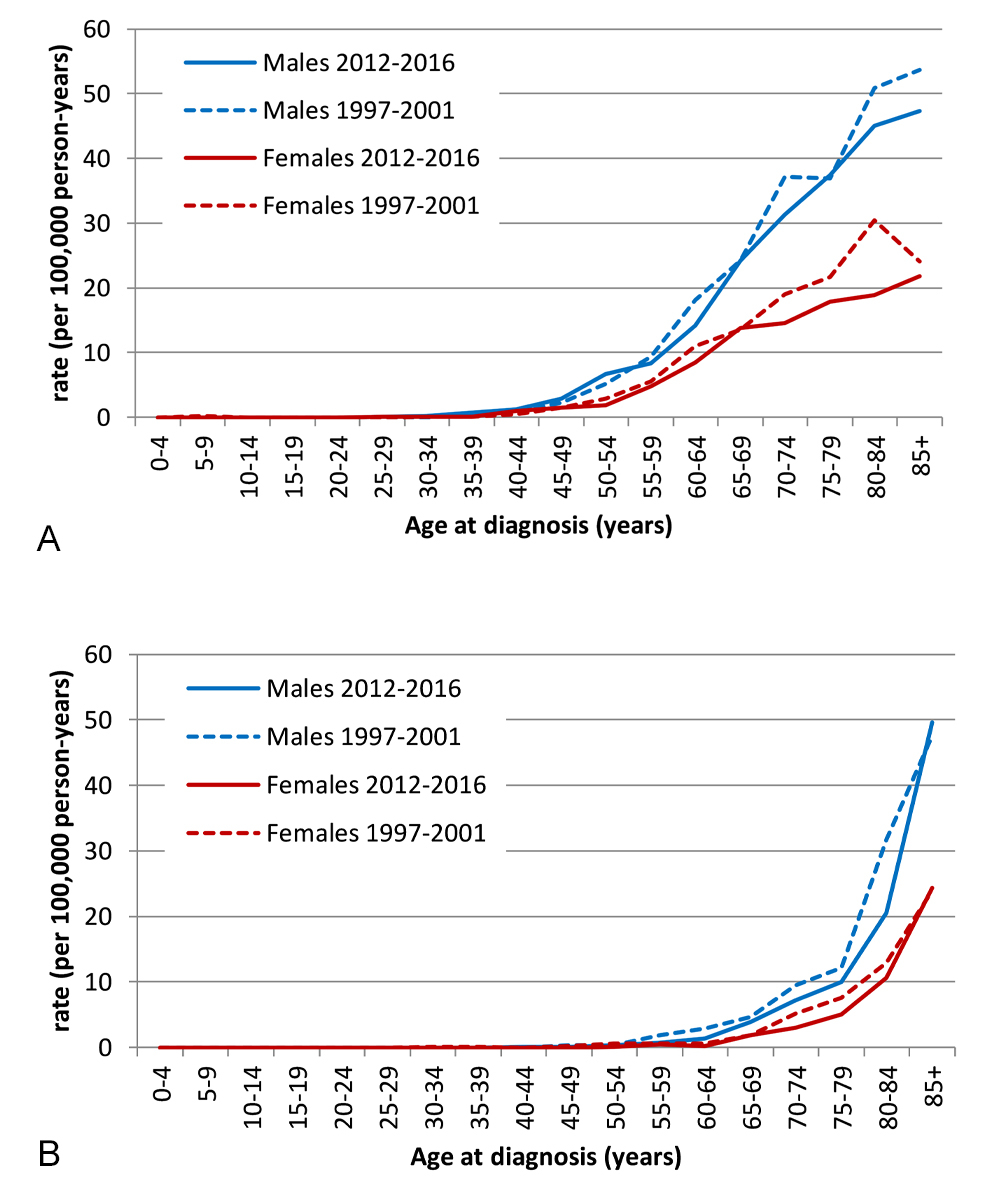
Figure 1 Age-specific incidence and mortality of chronic lymphocytic leukaemia / small lymphocytic lymphoma by sex, time periods 1997-–001 and 2012–2016.
(a) Incidence
(b) Mortality
DOI: https://doi.org/10.4414/smw.2021.20463
Chronic lymphocytic leukaemia (CLL) / small lymphocytic lymphoma (SLL) is the most prevalent leukaemia and the second most common B-cell neoplasm in Western countries [1, 2]. It affects mostly elderly persons with a median age at diagnosis of 72 years and a male:female ratio of 2:1.
Despite the double name, CLL/SLL is one disease, characterised by neoplastic monomorphic small mature B cells with a particular morphology and immunophenotype as defined by the current World Health Organization (WHO) classification [1, 3]. The WHO classification defines SLL as a subtype of CLL/SLL representing the nodal equivalent of CLL without leukaemic manifestation. About 10–20% of CLL/SLL cases are SLL.
Historically, CLL/SLL was first considered as one entity in the REAL classification of 1994. Earlier classifications usually categorised CLL and SLL as distinct diseases. SLL in particular was defined less stringently in classifications based solely on morphology and not including immunophenotypic criteria. Therefore, in some older classifications, SLL also included other B-cell neoplasms composed of small lymphocytes, such as marginal zonal lymphomas, Waldenström macroglobulinaemia, mantle cell lymphoma or T-cell proliferative disorders.
Irrespective of its presentation as the leukaemic or nodal variant, CLL/SLL is heterogeneous regarding the sequential somatic genetic lesions underlying leukaemogenesis, localisation, clinical manifestation and disease course. Many patients present in an asymptomatic stage not requiring immediate treatment at diagnosis [1, 4, 5].
Treatment of CLL/SLL has undergone considerable changes in Switzerland, as in many other countries. Mono- and combination chemotherapy, based on alkylating agents such as chlorambucil and cyclophosphamide since the 1960s and purine analogues such as fludarabine and cladribine since the 1980s [6, 7], was the mainstay of treatment until after 2000. After 2000, chemoimmunotherapies combining alkylating agents and/or purine analogues with anti-CD20 antibodies became standard, justified by improved clinical outcomes in randomised controlled trials [8–12]. Recently, new small molecules, such as the B-cell receptor kinase inhibitors, including the Bruton tyrosine kinase (BTK) inhibitor ibrutinib [13] and the phosphatidylinositol-3-kinase p110δ inhibitor idelalisib [14], as well as BCL-2 inhibitors such as venetoclax [15], improved outcomes in clinical trials.
Population-based studies from different Western countries reported improvement in survival: from Denmark between 1978 and 2013 [16], from Norway between 1952 and 2012 [17], from Germany and the USA between 2003 and 2011 [18]. So far, no recent population-based data have been published from Switzerland.
In this study, we analysed trends in incidence, mortality and survival of patients with CLL/SLL in Switzerland between 1997 and 2016. We investigated sex- and age-specific trends for age groups <65, 65–74 and ≥75 years. We were particularly interested to investigate whether the survival benefits reported from clinical trials translate into improved relative survival in CLL/SLL patients on a population level.
The National Agency for Cancer Registration (NACR) database provided incident CLL (ICD-O-code 9823/3) and SLL cases (ICD-O-code 9670/3) for the years 1997–2016 and corresponding vital status information. Cases were mutually exclusively coded as either CLL or SLL. NACR collects and harmonises cantonal cancer registry data and provides a central national database of cancer registration data in Switzerland. A detailed description of the organisation of cancer registration in Switzerland can be found elsewhere [19]. In 1997–2001, 13 out of 26 cantons conducted cancer registration (supplementary table S1 in the appendix), covering 57.8% of the Swiss population. As of 2016, this number increased to 23 cantons. However, at the time the analyses were carried out, three cantons (Geneva, Basel-Stadt and Basel-Landschaft) could not yet provide data for the incidence year 2016, resulting in population coverage of 85.2% for the last observation period. Incidence years covered by each canton are shown in table S1. Overall, 94.1% of all CLL/SLL cases were morphologically verified (table S2).
