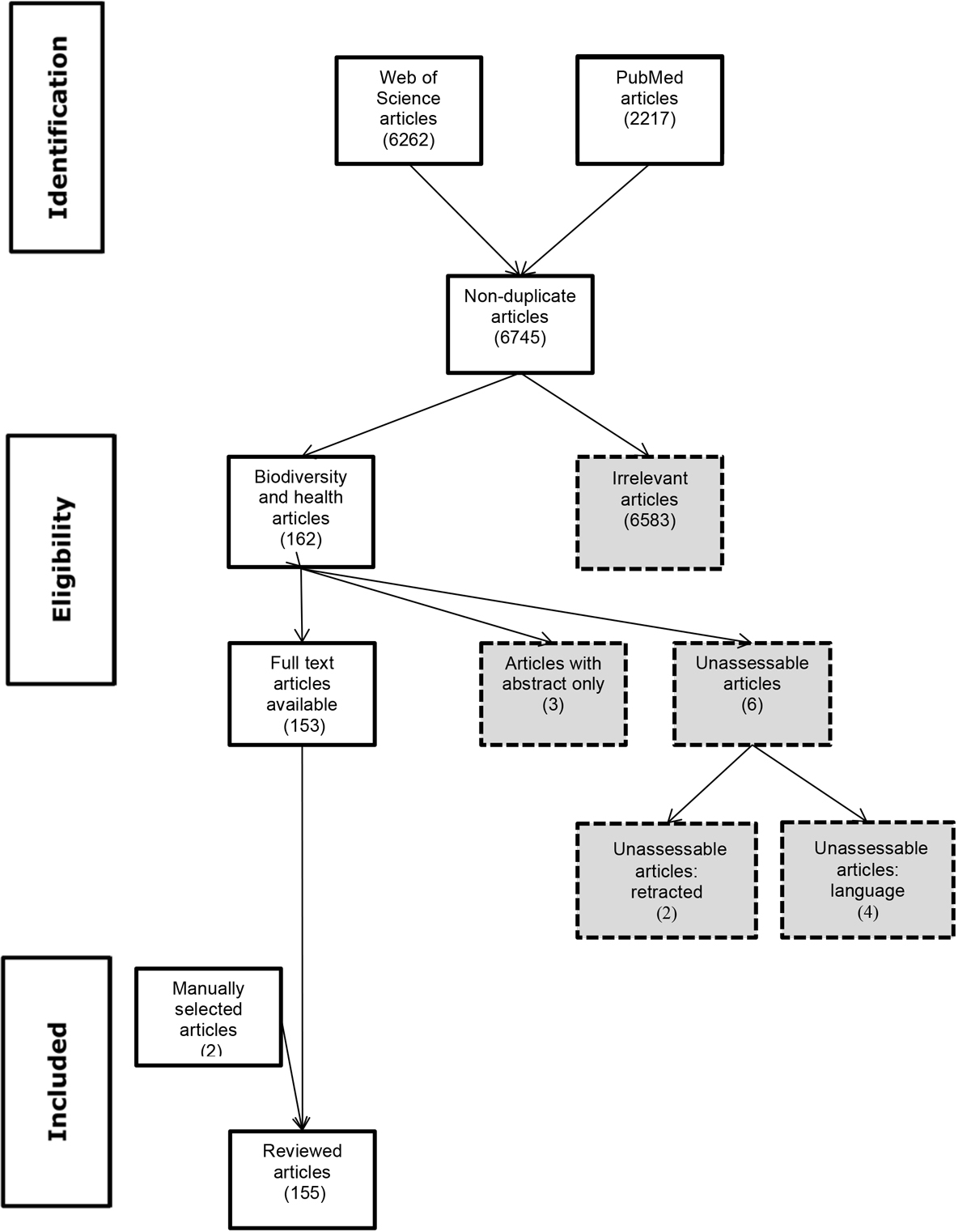
Figure 1 PRISMA flow diagram.
DOI: https://doi.org/10.4414/smw.2021.20485
Biodiversity has intrinsic value and a fundamental role in sustainable development and human health. Biodiversity is necessary for proper ecosystem functioning and provision of ecosystem services, but it is not yet well-delineated which specific processes are primarily important in sustaining human health [1, 2]. Huynen et al. [3] described “health functions” provided by ecosystems, one of which could be summarised as “disease regulation”. Earlier discussions relating biodiversity to human health centred on provisioning services, with both scientific and public health communities advocating preservation of biodiversity as crucial for future human health [4]. This line of argument focused more on conservation than on biodiversity itself [5]. The Millenium Ecosystem Assessment broadly defined ecosystem services and considered links to many facets of human well-being [6]. Available tools in the fields of ecology and economy were applied to people-nature relationships, framing them as stock-and-flow processes, but social science and local practitioner perspectives were marginalised [7]. The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), a joint global effort by government, academia and civil society, further refined a conceptual framework, extending the idea of ecosystem services to encompass nature’s contributions to people [8]. In view of the rapidly evolving landscape, we investigated the current body of scientific evidence for specific links between biodiversity and human infectious and non-communicable disease, aiming to characterise identifiable relationships or mechanisms. Reliable evidence would be valuable for decision makers to adequately inform effective sustainable environmental and health related policies.
Biodiversity: the variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part: this includes diversity within species, between species and of ecosystems [9].
Ecosystem services: the benefits people obtain from ecosystems; four categories are distinguished – supporting services, provisioning services, regulating services and cultural services [6].
Health: a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity [10].
Nature’s contribution to people: all the benefits that humanity – individuals, communities, societies, nations or humanity as a whole, in rural and urban settings – obtains from nature, encompassing ecosystem goods and services, which include provisioning, regulating and cultural services and nature’s gifts [11].
A search of the databases PubMed and Web of Science (WoS) on 16 April 2019 used key word algorithms to identify manuscripts related to biodiversity and health published from 2000 onwards. In PubMed, the search string was (biodiversity[Title/Abstract]) AND health[Title/Abstract]) OR (biodiversity[Title/Abstract]) AND (disease*[Title/Abstract] OR transmission[Title/Abstract]), resulting in 2217 hits. In WoS, the search string was (TS=(biodiversity AND health) OR (biodiversity AND (disease* OR transmission) AND LANGUAGE: (English), resulting in 6262 hits. EndNote™ bibliographic software was used to manage the citations. Duplicates were removed. Title and abstract review identified irrelevant studies. Inclusion criteria were studies investigating links between or mechanisms linking biodiversity and infectious disease, non-communicable disease, allergic/inflammatory disease and microbiomes. Exclusion criteria were irrelevant topic, based on title screening (e.g., land use, green space, medicinal plants, environmental/ecosystem services, invasive/introduced species, biodiversity conservation); health of non-human organisms; links connecting biodiversity and well-being / mental health, agricultural productivity, food security, human nutrition, health / air quality, health / noise mitigation, health / pharmaceutical development, health/water (fresh, ocean or wetland), and health/microclimates. Method studies (e.g., control efforts) and studies on the impact of climate change on biodiversity were excluded. Also excluded were studies with an abstract only, retracted studies and those in languages we were unable to translate (two Finnish, one Korean, one Chinese). The full text was reviewed for manuscripts fulfilling the inclusion criteria. Two additional studies were included following targeted review of included study bibliographies. The PRISMA flow diagram is depicted in figure 1. Atlas.ti™ 7 software was used to extract data for qualitative coding from the selected articles.

