
Figure 1 Proportion of patients by degree of residual right ventricular outflow tract (RVOT) stenosis and type of surgical correction. RV-PA = right ventricular-to-pulmonary artery
DOI: https://doi.org/10.4414/smw.2021.20491
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, with a prevalence of 3.9 per 10,000 live births and accounting for approximately 10% of all congenital heart disease [1, 2]. Although complete repair of TOF has been performed since the 1950s, there is still a debate about the optimal timing of elective repair [3, 4]. Currently, most paediatric cardiac surgery centres in developed countries perform elective repair of TOF between 3 and 11 months of age, reporting better outcomes in terms of morbidity and mortality [3–5]. Additional benefits of early repair include reduction in the duration of hypoxaemia and its adverse effects on cognitive and psychomotor development, prevention of sequelae of chronic severe right ventricular hypertrophy such as fibrosis, right ventricular dysfunction and arrhythmias, and establishment of adequate pulmonary blood flow necessary for normal lung development [2, 6, 7].
In many developing countries, because of poor access to medical care and lack of trained specialists, many children with TOF do not receive a timely diagnosis and therefore cannot benefit from early corrective surgery [8]. Instead, they are often operated on much later in childhood during humanitarian missions in their countries or through charitable organisations in specialized centres in developed countries [8, 9]. In collaboration with the Swiss humanitarian organisation Terre des Hommes, the Lausanne University Hospital has had a longstanding programme of performing late correction of TOF in such children.
The purpose of this study was to describe the early results of complete TOF repair in our institution in children older than one year of age, and to see how they compare with the existing literature.
We conducted a retrospective review of our surgical database, searching for all patients with a diagnosis of TOF who had a complete elective repair between January 2007 and December 2017 at the Lausanne University Hospital, a tertiary centre performing approximately 110 paediatric heart surgeries per year. Only patients older than one year at the time of surgery were included. Patients with pulmonary atresia, non-confluent pulmonary arteries or major aortopulmonary collateral arteries were excluded. The study protocol conformed to the ethical guidelines of the Helsinki Declaration as revised in 2013. The local ethics committee approved the study (EC-VD, ID 2018-01086) and written informed consent was waived.
For each patient, we searched the electronic medical record and collected the following data: date of birth, diagnosis, weight, height, date of surgery, type of surgical correction, surgical reintervention within 30 days of initial surgery, catheter intervention within 30 days of initial surgery, survival at 30 days after surgery, length of stay (LOS) in the intensive care unit (ICU), and LOS in the hospital. The patients’ preoperative echocardiographic reports were examined to record the diameters of the pulmonary valve annulus, main pulmonary artery, and right and left pulmonary arteries. The operation notes were examined in order to classify the type of surgical correction into three broad categories, namely valve-preserving surgery, transannular patch (TAP) or implantation of a valved right ventricle to pulmonary artery (RV-PA) conduit. The echocardiographic reports at 30 days after surgery were examined to record the maximum instantaneous gradient (MIG) across the right ventricular outflow tract (RVOT), as well as the degree of pulmonary insufficiency, which was qualitatively classified as trace, mild, moderate or severe. We defined significant residual RVOT stenosis as a MIG greater than 40 mm Hg, and significant pulmonary insufficiency as greater than mild.
Body mass index was calculated for each patient and its Z-score derived from the World Health Organization (WHO) / Centers for Disease Control and Prevention (CDC) data. The Z-scores for the echocardiographic diameters were derived using the Boston Children’s Hospital Z-Score Calculator (zscore.chboston.org).
Continuous variables are presented as median (interquartile range [IQR]) due to non-normal distribution of the data, and categorical variables are presented as absolute numbers (percentages). The Kruskal-Wallis test was used to compare the continuous variables, and Fisher's exact test was used to compare the categorical variables. Statistical analysis was performed using IBM/SPSS Statistics (version 26, IBM, Armonk, New York). A p-value <0.05 was considered statistically significant.
Between 2007 and 2017, 125 children over one year of age underwent complete repair of TOF in our centre. The patients’ characteristics are presented in table 1, and the results are presented in table 2, figure 1 and figure 2. There were no patients with documented chromosomal anomalies. Sixteen patients had a palliative Blalock-Taussig shunt prior to their complete repair, and three patients had a left anterior descending coronary artery originating from the right coronary artery. The median age at the time of complete repair was 4.4 years (IQR 3–5.7). The pulmonary valve was preserved in 82 patients (66%), a TAP was performed in 24 patients (19%), and 19 patients (15%) received a valved RV-PA conduit. There were no deaths at 30 days after surgery.