The Swiss Federal Statistical Office (SFSO) supplied mortality data, mid-year population estimates and cantonal death rates by age, sex and calendar year, covering all persons with permanent residence status in Switzerland. The SFSO codes death certificates and selects the underlying cause of death for the whole of Switzerland. Causes of death were coded according to the tenth revision of the International Classification of Diseases and Related Health Problems (ICD-10). Underlying cause of death is selected from the causes and conditions entered on the death certificate. When more than one cause or condition is entered on the certificate, ICD international rules for selecting the underlying cause of death apply [20]. We calculated mortality for the ICD codes C83.0 (small cell B-cell lymphoma) and C91.1 (chronic lymphocytic leukaemia of B-cell type).
No informed consent of the patients was necessary for this study. The data were collected and aggregated according to cantonal and state-wide provisions for the purpose of monitoring cancer burden, care and outcomes, and were provided and used for this study according to the respective legal regulations and approved by the Ethics Committee Zurich (KEK-ZH-Nr. 2014-0382, PB_2016–01643). The analyses were carried out by the National Institute for Cancer Registration and Epidemiology (NICER) until the end of 2019 and the National Agency for Cancer Registration (NACR) from 2020 onwards. Data transmission to third parties took place exclusively in aggregated form in accordance with the Swiss Cancer Registration Act (CRA) and Cancer Registration Ordinance (CRO) (CRA, Art. 23, par 1, 2; CRO, Art. 30, par 3, 4).
The expected number of cases for all of Switzerland were extrapolated by applying the observed sex-and age-specific incidence rates (five-year age intervals) to the population of all cantons, with the assumption of homogeneity of sex- and age-specific incidence rates across regions with and without cancer registration.
We calculated five-year age-specific, crude and age-standardised incidence and mortality rates with corresponding 95% confidence intervals (95% CIs) for consecutive five-year periods between 1997 and 2016: 1997–2001, 2002–2006, 2007–2011, and 2012–2016. For the main analyses, age-standardised rates were calculated using the direct method and the European Standard Population 2013 [21] as reference. To increase comparability with other studies, age-standardised rates were additionally calculated using the European Standard 1976, Segi’s World Standard and the new WHO Standard (WHO 2000–2025) [22]. The expected number of cases for all of Switzerland were extrapolated by applying the observed incidence rates to the population of all cantons under the assumption of homogeneity between regions with and without cancer registration. To assess whether increases in population coverage over time had an impact on observed incidence trends, we performed sensitivity analyses excluding cantons starting cancer registration after 1997.
Relative survival was calculated as the ratio of the observed survival of cancer cases and the expected survival of persons in the general population matching in age, sex, calendar year and region of residence [23, 24]. Observed survival was estimated based on transformation of the cumulative hazards. Expected survival was calculated using the Ederer II method [23]. We calculated observed and relative survival up to 10 years after diagnosis using period analysis [24]. We performed age-stratified analyses for the age categories <65 years, 65–74 years and 75+ years. Relative survival for all age groups combined were age-standardised using weights from the International Cancer Survival Standards (ICSS-Standard 1) [25]. To compare 5- and 10-year relative survival, we applied the significance test described by Parkin and Hakulinen [26].
Statistical analyses were performed by Stata/MP version 15.1 (STAT Corp., TX USA). All statistical tests are two-tailed and a p-value less than 0.05 was considered statistically significant.
Between 1997 and 2016, 6501 cases were reported in Switzerland. Based on the reported cases and the coverage of the registry, we expect a total of 9355 new cases for the same time period in Switzerland. The median age at diagnosis was 71 years in males (interquartile range 62–78) and 73 years in females (interquartile range 65–81). Twenty-eight percent of all patients were younger than 65 years at the time of diagnosis. The demographic characteristics of observed CLL/SLL cases are presented in table 1.
Table 1 Patient characteristics of chronic lymphocytic leukaemia / small lymphocytic lymphoma cases reported to Swiss cancer registries, 1997–2016.
| n | % | Median age at diagnosis (interquartile range) | |
|---|---|---|---|
| Overall | 6301 | 100.0% | 72 (63–79) |
| Sex | |||
| Males | 3723 | 59.1% | 71 (62–78) |
| Females | 2578 | 40.9% | 73 (65–81) |
| Age | |||
| <65 years | 1782 | 28.3% | – |
| 65–74 years | 1987 | 31.5% | – |
| 75–84 years | 2532 | 40.2% | – |
| Time period | – | ||
| 1997–2001 | 1201 | 19.0% | 72 (63–79) |
| 2002–2006 | 1473 | 23.4% | 71 (62–79) |
| 2007–2011 | 1562 | 24.8% | 72 (64–80) |
| 2012–2016 | 2065 | 32.8% | 71 (63–79) |
Population covered by cancer registration: 57.8% in 1997–2001, 59.0% in 2002–2006, 65.1% in 2007–2011 and 85.2% in 2012–2016
Annual case frequencies averaged 408 and 488 incident CLL/SLL cases for the time periods 1997–2001 and 2012–2016, respectively (table 2). Figure 1 depicts the corresponding rates by five-year age groups. In both sexes, the age-adjusted incidence peaked in the second time period and decreased thereafter (fig. 2). The sensitivity analyses excluding all cancer registries implemented after 1997 showed results similar to our main analyses (supplementary tables S6 and S7 in the appendix).
Table 2 Incidence and mortality of chronic lymphocytic leukaemia / small lymphocytic lymphoma in Switzerland, 1997–2016.
| 1997–2001 | 2002–2006 | 2007–2011 | 2012–2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
n*†
per year |
Rate‡ |
n*
per year |
Rate‡ |
n*
per year |
Rate‡ |
n*
per year |
Rate‡ | |||||
| Crude | Adjusted § | Crude | Adjusted § | Crude | Adjusted § | Crude | Adjusted § | |||||
| Incidence | ||||||||||||
| Overall | 409 | 5.8 | 7.0 | 495 | 6.8 | 7.8 | 483 | 6.2 | 7.0 | 488 | 5.9 | 6.4 |
| Sex | ||||||||||||
| Males | 231 | 6.7 | 9.6 | 290 | 8.1 | 10.8 | 281 | 7.3 | 9.4 | 305 | 7.5 | 8.9 |
| Females | 178 | 5.0 | 5.3 | 205 | 5.5 | 5.7 | 202 | 5.1 | 5.2 | 184 | 4.4 | 4.4 |
| Age | ||||||||||||
| <65 years | 117 | 2.0 | 2.3 | 147 | 2.4 | 2.6 | 129 | 2.0 | 2.1 | 139 | 2.0 | 2.1 |
| 65–74 years | 130 | 22.3 | 22.3 | 146 | 24.0 | 24.1 | 154 | 22.8 | 23.1 | 159 | 20.5 | 20.5 |
| 75+ years | 162 | 31.8 | 31.7 | 202 | 36.0 | 35.9 | 201 | 32.5 | 32.3 | 191 | 28.4 | 28.2 |
| Mortality¶ | ||||||||||||
| Overall | 136 | 1.9 | 2.4 | 151 | 2.0 | 2.4 | 159 | 2.1 | 2.3 | 152 | 1.9 | 2.0 |
| Sex | ||||||||||||
| Males | 74 | 2.1 | 3.6 | 84 | 2.3 | 3.7 | 87 | 2.3 | 3.3 | 87 | 2.1 | 3.0 |
| Females | 62 | 1.7 | 1.7 | 67 | 1.8 | 1.7 | 72 | 1.8 | 1.7 | 65 | 1.6 | 1.4 |
| Age | ||||||||||||
| <65 years | 15 | 0.2 | 0.3 | 14 | 0.2 | 0.2 | 12 | 0.2 | 0.2 | 9 | 0.1 | 0.1 |
| 65–74 years | 29 | 5.0 | 5.0 | 30 | 4.9 | 4.9 | 30 | 4.4 | 4.5 | 30 | 3.8 | 3.9 |
| 75+ years | 92 | 18.2 | 18.4 | 107 | 19.1 | 19.1 | 117 | 18.9 | 18.7 | 114 | 16.9 | 16.5 |
* Incidence: mean annual case frequency extrapolated to the whole Swiss population from cases observed in the cancer registries. † Discrepancies between the sum and total number of estimated incidence cases are caused by rounding errors. Mortality: mean annual case frequency derived from nationwide cause of death statistics. ‡ per 100,000 person-years. § European Standard 2013 ¶ Mortality: mean annual case frequency derived from nationwide cause of death statistics.