Figure 1 PRISMA flow diagram.
The 155 articles (see supplementary table S1 in the appendix) selected for this review focused specifically on biodiversity as it relates to human health in the context of infectious disease (92 papers) and non-communicable disease (36 papers). Twenty-seven manuscripts did not separate the two disease categories. Sixty-two papers were review articles, and the remainder were investigative studies, further categorised as observational (23), modeling (26), laboratory (3) or field (2) experimental, ecological (7) or not classifiable (32).
We identified manuscripts on the topic of infectious disease, including both outbreaks and emergence of directly transmitted and vector-borne infections, relevant to biodiversity. Selected studies investigated the zoonoses bartonellosis [12], bovine tuberculosis [13, 14], echinococcosis [15], hantavirus [16–22], leptospirosis [23], Lyme disease [18–22] and West Nile virus [18, 21, 24–27], and vector-borne diseases Chaga’s disease [28], malaria [18, 29, 30], schistosomiasis [12, 18, 31] and tick-borne encephalitis [32].
In 92 studies investigating links between biodiversity and infectious disease, two main mechanisms are proposed to influence transmission, dilution and amplification. The dilution hypothesis argues that an increase in species diversity leads to a decrease in pathogen prevalence. The assumption, at the local level, is that an increase in the number of non-host species decreases the number of intra-species encounters between infected and susceptible hosts, leading to a decrease in pathogen transmission rate and prevalence [33, 34]. Theoretical models, laboratory experiments, and observational and experimental field studies provide support to validate this hypothesis [35], but the generalisability of the concept is robustly debated [36–39]. The amplification effect is the converse of the dilution hypothesis, that there is a positive correlation between species diversity and disease risk / infection prevalence [13].
A number of drivers are postulated to influence these two different mechanisms. Several impact community structure and assemblage, including encounter reduction [13, 16], susceptible host regulation through interspecies competition or predation, which limits the abundance of competent hosts [13], and competition for food [12, 31]. All of these factors ultimately regulate host abundance and population density [40]. Community assemblage is complex, encompassing species richness (number of species), species evenness (relative abundance of the different species) and species composition (specific identities of species) [33]. It is suggested that species loss is not a random process. Species that are less able to adapt to changes in their environment could be more likely to disappear, whereas those that are resilient should increase to dominate local host communities [41], acting as vectors and pathogen reservoirs [42]. Host competence, the ability of a host to transmit disease, is an influential driver, which impacts patterns of transmission [12]. Biologists differentiate between low competence (alternative) hosts and decoy (completely unsuitable) hosts, which result in wasted transmission opportunities [12, 21, 43].
The controversy about the postulated hypothesis of the relationship of health and biodiversity is also partially due to important overlaying phenomena that mutually affect each other and are difficult to disentangle [44, 45]. These are, among others, human and livestock population growth (particularly for chickens and pigs), habitat destruction and deforestation [46], (illegal) wildlife trade, low biosecurity and poor animal welfare in livestock production. The recent spill-over and rapid spread of SARS-Cov2 into Danish captive mink [47], kept under extremely crowded and unsanitary conditions, could be considered as a negative example, thereby supporting the dilution hypothesis, but is clearly also related to intensive livestock production. The associated culling of 17 million mink in Denmark reflects animal mass killing and sheds an appalling light on current humane standards in animal production. Similarly, the so called “palm oil hypothesis” argues that the deforestation and planting of oil palms dislocated bats from their natural habitat and exposed oil palm plantation workers to the risk of Ebola spill-over, whereas the same populations would have hardly been exposed to bats in a pristine rain forest in the same area [46].
It is widely agreed that scale has an outsized impact in the context of infectious disease. A recent review considered drivers of Plasmodium knowlesi transmission over multiple spatial scales from molecular to regional [29]. Identified factors influencing spread of disease were parasite-host evolutionary dynamics, diversity, abundance and range of host and vector species, and the spatial and temporal overlap between them. Untangling and understanding the causal mechanisms that generate diversity-disease relationships is only possible when collected data relate spatially and temporally to the relevant outcomes [27, 48–50], highlighting the importance of local scale. For instance, although species diversity may increase disease risk on a local scale (amplification), the mechanism of encounter reduction could operate at larger scales, resulting in an overall dilution effect [51]. Johnson and Thieltges [12] illustrate the issue of divergence of spatial scale with two examples. Trematode miracidia are short lived and demonstrate significant spatial heterogeneity within a single wetland ecosystem, whereas tick vectors are comparatively long lived and travel on vertebrate hosts, vastly increasing their spatial scale of transmission. Habitat properties (land use, fragmentation) play an additional role in driving the mechanisms of dilution versus amplification [20], further supporting the argument for careful consideration of scale. Confounding becomes even more important when incidence data are collected from larger spatial scales than those in which transmission occurs [52], and often studies overlook spatial and temporal divergence [50].
Much of the research on the effect of biodiversity on infectious disease transmission has been on vector-borne diseases that are tick or mosquito transmitted. The inclusion of disease vectors makes transmission systems less tractable. This is because the dynamic interactions of (multiple) reservoir wildlife hosts, insect or arthropod vectors, livestock, companion animals and humans are highly complex and there are very few datasets that address their spatio-temporal dynamics in relation to biodiversity.
Directly transmitted diseases have received less attention, although they are easier to analyse from a mathematical perspective. We refer here to a recent study on Sin Nombre hantavirus modelled infection dynamics, using a multisite dataset to show that dilution and amplification occur at the same time in the same host-pathogen system [17]. There was an amplification effect, an increase in transmission rate, but overall net dilution, because the effect of diversity on the reservoir host population density was stronger. The authors conclude that how biodiversity affects individual mechanisms driving prevalence and their relative strengths must be considered in order to make generalisable predictions.
A closer look at the related mathematical approach allows a better understanding of the phenomena involved. The authors use a susceptible-infectious-recovered framework which conceptualises the drivers of the transmission, as depicted in equations 1 and 2 and figure 2, as frequency- and density-dependent processes.

