, G. Natalucci, M. Adams, U. Jochumsen, S. Böttger); and University Children's Hospital Zurich, Department of Intensive Care and Neonatology (B. Frey, V. Bernet, C. Hagmann, B. Brotschi).
All authors were involved in the conception of the study. BG and MK designed the study in detail. BG was responsible for acquisition of data, data analysis and interpretation, search and review of literature, and drafting the manuscript. VR, AB, and KS helped with the acquisition of data. MA performed all statistical analyses of the study, interpreted results and critically revised the manuscript. BB and CH supervised design of the study, data analysis and interpretation, search and review of literature, and critical review of manuscript. All authors have read and approved the final manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Hagmann CF , Brotschi B , Bernet V , Latal B , Berger TM , Robertson NJ . Hypothermia for perinatal asphyxial encephalopathy. Swiss Med Wkly. 2011;141:w13145 .
2 Brotschi B , Grass B , Ramos G , Beck I , Held U , Hagmann C , et al.; National Asphyxia Cooling Register Group. The impact of a register on the management of neonatal cooling in Switzerland. Early Hum Dev. 2015;91(4):277–84. doi:.https://doi.org/10.1016/j.earlhumdev.2015.02.009
3 Sarkar S , Barks J . Management of neonatal morbidities during hypothermia treatment. Semin Fetal Neonatal Med. 2015;20(2):97–102. doi:.https://doi.org/10.1016/j.siny.2015.01.007
4 Giesinger RE , Bailey LJ , Deshpande P , McNamara PJ . Hypoxic-Ischemic Encephalopathy and Therapeutic Hypothermia: The Hemodynamic Perspective. J Pediatr. 2017;180:22–30.e2. doi:.https://doi.org/10.1016/j.jpeds.2016.09.009
5 Fitzgerald MP , Massey SL , Fung FW , Kessler SK , Abend NS . High electroencephalographic seizure exposure is associated with unfavorable outcomes in neonates with hypoxic-ischemic encephalopathy. Seizure. 2018;61:221–6. doi:.https://doi.org/10.1016/j.seizure.2018.09.003
6 Rao R , Lee KS , Zaniletti I , Yanowitz TD , DiGeronimo R , Dizon MLV , et al. Antimicrobial therapy utilization in neonates with hypoxic-ischemic encephalopathy (HIE): a report from the Children’s Hospital Neonatal Database (CHND). J Perinatol. 2020;40(1):70–8. doi:.https://doi.org/10.1038/s41372-019-0527-2
7 Jacobs SE , Berg M , Hunt R , Tarnow-Mordi WO , Inder TE , Davis PG . Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;(1):CD003311. doi:.https://doi.org/10.1002/14651858.CD003311.pub3
8 Azzopardi DV , Strohm B , Edwards AD , Dyet L , Halliday HL , Juszczak E , et al.; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58. doi:.https://doi.org/10.1056/NEJMoa0900854
9 Shankaran S , Laptook AR , Ehrenkranz RA , Tyson JE , McDonald SA , Donovan EF , et al.; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. doi:.https://doi.org/10.1056/NEJMcps050929
10 Azzopardi D , Strohm B , Linsell L , Hobson A , Juszczak E , Kurinczuk JJ , et al.; UK TOBY Cooling Register. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK--analysis of national data. PLoS One. 2012;7(6):e38504. doi:.https://doi.org/10.1371/journal.pone.0038504
11 Diederen CMJ , van Bel F , Groenendaal F . Complications During Therapeutic Hypothermia After Perinatal Asphyxia: A Comparison with Trial Data. Ther Hypothermia Temp Manag. 2018;8(4):211–5. doi:.https://doi.org/10.1089/ther.2017.0046
12 Mansky T , Völzke T , Nimptsch U . Improving outcomes using German Inpatient Quality Indicators in conjunction with peer review procedures. Z Evid Fortbild Qual Gesundhwes. 2015;109(9-10):662–70. doi:.https://doi.org/10.1016/j.zefq.2015.10.014
13 Thompson CM , Puterman AS , Linley LL , Hann FM , van der Elst CW , Molteno CD , et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757–61. doi:.https://doi.org/10.1111/j.1651-2227.1997.tb08581.x
14 Sarnat HB , Sarnat MS . Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. doi:.https://doi.org/10.1001/archneur.1976.00500100030012
15 Thompson CM , Puterman AS , Linley LL , Hann FM , van der Elst CW , Molteno CD , et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757–61. doi:.https://doi.org/10.1111/j.1651-2227.1997.tb08581.x
16 Donabedian A . The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. doi:.https://doi.org/10.1001/jama.1988.03410120089033
17 Bell MJ , Ternberg JL , Feigin RD , Keating JP , Marshall R , Barton L , et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi:.https://doi.org/10.1097/00000658-197801000-00001
18R: The R Project for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, Available from: https://www.r-project.org/ [last accessed 2020 Feb 6].