Figure 1 Age-specific incidence and mortality of chronic lymphocytic leukaemia / small lymphocytic lymphoma by sex, time periods 1997-–001 and 2012–2016.
(a) Incidence
(b) Mortality

Figure 2 Age-standardised incidence and mortality of chronic lymphocytic leukaemia / small lymphocytic lymphoma by sex and time period
(a) Incidence
(b) Mortality
Sex-specific incidence rates were around twice as high for males as for females across all age groups (fig. 1). For both sexes, the age-specific incidence rate first increases almost exponentially but flattens thereafter with a more linear increase after around 60 years of age. Similarly, the male:female ratios of age-adjusted incidence rate varied only slightly across time periods (mean 1.88, range 1.81–2.02; fig. 2).
Incidence rates age-adjusted for common standard populations are provided in the appendix (table S4).
Age-standardised rates of mortality also followed a downward trend (table 2), from 2.4 deaths per 100,000 person-years in 1997–2001 to 2.0 in 2012–2016.
Mortality rates for males were around twice as high as for females across all age groups. Both sex-specific mortality rates grew almost exponentially with increasing age, reaching about 50 per 100,000 person-years for males and about 25 per 100,000 person-years for females aged 85 years or older (fig. 1).
Crude and age-standardised incidence and mortality rates were strikingly higher in males than in females across all time periods. Age-adjusted mortality (mean 2.09, range 1.94–2.18) varied only slightly by time period, but the sex ratio was higher for mortality than for incidence for all time periods (fig. 2).
Mortality rates age-adjusted for common standard populations are provided in the appendix (table S5)for readers interested in comparing our data with other populations.
Five-year age-standardised relative survival increased from 77.9% (95% CI 74.2–81.2%) in 1997–2001 to 83.6% (95% CI 81.0–85.8%) in 2012–2016 (p = 0.009). Ten-year age-standardised relative survival rose from 55.6% (95% CI 50.5–60.3%) in 1997–2001 to 64.2% (95% CI 60.4–67.7%) in 2012–2016 (p = 0.005; supplementary fig. S1 in the appendix). These differences resulted from significant improvement between the first and second time period (p = 0.017 for 5-year relative survival and p = 0.007 for 10-year relative survival) without significant improvement in pairwise comparisons of the subsequent time periods. Observed and relative survival were better in younger patients (fig. 3). But improvement over time was significant only for the middle age group: in patients diagnosed below age 65 years 5-year relative survival improved from 88.8% (95% CI 84.0–92.5%) in 1997–2001 to 92.9% (95% CI 89.5–95.5%; p = 0.117) in 2012–2016; in the age group 65–74 years relative survival increased from 78.7.0% (95% CI 72.584.2%) to 87.3% (95% CI 82.8–91.0; p = 0.020); and for those aged 75 years or older 5-year relative survival increased from 61.6% (95% CI 53.4–69.6%) to 65.2% (95% CI 59.0–71.2; p = 0.480) (figs 3 and 4 ). Ten years after diagnosis, patients diagnosed between the ages of 65 and 74 years again showed substantial improvements in relative aurvival, but not the other two age groups: in those with age below 65 years from 71.0% (95% CI 63.9–77.3%) in 1997–2001 to 78.4% (95% CI 73.1–83.0%) in 2012–2016 (p = 0.081); age 65–74 years from 51.2% (95% CI 43.1–59.3%) to 67.5% (95% CI 60.8–73.8; p = 0.002); age group 75+ years 35.1% (95% CI 24.7–47.4%) in 1997–2001 and 36.9% (95% CI 29.2–45.5%) in 2012–2016 (p = 0.793) (fig. 3). Relative survival curves declined steadily over the whole observation time without evidence of a plateau. Detailed observed and relative survival by age group and period are provided in the appendix (table S6), as well as age-standardised survival curves (fig. S1).
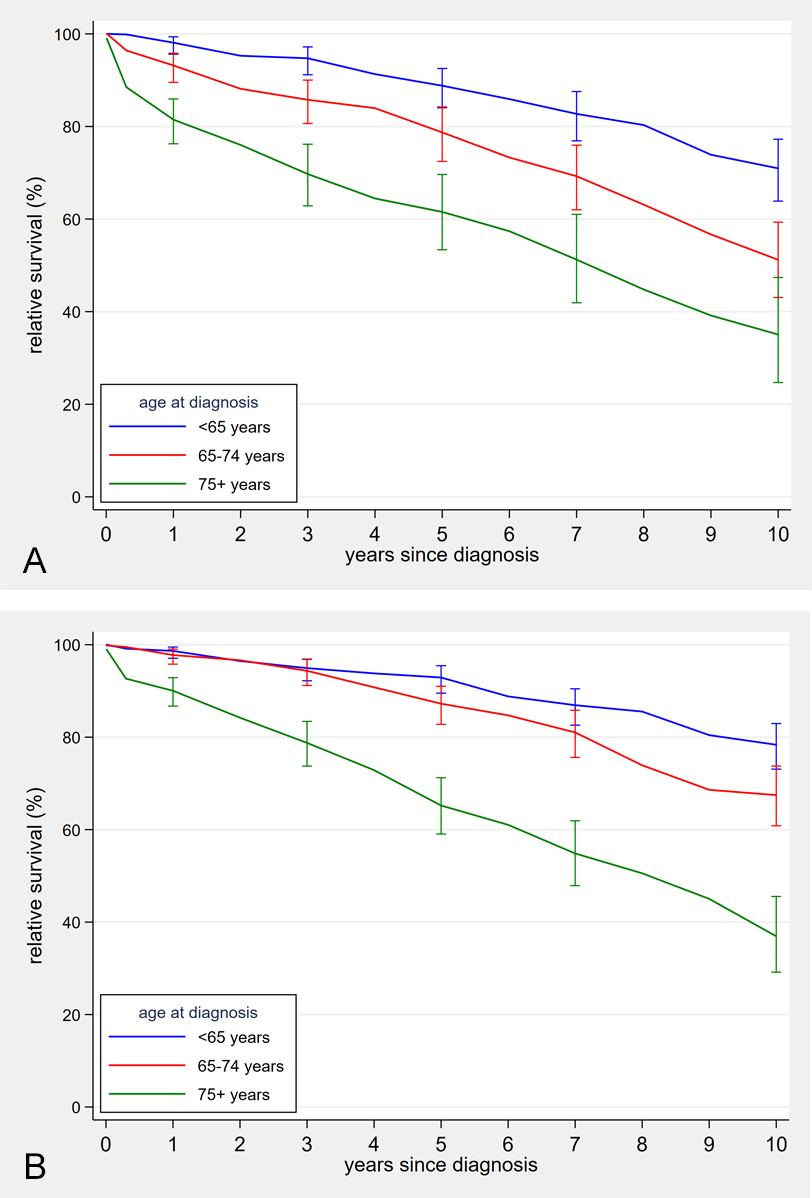
Figure 3 Age-specific relative survival curves of chronic lymphocytic leukaemia / small lymphocytic lymphoma patients, 1997–2001 and 2012–2016.
