Role of myeloid-derived suppressor cells in hormone-dependent cancers
DOI: https://doi.org/10.4414/smw.2021.20483
Siddhartha
Mukherjeeab, Angela R.
Eliaab, Arianna
Calcinottoab
a Institute of Oncology Research (IOR), Oncology Institute of Southern Switzerland, Bellinzona, Switzerland
b Università della Svizzera Italiana (USI), Faculty of Biomedical Sciences, Lugano, Switzerland
Summary
Tumour-infiltrating myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of myeloid cells. The main feature of MDSCs is their ability to suppress T-cell activation and function, which leads to immunosuppressive activity in the tumour microenvironment. Higher numbers of circulating and tumour-infiltrating MDSCs have been observed in a large number of patients with various types of tumour, and are linked to poor prognosis, especially in hormone-driven tumours. Recently, it has been demonstrated that the recruitment of MDSCs in prostate cancer confers resistance to canonical endocrine therapies, opening a new approach to the treatment of hormone-driven cancer patients.
Introduction
Hormone-dependent cancers rely on hormones for their continuous growth and survival. Examples of hormone-driven cancers are prostate, breast and ovarian cancers. Over the years, a growing body of literature has reported that hormone-driven cancers are poorly immunogenic and their tumour microenvironment is highly infiltrated by myeloid cells with immunosuppressive activity [1–6]. One of the most represented myeloid populations in these tumours are myeloid-derived suppressor cells (MDSCs) [2, 4, 7]. Higher numbers of circulating and tumour-infiltrating MDSCs have been observed in a large number of patients with various types of tumour, and are linked to poor prognosis, especially in hormone-driven tumours [1, 6–8]. For more than the last two decades, MDSCs have captured the spotlight owing to their ability to suppress T-cell activation and function, which leads to immunosuppressive activity in the tumour microenvironment [9]. Scientists have been investigating MDSCs in both humans and mice as a major antagonist of antitumour immunity. These cells are being recognised as immunosuppressive as they accumulate in almost every type of cancer patient [10, 11]. However, novel roles played by MDSCs in the tumour microenvironment have recently been defined. This review will summarise the role of MDSCs in hormone-dependent cancers and will provide a detailed insight into some of the recent studies that have provided significant evidence on unexpected functions played by MDSCs to support tumourigenesis.
Origin of MDSCs
In cancer, the physiological process of myelopoiesis within the bone marrow is dysregulated. MDSCs generally are generated in the bone marrow through a mechanism involving granulocyte-monocyte colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-10 and tumour necrosis factor-alpha (TNF-α) [12]. During tumourigenesis, these factors are overproduced and favour the generation of a very heterogeneous population of granulocytes, which are recruited within the tumour, and can support tumour progression and cancer cell proliferation [13]. As yet there is no clear picture that distinguishes subpopulations of tumour-infiltrating granulocytic populations, polymorphonuclear, in a tumour. This population, also called immature myeloid cells [14], includes tumour-associated neutrophils [3, 15], immunosuppressive neutrophils [16] and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) [2, 4, 7]. Here, we focus on PMN-MDSC biology. PMN-MDSC is a term introduced to scientific literature more than 10 years ago describing a group of immature myeloid cells with potent immune regulatory activity [17]. The current preponderant view is that PMN-MDSCs differentiate along the same pathways as neutrophils [18]. Recently, in addition to pluripotent hematopoietic stem cells, multipotent common myeloid progenitors and granulocyte-macrophage progenitors, several populations of committed granulocytic precursors were identified [19–21]. Moreover, precursors that belong to the monocytic lineage, termed monocyte-like precursors of granulocytes has been defined as an abnormal mechanism of PMN-MDSC accumulation in cancer (fig. 1A) [22].