Table 1 Patient characteristics.
| All patients | Preserved PV | TAP | RV-PA conduit | p-value | |
|---|---|---|---|---|---|
| Number of patients | 125 (100) | 82 (66) | 24 (19) | 19 (15) | |
| Age (years) | 4.4 (3–5.7) | 4.3 (3–5.7) | 4.3 (2.8–5.2) | 4.6 (3–6.3) | 0.83 |
| Weight (kg) | 14.4 (11–16.2) | 14.4 (11.4–16.1) | 14 (10.5–15.5 | 13 (11–18.5) | 0.75 |
| Height (cm) | 97 (90–108) | 97.5 (91–108) | 97 (86.8–106) | 95 (88.5–113.5) | 0.86 |
| BMI Z-score | −1 (−2.5, −0.1) | −1.02 (−2.7, −0.1) | −1.1 (−2.5, −0.0) | −1 (−1.6, −0.2) | 0.94 |
| Prior BTS | 16 (13) | 13 (16) | 1 (4) | 2 (11) | |
| LAD from RCA | 3 (2) | 1 (1) | 0 | 2 (11) | |
| PVa Z-score | −2.4 (−2.9, −1.7) | −2.2 (−2.6, −1.5) | −2.7 (−3.1, −2.3) | −2.9 (−3.1, −2.6) | 0.00001* |
| MPA Z-score | −2.6 (−3.2, −1.6) | −2.4 (−3.2, −1.5) | −2.7 (−3.3, −2.5) | −3.1 (−4.1, −1.4) | 0.07 |
| RPA Z-score | −1.3 (−2.3, −0.6) | −1.2 (−2.1, −0.3) | −2.3 (−2.9, −1.5) | −1.6 (−1.9, −1.1) | 0.02† |
| LPA Z-score | −1.7 (−2.6, −0.7) | −1.5 (−2.5, −0.6) | −1.9 (−2.5, −1.1) | −2.4 (−2.8, −2.1) | 0.01‡ |
BMI = body mass index; BTS = Blalock-Taussig shunt; LAD = left anterior descending coronary artery; RCA = right coronary artery; LPA = left pulmonary artery; MPA = main pulmonary artery; PV = pulmonary valve; PVa = pulmonary valve annulus; RPA = right pulmonary artery; RV-PA = right ventricular-to-pulmonary artery; TAP = transannular patch Data are presented as number (%) or median (interquartile range) * Preserved PV compared with TAP and RV-PA conduit † TAP compared to preserved PV and RV-PA conduit ‡ RV-PA conduit compared to preserved PV p-values <0.05 are in bold type
Table 2 Patient outcomes.
| All patients | Preserved PV | TAP | RV-PA conduit | p-value | |
|---|---|---|---|---|---|
| Number of patients | 125 (100) | 82 (66) | 24 (19) | 19 (15) | |
| Mortality | 0 | 0 | 0 | 0 | |
| Reinterventions | 12 (9.6) | 7 (8.5) | 4 (16.7) | 1 (5.2) | |
| Surgery | 8 | 6 | 2 | 0 | |
| Catheterisation | 4 | 1 | 2 | 1 | |
| ICU LOS (days) | 7 (5–9) | 6 (5–8) | 7 (6–9.8) | 6 (6–12) | 0.12 |
| Hospital LOS (days) | 12 (10–16) | 12 (9–15.8) | 10 (9.8–20.3) | 12 (10–26) | 0.33 |
| Residual MIG (mm Hg) | 21.7 (5–30) | 22 (10.4–30) | 15.4 (5–31) | 26 (12–30) | 0.56 |
| Residual MIG >40 mm Hg | 9 (7.2) | 3 (3.7) | 4 (16.7) | 2 (10.5) | |
| PI ≥moderate | 24 (19.2) | 10 (12.2) | 12 (50) | 2 (10.5) |
ICU = intensive care unit; LOS = length of stay; MIG = maximum instantaneous gradient; PI = pulmonary insufficiency; PV = pulmonary valve; RV-PA = right ventricular-to-pulmonary artery; TAP = transannular patch. Data are presented as number (%) or median (interquartile range)

Figure 1 Proportion of patients by degree of residual right ventricular outflow tract (RVOT) stenosis and type of surgical correction. RV-PA = right ventricular-to-pulmonary artery