(a) Time period 1997–2001
(b) Time period 2012–2016
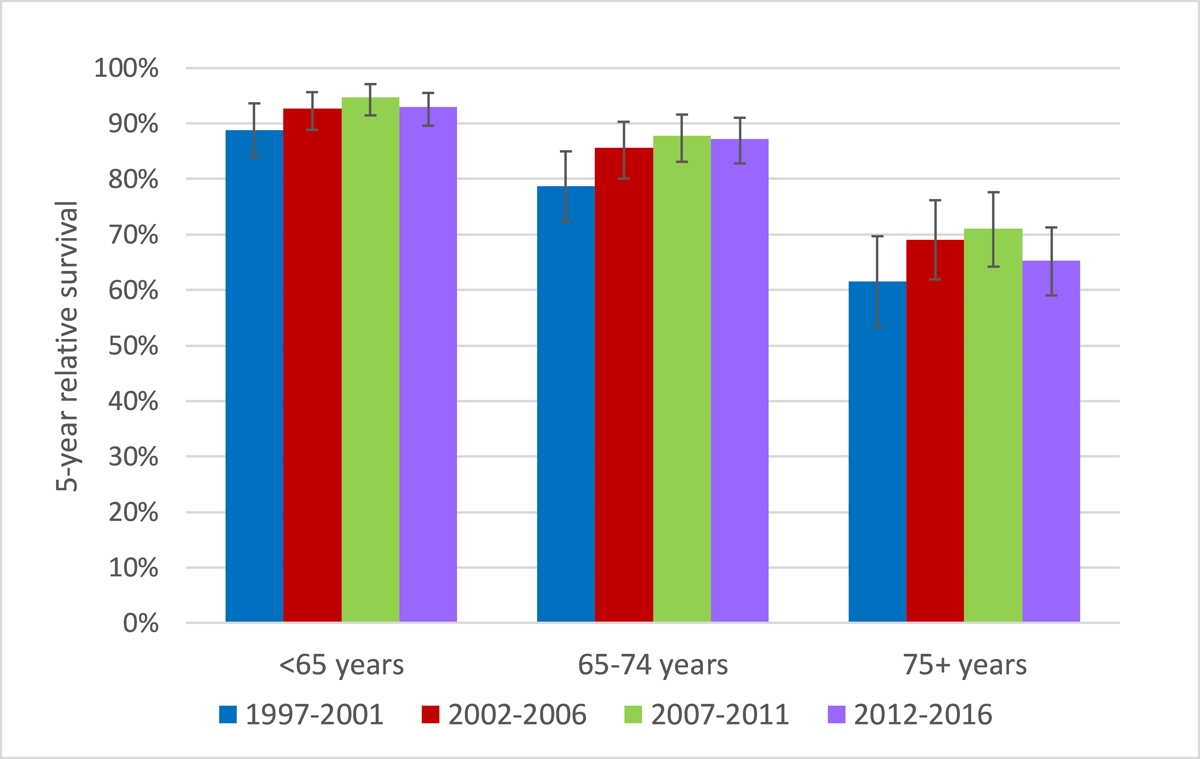
Figure 4 Relative 5-year survival after chronic lymphocytic leukaemia / small lymphocytic lymphoma diagnosis by age-group and time period
In this study we aimed to provide estimates and trends of age-standardised, age- and sex-specific incidence rates, mortality rates and time-dependent survival probability for patients diagnosed with CLL/SLL. These are the first published population-based data from CLL patients in Switzerland over a long, recent time period.
Compared with other western countries, we find no obvious deviation in age-adjusted incidence for our data. This estimation is very rough, and thorough comparisons between registry data from different countries is very limited because of methodological variations: the selection of diagnosis codes in particular is not consistent between countries. CLL and SLL are not always seen as one entity.
For instance, the International Agency for Research on Cancer (IARC) reports age-standardised incidence rates (WHO world standard population) across selected European registries from 2008 to 2012 for males of between 2.4 and 8.8 and for females of between 1.0 and 5.9 [27]. This selection was based on ICD-10 and included cases with code C91 (lymphoid leukaemias), which on one hand is not limited to CLL and on the other hand does not include all cases of SLL. Furthermore, CLL incidence in the Netherlands between 1989 and 2008 was reported to be slightly lower than ours: 5.1 per 100,000 person-years for males and 2.3 to 2.5 for females (European standard population 1976). Aside from ICD-O codes 9670 and 9823, the case selection in this study included additional codes (9592, 9800, 9820 and 9823) [28]. In Germany, for ICD-O codes 9823/3 and 9670/3 between 2001 and 2010 incidence rates for men were 5.67 per 100,000 person-years, for females 2.94, and for both sexes 4.12 (European standard population 1976) [29].
The increase in absolute incidence counts is explainable by demographic changes. The skewed male:female ratio with higher incidence in males is also known from other studies.
The change in diagnostic criteria is likely the main reason for the lower incidence rates in recent years: in the NCI 1996 criteria, a cut off of >5 × 109/l B cells in peripheral blood was required whereas IWCLL 2008 adjusted this cell compartment to monoclonal B lymphocytes and introduced the term monoclonal B-cell lymphocytosis for those individuals with lower blood count and CLL immunophenotype. This change might have led to reclassification of newly diagnosed patients, who would formerly have been diagnosed with CLL/SLL, as monoclonal B-cell lymphocytosis.
In addition, reporting delay and underreporting may underestimate the true incidence rate. Reporting delay is an issue for the estimation of CLL incidence, well known from other cancer registries [30]. Reporting delay and underreporting might be a reason for lack of completeness of lymphoid leukaemias in Swiss cancer registries. An evaluation of completeness among these registries has shown high completeness for most cancer types except for lymphoid leukaemia [31].
Whereas reporting delay mainly affects recent years, underreporting would bias the incidence rate toward lower values across all time periods. The reason for these biases with CLL are unclear. We speculate that diagnostic procedures and the relatively long lasting (often several years) asymptomatic phase before treatment may be key players. In contrast to most neoplastic diseases, histological confirmation is not necessary to diagnose CLL. In line with current guidelines [1, 32, 33] and from our experience in clinical practice, we expect that most CLL cases are diagnosed from blood cell counts and flow cytometric immunophenotyping of peripheral blood. Therefore, in these cases reporting relies solely on the diagnostic facilities performing flow cytometry and the treating physician. Assuming that pathology institutes report neoplastic diseases consistently, we hypothesise that flow cytometry facilities and physicians were less well implemented in the reporting process during the analysed time period. The cancer registry classifies verification of the diagnosis into the categories morphologically, cytologically or others (table S2). SLL diagnosis was consistently histologically verified in more than 90% of cases across all time periods. In contrast, the fraction of histologically verified CLL cases dropped from 61% in the first to 40% in the last time period. The fraction of cytologically verified diagnoses increased from 32% in the first to 56% in the last time period. The fraction of histologically verified CLL cases in the last time period is still surprisingly high. Given these numbers and our experience of the clinical background, we question whether all cytologically verified cases have been reported. Interestingly, in a study on population-based data from Norway the ratio of case numbers based on immunophenotyping and cytology compared with histology was much higher [17].
In clinical practice, histological confirmation is often not done at diagnosis in asymptomatic states, but sometimes at progression before initiation of treatment. In a relevant number of cases, CLL might have been reported at the time of first treatment but not when the disease was first diagnosed.
Age-adjusted mortality rates significantly decreased over time for males and females. As the Swiss cancer registry data are not linked to information about therapy, we cannot verify whether improved treatments had an impact on reduction of mortality.
The relative reduction in age-adjusted mortality was more in the youngest age-group. This finding might be explained by the introduction of fludarabine, cyclophosphamide and rituximab, which was probably the most effective treatment used for this age group in the time frame investigated. Furthermore, fewer treatment-related deaths might have reduced mortality.
The ratio of sex-specific age-adjusted mortality rates, with more than two-fold higher age-adjusted mortality rates for males compared to females, is even higher than the male:female ratio of age-adjusted incidence rates, indicating that the increased mortality in males is not attributable solely to the higher disease incidence in males. Sex disparity in CLL outcome is well known from epidemiological and clinical studies, with fewer adverse prognostic markers (e.g., Binet stage, unmutated immunoglobulin heavy chain variable [IGHV] genes, β2-microglobulin levels, CD38 expression, Zap-70 positivity, TP53 aberrations and 11q deletions) and better response to treatment (e.g., to chlorambucil- and fludarabine-based regimens) in females [34]. The reason for this sex disparity is not entirely understood, but it seems that biological factors mainly contribute to these differences. For instance, effects of sexual hormones [35], sex chromosome-dependent differences in microRNAs [36] and different methylation patterns in CLL cells [37] have been observed.
There was a trend to improved survival in the two younger age groups, but improvement was significant in the 65–74 age group only.
We did not observe a shift of median age at diagnosis nor from incident cases toward lower ages, which might indicate lead-time bias due to earlier diagnosis in the asymptomatic state. But our data might be not sensitive enough to detect earlier diagnosis. We have no information on disease stages at time of diagnosis.