Equations 1 and 2
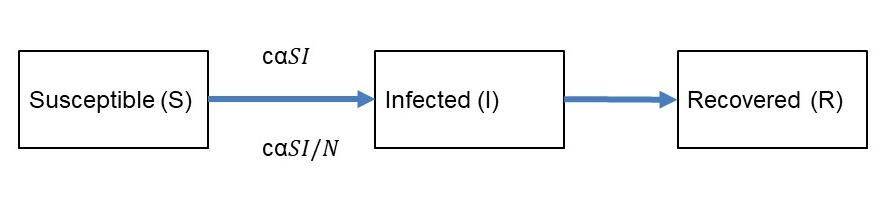
Figure 2 Flow chart of the transmission of an infectious disease in a given population.
Density-dependent transmission is thereby proportional to the contact rate c between susceptible (S) and infected (I) individuals and the probability of transmission (transmissibility) α. The transmission rate β = c α is considered as the product of the contact rate and the transmissibility of a pathogen. Frequency dependence is observed if the transmission depends on the frequency of infected among the total population N = (S + I + R) as (I/N). From these equations, we can infer the relationship of biodiversity and disease transmission in figure 3. Dilution will occur if the host population density decreases and if contact rates and/or transmissibility, which is also dependent on host susceptibility, are decreased. An amplification would occur if increased species diversity leads to an increase of host density, contact rates and/or transmissibility. In conclusion, the dilution effect is confirmed if the host density drives the transmission process over the transmissibility of the pathogen. It has an important spatial or contextual aspect, showing that smaller scale transmission dynamics can indicate an amplification effect if transmissibility dominates over host density. This example shows that biodiversity is clearly related to the transmission of infectious diseases, but that this relationship is complex and scaled. It has to be disentangled for every specific pathogen and ecosystem in relation to humans in relation to urban and agro-ecosystems.
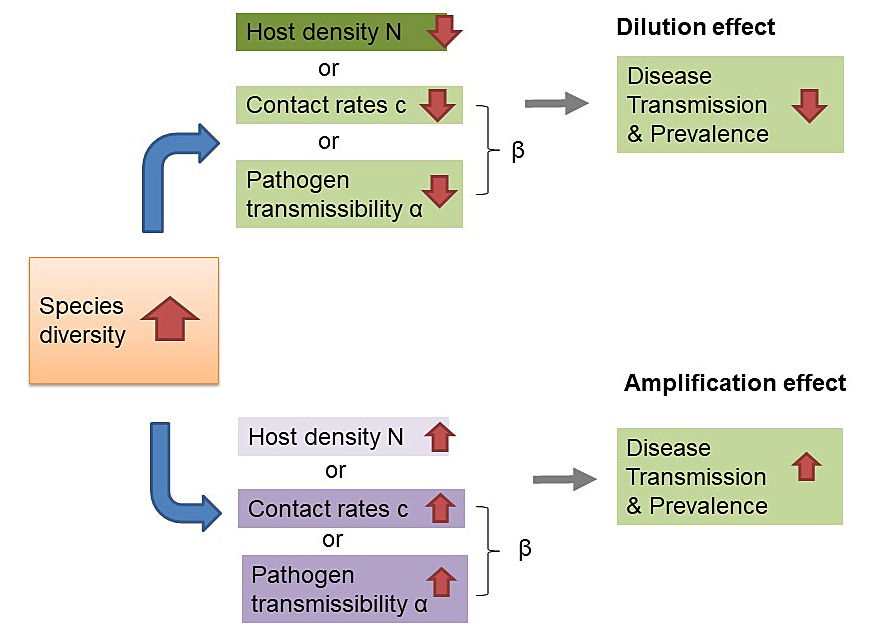
Figure 3 Graphical depiction of how species diversity could affect transmission and prevalence of a directly transmitted disease (adapted from Luis et al. [17]).
Human exposure risk from infectious diseases of animal origin (zoonoses) can be estimated from animal-human transmission models. To our knowledge, very few such models exist and almost none address wildlife to human transmission. The so-called zoonotic potential of an infectious disease, the risk of transmission from animals to humans, can be estimated by the fraction of the transmission rates between animals βa and between animals and humans βah as βah/βa. For example the zoonotic potential of Brucella melitensis from small ruminants to humans in Mongolia is 1/15, whereas for Brucella abortus from cattle to humans it is 1/110. This shows that, in the same ecosystem, the risk of transmission of B. melitensis to humans is almost ten times higher than for B. abortus [53]. The risk of human rabies exposure from dogs in Chad in Central Africa is 1/400 [54] and the risk of transmission of bovine tuberculosis (Mycobacterium bovis) from cattle to humans is <1/1000 in Morocco [55]. These examples show how variable human exposure to directly transmitted zoonoses (stage 2) is and how dependent it is from a given agro-ecosystem. Wildlife reservoirs play a role in some of them [56, 57]. For example for M. bovis, the badger (Meles meles) is a reservoir host in the United Kingdom, which leads to continuous re-infection of cattle on pasture, and makes elimination of the disease almost impossible. Similarly, wild boar (Sus scrofa) and red deer (Cervus elaphus) carry M. bovis in some continental European countries. One could argue, as an example for the biodiversity-health link, that the introduction of large predators, such bears or wolves, could reduce red deer populations and hence reduce the risk of transmission of M. bovis to cattle, but this requires more research.
We identified 36 manuscripts relevant to the effects of biodiversity on non-communicable diseases, within the broad grouping of allergic and inflammatory disease (including asthma), cardiovascular disease, and diabetes, and the relationship of microbiomes to health. Numerous studies suggest that microbe-rich environments are protective against inflammatory and autoimmune disease [58, 59], but recent work proposes that declining biodiversity more likely contributes to human immune dysfunction [60, 61]. The hygiene hypothesis proposes that modern lifestyles do not expose people to the microbial diversity (“old friends” or “keystone bacteria”) with which the human immune system evolved and which it requires for normal maturation [2, 62, 63]. This extends to the biodiversity hypothesis, that lack of exposure to natural environments and associated microbial diversity leads to dysbiosis in the human commensal microbiota, immune dysfunction and clinical disease [60, 64, 65]. Support for this hypothesis comes from work suggesting that the gut microbiome interacts with the immune system to maintain immune function [66], because factors in the neonatal period, such as Caesarean delivery, breastfeeding and antibiotic use, are associated with increased incidence of asthma and allergic disease. Debate continues regarding the relative importance of sources of microbial exposure, including microbe diversity and key species, during both early development and later life [60, 61, 65]. Metagenomics sequencing indicates that heritability also plays a role in the intestinal microbiome, in contrast to that of the skin [67].