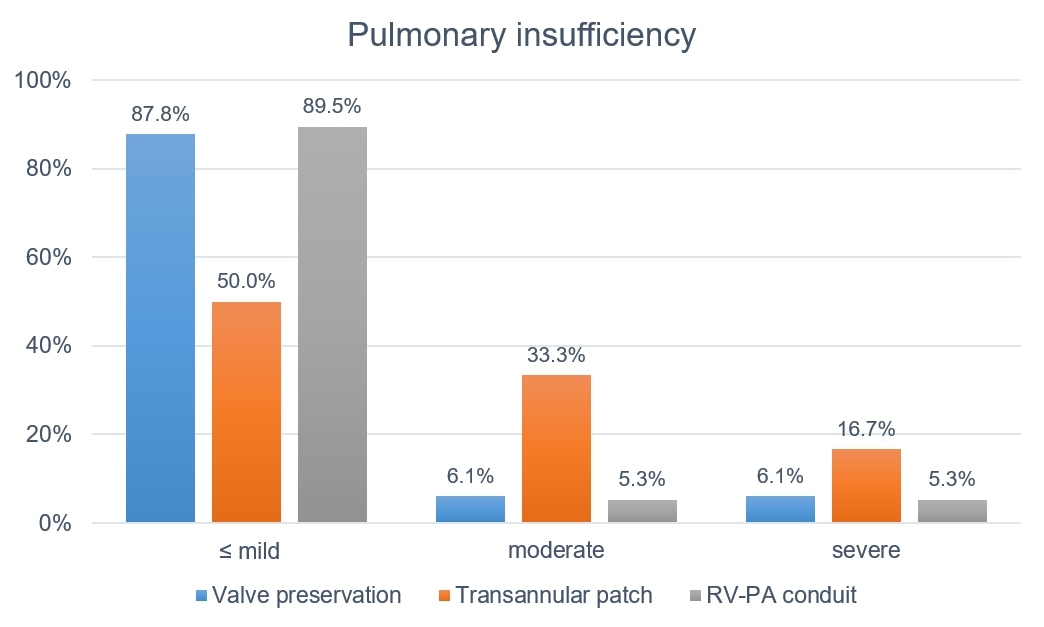
Figure 2 Proportion of patients by degree of pulmonary insufficiency and type of surgical correction. RV-PA = right ventricular-to-pulmonary artery
Six patients with a preserved pulmonary valve required surgical reintervention within 30 days, two for a significant residual ventricular septal defect, one for pacemaker implantation due to complete atrioventricular block, one for residual infundibular stenosis, one for residual supravalvar pulmonary stenosis and one for left pulmonary artery stenosis after unsuccessful percutaneous balloon dilatation. Among patients with a TAP, two required surgical reintervention, one for residual pulmonary annulus stenosis and one for a significant residual ventricular septal defect. In addition, two patients required cardiac catheterisation for bilateral percutaneous balloon dilatation of pulmonary artery stenosis. No patient with a valved RV-PA conduit required surgical reintervention, and only one needed a percutaneous balloon dilatation of left pulmonary artery stenosis. There was no statistically significant difference in the reintervention rate between the types of surgical correction (p >0.05 for all contingency tables).
The median LOS in the ICU for all patients was 7 days (IQR 5–9) and the median LOS in the hospital for all patients was 12 days (IQR 10–16). There was no statistically significant difference in the ICU or hospital LOS between the types of surgical correction (p >0.05).
The median residual MIG across the RVOT at 30 days after surgery, for all patients, was 21.7 mm Hg (IQR 5–30). Overall, nine patients (7.2%) had significant residual RVOT stenosis, three (3.7%) after valve-preserving surgery, four (16.7%) after TAP, and two (10.5%) after implantation of a valved RV-PA conduit. There was a statistically significant difference between patients with valve-preserving surgery and TAP (p = 0.045). Significant pulmonary insufficiency at 30 days after surgery was seen in 19.2% of all patients, with 12.2% in patients with valve-preserving surgery, 50% in patients with a TAP, and 10.5% in patients with implantation of a valved RV-PA conduit. Patients with a TAP had significantly more pulmonary insufficiency than patients with either valve-preserving surgery (p = 0.0002) or implantation of a valved RV-PA conduit (p = 0.0087).
Late complete repair of TOF has become rare in developed countries as most patients today are operated on before one year of age with excellent short- and long-term outcomes [10–12]. We sought to analyse the early results of complete repair of TOF in a cohort of children older than one year of age, referred to our centre from developing countries as part of an ongoing humanitarian project.