Advances in treatment in the earlier time periods brought significant survival improvement: at this time, anti-CD20 antibodies and combination therapy were introduced into CLL treatment. Given the results from randomised controlled trials, we would expect that the introduction of fludarabine, cyclophosphamide and rituximab for younger patients might have contributed to the higher relative survival rates in those aged below 65 years [9, 12]. As mentioned before, we do not have linked data on treatments to verify this hypothesis. The years of first approval in Switzerland of drugs commonly used for treatment of CLL/SLL may provide a rough frame work of time points when they might have been used: chlorambucil 1957, cyclophosphamide 1960, fludarabine 1995, rituximab 1997, alemtuzumab 2001, bendamustine 2009, ofatumumab 2011, obinutuzumab 2014, ibrutinib 2014, idelalisib 2015, and venetoclax 2018[38, 39]. When we extrapolate the possible use of each of these drugs, we have to keep in mind that not all of them were first approved for treatment of CLL/SLL, but rather for other neoplasms, particularly other B-cell neoplasms. Unfortunately, we cannot reliably infer whether they have been used as monotherapy or in combination regimens. Furthermore, some of these drugs may have been used in clinical trials or off-label in named-patient programmes before approval. Interestingly, the survival improvement was more pronounced in the middle aged. It remains speculative whether chemoimmunotherapy is responsible for this improvement, even though more intensive treatment usually leads to higher toxicity and mortality in this age group [12]. Maybe the introduction of the relatively low toxicity antibody monotherapy in this age group, either as a replacement of chemotherapy in relapsed or unfit patients or as an additional line of treatment after chemotherapy, had a significant impact on survival. To our knowledge, comparison of overall survival in elderly CLL patients treated with monoclonal antibody monotherapy versus chemotherapy has not been investigated in prospective comparative trials. Furthermore, improvements in supportive care or more thorough risk stratification for treatment might have been beneficial for survival. Newer drugs, such as B-cell receptor kinase inhibitors and BCL-2 inhibitors, that demonstrated a survival advantage compared to chemoimmunotherapy in prospective randomised trials [15, 40] did not add to the observed survival improvement between the first and the second time periods, since they were introduced later.
In the other two age groups, the largest gain in relative survival for 5-year survival, even if not significant, was also between the two first time periods. For 10-year survival, those aged <65 years at diagnosis demonstrated the highest increase between 2002–2006 and 2007–2011, whereas those aged 75 years or older again had most improved survival from 1997–2001 to 2002–2006. The relative survival in the later time periods might be underestimated owing to selective reporting of more severe cases. One possibility for such a bias is the aforementioned change in diagnostic criteria for CLL, which might have affected the last two time periods. Furthermore, selective reporting delay for asymptomatic or low-risk cases might particularly affect the latest time period.
Females demonstrated better relative survival than males in all time periods. Relative survival seems to diverge between sexes with increasing survival time (data not shown). In contrast, no clear tendency by calendar time is visible, suggesting that advances in disease management had no impact on differences in survival by sex. We assume that reasons for better relative survival in females are biological differences mentioned in the former section.
Given the relatively long survival and the putative ongoing need for treatment, patients require lifelong follow-up with or without treatment. Presumably the intensity and cost of care heavily depends on the morbidity and treatment applied. Unfortunately, such data are not available from the registry. Population-based treatment-free intervals or time to next treatment combined with information about disability might provide better estimates of healthcare costs than incidence, mortality and survival rates alone.
This is the first population-based report on trends in incidence, mortality and survival for CLL/SLL patients in Switzerland.
Population coverage was not stable over time but increased over time, reaching a coverage over 70% percent in the last time period.
The incidence rates presented here likely underestimate true incidences in recent years because of reporting delay and underreporting. Currently, NICER provides no model to estimate incidence rates adjusted for reporting delay.
The registry data do not include information about treatment. Therefore, we cannot directly infer how much the development of treatment practice has contributed to improvement in survival. Furthermore, the registry does not include any information about disease stage or biomarkers, such as IGHV mutational status or TP53 aberrations.
Our selection of five-year time intervals for calendar time periods and the grouping into three age groups for age-specific analysis may blur more detailed trends and age differences. Unfortunately, statistical inference from a finer resolution is limited due to case numbers.
There was no evidence of change in age-adjusted incidence rates during the observed time period. The estimated age-adjusted incidence rates are within the range of incidence rates reported from other European countries. Mortality significantly improved. Overall, age-adjusted survival improved with the greatest improvement for those aged 65–74 years at diagnosis. CLL/SLL has striking sex differences, with higher incidence rates, higher mortality and shorter survival for males. We assume that the survival improvement is due to advances in treatment, even though the data presented are not linked to individual treatment information. Biases from reporting delay, underreporting and changes in diagnostic criteria are major limitations and hamper more accurate interpretation of the registry data.
The Swiss cancer data used in these analyses was supplied by National Agency for Cancer Registration (NACR) (operated by the National Institute for Cancer Epidemiology and Registration [NICER]) and its partner registries in cantons (alphabetical order) Aargau, Basel, Bern, Central Switzerland, East Switzerland, Fribourg, Geneva, Glarus and Graubuenden, Neuchâtel and Jura, Ticino, Valais, Vaud, Zuerich and Zug.
Members of the NICER Working Group for these analyses include (alphabetical order of cantons): Aargau – M. Adam; Basel – K. Staehelin; Bern – A. Perren; Central Switzerland - J. Diebold; East Switzerland – M. Mousavi; Fribourg – Yvan Bergeron; Geneva - Elisabetta Rapiti; Glarus and Graubuenden – M. Mousavi; Neuchâtel and Jura – J.-L. Bulliard and M. Maspoli; Ticino – A. Bordoni; Valais – I. Konzelmann; Vaud – J.-L. Bulliard; Zurich and Zug - S. Rohrmann.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
MA has participated on advisory boards of AbbVie and Janssen-Cilag, received travel support from AbbVie, German CLL Study Group (GCLLSG), Gilead, Mundipharma, Novartis and Roche. AF and VA have no conflict of interest to disclose.
1Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th Edition. ed: International Agency for Research on Cancer; 2017.
2Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2015, National Cancer Institute Bethesda, MD: National Cancer Institute; 2018 [updated April 2018; cited 2018 November 2]. Available from: https://seer.cancer.gov/csr/1975_2015/.
3 Santos FPS O’Brien S . Small lymphocytic lymphoma and chronic lymphocytic leukemia: are they the same disease? Cancer J. 2012;18(5):396–403. doi:.https://doi.org/10.1097/PPO.0b013e31826cda2d
4 Kipps TJ Stevenson FK Wu CJ Croce CM Packham G Wierda WG Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3(1):16096. doi:.https://doi.org/10.1038/nrdp.2016.96
5 Hallek M Shanafelt TD Eichhorst B . Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–37. doi:.https://doi.org/10.1016/S0140-6736(18)30422-7
6 Grever MR Kopecky KJ Coltman CA Files JC Greenberg BR Hutton JJ Fludarabine monophosphate: a potentially useful agent in chronic lymphocytic leukemia. Nouv Rev Fr Hematol. 1988;30(5-6):457–9.