Recent findings on the effects of chronic malnutrition in children provide further support to the importance of microbial diversity in the gastrointestinal microbiome, relating the macro-level effect of lacking food diversity [68] and quantity to resulting dysbiosis [69]. The resulting chronic inflammation of the gut leads to stunting, with consequent long-standing complex processes resulting in impaired child growth, long after adequate nutrition is made available again. The microbial dysbiosis further facilitates colonisation with pathogenic or potentially pathogenic bacteria, such as Escherichia coli / Shigella sp. and Campylobacter sp. This work led to a revision of the Koch’s postulates of the single microbial origin of a specific infectious disease, with the emergence of the “ecological Koch’s postulates”. The ecological Koch’s postulates recognise that the gut harbours a full ecosystem of microorganisms, forming an entity that can lead ultimately to disease, as a disease-promoting ecosystem (dysbiosis). Remarkably, dysbiotic microbiota can be found in similar composition, can be retrieved from an affected host and can be transmitted to germ-free hosts where they remain fairly stable [70].
There is strong evidence that a child’s early environment, including maternally transferred prenatal signals, affects immune maturation, which modifies later disease risk [61]. Microbiota of the gut, skin and respiratory tract activate innate and regulatory networks, which contribute to healthy immune function [60, 71, 72]. Experimental work supports the idea that early postnatal colonisation of the intestine with microbiota self-induces temporal activation of bacterial sensors, which influence intestinal barrier function and humoral immunity [73]. Commensal microbes use toll-like receptor signals to maintain mucosal homeostasis [74], and regulation of intestinal permeability is affected by microbial shifts associated with low-grade inflammation [62]. Animal models indicate that cellular communication occurs through protein inducers; however, current understanding of the role of microbial colonisation dynamics is limited [75].
Bi-directional axes communicate between the gut microbiome and the brain, as well as between the skin microbiome and the lung, including direct and indirect immune, humoral and neural mechanisms [59, 71, 76]. The nervous system and pulmonary immune responses play important signalling roles related to normal function versus disease states, but current experimental studies only investigated single microbial niches, whereas it is highly likely that microbial complexity affects multiple niches [59, 77].
Expanding on the biochemical, immunological and microbial effects of biodiversity on non-communicable diseases, there is growing body of knowledge on the positive effects of nature on human mental health and wellbeing [78]. The Intergovernmental Science-Policy Platform for Biodiversity and Ecosystem Services (IPBES) has named these benefits and services as Nature’s Contributions to People (NCP) [79]. Besides the material benefits of nature, such as food and building material, there are numerous non-material benefits. For example, good access to vegetated areas is related to better cognitive function, fewer symptoms of depression, lower stress and lower risk of psychiatric disorders. A most recent and compelling example is that bird species richness is positively associated with life satisfaction across Europe [80].
A main challenge in assessing links between biodiversity and health is the vast heterogeneity encountered. There is wide variation in the definitions used for health and, particularly, for biodiversity [2, 33, 60], often related to the level of variability considered, which makes comparisons difficult or impossible. Few studies considered direct measures, with nearly all looking at indirect proxy indicators for biodiversity [3, 14, 27, 50]. A third important heterogeneity was found in study type, which encompassed modelling studies [26], including multi-host and network-based, and comparative observational studies [23]. Laboratory-based experiments [3] and applied field studies in natural systems [2] were few and had small sample sizes (table S1 in the appendix).
Complexity was an often cited theme in the studies and review articles included. An understanding of relevant ecology is considered crucial, but generally remains incomplete owing to the myriad interactions and dynamic nature of the systems [49, 81]. Use of multi-host species epidemiological models indicates that finding generalisable diversity-disease patterns across host-pathogen systems in the field is more difficult than previously appreciated [40]. Disease transmission dynamics can be both density and frequency dependent, with consideration of biology and behaviour of hosts, vectors and pathogens [12, 15, 16, 81, 82], resulting in a high degree of variation and complexity. For example, Dobson and Auld [83] differentiate between biting insects that typically take multiple small blood meals and most ticks, which typically take only three large blood meals throughout adult life. In the context of dilution, wasted bites are far more influential in disease transmission for tick-borne than for insect-borne pathogens. Human behaviour adds additional complexity [84], which is more widely represented by social context [49] and global anthropogenic trends with environmental impacts, such climate change, nutrient pollution, protracted political/armed conflict and economic collapse [42, 85]. Untangling specific interactions in a temporal and spatial context and understanding transmission dynamics remains a major challenge.
Several studies noted uncertainty [71, 84, 86], lack of convincing field evidence [19], lack of validation criteria [87] or failure to consider confounding [85]. Others described limitations precluding determination of causality, including the need for temporal studies [23], the difficulty in decoupling change in socioeconomic status from health status changes [2], and the likely non-linearity of diversity-disease risk relationships [3, 30, 52]. Investigators noted that evidence was mixed [88] or even contrasting [87]. Both complexity of transmission dynamics [25, 37, 89] and spatial scale were suggested as explanatory [27]. For example, an important distinction is the concept of hazard, such as the presence of a pathogen in an environment, versus risk, such as the probability of being infected. Risk is relevant from a health perspective. Biodiversity drivers, notably those influenced by anthropogenic change, for example, land use, likely act differently in transmission of endemic pathogens than they do for disease emergence in humans [85]. A recent review of disease ecology studies catalogued sources of uncertainty into six broad overlapping categories: intrinsic biological factors associated with host-pathogen interaction; demographic misclassification of hosts; incomplete taxonomic knowledge on host-pathogen systems; mismatch in sampling scales; imprecision in diagnostic methods; and additional environmental modifying effects within each of the categories [90]. The authors concluded that appropriate sampling and analytical methods could account for or minimise the influence of uncertainty. An additional criticism noted was publication bias [87], identified as bias towards publishing reports of a negative relationship between biodiversity and disease [91].
None of 155 reviewed studies documented causal evidence for a mechanism linking biodiversity and human health.
Dilution and amplification are the two main mechanisms thought to influence the effect of biodiversity on transmission. Numerous natural and anthropogenic drivers regulate host abundance and population density. Scale, both spatial and temporal, highly impacts diversity-disease relationships.
The “biodiversity hypothesis” states that lack of exposure to microbial diversity in natural environments leads to imbalance of human commensal microbiota, immune dysfunction and clinical disease. There is strong evidence that a child’s early environment, including maternally transferred prenatal signals, affects immune maturation, modifying later disease risk.
There are widely heterogeneous definitions, approaches and methods used between biodiversity and health research areas. Complexity of ecology and variability of human demographic, socioeconomic and cultural systems contribute to uncertainty in research results.
None of the 155 studies reviewed documented causal evidence for a mechanism linking biodiversity and human health. A main finding is the variability and complexity of the links between biodiversity and health, depending on disease system, local ecology and probably also human social context [27, 49], although the latter was not a focus of this review. The interactive relationships between biodiversity, disease risk/transmission and anthropogenic change are important, and, although difficult to account for, must be considered [49, 85]. The relevance of local scale [92] is perhaps not a surprising finding, but may counter current thinking that “bigger data” is necessarily better.