The reported operative mortality of late complete repair of TOF in children ranges from 0% to 9% [8, 9, 13–15]. Butera et al. analysed the results of 22 patients with TOF who underwent complete repair in Cameroon by a visiting western surgical team at a mean age of 9.2 ± 6.5 years [8]. Two patients (9%) died during the early postoperative period. In a study by Benbrik et al., 47 children from developing countries had complete repair of TOF at a mean age of 4.8 ± 3.2 years, and two patients (4.2%) died postoperatively [13]. A study by Raj et al. showed an in-hospital mortality of 2%, with only one death among 50 children undergoing complete repair in a developing country at a median age of 6 years (range 1–14 years) [14]. Waqar et al. reported on a large cohort of 307 children in a developing country with primary repair at a mean age of 9.6 ± 4.9 years. The 30-day mortality was 1.3% (4 patients) [15]. In another Swiss study, Heinisch et al. reported no in-hospital mortality among 25 children who underwent complete repair of TOF at a mean age of 5.9 ± 3.5 years [9]. In our study, 125 children had complete repair of TOF at a median age of 4.4 years (IQR 3–5.7), with no mortality at 30 days after surgery. These are excellent results compared with the studies mentioned above and comparable to those of early complete repair of TOF [16].
Eleven patients in our study required 12 surgical or catheter-based interventions within 30 days of initial surgery, resulting in a reintervention rate of 9.6%. This is comparable to the reintervention rate of 8.5% reported by Benbrik et al. in a similar cohort, and lower than the reintervention rate of 13.3% reported in patients undergoing complete repair of TOF in infancy [13, 17]. Although there was no statistically significant difference between the types of surgical correction, patients who underwent repair with a TAP had the highest rate of reintervention at 16.7%.
The majority (66%) of patients in our cohort underwent valve-preserving surgery. Heinisch et al. reported an exceedingly high rate of valve preservation in their cohort at 96%, which they attributed to unusually favourable pulmonary valve morphology and pulmonary annulus size in almost all of their patients [9]. In the other studies of complete repair of TOF in children older than one year of age, the rate of valve-preserving surgery was comparable to the rate in our study, ranging from 30% to 66.5% [8, 13–15]. The reported rate of valve preservation among children undergoing complete repair in infancy ranges from 22% to 80%, which is also comparable to our cohort [18–21]. Interestingly, 15% of patients in our study had implantation of a valved RV-PA conduit, whereas no conduits seem to have been used at initial surgery in the other studies of late complete repair. Two of these cases may have been due to a left anterior descending artery originating from the right coronary artery and crossing the RVOT, although we could not identify this as the explicit reason from the operation notes. Upon closer examination of our data, we found that the proportion of patients who had implantation of a valved RV-PA conduit decreased from 34.1% (15 out of 44 patients) before 2011 to just 4.9% (4 out of 81 patients) after 2011, whereas the proportion of those who had a TAP increased from 4.5% (2 out of 44 patients) to 27.2% (22 out of 81 patients) and the proportion of valve-preserving surgery was essentially unchanged (61.4% vs. 67.9%) between the two time periods. Since there was no significant difference between patients with valved RV-PA conduit and TAP in any of the relevant characteristics (patient size, pulmonary valve annulus or main pulmonary artery Z-score), we attribute the overall high use of the conduit in our patient population to an institutional bias against the use of a TAP prior to 2011, in cases where the pulmonary valve could not be preserved.
The median ICU LOS in our study was 7 days (IQR 5–9) and the median hospital LOS was 12 days (IQR 10–16). All of the other similar studies on late complete repair of TOF in children reported shorter lengths of stay, with ICU LOS ranging from a mean of 1.8 ± 1.2 days to 5 ± 3 days and hospital LOS ranging from a mean of 7.6 ± 3.8 days to 15 ± 7 days [8, 9, 13, 15]. Outcome studies on complete repair of TOF in infancy show either comparable or shorter ICU and hospital LOS compared with our study [16, 17, 21, 22]. We found no patient-related factors that could explain the higher ICU and hospital LOS in our study. We therefore hypothesise that the most likely explanation, admittedly, is a tendency towards a very conservative approach to postoperative management of these patients in our institution.