7 Piro LD Carrera CJ Beutler E Carson DA . 2-Chlorodeoxyadenosine: an effective new agent for the treatment of chronic lymphocytic leukemia. Blood. 1988;72(3):1069–73. doi:.https://doi.org/10.1182/blood.V72.3.1069.bloodjournal7231069
8 Keating MJ O’Brien S Albitar M Lerner S Plunkett W Giles F Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88. doi:.https://doi.org/10.1200/JCO.2005.12.051
9 Hallek M Fischer K Fingerle-Rowson G Fink AM Busch R Mayer J International Group of Investigators German Chronic Lymphocytic Leukaemia Study Group . Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74. doi:.https://doi.org/10.1016/S0140-6736(10)61381-5
10 Robak T Dmoszynska A Solal-Céligny P Warzocha K Loscertales J Catalano J Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1756–65. doi:.https://doi.org/10.1200/JCO.2009.26.4556
11 Goede V Fischer K Busch R Engelke A Eichhorst B Wendtner CM Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10. doi:.https://doi.org/10.1056/NEJMoa1313984
12 Eichhorst B Fink A-M Bahlo J Busch R Kovacs G Maurer C international group of investigators German CLL Study Group (GCLLSG) . First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–42. doi:.https://doi.org/10.1016/S1470-2045(16)30051-1
13 Byrd JC Furman RR Coutre SE Flinn IW Burger JA Blum KA Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi:.https://doi.org/10.1056/NEJMoa1215637
14 Furman RR Sharman JP Coutre SE Cheson BD Pagel JM Hillmen P Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi:.https://doi.org/10.1056/NEJMoa1315226
15 Seymour JF Kipps TJ Eichhorst B Hillmen P D’Rozario J Assouline S Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2018;378(12):1107–20. doi:.https://doi.org/10.1056/NEJMoa1713976
16 da Cunha-Bang C Simonsen J Rostgaard K Geisler C Hjalgrim H Niemann CU . Improved survival for patients diagnosed with chronic lymphocytic leukemia in the era of chemo-immunotherapy: a Danish population-based study of 10455 patients. Blood Cancer J. 2016;6(11):e499. doi:.https://doi.org/10.1038/bcj.2016.105
17 Lenartova A Johannesen TB Tjønnfjord GE . National trends in incidence and survival of chronic lymphocytic leukemia in Norway for 1953-2012: a systematic analysis of population-based data. Cancer Med. 2016;5(12):3588–95. doi:.https://doi.org/10.1002/cam4.849
18 Pulte D Castro FA Jansen L Luttmann S Holleczek B Nennecke A GEKID Cancer Survival Working Group . Trends in survival of chronic lymphocytic leukemia patients in Germany and the USA in the first decade of the twenty-first century. J Hematol Oncol. 2016;9(1):28. doi:.https://doi.org/10.1186/s13045-016-0257-2
19 Arndt V . Population-based cancer registration and research in Switzerland: examples, limitations and perspectives. Swiss Cancer Bulletin. 2016;36(4):354–9.
20World Health Organization. International statistical classification of diseases and related health problems. 10th revision, Fifth edition, 2016 ed. Geneva: World Health Organization; 2015.
21Pace M, Cayotte E, Agafitei L, Zupanic T, Wojtyniak B, Gissler M, et al. Revision of the European Standard Population: report of Eurostat's task force: 2013 edition. Luxembourg: Publications Office; 2013.
22Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. GPE Discussion Paper Series: No. 31. Age standardization of rates: a new WHO standard. World Health Organization; 2000.
23 Dickman PW Coviello E . Estimating and modeling relative survival. Stata J. 2015;15(1):186–215. doi:.https://doi.org/10.1177/1536867X1501500112
24 Hockey R Tooth L Dobson A . Relative survival: a useful tool to assess generalisability in longitudinal studies of health in older persons. Emerg Themes Epidemiol. 2011;8(1):3. doi:.https://doi.org/10.1186/1742-7622-8-3
25 Corazziari I Quinn M Capocaccia R . Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40(15):2307–16. doi:.https://doi.org/10.1016/j.ejca.2004.07.002
26Parkin D, Hakulinen T. Analysis of Survival. Cancer Registration: Principles and Methods. Lyon: International Agency for Research on Cancer (IACR); 1991. p. 159-76.
27Cancer Incidence in Five Continents. CI5plus: IARC CancerBase No. 9 [Internet]. International Agency for Research on Cancer. 2018. Available from: http://ci5.iarc.fr
28 van den Broek EC Kater AP van de Schans SAM Karim-Kos HE Janssen-Heijnen MLG Peters WG Chronic lymphocytic leukaemia in the Netherlands: trends in incidence, treatment and survival, 1989-2008. Eur J Cancer. 2012;48(6):889–95. doi:.https://doi.org/10.1016/j.ejca.2011.06.053
29 Nennecke A Wienecke A Kraywinkel K . Inzidenz und Überleben bei Leukämien in Deutschland nach aktuellen standardisierten Kategorien [Leukemia incidence and survival in Germany according to current standardized categories]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014;57(1):93–102. Article in German. doi:.https://doi.org/10.1007/s00103-013-1869-0
30 Dores GM Anderson WF Curtis RE Landgren O Ostroumova E Bluhm EC Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007;139(5):809–19. doi:.https://doi.org/10.1111/j.1365-2141.2007.06856.x
31 Lorez M Bordoni A Bouchardy C Bulliard J-L Camey B Dehler S Evaluation of completeness of case ascertainment in Swiss cancer registration. Eur J Cancer Prev. 2017;26:S139–46. doi:.https://doi.org/10.1097/CEJ.0000000000000380
32 Hallek M Cheson BD Catovsky D Caligaris-Cappio F Dighiero G Döhner H International Workshop on Chronic Lymphocytic Leukemia . Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi:.https://doi.org/10.1182/blood-2007-06-093906
33 Hallek M Cheson BD Catovsky D Caligaris-Cappio F Dighiero G Döhner H iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60. doi:.https://doi.org/10.1182/blood-2017-09-806398
34 Catovsky D Wade R Else M . The clinical significance of patients’ sex in chronic lymphocytic leukemia. Haematologica. 2014;99(6):1088–94. doi:.https://doi.org/10.3324/haematol.2013.101378
35 Allain EP Venzl K Caron P Turcotte V Simonyan D Gruber M Sex-dependent association of circulating sex steroids and pituitary hormones with treatment-free survival in chronic lymphocytic leukemia patients. Ann Hematol. 2018;97(9):1649–61. doi:.https://doi.org/10.1007/s00277-018-3356-z
36 Carè A Bellenghi M Matarrese P Gabriele L Salvioli S Malorni W . Sex disparity in cancer: roles of microRNAs and related functional players. Cell Death Differ. 2018;25(3):477–85. doi:.https://doi.org/10.1038/s41418-017-0051-x
37 Lin S Liu Y Goldin LR Lyu C Kong X Zhang Y Sex-related DNA methylation differences in B cell chronic lymphocytic leukemia. Biol Sex Differ. 2019;10(1):2. doi:.https://doi.org/10.1186/s13293-018-0213-7
38List of authorised medicines: Swissmedic. Swiss Agency for Therapeutic Products; 2020 [updated 2020 October 31; cited 2020 November 30]. Available from: https://www.swissmedic.ch/dam/swissmedic/en/dokumente/internetlisten/zugelassene_arzneimittel_ham.xlsx.download.xlsx/Zugelassene_Arzneimittel_HAM.xlsx.
39PharmaWiki. Medikamente und Gesundheit: PharmaWiki GmbH; [cited 2020 November 30]. Available from: https://www.pharmawiki.ch/wiki/.
40 Shanafelt TD Wang XV Kay NE Hanson CA O’Brien S Barrientos J Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med. 2019;381(5):432–43. doi:.https://doi.org/10.1056/NEJMoa1817073
Table S1Swiss cantons providing incidence data to the current analyses, with time periods.
| Cantons | Time period |
|---|---|
| Aargau | 2013–2016 |
| Basel-City* | 1997–2015 |
| Basel-Country* | 1997–2015 |
| Bern | 2014–2016 |
| Fribourg | 2006–2016 |
| Geneva | 1997–2015 |
| Glarus† | 1997–2016 |
| Graubünden† | 1997–2016 |
| Jura‡ | 2005–2016 |
| Lucerne§ | 2010–2016 |
| Neuchâtel‡ | 1997–2016 |
| Nidwalden§ | 2011–2016 |
| Obwalden§ | 2011–2016 |
| St Gallen¶ | 1997–2016 |
| Appenzell-Ausserrhoden¶ | 1997–2016 |
| Appenzell-Innerrhoden¶ | 1997–2016 |
| Ticino | 1997–2016 |
| Thurgau¶ | 2012–2016 |
| Uri§ | 2011–2016 |
| Vaud | 1997–2016 |
| Valais | 1997–2016 |
| Zug‖ | 2011–2016 |
| Zurich‖ | 1997–2016 |
Managed by one cancer registry (CR): * CR Basel-City and Basel-Country; † CR Glarus-Graubünden; ‡ CR Neuchâtel and Jura; § CR Central Switzerland; ¶ CR East Switzerland; ‖ CR Zurich and Zug
Table S2Type of verification of the diagnosis by time period and diagnosis code.