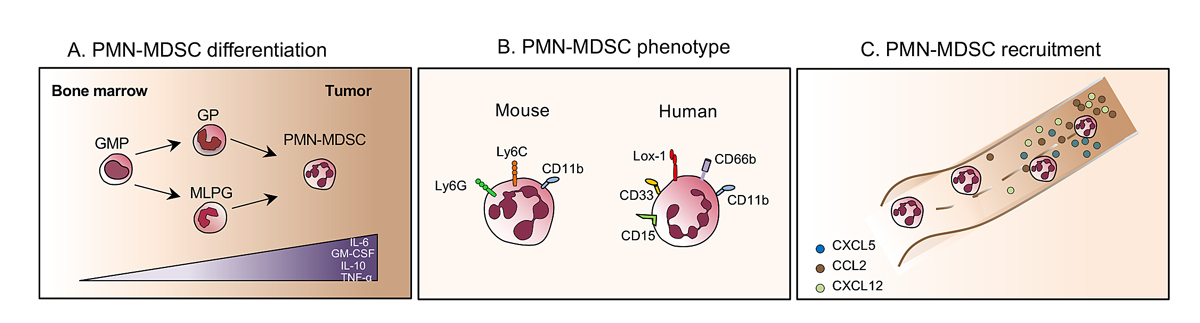
Figure 1 Development and recruitment of PMN-MDSCs. (A) During tumourigenesis GM-CSF, interleukin (IL)-6, IL-10 and tumour necrosis factor alpha (TNF-α) drive the differentiation of granulocyte-macrophage progenitors (GMPs), granulocytic precursors (GPs) and monocyte-like precursors of granulocytes (MLPG) into polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC). (B) Surface markers expressed by mouse (left) and human (right) PMN-MDCSs. (C) PMN-MDSCs are recruited to the tumour following the tumour-derived chemokine gradient.
Markers of PMN-MDSCs in mice are the expression of CD11b and the two-epitopes of the Gr1 molecule: Ly6G and ly6C. Indeed, murine PMN-MDSCs are defined as CD11b positive, Ly6Clow, Ly6Gbright [23]. In humans, PMN-MDSCs are most commonly defined as CD11b+CD15+ CD14− or CD11b+CD66b+CD14− [23]. They express the common myeloid marker CD33 but lack the expression of the major histocompatibility complex (MHC) class II molecule human leucocyte antigen (HLA)-DR [24]. Currently, PMN-MDSCs can be distinguished from proinflammatory neutrophils by the expression of lectin-type oxidised low-density lipoprotein (LDL) receptor-1 (Lox-1) and by density gradient. Lox-1 expression intensity has been associated with higher immunosuppressive activity (fig. 1B) [25].
The recruitment of PMN-MDSCs to the tumour microenvironment is still not fully understood. PMN-MDSC recruitment to the tumour bed is associated with interactions between tumour-derived chemokines and their receptors expressed on PMN-MDSCs. Recent studies have demonstrated the contribution of these tumour-derived chemokines, such as CC-chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 5 (CXCL5) and CXCL12, and their respective receptors CCR2, CCR5 and CXCR4, to the recruitment of PMN-MDSCs to the tumour microenvironment [1, 13]. These chemokines are highly redundant, irrespective of the cancer type, and help to provide a continuous supply of PMN-MDSCs to the tumour (fig. 1C).
Function of MDSCs
Most studies have shown that the immunosuppressive functions of MDSCs require direct cell-cell contact, which suggests that they act either through cell-surface receptors and/or through the release of short-lived soluble mediators [26]. MDSCs regulate immunosuppression through various mechanisms. The best-recognised mechanism is associated with the metabolism of L-arginine. MDSCs express high levels of both arginase and nitric oxide synthase (iNOS), which are two important enzymes involved in L-arginine metabolism. In detail, iNOS uses L-arginine to generate nitric oxide (NO) and arginase to convert L-arginine into urea and L-ornithine. The increased activity of arginase in MDSCs leads to enhanced L-arginine catabolism, which depletes this nonessential amino acid from the microenvironment. The shortage of L-arginine in the tumour microenvironment inhibits T-cell proliferation through several mechanisms, including decreasing their CD3ζ expression [27]. In addition, NO suppresses T-cell function through a variety of mechanisms that involve the inhibition of Janus kinase 3 (JAK3) and signal transducer and activator of transcription-5 (STAT5) in T lymphocytes, the inhibition of MHC class II expression and the induction of T-cell apoptosis [28, 29].