Based on the reviewed body of evidence linking biodiversity to human infectious and non-communicable disease, a future research agenda to determine the net effects of diversity must include integrative approaches to combine different perspectives [27, 52]. A recent study on Chaga’s disease developed two species-distribution models and combined them with national health data to analyse a causality assumption and investigate consistency across scales [28]. Although species composition better predicted the observed pattern of Chaga’s disease occurrence than did species richness, only 5% of the variability was explained. The authors concluded that macroecology of infectious diseases must go further than analysis of biodiversity patterns and consider human infection as part of the ecological system. As we described above for directly transmitted infectious diseases, human exposure can be quite variable and is dependent on a given agro-ecosystem. Species distribution models must be appropriately utilised to accurately predict infection rates. Utilising landscape scale epidemiology would allow consideration of local scale, transmission dynamics and biogeographical regionality [49]. However, observational approaches need to be combined with experimental manipulation in communities and models that include community feedbacks across gradients incorporating natural ecosystems [36, 84]. Cost-effectiveness and cost-benefit analyses are often missing, yet crucially needed by policy makers in order to make key decisions [18]. Innovative, strategic responses will require interdisciplinary approaches. The Rockefeller Foundation-Lancet Commission on planetary health reviewed the scientific basis linking human health to the underlying integrity of the Earth’s natural systems and identified substantial gaps in knowledge related to improving planetary health, including human health [93]. Their report concluded with recommendations to develop a holistic integrated research agenda, including proposals to address conceptual, research/informational and governance challenges. Our review indicates that this scientific knowledge gap still exists, in particular regarding scientific evidence for specific links between biodiversity and human infectious and non-communicable disease to characterise identifiable relationships.
Although studies on the interface between biodiversity and health have increased understanding of how changes in biological diversity affect health outcomes, there is still not sufficient reliable evidence to robustly inform environmental and health policies [18, 94]. Although many policy decisions are made at national level, in order to be an effective lever for improving public health, engagement must occur across levels because of the relevance of local scale [50, 61]. Contextual adaptation is imperative. Dialogue must be across disciplines, effectively including the diversity of knowledge of all stakeholders, to adequately address the complexity of relationships between biodiversity and health. The inclusive IPBES conceptual framework, utilising the “nature’s contributions to people” approach, specifically acknowledges the range of existing views [11]. Blending a generalising perspective with a context-specific perspective allows for co-construction of knowledge among disciplines and knowledge systems [95], which can be applied to generate more legitimate practices and translate into sustainable, equitable policies. In December 2017, the Executive Secretary of the Convention on Biological Diversity produced technical guidelines [96], which support consideration of biodiversity and ecosystem management in application of One Health approaches. The guidelines include a “State of Knowledge Review” detailing specific recommendations in 12 areas where health and biodiversity connect, and providing a road map for the future. Our review indicates that the scientific evidence on causal mechanisms between biodiversity and human infectious and non-communicable diseases is to date wholly inadequate. The post-2020 biodiversity framework should surely include resources to consider such linkages, and we suggest recommendations for action.
Develop evidence-based answers to open questions in interdisciplinary research programs combining different perspectives through integrative approaches [27, 52].
Harmonise methodologies and assessment methods. Include epidemiological and longitudinal studies [84].
Analyse the cost-effectiveness and cost-benefit ratios of the impact of biodiversity on human health from a life-course perspective to support decision-makers in politics and business [18].
Develop innovative, strategic solutions to improve the knowledge base through interdisciplinary contextual approaches [93].
Maintain dialogue across disciplines and include diversity of knowledge of all stakeholders to address complexity of relationships between biodiversity and health.
Emphasise the contribution of biodiversity in addressing priority health issues and identify facts and synergies on health benefits and risks.
Adapt communication on health benefits of biodiversity to the interests of different stakeholders.
Promote knowledge development in relevant disciplines and transdisciplinary approaches to integrate into sustainable policies.
Highlight links between climate change, human health and biodiversity policies and develop cross-sectoral approaches to exploit synergies.
Develop political strategies across different spatial levels since decisions are often made nationally, but local scale impacts public health [50, 61].
The appendix is available in the pdf version of this article.
The authors are grateful to Oliver Balmer and Jan Hattendorf for guidance on the method and critical review of the manuscript.
No financial support and no other potential conflict of interest relevant to this article were reported.
1 Aerts R Honnay O Van Nieuwenhuyse A . Biodiversity and human health: mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br Med Bull. 2018;127(1):5–22. doi:.https://doi.org/10.1093/bmb/ldy021
2 Hough RL . Biodiversity and Human Health: Evidence for Causality? Biodivers Conserv. 2014;23(2):267–88. doi:.https://doi.org/10.1007/s10531-013-0614-1
3 Huynen MM Martens P De Groot RS . Linkages between biodiversity loss and human health: a global indicator analysis. Int J Environ Health Res. 2004;14(1):13–30. doi:.https://doi.org/10.1080/09603120310001633895
4 Chivian E Bernstein AS . Embedded in nature: human health and biodiversity. Environ Health Perspect. 2004;112(1):A12–3. doi:.https://doi.org/10.1289/ehp.112-a12
5 Ostfeld RS Keesing F . Biodiversity and Disease Risk: The Case of Lyme Disease. Conserv Biol. 2000;14(3):722–8. doi:.https://doi.org/10.1046/j.1523-1739.2000.99014.x
6Assessment ME. Ecosystems and Human Well-Being: Synthesis. Washington, DC: Island Press; 2005.
7 Norgaard RB . Ecosystem Services: From Eye-Opening Metaphor to Complexity Blinder. Ecol Econ. 2010;69(6):1219–27. doi:.https://doi.org/10.1016/j.ecolecon.2009.11.009
8 Díaz S Demissew S Carabias J Joly C Lonsdale M Ash N The IPBES Conceptual Framework — Connecting Nature and People. Curr Opin Environ Sustain. 2015;14:1–16. doi:.https://doi.org/10.1016/j.cosust.2014.11.002
9United Nations. Convention on Biodiversity. Rio de Janeiro: United Nations; 1992. p. 214.
10WHO. Constitution of the World Health Organization. Geneva, Switzerland: World Health Organization; 1948.