| Time period | CLL (ICD-O-code 9823/3), n (%) | SLL (ICD-O-code 9670/3), n (%) | ||||
|---|---|---|---|---|---|---|
| Morphologically verified | Other | Morphologically verified | Other | |||
| Cytological | Histological | Cytological | Histological | |||
| 1997–2001 | 317 (32.4) | 596 (60.9) | 66 (6.7) | 8 (3.6) | 213 (96.0) | 1 (0.5) |
| 2002–2006 | 466 (37.3) | 689 (55.1) | 96 (7.7) | 21 (9.5) | 200 (90.1) | 1 (0.5) |
| 2007–2011 | 591 (45.1) | 641 (48.9) | 80 (6.1) | 15 (6.0) | 235 (94.0) | 0 (0.0) |
| 2012–2016 | 979 (56.4) | 685 (39.5) | 71 (4.1) | 16 (4.9) | 312 (94.6) | 2 (0.6) |
| CLL = chronic lymphatic leukaemia; SLL = small lymphocytic lymphoma | ||||||
Table S3Age-adjusted incidence of chronic lymphocytic leukaemia / small lymphocytic lymphoma in Switzerland, 1997–2016.
| Standard | 1997–2001 | 2002–2006 | 2007–2011 | 2012–2016 |
|---|---|---|---|---|
| Rate * (95% CI) | Rate * (95% CI) | Rate * (95% CI) | Rate * (95% CI) | |
| Males | ||||
| Euro 2013 | 9.6 (8.8–10.3) | 10.8 (10.0–11.5) | 9.4 (8.8–10.1) | 8.9 (8.4–9.4) |
| Euro 1976 | 6.0 (5.5–6.4) | 6.8 (6.3–7.3) | 5.8 (5.4–6.2) | 5.6 (5.3–5.9) |
| WHO World | 4.5 (4.2–4.9) | 5.2 (4.8–5.5) | 4.4 (4.1–4.7) | 4.3 (4.0–4.5) |
| Segi’s World | 3.9 (3.6–4.3) | 4.5 (4.2–4.8) | 3.8 (3.6–4.1) | 3.7 (3.5–3.9) |
| Females | ||||
| Euro 2013 | 5.3 (4.9–5.8) | 5.7 (5.2–6.1) | 5.2 (4.8–5.6) | 4.4 (4.1–4.7) |
| Euro 1976 | 3.3 (3.0–3.7) | 3.6 (3.3–3.9) | 3.2 (3.0–3.5) | 2.8 (2.6–3.0) |
| WHO World | 2.6 (2.3–2.8) | 2.7 (2.5–2.9) | 2.5 (2.3–2.7) | 2.1 (2.0–2.3) |
| Segi’s World | 2.2 (2.0–2.5) | 2.3 (2.1–2.6) | 2.1 (2.0–2.3) | 1.9 (1.7–2.0) |
| Both sexes | ||||
| Euro 2013 | 7.0 (6.6–7.4) | 7.8 (7.4–8.2) | 7.0 (6.6–7.4) | 6.4 (6.1–6.7) |
| Euro 1976 | 4.4 (4.2–4.7) | 5.0 (4.7–5.3) | 4.4 (4.1–4.6) | 4.1 (3.9–4.3) |
| WHO World | 3.4 (3.2–3.6) | 3.8 (3.6–4.0) | 3.3 (3.1–3.5) | 3.1 (3.0–3.3) |
| Segi’s World | 3.0 (2.8–3.2) | 3.3 (3.1–3.5) | 2.9 (2.8–3.1) | 2.7 (2.6–2.9) |
CI = confidence interval * per 100,000 person-years
Table S4Age-adjusted mortality of chronic lymphocytic leukaemia / small lymphocytic lymphoma in Switzerland, 1997–2016.
| Standard | 1997–2001 | 2002–2006 | 2007–2011 | 2012–2016 |
|---|---|---|---|---|
| Rate * (95% CI) | Rate * (95% CI) | Rate * (95% CI) | Rate * (95% CI) | |
| Males | ||||
| Euro 2013 | 3.6 (3.2–4.0) | 3.7 (3.3–4.0) | 3.3 (3.0–3.6) | 3.0 (2.7–3.3) |
| Euro 1976 | 1.8 (1.6–2.0) | 1.8 (1.7–2.0) | 1.6 (1.5–1.8) | 1.4 (1.3–1.6) |
| WHO World | 1.3 (1.2–1.5) | 1.4 (1.2–1.5) | 1.2 (1.1–1.3) | 1.1 (1.0–1.2) |
| Segi’s World | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 1.0 (0.9–1.1) | 0.8 (0.7–0.9) |
| Females | ||||
| Euro 2013 | 1.7 (1.5–1.9) | 1.7 (1.5–1.9) | 1.7 (1.5–1.8) | 1.4 (1.2–1.6) |
| Euro 1976 | 0.9 (0.8–1.0) | 0.8 (0.8–1.0) | 0.8 (0.7–0.9) | 0.7 (0.6–0.8) |
| WHO World | 0.7 (0.6–0.7) | 0.6 (0.6–0.7) | 0.6 (0.5–0.7) | 0.5 (0.4–0.5) |
| Segi’s World | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.5 (0.4–0.5) | 0.4 (0.3–0.4) |
| Both sexes | ||||
| Euro 2013 | 2.4 (2.2–2.6) | 2.4 (2.3–2.6) | 2.3 (2.2–2.5) | 2.0 (1.9–2.2) |
| Euro 1976 | 1.2 (1.1–1.3) | 1.2 (1.2–1.3) | 1.2 (1.1–1.2) | 1.0 (0.9–1.1) |
| WHO World | 0.9 (0.9–1.0) | 0.9 (0.9–1.0) | 0.9 (0.8–0.9) | 3.1 (1,4–5.3) |
| Segi’s World | 0.7 (0.7–0.8) | 0.7 (0.7–0.8) | 0.7 (0.6–0.7) | 0.7 (0.7–0.8) |
CI = confidence interval * per 100,000 person-years
Table S5Observed and relative survival of chronic lymphocytic leukaemia / small lymphocytic lymphoma patients by age-group and period.
| 1-year survival | 3-year survival | 5-year survival | 10-year survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OS | RS* (95% CI) | OS | RS* (95% CI) | OS | RS* (95% CI) | OS | RS* (95% CI) | ||
| <65 years | |||||||||
| 1997–2001 | 97.5 | 98.1 (95.6–99.4) | 92.7 | 94.7 (91.2–97.2) | 85.3 | 88.8 (84.0–92.5) | 64.0 | 71.0 (63.9–77.3) | |
| 2002–2006 | 98.5 | 99.1 (97.3–99.9) | 94.2 | 96.1 (93.0–98.1) | 89.5 | 92.7 (88.8–95.6) | 66.6 | 72.9 (66.6–78.4) | |
| 2007–2011 | 99.2 | 99.7 (98.1–100.3) | 96.1 | 97.7 (95.3–99.3) | 91.8 | 94.7 (91.4–97.1) | 75.3 | 81.5 (76.1–86.2) | |
| 2012–2016 | 98.3 | 98.7 (97.1–99.5) | 93.6 | 94.9 (92.2–96.9) | 90.4 | 92.9 (89.5–95.5) | 73.0 | 78.4 (73.1–83.0) | |
| 65–74 years | |||||||||
| 1997–2001 | 91.3 | 93.2 (89.5–95.8) | 80.2 | 85.8 (80.6–90.0) | 69.5 | 78.7 (72.5–84.2) | 37.2 | 51.2 (43.1–59.3) | |
| 2002–2006 | 95.4 | 97.1 (94.4–98.8) | 84.6 | 89.7 (85.1–93.3) | 76.9 | 85.6 (80.1–90.3) | 52.3 | 69.9 (61.9–77.5) | |
| 2007–2011 | 95.3 | 96.7 (94.2–98.4) | 87.5 | 92.2 (88.5–95.1) | 79.9 | 87.8 (83.2–91.6) | 51.0 | 65.7 (58.7–72.4) | |
| 2012–2016 | 96.5 | 97.8 (95.8–99.1) | 90.3 | 94.4 (91.2–96.8) | 80.3 | 87.3 (82.8–91.0) | 53.9 | 67.5 (60.8–73.8) | |
| 75+ years | |||||||||
| 1997–2001 | 75.6 | 81.5 (76.3–86.0) | 54.9 | 69.7 (62.9–76.2) | 39.9 | 61.6 (53.4–69.6) | 12.0 | 35.1 (24.7–47.4} | |
| 2002–2006 | 81.9 | 87.9 (83.9–91.4) | 61.8 | 77.1 (71.3–82.5) | 46.9 | 69.1 (61.9–76.1) | 17.8 | 46.6 (35.9–58.4) | |
| 2007–2011 | 86.4 | 92.1 (88.6–95.0) | 65.2 | 80.3 (74.8–85.4) | 49.1 | 71.1 (64.3–77.6) | 17.9 | 43.9 (34.8–53.9) | |
| 2012–2016 | 84.5 | 90.1 (86.7–92.9) | 64.4 | 78.8 (73.7–83.4) | 45.9 | 65.2 (59.0–71.2) | 15.8 | 36.9 (29.2–45.5) | |
| All ages† | |||||||||
| 1997–2001 | 89.4 | 91.9 (89.9–93.5) | 78.1 | 84.8 (81.8–87.3) | 67.7 | 77.9 (74.2–81.2) | 42.3 | 55.6 (50.5–60.3) | |
| 2002–2006 | 92.9 | 95.3 (93.8–96.5) | 82.0 | 88.5 (86.0–90.6) | 73.4 | 83.5 (80.3–86.3) | 49.2 | 64.9 (60.0–69.3) | |
| 2007–2011 | 94.4 | 96.6 (95.2–97.6) | 84.6 | 91.0 (88.7–92.8) | 76.0 | 85.7 (82.9–88.1) | 52.7 | 66.7 (62.6–70.5) | |
| 2012–2016 | 93.8 | 96.0 (94.7–96.9) | 84.4 | 90.2 (88.2–91.9) | 75.1 | 83.6 (81.0–85.8) | 52.1 | 64.2 (60.4–67.6) | |
CI = confidence interval; OS = observed survival; RS = relative survival * presented in percentages † age-standardised observed and relative survival
Table S6Patient characteristics of chronic lymphocytic leukaemia / small lymphocytic lymphoma cases reported to Swiss cancer registries, 1997–2016 (sensitivity analyses).