Another important factor that contributes to the suppressive activity of MDSCs is reactive the presence of oxygen species (ROS). Increased production of ROS has emerged as one of the main characteristics of MDSCs [30]. This idea is supported by studies in which inhibition of ROS production by MDSCs isolated from mice and patients with cancer completely abrogated the suppressive effect of these cells in vitro [31]. MDSCs are highly influenced by the tumour microenvironment: transforming growth factor-beta (TGFβ), IL-10, IL-6, IL-3, platelet-derived growth factor (PDGF) and GM-CSF induce the production of ROS [32]. More recently, it has emerged that peroxynitrite (ONOO−) is a crucial mediator of MDSC-mediated suppression of T-cell function. Peroxynitrite is a product of a chemical reaction between NO and superoxide anion (O2
−) and is one of the most powerful oxidants produced in the body; it induces nitration and nitrosylation of different amino acids. Several studies confirm that high levels of peroxynitrite are associated with tumour progression in many types of cancer, which has been linked with T-cell unresponsiveness [33]. Peroxynitrite production by MDSCs during direct contact with T cells results in nitration of the T-cell receptor and CD8 molecules, which alters the specific peptide binding of the T cells and renders them unresponsive to antigen-specific stimulation [34]. PMN-MDSCs rely more on the expression of ROS and arginase to mediate immunosuppression [35]. As a consequence of their high immunosuppressive activity, the high frequency of PMN-MDSCs in tumours corresponds to enhanced immunosuppression [36].
Emerging studies and the development of new genetic tools have provided cancer research with new insights into the profound influence of these dynamic cells, by uncovering distinct capabilities for PMN-MDSCs throughout each step of carcinogenesis: from primary tumour growth to metastasis to therapy resistance [37, 38]. Indeed, MDSCs have been shown to mediate protumoural functions by promoting tumour angiogenesis, and driving tumour cell invasion and metastasis. Furthermore, previous reports have proposed that MDSCs play a major role in epithelial-to-mesenchymal transition or creating “pre-metastatic niches” that lead to metastatic activity [1, 13, 23]. These functions will be discussed in detail in the following paragraphs.
MDSCs and prostate cancer
Prostate cancer is one of the leading causes of cancer incidence and the second leading cause of cancer-related deaths amongst males in the United States. For organ-confined disease, the initial treatment is prostatectomy or radiation, which usually is curative. Androgen-deprivation therapy has become the main prostate cancer therapy for patients at various stages of the disease [38]. However, a considerable fraction of patients receiving such treatments ultimately progress to more aggressive disease, developing castration-resistant prostate cancer (CRPC) [38]. The prognosis for patients with CRPC remains poor and treatments for these patients remain a major unmet medical need [38]. Recently, Calcinotto et al. [38] reported in Nature an unexpected role played by tumour-infiltrating immune cells (summarised in fig. 2). They found that myeloid cells can drive therapy resistance in CRPC, which progresses despite low androgen levels. This discovery opened several possibilities for new adjuvant therapeutic options to broaden our understanding of the wide range of interactions in the prostate cancer microenvironment. As the androgen receptor is considered a regulator of gene expression, the absence of androgen-mediated signalling through androgen-deprivation therapy could result in the prevention of prostate cancer progression [39, 40].