19 Weeke LC , Boylan GB , Pressler RM , Hallberg B , Blennow M , Toet MC , et al.; NEonatal seizure treatment with Medication Off -patent (NEMO)consortium. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur J Paediatr Neurol. 2016;20(6):855–64. doi:.https://doi.org/10.1016/j.ejpn.2016.06.003
20 Grass B , Crosdale B , Keyzers M , Deshpande P , Hahn C , Ly LG , et al. Implementation of amplitude-integrated electroencephalography in tertiary Canadian Neonatal Intensive Care Units-a longitudinal study. Paediatr Child Health. 2020;25(8):511–7. doi:.https://doi.org/10.1093/pch/pxz091
21 Bashir RA , Espinoza L , Vayalthrikkovil S , Buchhalter J , Irvine L , Bello-Espinosa L , et al. Implementation of a Neurocritical Care Program: Improved Seizure Detection and Decreased Antiseizure Medication at Discharge in Neonates With Hypoxic-Ischemic Encephalopathy. Pediatr Neurol. 2016;64:38–43. doi:.https://doi.org/10.1016/j.pediatrneurol.2016.07.007
22 Glass HC , Ferriero DM , Rowitch DH , Shimotake TK . The neurointensive nursery: concept, development, and insights gained. Curr Opin Pediatr. 2019;31(2):202–9. doi:.https://doi.org/10.1097/MOP.0000000000000733
23 Novak CM , Eke AC , Ozen M , Burd I , Graham EM . Risk Factors for Neonatal Hypoxic-Ischemic Encephalopathy in the Absence of Sentinel Events. Am J Perinatol. 2019;36(1):27–33. doi:.https://doi.org/10.1055/s-0038-1639356
24 Wu YW , Escobar GJ , Grether JK , Croen LA , Greene JD , Newman TB . Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290(20):2677–84. doi:.https://doi.org/10.1001/jama.290.20.2677
25 Black C , Roos NP . Administrative data. Baby or bathwater? Med Care. 1998;36(1):3–5. doi:.https://doi.org/10.1097/00005650-199801000-00002
26 Lilford R , Mohammed MA , Spiegelhalter D , Thomson R . Use and misuse of process and outcome data in managing performance of acute medical care: avoiding institutional stigma. Lancet. 2004;363(9415):1147–54. doi:.https://doi.org/10.1016/S0140-6736(04)15901-1
27Bundesamt für Gesundheit (BAG). Qualitätsindikatoren der Schweizer Akutspitäler 2016. Bern: Bundesamt für Gesundheit; 2016.
Arrhythmia
Arrhythmias identified on ECG, except Sinusbradycardia
CFM findings (classification of Linda de Vries)
Coagulopathy
Any disorder requiring treatment in order to maintain or recover normal haemostasis according to unit's policy
Delivery complications
This can include prolapsed cord, abruption, shoulder dystocia, ruptured uterus, head entrapment etc
Diabetes
Existing diagnosis of diabetes or gestational diabetes requiring treatment
Head entrapment
Severely delayed second stage during breech delivery, vaginally or at caesarean section
Hypoglycaemia (infant)
Blood glucose< 2.5 mmol/L