| n | % | Median age at diagnosis (interquartile range) | |
|---|---|---|---|
| Overall | 5446 | 100.0% | 72 (63–80) |
| Sex | |||
| Males | 3169 | 58.2% | 71 (62–78) |
| Females | 2277 | 41.8% | 74 (65–81) |
| Age | |||
| <65 years | 1524 | 28.0% | – |
| 65–74 years | 1696 | 31.1% | – |
| 75–84 years | 2226 | 40.9% | – |
| Time period | – | ||
| 1997–2001 | 1201 | 22.1% | 72 (63–79) |
| 2002–2006 | 1452 | 26.7% | 71.5 (62–79) |
| 2007–2011 | 1428 | 26.2% | 72 (64–80) |
| 2012–2016 | 1365 | 25.1% | 72 (63–80) |
Population covered by cancer registration: 57.8% in 1997–2001, 57.9% in 2002–2006, 58.2% in 2007–2011, and 56.1% in 2012–2016
Table S7Incidence of chronic lymphocytic leukaemia / small lymphocytic lymphoma in Switzerland, 1997–2016 (sensitivity analyses).
| 1997–2001 | 2002–2006 | 2007–2011 | 2012–2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
n*
per year |
Rate† |
n*
per year |
Rate† |
n*
per year |
Rate† |
n*
per year |
Rate† | |||||
| Crude | Adjusted ‡ | Crude | Adjusted ‡ | Crude | Adjusted ‡ | Crude | Adjusted ‡ | |||||
| Incidence | ||||||||||||
| Overall | 409 | 5.8 | 7.0 | 496 | 6.8 | 7.8 | 490 | 6.3 | 7.1 | 491 | 5.9 | 6.4 |
| Sex | ||||||||||||
| Males | 231 | 6.7 | 9.6 | 291 | 8.1 | 10.8 | 283 | 7.4 | 9.5 | 302 | 7.4 | 8.8 |
| Females | 178 | 5.0 | 5.3 | 205 | 5.5 | 5.7 | 207 | 5.3 | 5.4 | 189 | 4.6 | 4.5 |
| Age | ||||||||||||
| <65 years | 117 | 2.0 | 2.3 | 146 | 2.4 | 2.6 | 129 | 2.0 | 2.1 | 140 | 2.0 | 2.1 |
| 65-74 years | 130 | 22.3 | 22.3 | 145 | 23.9 | 24.0 | 157 | 23.3 | 23.6 | 154 | 19.9 | 20.0 |
| 75+ years | 162 | 31.8 | 31.7 | 205 | 36.4 | 36.4 | 204 | 32.9 | 32.6 | 197 | 29.2 | 29.0 |
* Incidence: mean annual case frequency extrapolated to the whole Swiss population from cases observed in the cancer registries. † per 100,000 person-years. ‡ European Standard 2013.
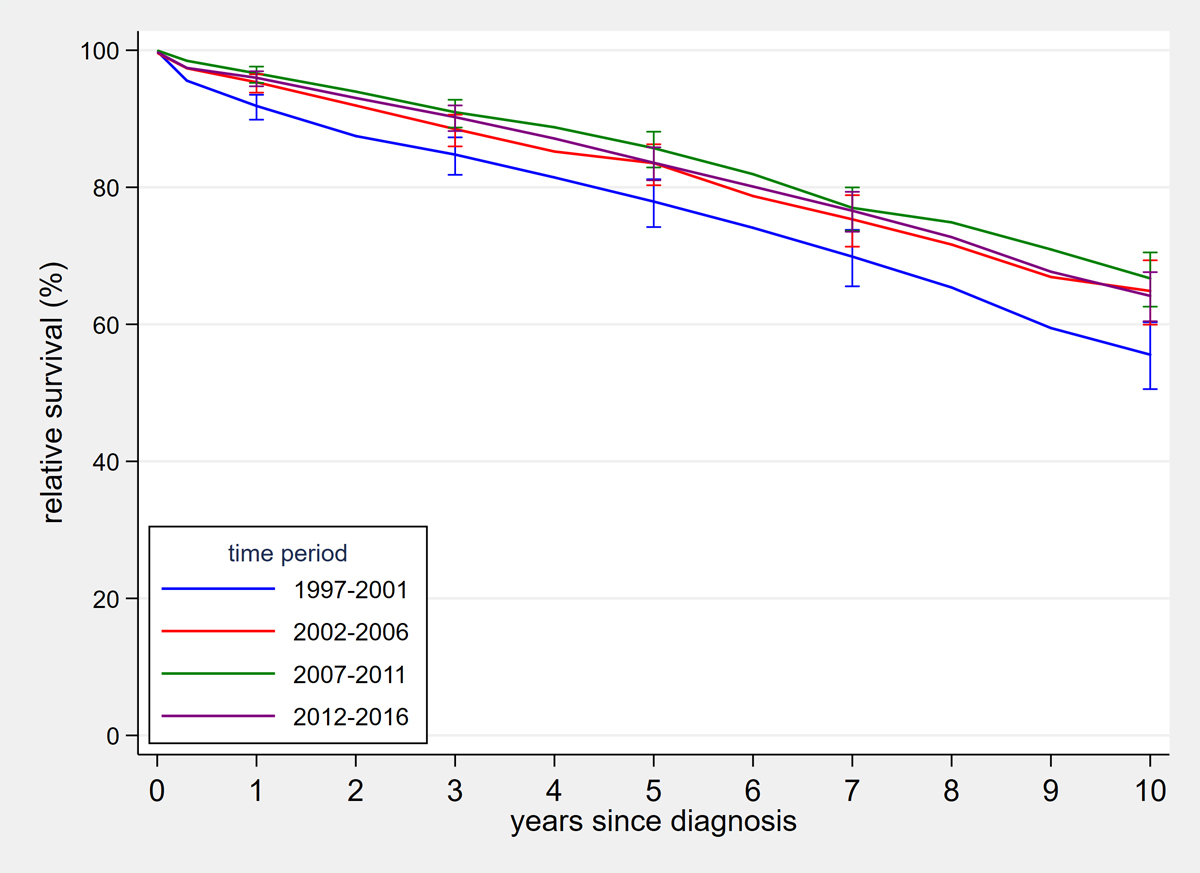
Figure S1 Age-standardised relative survival curves of chronic lymphocytic leukaemia / small lymphocytic lymphoma patients by time period.