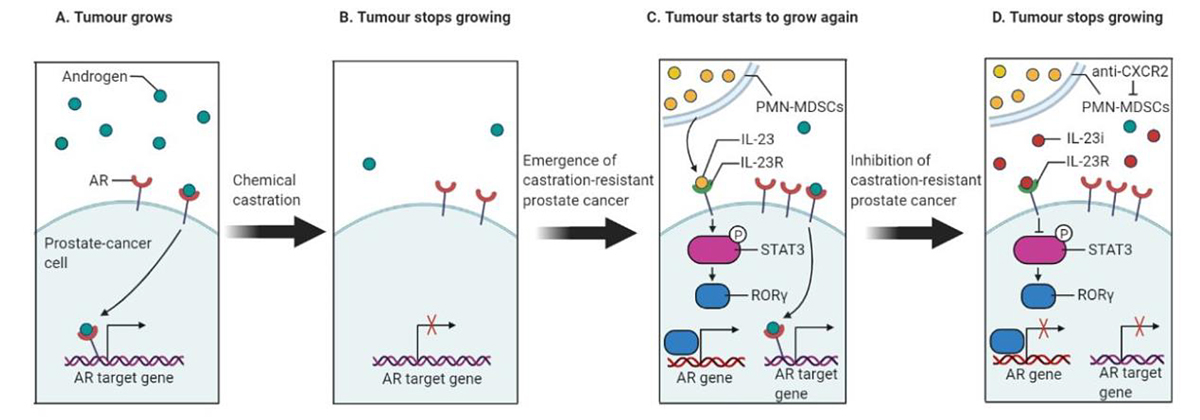
Figure 2 PMN-MDSCs drive therapy resistance in castration-resistant prostate cancer. (A) Androgen receptor (AR) binding drives the transcription of genes that are required to promote the growth of prostate cancer. (B) Chemical castration is used to lower androgen levels in prostate cancer patients. Nevertheless, the subsequent deceleration of tumour growth is transient. (C) The authors showed that in castration-resistant conditions, PMN-MDSCs contribute to the failure of therapy. PMN-MDSCs secrete IL-23, which binds to the IL-23 receptor (IL-23R) on tumour cells triggering a pathway that is mediated by RORγ and STAT3 proteins (here STAT3 gets phosphorylated; “P” is a phosphate group). This pathway drives the expression of androgen receptors, which in turn helps the transcription of androgen-dependent genes, which promotes the growth of prostate cancer. (D) Inhibition of IL-23/IL23R axis using an IL-23 inhibitor (IL-23i) blocks prostate tumour growth by repressing the downstream pathway mediated by RORγ and STAT3, thus resulting in a downregulation of the androgen receptor target genes.
CRPC depends on a vast spectrum of mechanisms [41] and causes disease progression in patients. The identification of mechanisms associated with disease progression has led to the development of associated treatments. Nearly half of CRPCs have been observed to have an increase in the copy number of the androgen receptor gene [42]. Accumulated data provide a strong correlation between androgen receptor expression and CRPC progression, which has pushed biomedical research a step forward towards the generation of androgen receptor inhibitory drugs [43]. In addition, other inhibitory drugs are being developed, to block androgen biosynthesis via an increase of androgen synthesising enzymes [44]. Despite of these drug-driven inhibitions of androgen regulation in CRPC patients, resistance to these clinical drug molecules can still be acquired. Henceforth, patients receiving these canonical drug-driven treatments are in dire need of additional clinical strategies. Calcinotto and colleagues investigated the role of immune cell populations behind the progression of CRPC. Prostate tumour biopsies from patients with advanced CRPC were enriched in PMN-MDSCs compared with biopsies collected from patients still sensitive to androgen-deprivation treatments. As research models, the authors used human samples and mouse models of prostate cancer to investigate the direct contribution of PMN-MDSCs to castration resistance. In mice, the authors saw an increase in the recruitment of PMN-MDSCs in tumours collected from castration-resistant mice. Moreover, they found that the conditioned medium of PMN-MDSCs generated in vitro helped androgen-dependent prostate-cancer cell lines sustain their proliferation and survival under androgen deprivation. The secretome of PMN-MDSCs increased the transcription of androgen receptor target genes, whose expression in physiological conditions is driven by the androgen receptor. In addition, pharmacological inhibition of MDSCs delayed the emergence of castration resistance in mice. It was evident that a factor released by PMN-MDSCs promoted the appearance of CRPC. Strikingly, they found overexpression of IL-23 and its receptor upon castration in the tumours of castrated mice and CRPC patients. Based on the robust data, Calcinotto et al. proposed that signalling mediated by IL-23 secreted by PMN-MDSCs on prostate cancer cells could be a major reason for the promotion of the development of CRPC. By blocking IL-23-mediated signalling in mice through pharmacological and/or genetic approaches, the authors showed a delay in the development of CRPC. Their experimental model clearly demonstrated a significant increase in the expression of androgen receptors and the genes that are transcriptionally dependent on androgen receptor, via the activation of the STAT3 – retinoic acid-related orphan receptor gamma (RORγ) pathway by IL-23 (fig. 2). Treatment aimed at blocking IL-23, such as blocking antibodies, reverts the efficacy of androgen-deprivation therapy in CRPC animals. IL-23-specific antibodies, which block the effect of IL-23, have been approved for a clinical trial in patients with autoimmune conditions. This will clearly pave the way for a possible treatment of CRPC.