The incidence of significant residual RVOT stenosis at 30 days after repair in our study was 7.2%. In contrast, Butera et al. reported no significant residual RVOT stenosis in their study, and Waqar et al. diagnosed moderate RVOT obstruction (pressure gradient >40 mm Hg) after surgery in 5.2% of their patients [8, 15]. In our study, marked residual RVOT stenosis was significantly higher in patients with a TAP compared with those with valve-preserving surgery. We hypothesise that this was due to insufficient patch enlargement of more hypoplastic pulmonary annuli and main pulmonary arteries in the former group.
Moderate or severe pulmonary insufficiency at 30 days after repair was seen in 19.2% of our patients and was significantly higher in those with a TAP. This compares favourably to the results of Raj. et al and Waqar et al., who reported moderate or severe pulmonary insufficiency postoperatively in 56% and 37.1% of their patients, respectively, even though the rates of TAP repair in their studies were very similar to ours [14, 15].
Our study has two main limitations, inherent to its design. First, since we were interested in the results of complete TOF repair only in children older than one year of age, we do not know how these results compare to those of children who underwent complete repair in our centre during the same period in their infancy. Comparing our results to the results of early repair published from other centres may therefore not be relevant. However, the patient population we studied is arguably very different from patients with TOF operated in developed countries during their infancy, and a comparison of the two may therefore not even be pertinent. Second, we could only analyse the early results of repair because most patients return to their home country after their routine postoperative evaluation at 30 days after surgery. Thus, we do not know the long-term incidence of reintervention or the long-term outcome of significant residual RVOT obstruction and pulmonary insufficiency in our cohort.
Compared with the existing literature on complete repair of TOF in children older than one year of age, this study showed good early results in our institution with no mortality, similar rates of reintervention and valve-preserving surgery, higher mean ICU and hospital LOS, higher incidence of significant residual RVOT stenosis, and lower incidence of significant pulmonary insufficiency. Repair with a TAP was a risk factor for significant residual RVOT stenosis and significant pulmonary insufficiency.
No financial support and no other potential conflict of interest relevant to this article were reported.
1 Huehnergarth KV , Gurvitz M , Stout KK , Otto CM . Repaired tetralogy of Fallot in the adult: monitoring and management. Heart. 2008;94(12):1663–9. doi:.https://doi.org/10.1136/hrt.2008.147249
2Gatzoulis MA. Tetralogy of Fallot. In: Gatzoulis MA, Webb GD, Daubeney PEF eds.; Diagnosis and Management of Adult Congenital Heart Disease. Edinburgh: Churchill Livingstone; 2003. pp 315–26.
3 Van Arsdell GS , Maharaj GS , Tom J , Rao VK , Coles JG , Freedom RM , et al. What is the optimal age for repair of tetralogy of Fallot? Circulation. 2000;102(19, Suppl 3):III123–9. doi:.https://doi.org/10.1161/01.CIR.102.suppl_3.III-123
4 Cunningham MEA , Donofrio MT , Peer SM , Zurakowski D , Jonas RA , Sinha P . Optimal Timing for Elective Early Primary Repair of Tetralogy of Fallot: Analysis of Intermediate Term Outcomes. Ann Thorac Surg. 2017;103(3):845–52. doi:.https://doi.org/10.1016/j.athoracsur.2016.07.020
5 Steiner MB , Tang X , Gossett JM , Malik S , Prodhan P . Timing of complete repair of non-ductal-dependent tetralogy of Fallot and short-term postoperative outcomes, a multicenter analysis. J Thorac Cardiovasc Surg. 2014;147(4):1299–305. doi:.https://doi.org/10.1016/j.jtcvs.2013.06.019
6 Miller SP , McQuillen PS , Hamrick S , Xu D , Glidden DV , Charlton N , et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–38. doi:.https://doi.org/10.1056/NEJMoa067393