11 Díaz S Pascual U Stenseke M Martín-López B Watson RT Molnár Z Assessing nature’s contributions to people. Science. 2018;359(6373):270–2. doi:.https://doi.org/10.1126/science.aap8826
12 Johnson PT Thieltges DW . Diversity, decoys and the dilution effect: how ecological communities affect disease risk. J Exp Biol. 2010;213(6):961–70. doi:.https://doi.org/10.1242/jeb.037721
13 Huang ZY de Boer WF van Langevelde F Xu C Ben Jebara K Berlingieri F Dilution Effect in Bovine Tuberculosis: Risk Factors for Regional Disease Occurrence in Africa. Proc Biol Sci. 2013;280(1765):624. doi:. https://doi.org/10.1098/rspb.2013.0624
14 Sintayehu DW Heitkönig IMA Prins HHT Tessema ZK DE Boer WF . Effect of host diversity and species assemblage composition on bovine tuberculosis (bTB) risk in Ethiopian cattle. Parasitology. 2017;144(6):783–92. doi:.https://doi.org/10.1017/S0031182016002511
15 Giraudoux P Raoul F Pleydell D Li T Han X Qiu J Drivers of Echinococcus multilocularis transmission in China: small mammal diversity, landscape or climate? PLoS Negl Trop Dis. 2013;7(3):e2045. doi:.https://doi.org/10.1371/journal.pntd.0002045
16 Dizney LJ Ruedas LA . Increased host species diversity and decreased prevalence of Sin Nombre virus. Emerg Infect Dis. 2009;15(7):1012–8. doi:.https://doi.org/10.3201/eid1507.081083
17 Luis AD Kuenzi AJ Mills JN . Species diversity concurrently dilutes and amplifies transmission in a zoonotic host-pathogen system through competing mechanisms. Proc Natl Acad Sci USA. 2018;115(31):7979–84. doi:.https://doi.org/10.1073/pnas.1807106115
18 Pongsiri MJ Roman J Ezenwa VO Goldberg TL Koren HS Newbold SC Biodiversity Loss Affects Global Disease Ecology. Bioscience. 2009;59(11):945–54. doi:.https://doi.org/10.1525/bio.2009.59.11.6
19 Bouchard C Beauchamp G Leighton PA Lindsay R Bélanger D Ogden NH . Does high biodiversity reduce the risk of Lyme disease invasion? Parasit Vectors. 2013;6(1):195. doi:.https://doi.org/10.1186/1756-3305-6-195
20 Ehrmann S Ruyts SC Scherer-Lorenzen M Bauhus J Brunet J Cousins SAO Habitat properties are key drivers of Borrelia burgdorferi (s.l.) prevalence in Ixodes ricinus populations of deciduous forest fragments. Parasit Vectors. 2018;11(1):23. doi:.https://doi.org/10.1186/s13071-017-2590-x
21 Ostfeld RS . Biodiversity loss and the rise of zoonotic pathogens. Clin Microbiol Infect. 2009;15(Suppl 1):40–3. doi:.https://doi.org/10.1111/j.1469-0691.2008.02691.x
22 Ruyts SC Landuyt D Ampoorter E Heylen D Ehrmann S Coipan EC Low probability of a dilution effect for Lyme borreliosis in Belgian forests. Ticks Tick Borne Dis. 2018;9(5):1143–52. doi:.https://doi.org/10.1016/j.ttbdis.2018.04.016
23 Derne BT Fearnley EJ Lau CL Paynter S Weinstein P . Biodiversity and leptospirosis risk: a case of pathogen regulation? Med Hypotheses. 2011;77(3):339–44. doi:.https://doi.org/10.1016/j.mehy.2011.05.009
24 Ezenwa VO Godsey MS King RJ Guptill SC . Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc Biol Sci. 2006;273(1582):109–17. doi:.https://doi.org/10.1098/rspb.2005.3284
25 Roche B Rohani P Dobson AP Guégan JF . The impact of community organization on vector-borne pathogens. Am Nat. 2013;181(1):1–11. doi:.https://doi.org/10.1086/668591
26 Werden L Barker IK Bowman J Gonzales EK Leighton PA Lindsay LR Geography, deer, and host biodiversity shape the pattern of Lyme disease emergence in the Thousand Islands Archipelago of Ontario, Canada. PLoS One. 2014;9(1):e85640. doi:.https://doi.org/10.1371/journal.pone.0085640
27 Wood CL Lafferty KD . Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28(4):239–47. doi:.https://doi.org/10.1016/j.tree.2012.10.011
28 Eduardo AA Santos LABO Rebouças MC Martinez PA . Patterns of vector species richness and species composition as drivers of Chagas disease occurrence in Brazil. Int J Environ Health Res. 2018;28(6):590–8. doi:.https://doi.org/10.1080/09603123.2018.1497776
29 Davidson G Chua TH Cook A Speldewinde P Weinstein P . Defining the ecological and evolutionary drivers of Plasmodium knowlesi transmission within a multi-scale framework. Malar J. 2019;18(1):66. doi:.https://doi.org/10.1186/s12936-019-2693-2
30 Laporta GZ Lopez de Prado PI Kraenkel RA Coutinho RM Sallum MA . Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl Trop Dis. 2013;7(3):e2139. doi:.https://doi.org/10.1371/journal.pntd.0002139
31 Johnson PTJ Lund PJ Hartson RB Yoshino TP . Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proc Biol Sci. 2009;276(1662):1657–63. doi:.https://doi.org/10.1098/rspb.2008.1718
32 Palo RT . Tick-borne encephalitis transmission risk: its dependence on host population dynamics and climate effects. Vector Borne Zoonotic Dis. 2014;14(5):346–52. doi:.https://doi.org/10.1089/vbz.2013.1386
33Ostfeld RS, Keesing F. Effects of Host Diversity on Infectious Disease. In: Futuyma DJ, editor. Annual Review of Ecology, Evolution, and Systematics. 2012;43:157–82.