Another important study by Garcia et al., using an endogenous Pten-null mouse model, demonstrated that the prostate tumour microenvironment is enriched with GR1+ CD11b+ MDSCs, which are solely responsible for disease initiation and progression [45]. Di Mitri et al. found that tumours derived from Pten-null mice are enriched in MDSCs (defined as CD11b+ Gr1+ cells) and help to sustain tumour growth by bypassing senescence in a paracrine manner [46]. On the other hand, Wang et al. explored the potential of MDSCs in antagonising T-cell proliferation upon the activation of yes-associated protein (YAP) signalling in prostate tumours [47, 48].
MDSCs and breast cancer
Breast cancer is the second most common cancer overall, impacting millions of women each year. Nearly 2 million new cases and 606,520 deaths were estimated to occur in the United States alone in 2020 [49], and the rate of invasive breast cancer is increasing in nearly every region globally at an alarming rate [50]. Breast cancer is a heterogeneous group of diseases with different histopathological characteristics and high genetic variability, and is therefore characterised by varying prognostic outcomes [51]. Clinically, breast cancer can be categorised into three basic therapeutic groups [52]: triple-negative breast cancers, which lack expression of oestrogen and progesterone receptors and HER2, and are also known as basal-like breast cancers [53–55]; the HER2 amplified group, which dramatically overexpresses the HER2 receptor tyrosine kinase; the oestrogen receptor positive group, which are also referred to as luminal-like breast cancers. Tumours of the oestrogen receptor positive group, which are the most numerous, rely on oestrogens to promote proliferation and inhibit apoptosis. Patients with these tumours receive anti-hormonal therapy, but often experience therapy resistance and relapse.
Besides their immunosuppressive function, PMN-MDSCs actively shape the breast tumour microenvironment through complex cross-talk between cancer cells and the surrounding stroma, resulting in increased angiogenesis, tumour invasion and metastasis [47, 56]. Breast cancer cells express various cytokines, including GM-CSF and IL-6, that impact myeloid cell differentiation, promoting PMN-MDSCs during tumour development [8, 57]. PMN-MDSCs accumulate in the blood and immune organs of breast cancer patients, develop immunosuppressive activity and alter tumour progression either by infiltrating tumours [58] or homing in to distant organs to establish premetastatic niches [2]. Numerous research findings have demonstrated the occupancy and escalation of PMN-MDSCs in different tumour microenvironments that lead to poor prognostic outcomes in advanced-stage breast cancer patients [4, 5, 7, 59]. Furthermore, circulating MDSCs have been monitored in a large number of breast cancer patients with poor prognosis [4, 7, 60, 61]. Bosiljcic et al. showed that a higher number of immunosuppressive MDSCs (CD11b+ GR1+ cells) was significantly found in the spleen and lungs of 4T1 and 4T07 tumour-bearing mice, where, notably, the higher level of MDSC accumulation in mice organs was associated with the production of G-CSF [62].