Hyperglycaemia
Blood glucose > 10 mmol/L
Hypotension
Hypotension requiring treatment which defined by unit policies
Illicit drug use
Recorded drug or alcohol use that may lead to social, occupational, psychological, or physical problems
Late onset sepsis (>72h after birth) confirmed by blood or CSF culture
Any evidence of infection requiring antibiotic therapy which is confirmed on culture
Major cerebral anomaly
Including evidence of parenchymal haemorrhage as determined by ultrasound, ventricular dilatation (defined as >97th centile for gestational age) or the presence of porencephalic cysts or cystic leukomalacia
Maternal seizure
Convulsions due to eclampsia or other causes, e.g. epilepsy
Meconium aspiration syndrome
The presence of meconium stained amniotic fluid at birth and severe respiratory distress within 1 hour of birth and compatible x-ray changes
Necrotising enterocolitis
Infants with abdominal distension, blood in stoolstogether with abdominal x-ray showing bowel oedema, pneumatosis or pneumoperitoneum, Bell stag 2 or 3
Placental abruption
Separation of a normally situated placenta after 28th week of pregnancy
Placenta praevia
Placenta partially or wholly covering the internal cervical os
Pre-eclampsia
Hypertension greater than 140/90mmHg during pregnancy
Pregnancy complications
This can include: pre-eclampsia, maternal seizure, thyroid disorder, diabetes, placenta praevia, known illicit drug use
Prolapsed cord
Cord presentation following rupture of membranes
Pulmonary airleak
Any radiologically confirmed airleak serious enough to affect management (including pneumothorax, pulmonary interstitial emphysema, pneumopericardium, pneumoperitoneum, pneumomediastinum)
Pulmonary haemorrhage
Copious bloody secretions with clinical deterioration requiring changes in ventilatory management
Pulmonary hypertension
Severe hypoxaemia disproportionate to the severity of lung disease, evidence of a right to left shunt and other findings suggesting PHT in echocardiography and the need for medication
Renal failure
Renal failure requiring dialysis
Ruptured uterus
Spontaneous full-thickness tear in the uterine wall due to existing scar, obstructed labour
Seizures
Clinical or subclinical identified on aEEG/EEG
Sepsis
Any evidence of infection requiring antibiotic therapy which is confirmed on culture
Shoulder dystocia
Failure of the shoulders to rotate into the anteroposterior diameter of the pelvis following delivery of the head, resulting in a substantial delay in delivery
Maternal thyroid disorder
Thyroid dysfunction requiring treatment during pregnancy
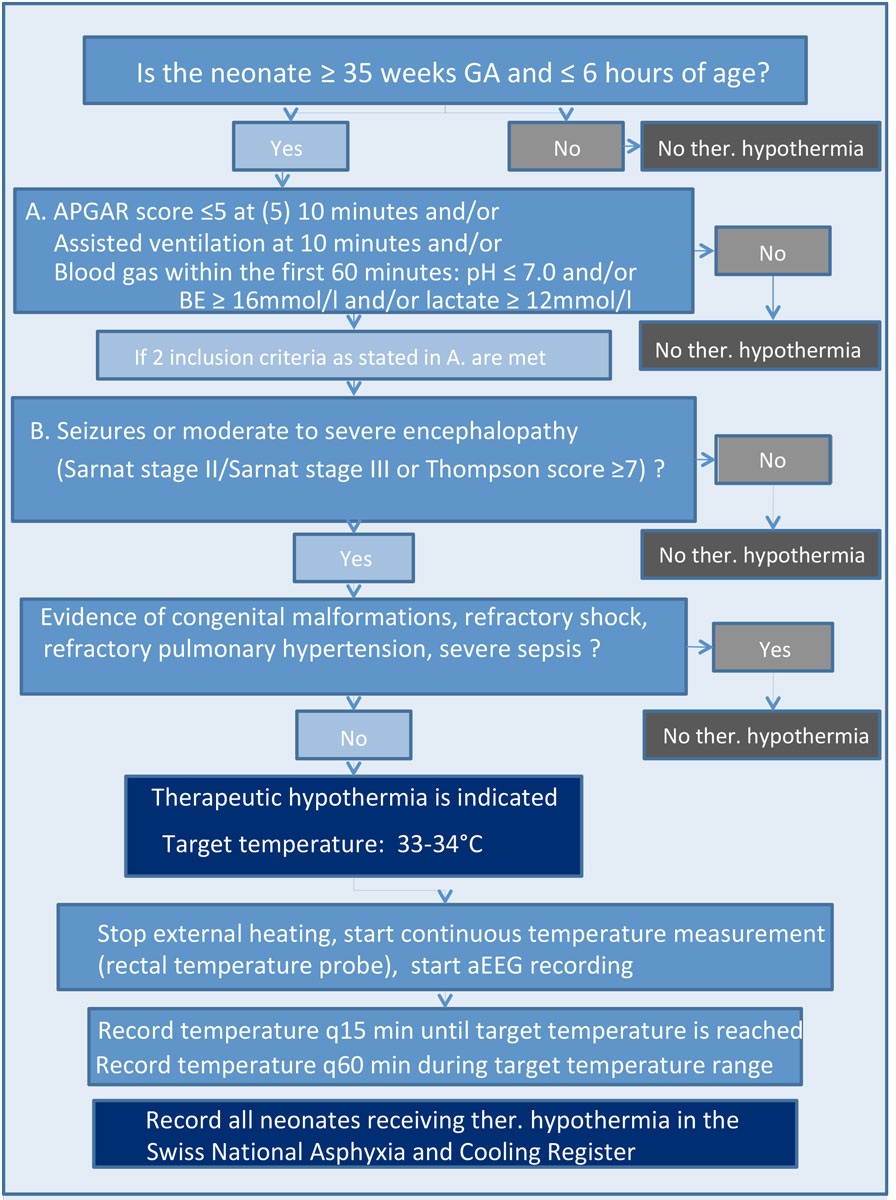
Figure S1 Eligibility of neonates with HIE for therapeutic hypothermia.