7 Walsh EP , Rockenmacher S , Keane JF , Hougen TJ , Lock JE , Castaneda AR . Late results in patients with tetralogy of Fallot repaired during infancy. Circulation. 1988;77(5):1062–7. doi:.https://doi.org/10.1161/01.CIR.77.5.1062
8 Tchoumi JCT , Ambassa JC , Giamberti A , Cirri S , Frigiola A , Butera G . Late surgical treatment of tetralogy of Fallot. Cardiovasc J Afr. 2011;22(4):179–81. doi:.https://doi.org/10.5830/CVJA-2010-057
9 Heinisch PP , Guarino L , Hutter D , Bartkevics M , Erdoes G , Eberle B , et al. Late correction of tetralogy of Fallot in children. Swiss Med Wkly. 2019;149:w20096. doi:.https://doi.org/10.4414/smw.2019.20096
10 Kirsch RE , Glatz AC , Gaynor JW , Nicolson SC , Spray TL , Wernovsky G , et al. Results of elective repair at 6 months or younger in 277 patients with tetralogy of Fallot: a 14-year experience at a single center. J Thorac Cardiovasc Surg. 2014;147(2):713–7. doi:.https://doi.org/10.1016/j.jtcvs.2013.03.033
11 Ooi A , Moorjani N , Baliulis G , Keeton BR , Salmon AP , Monro JL , et al. Medium term outcome for infant repair in tetralogy of Fallot: Indicators for timing of surgery. Eur J Cardiothorac Surg. 2006;30(6):917–22. doi:.https://doi.org/10.1016/j.ejcts.2006.08.022
12 Kantorova A , Zbieranek K , Sauer H , Lilje C , Haun C , Hraska V . Primary early correction of tetralogy of Fallot irrespective of age. Cardiol Young. 2008;18(2):153–7. doi:.https://doi.org/10.1017/S1047951108001960
13 Benbrik N , Romefort B , Le Gloan L , Warin K , Hauet Q , Guerin P , et al. Late repair of tetralogy of Fallot during childhood in patients from developing countries. Eur J Cardiothorac Surg. 2015;47(3):e113–7. doi:.https://doi.org/10.1093/ejcts/ezu469
14 Raj R , Puri GD , Jayant A , Thingnam SKS , Singh RS , Rohit MK . Perioperative echocardiography-derived right ventricle function parameters and early outcomes after tetralogy of Fallot repair in mid-childhood: a single-center, prospective observational study. Echocardiography. 2016;33(11):1710–7. doi:.https://doi.org/10.1111/echo.13333
15 Waqar T , Riaz MU , Mahar T . Tetralogy of Fallot repair in patients presenting after Infancy: A single surgeon experience. Pak J Med Sci. 2017;33(4):984–7. doi:.https://doi.org/10.12669/pjms.334.12891
16 Yang S , Wen L , Tao S , Gu J , Han J , Yao J , et al. Impact of timing on in-patient outcomes of complete repair of tetralogy of Fallot in infancy: an analysis of the United States National Inpatient 2005-2011 database. BMC Cardiovasc Disord. 2019;19(1):46. doi:.https://doi.org/10.1186/s12872-019-0999-1
17 Bakhtiary F , Dähnert I , Leontyev S , Schröter T , Hambsch J , Mohr FW , et al. Outcome and incidence of re-intervention after surgical repair of tetralogy of fallot. J Card Surg. 2013;28(1):59–63. doi:.https://doi.org/10.1111/jocs.12030
18 Stewart RD , Backer CL , Young L , Mavroudis C . Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. Ann Thorac Surg. 2005;80(4):1431–8, discussion 1438–9. doi:.https://doi.org/10.1016/j.athoracsur.2005.04.016
19 Simon BV , Swartz MF , Egan M , Cholette JM , Gensini F , Alfieris GM . Use of a Dacron Annular Sparing Versus Limited Transannular Patch With Nominal Pulmonary Annular Expansion in Infants With Tetralogy of Fallot. Ann Thorac Surg. 2017;103(1):186–92. doi:.https://doi.org/10.1016/j.athoracsur.2016.05.056
20 Hickey E , Pham-Hung E , Halvorsen F , Gritti M , Duong A , Wilder T , et al. Annulus-Sparing Tetralogy of Fallot Repair: Low Risk and Benefits to Right Ventricular Geometry. Ann Thorac Surg. 2018;106(3):822–9. doi:.https://doi.org/10.1016/j.athoracsur.2017.11.032
21 Woldu KL , Arya B , Bacha EA , Williams IA . Impact of neonatal versus nonneonatal total repair of tetralogy of fallot on growth in the first year of life. Ann Thorac Surg. 2014;98(4):1399–404. doi:.https://doi.org/10.1016/j.athoracsur.2014.05.034
22 Tamesberger MI , Lechner E , Mair R , Hofer A , Sames-Dolzer E , Tulzer G . Early primary repair of tetralogy of fallot in neonates and infants less than four months of age. Ann Thorac Surg. 2008;86(6):1928–35. doi:.https://doi.org/10.1016/j.athoracsur.2008.07.019
No financial support and no other potential conflict of interest relevant to this article were reported.