34 Schmidt KA Ostfeld RS . Biodiversity and the Dilution Effect in Disease Ecology. Ecology. 2001;82(3):609–19. doi:.https://doi.org/10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2
35 Civitello DJ Cohen J Fatima H Halstead NT Liriano J McMahon TA Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc Natl Acad Sci USA. 2015;112(28):8667–71. doi:.https://doi.org/10.1073/pnas.1506279112
36 Levi T Massey AL Holt RD Keesing F Ostfeld RS Peres CA . Does biodiversity protect humans against infectious disease? Comment. Ecology. 2016;97(2):536–42. doi:.https://doi.org/10.1890/15-354.1
37 Randolph SE Dobson AD . Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139(7):847–63. doi:.https://doi.org/10.1017/S0031182012000200
38 Wood CL Lafferty KD DeLeo G Young HS Hudson PJ Kuris AM . Does biodiversity protect humans against infectious disease? Ecology. 2014;95(4):817–32. doi:.https://doi.org/10.1890/13-1041.1
39 Wood CL Lafferty KD DeLeo G Young HS Hudson PJ Kuris AM . Does biodiversity protect humans against infectious disease? [Reply]. Ecology. 2016;97(2):543–6. doi:.https://doi.org/10.1890/15-1503.1
40 Mihaljevic JR Joseph MB Orlofske SA Paull SH . The scaling of host density with richness affects the direction, shape, and detectability of diversity-disease relationships. PLoS One. 2014;9(5):e97812. doi:.https://doi.org/10.1371/journal.pone.0097812
41 Ostfeld R Keesing F . The Function of Biodiversity in the Ecology of Vector-Borne Zoonotic Diseases. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2000;78(12):2061–78. doi:.https://doi.org/10.1139/z00-172
42 Keesing F Belden LK Daszak P Dobson A Harvell CD Holt RD Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–52. doi:.https://doi.org/10.1038/nature09575
43 Keesing F Ostfeld RS . Is biodiversity good for your health? Science. 2015;349(6245):235–6. doi:.https://doi.org/10.1126/science.aac7892
44 Butler CD . Infectious disease emergence and global change: thinking systemically in a shrinking world. Infect Dis Poverty. 2012;1(1):5. doi:.https://doi.org/10.1186/2049-9957-1-5
45 Butler CD . Plagues, Pandemics, Health Security and War on Nature. J Hum Secur. 2020;16(1):53–7. doi:.https://doi.org/10.12924/johs2020.16010053
46 Wallace RG Gilbert M Wallace R Pittiglio C Mattioli R Kock R . Did Ebola Emerge in West Africa by a Policy-Driven Phase Change in Agroecology? Environ Plann A. 2014;46:2533–42. doi:.https://doi.org/10.1068/a4712com
47 Koopmans M . SARS-CoV-2 and the human-animal interface: outbreaks on mink farms. Lancet Infect Dis. 2021;21(1):18–9. doi:.https://doi.org/10.1016/S1473-3099(20)30912-9
48 Johnson PT Preston DL Hoverman JT Henderson JS Paull SH Richgels KL Species diversity reduces parasite infection through cross-generational effects on host abundance. Ecology. 2012;93(1):56–64. doi:.https://doi.org/10.1890/11-0636.1
49 Salkeld DJ Padgett KA Jones JH . A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013;16(5):679–86. doi:.https://doi.org/10.1111/ele.12101
50 Wood CL McInturff A Young HS Kim D Lafferty KD . Human infectious disease burdens decrease with urbanization but not with biodiversity. Philos Trans R Soc Lond B Biol Sci. 2017;372(1722): 20160122. doi:.https://doi.org/10.1098/rstb.2016.0122
51 Huang ZY VAN Langevelde F Estrada-Peña A Suzán G DE Boer WF . The diversity-disease relationship: evidence for and criticisms of the dilution effect. Parasitology. 2016;143(9):1075–86. doi:.https://doi.org/10.1017/S0031182016000536
52 Kilpatrick AM Salkeld DJ Titcomb G Hahn MB . Conservation of biodiversity as a strategy for improving human health and well-being. Philos Trans R Soc Lond B Biol Sci. 2017;372(1722): 20160122. doi:.https://doi.org/10.1098/rstb.2016.0131
53 Zinsstag J Roth F Orkhon D Chimed-Ochir G Nansalmaa M Kolar J A model of animal-human brucellosis transmission in Mongolia. Prev Vet Med. 2005;69(1-2):77–95. doi:.https://doi.org/10.1016/j.prevetmed.2005.01.017
54 Zinsstag J Dürr S Penny MA Mindekem R Roth F Menendez Gonzalez S Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc Natl Acad Sci USA. 2009;106(35):14996–5001. doi:.https://doi.org/10.1073/pnas.0904740106
55 Abakar MF Yahyaoui Azami H Justus Bless P Crump L Lohmann P Laager M Transmission dynamics and elimination potential of zoonotic tuberculosis in morocco. PLoS Negl Trop Dis. 2017;11(2):e0005214. doi:.https://doi.org/10.1371/journal.pntd.0005214
56Tschopp R. Bovine Tuberculosis in Ethiopian Local Cattle and Wildlife: Epidemiology, Economics and Ecosystems. Doctoral Thesis, University of Basel, Faculty of Science. 2010.
57 Tschopp R Berg S Argaw K Gadisa E Habtamu M Schelling E Bovine tuberculosis in Ethiopian wildlife. J Wildl Dis. 2010;46(3):753–62. doi:.https://doi.org/10.7589/0090-3558-46.3.753
58 Karvonen AM Hyvärinen A Gehring U Korppi M Doekes G Riedler J PASTURE Study Group . Exposure to microbial agents in house dust and wheezing, atopic dermatitis and atopic sensitization in early childhood: a birth cohort study in rural areas. Clin Exp Allergy. 2012;42(8):1246–56. doi:.https://doi.org/10.1111/j.1365-2222.2012.04002.x
59 Schaub B Vercelli D . Environmental protection from allergic diseases: From humans to mice and back. Curr Opin Immunol. 2015;36:88–93. doi:.https://doi.org/10.1016/j.coi.2015.07.004
60 Haahtela T Holgate S Pawankar R Akdis CA Benjaponpitak S Caraballo L WAO Special Committee on Climate Change and Biodiversity . The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6(1):3. doi:.https://doi.org/10.1186/1939-4551-6-3
61 von Hertzen L Beutler B Bienenstock J Blaser M Cani PD Eriksson J Helsinki alert of biodiversity and health. Ann Med. 2015;47(3):218–25. doi:.https://doi.org/10.3109/07853890.2015.1010226
62 Logan AC Jacka FN Prescott SL . Immune-Microbiota Interactions: Dysbiosis as a Global Health Issue. Curr Allergy Asthma Rep. 2016;16(2):13. doi:.https://doi.org/10.1007/s11882-015-0590-5
63 Rook GA . Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci USA. 2013;110(46):18360–7. doi:.https://doi.org/10.1073/pnas.1313731110
64 Hanski I von Hertzen L Fyhrquist N Koskinen K Torppa K Laatikainen T Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci USA. 2012;109(21):8334–9. doi:.https://doi.org/10.1073/pnas.1205624109
65 Karkman A Lehtimäki J Ruokolainen L . The ecology of human microbiota: dynamics and diversity in health and disease. Ann N Y Acad Sci. 2017;1399(1):78–92. doi:.https://doi.org/10.1111/nyas.13326
66 Riiser A . The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11(1):35. doi:.https://doi.org/10.1186/s13223-015-0102-0
67 Wollina U . Microbiome in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:51–6. doi:.https://doi.org/10.2147/CCID.S130013
68 Osman KA Zinsstag J Tschopp R Schelling E Hattendorf J Umer A Nutritional status and intestinal parasites among young children from pastoralist communities of the Ethiopian Somali region. Matern Child Nutr. 2020;16(3):e12955. doi:.https://doi.org/10.1111/mcn.12955
69 Vonaesch P Morien E Andrianonimiadana L Sanke H Mbecko JR Huus KE Afribiota Investigators . Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc Natl Acad Sci USA. 2018;115(36):E8489–98. doi:.https://doi.org/10.1073/pnas.1806573115
70 Vonaesch P Anderson M Sansonetti PJ . Pathogens, microbiome and the host: emergence of the ecological Koch’s postulates. FEMS Microbiol Rev. 2018;42(3):273–92. doi:.https://doi.org/10.1093/femsre/fuy003
71 Aziz Q Doré J Emmanuel A Guarner F Quigley EM . Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil. 2013;25(1):4–15. doi:.https://doi.org/10.1111/nmo.12046
72 Prescott SL Larcombe DL Logan AC West C Burks W Caraballo L The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ J. 2017;10(1):29. doi:.https://doi.org/10.1186/s40413-017-0160-5
73 Dimmitt RA Staley EM Chuang G Tanner SM Soltau TD Lorenz RG . Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. J Pediatr Gastroenterol Nutr. 2010;51(3):262–73. doi:.https://doi.org/10.1097/MPG.0b013e3181e1a114
74Shanahan F. Linking Lifestyle with Microbiota and Risk of Chronic Inflammatory Disorders. In: Rook GAW, editor. Hygiene Hypothesis and Darwinian Medicine. Progress in Inflammation Research Series. Cham, Switzerland: Birkhaüser; 2009. pp 93–102.