Several results support the role of PMN-MDSCs during the metastatic process. Danilin et al. demonstrated the role of MDSCs in promoting bone metastasis in breast cancer mouse models [63]. They also provided evidence regarding the interaction between MDSCs and the breast tumour microenvironment beyond their immunosuppressive activity, by using orthotopic and intracardiac breast cancer mouse models [63]. Furthermore, Ma et al. showed an increase in the level of TGF-β1 in a breast cancer mouse model with lung metastasis in postoperative conditions [64]. Importantly, the recruitment of MDSCs to the microenvironment is associated with the inhibition of anti-tumour immune responses through suppressive cytokines such as TGF-β1, vascular endothelial growth factor (VEGF) and IL-10 [64].
MDSCs and ovarian cancer
Ovarian cancer is one of the major causes of cancer-related deaths in women, putting it in the fifth position among other gynaecological cancers [65]. Despite standard first-line chemotherapy, patients with advanced ovarian cancer experience tumour relapse within a few years after treatment. Recently, there has been compelling evidence of a correlation between poor prognosis in ovarian cancer and MDSCs [66, 67]. For example, Baert et al., using an ID8-fLuc ovarian cancer mouse model, demonstrated that MDSCs play a major role in immunosuppression [66]. Likewise, Horikawa et al. demonstrated in an ovarian cancer murine model that VEGF plays a significant role in suppressing CD8 T-cell activity by stimulating the accumulation of MDSCs in the tumour microenvironment [67]. Furthermore, a few studies have revealed the importance of cytokines in the recruitment of MDSCs in the ovarian tumour microenvironment [6, 68, 69]. As an example, Alfaro et al. showed that IL-8 has an impact on the recruitment of PMN-MDSCs in the tumour microenvironment of ovarian cancer [68]. Importantly, Obermajer et al. showed that prostaglandin E2 mediates a tumour-associated inflammatory function by promoting the tumour infiltration of immunosuppressive monocytic MDSCs through the CXCR4-CXCL12/SDF-1 migratory axis [70].
Apart from their immunosuppressive activity, MDSCs also have a robust impact in promoting two of the dreadful hallmarks of cancer, angiogenesis and metastasis, in ovarian cancer [71]. In this regard, Cui et al. found that MDSCs promote “stemness”-like activity by targeting corepressor C-terminal-binding protein 2 (CtBP2), which leads to metastasis formation, and demonstrated, with a mechanistic approach, the clinical relevance of cross-talk among MDSCs, microRNA101 and CtBP2 in ovarian cancer patients [72]. Overall, we can assert that MDSCs play a key role in promoting ovarian cancer tumorigenesis through both immunosuppressive (suppression of T-cell response) and non-immunosuppressive mechanisms (metastasis formation, angiogenesis), which would lay the path for future studies combining immunotherapy with conventional anticancer therapies to target not only ovarian cancer, but most other cancer types as well.
Targeting of MDSCs
Clinical data indicate that increased MDSC counts in peripheral blood also impact prognosis and treatment outcome [3, 4, 7, 36, 60, 61]. Some chemotherapies can critically suppress MDSC counts and it is postulated that this may be vital to the benefit from these drugs [73–75]. However, following anticancer treatment, the frequency of MDSCs does not decline to the level seen in tumour-free mice and healthy human subjects. Moreover, tumour recurrence after treatment correlates with re-expansion of MDSCs [76]. A few studies have investigated the development of MDSC inhibitors, most of them focused on inhibiting the STAT3 signalling pathway [77, 78] or kinases involved in MDSC function [79]. However, none of them has progressed beyond the pre-clinical phase. Recently, MDSCs displayed a significantly higher sensitivity to cabozantinib and BEZ235. Cabozantinib or BEZ235 treatment alleviated the suppressive activity of intratumoural MDSCs on CD4+ and CD8+ T-cell proliferation through inhibition of PI3K signalling in a mouse model of castration-resistant prostate cancer when combined with immune-checkpoint blockers [80]. Recent studies have described the contribution of CXCR2 in the recruitment of MDSCs to the tumour bed. Pharmacological inhibition of CXCR2 signalling was broadly explored in combination with checkpoint blockers in pancreatic cancer [81] and prostate cancer [80] and even proposed for the treatment of castration resistant prostate cancer patients [38, 46]. First, a short peptide CXCR2 “pepducin” (1/2i-pal) was used to inhibit CXCR2 signalling by interfering with its ability to couple to intracellular signal-transduction molecules [82, 83]. Later, several small-molecule inhibitors of CXCR2 were developed, such as AZD5069 [38, 81, 84], AZ13381758 [84], SX-682 [80].