75 Ruokolainen L Fyhrquist N Haahtela T . The rich and the poor: environmental biodiversity protecting from allergy. Curr Opin Allergy Clin Immunol. 2016;16(5):421–6. doi:.https://doi.org/10.1097/ACI.0000000000000304
76 Doré J Simrén M Buttle L Guarner F . Hot topics in gut microbiota. United European Gastroenterol J. 2013;1(5):311–8. doi:.https://doi.org/10.1177/2050640613502477
77 Mosca A Leclerc M Hugot JP . Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front Microbiol. 2016;7:455. doi:.https://doi.org/10.3389/fmicb.2016.00455
78Swiss Academy of Sciences. Biodiversity, a Guarantee for Health? Swiss Academies Factsheet; 2019.
79 Díaz S Pascual U Stenseke M Martín-López B Watson RT Molnár Z Assessing nature’s contributions to people. Science. 2018;359(6373):270–2. doi:.https://doi.org/10.1126/science.aap8826
80 Methorst J Rehdanz K Mueller T Hansjürgens B Bonn A Böhning-Gaese K . The Importance of Species Diversity for Human Well-Being in Europe. Ecol Econ. 2020;181:106917. doi:. https://doi.org/10.1016/j.ecolecon.2020.106917
81 Roche B Dobson AP Guégan JF Rohani P . Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Philos Trans R Soc Lond B Biol Sci. 2012;367(1604):2807–13. doi:.https://doi.org/10.1098/rstb.2011.0364
82 Dizney L Dearing MD . Behavioural differences: a link between biodiversity and pathogen transmission. Anim Behav. 2016;111:341–7. doi:.https://doi.org/10.1016/j.anbehav.2015.11.006
83 Dobson AD Auld SK . Epidemiological Implications of Host Biodiversity and Vector Biology: Key Insights from Simple Models. Am Nat. 2016;187(4):405–22. Published online March 31, 2016. doi:.https://doi.org/10.1086/685445
84 Ostfeld RS Keesing F . Is Biodiversity Bad for Your Health? Ecosphere. 2017;8(3):e01676. doi:.https://doi.org/10.1002/ecs2.1676
85 Hosseini PR Mills JN Prieur-Richard AH Ezenwa VO Bailly X Rizzoli A Does the impact of biodiversity differ between emerging and endemic pathogens? The need to separate the concepts of hazard and risk. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160129. doi:.https://doi.org/10.1098/rstb.2016.0129
86 Brooks CP Zhang H . A null model of community disassembly effects on vector-borne disease risk. J Theor Biol. 2010;264(3):866–73. doi:.https://doi.org/10.1016/j.jtbi.2010.03.016
87 Tischer CG Heinrich J . Exposure assessment of residential mould, fungi and microbial components in relation to children’s health: achievements and challenges. Int J Hyg Environ Health. 2013;216(2):109–14. doi:.https://doi.org/10.1016/j.ijheh.2012.05.002
88 States SL Brinkerhoff RJ Carpi G Steeves TK Folsom-O’Keefe C DeVeaux M Lyme disease risk not amplified in a species-poor vertebrate community: similar Borrelia burgdorferi tick infection prevalence and OspC genotype frequencies. Infect Genet Evol. 2014;27:566–75. doi:.https://doi.org/10.1016/j.meegid.2014.04.014
89 Miller E Huppert A . The effects of host diversity on vector-borne disease: the conditions under which diversity will amplify or dilute the disease risk. PLoS One. 2013;8(11):e80279. doi:.https://doi.org/10.1371/journal.pone.0080279
90 Lachish S Murray KA . The Certainty of Uncertainty: Potential Sources of Bias and Imprecision in Disease Ecology Studies. Front Vet Sci. 2018;5:90. doi:.https://doi.org/10.3389/fvets.2018.00090
91 Salkeld DJ Padgett KA Jones JH Antolin MF . Public health perspective on patterns of biodiversity and zoonotic disease. Proc Natl Acad Sci USA. 2015;112(46):E6261. Published online October 29, 2015. doi:.https://doi.org/10.1073/pnas.1517640112
92 Morand S Jittapalapong S Suputtamongkol Y Abdullah MT Huan TB . Infectious diseases and their outbreaks in Asia-Pacific: biodiversity and its regulation loss matter. PLoS One. 2014;9(2):e90032. doi:.https://doi.org/10.1371/journal.pone.0090032
93 Whitmee S Haines A Beyrer C Boltz F Capon AG de Souza Dias BF Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation-Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. doi:.https://doi.org/10.1016/S0140-6736(15)60901-1
94 Lovell R Wheeler BW Higgins SL Irvine KN Depledge MH . A systematic review of the health and well-being benefits of biodiverse environments. J Toxicol Environ Health B Crit Rev. 2014;17(1):1–20. doi:.https://doi.org/10.1080/10937404.2013.856361
95 Tengö M Hill R Malmer P Raymond CM Spierenburg M Danielsen F Weaving Knowledge Systems in Ipbes, Cbd and Beyond—Lessons Learned for Sustainability. Curr Opin Environ Sustain. 2017;26-27:17–25. doi:.https://doi.org/10.1016/j.cosust.2016.12.005
96Convention on Biological Diversity, Subsidiary Body on Scientific, Technical and Technological Advice. Twenty-first meeting. Montreal, Canada, 11–14 December 2017. Guidance on Integrating Biodiversity Considerations into One Health Approaches. Based on CBD/SBSTTA/21/9.
No financial support and no other potential conflict of interest relevant to this article were reported.