Concluding remarks
Several sets of evidence at preclinical and clinical levels demonstrate the prominent role played by MDSCs in supporting tumourigenesis. They promote tumour progression and metastasis, and create an immunosuppressive tumour microenvironment. Many questions relating to the polyhedric protumoural role of MDSC in cancers are still open. Despite the fact that MDSCs expand and play an integral role in promoting hormone-dependent tumour progression, the molecular mechanisms behind the role of MDSCs in T-cell suppression and resistance to anti-hormonal therapies still need to be elucidated. The identity of MDSCs, typically defined as immature myeloid cells, is highly controversial as they can be defined only functionally.
One of the major achievements in the field will be better characterisation of human tumour-infiltrating MDSCs, in order to define subsets specifically infiltrating each cancer type. Moreover, the results of a deeper characterisation of surface markers expressed by pathogenic MDSCs will provide new checkpoints to target, potentially providing innovative targets for immunotherapy. The use of blockers of MDSCs for the treatment of hormone-driven cancer to inhibit the protumoural functions of these cells in the tumour microenvironment is under clinical evaluation. This approach will open a new approach to the treatment of hormone-driven cancers. In different tumour contexts, research has demonstrated the value of targeting MDSCs in combination with immune checkpoint inhibitors and other forms of immunotherapy to enhance their efficacy. This strategy was shown to improve overall survival in patients with several types of cancer. Similarly, in hormone-driven cancers, a combined approach targeting the anti-hormonal therapy with anti-MDSC therapies may be beneficial.
References
1
Talmadge
JE
,
Gabrilovich
DI
. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–52. doi:.https://doi.org/10.1038/nrc3581
2
Kim
IS
,
Gao
Y
,
Welte
T
,
Wang
H
,
Liu
J
,
Janghorban
M
, et al.
Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol. 2019;21(9):1113–26. doi:.https://doi.org/10.1038/s41556-019-0373-7
3
Coffelt
SB
,
Kersten
K
,
Doornebal
CW
,
Weiden
J
,
Vrijland
K
,
Hau
CS
, et al.
IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8. doi:.https://doi.org/10.1038/nature14282
4
Wculek
SK
,
Malanchi
I
. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–7. doi:.https://doi.org/10.1038/nature16140
5
Wellenstein
MD
,
Coffelt
SB
,
Duits
DEM
,
van Miltenburg
MH
,
Slagter
M
,
de Rink
I
, et al.
Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature. 2019;572(7770):538–42. doi:.https://doi.org/10.1038/s41586-019-1450-6
6
Gonzalez-Aparicio
M
,
Alfaro
C
. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role?
J Immunol Res. 2019;2019:6252138. doi:.https://doi.org/10.1155/2019/6252138
7
Yu
J
,
Du
W
,
Yan
F
,
Wang
Y
,
Li
H
,
Cao
S
, et al.
Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–97. doi:.https://doi.org/10.4049/jimmunol.1201449
8
Casbon
AJ
,
Reynaud
D
,
Park
C
,
Khuc
E
,
Gan
DD
,
Schepers
K
, et al.
Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA. 2015;112(6):E566–75. doi:.https://doi.org/10.1073/pnas.1424927112
9
Gabrilovich
DI
,
Bronte
V
,
Chen
SH
,
Colombo
MP
,
Ochoa
A
,
Ostrand-Rosenberg
S
, et al.
The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425
, author reply 426. doi:.https://doi.org/10.1158/0008-5472.CAN-06-3037
10
Messmer
MN
,
Netherby
CS
,
Banik
D
,
Abrams
SI
. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64(1):1–13. doi:.https://doi.org/10.1007/s00262-014-1639-3
11
Solito
S
,
Marigo
I
,
Pinton
L
,
Damuzzo
V
,
Mandruzzato
S
,
Bronte
V
. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319(1):47–65. doi:.https://doi.org/10.1111/nyas.12469
12
Barreda
DR
,
Hanington
PC
,
Belosevic
M
. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28(5):509–54. doi:.https://doi.org/10.1016/j.dci.2003.09.010
13
Marvel
D
,
Gabrilovich
DI
. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–64. doi:.https://doi.org/10.1172/JCI80005
14
Kusmartsev
S
,
Gabrilovich
DI
. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55(3):237–45. doi:.https://doi.org/10.1007/s00262-005-0048-z
15
Fridlender
ZG
,
Sun
J
,
Kim
S
,
Kapoor
V
,
Cheng
G
,
Ling
L
, et al.
Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94. doi:.https://doi.org/10.1016/j.ccr.2009.06.017
16
Casbon
AJ
,
Reynaud
D
,
Park
C
,
Khuc
E
,
Gan
DD
,
Schepers
K
, et al.
Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA. 2015;112(6):E566–75. doi:.https://doi.org/10.1073/pnas.1424927112
17
Gabrilovich
DI
,
Bronte
V
,
Chen
SH
,
Colombo
MP
,
Ochoa
A
,
Ostrand-Rosenberg
S
, et al.
The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425
, author reply 426. doi:.https://doi.org/10.1158/0008-5472.CAN-06-3037
18
Gabrilovich
DI
,
Ostrand-Rosenberg
S
,
Bronte
V
. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi:.https://doi.org/10.1038/nri3175
19
Waight
JD
,
Netherby
C
,
Hensen
ML
,
Miller
A
,
Hu
Q
,
Liu
S
, et al.
Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123(10):4464–78. doi:.https://doi.org/10.1172/JCI68189
20
Yáñez-Mó
M
,
Siljander
PR
,
Andreu
Z
,
Zavec
AB
,
Borràs
FE
,
Buzas
EI
, et al.
Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi:.https://doi.org/10.3402/jev.v4.27066
21
Evrard
M
,
Kwok
IWH
,
Chong
SZ
,
Teng
KWW
,
Becht
E
,
Chen
J
, et al.
Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity. 2018;48(2):364–379.e8. doi:.https://doi.org/10.1016/j.immuni.2018.02.002
22
Mastio
J
,
Condamine
T
,
Dominguez
G
,
Kossenkov
AV
,
Donthireddy
L
,
Veglia
F
, et al.
Identification of monocyte-like precursors of granulocytes in cancer as a mechanism for accumulation of PMN-MDSCs. J Exp Med. 2019;216(9):2150–69. doi:.https://doi.org/10.1084/jem.20181952
23
Bronte
V
,
Brandau
S
,
Chen
SH
,
Colombo
MP
,
Frey
AB
,
Greten
TF
, et al.
Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7(1):12150. doi:.https://doi.org/10.1038/ncomms12150
24
Nagaraj
S
,
Nelson
A
,
Youn
JI
,
Cheng
P
,
Quiceno
D
,
Gabrilovich
DI
. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res. 2012;72(4):928–38. doi:.https://doi.org/10.1158/0008-5472.CAN-11-2863
25
Condamine
T
,
Dominguez
GA
,
Youn
JI
,
Kossenkov
AV
,
Mony
S
,
Alicea-Torres
K
, et al.
Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1(2):aaf8943. doi:.https://doi.org/10.1126/sciimmunol.aaf8943
26
Gabrilovich
DI
,
Nagaraj
S
. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi:.https://doi.org/10.1038/nri2506
27
Rodriguez
PC
,
Zea
AH
,
Culotta
KS
,
Zabaleta
J
,
Ochoa
JB
,
Ochoa
AC
. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J Biol Chem. 2002;277(24):21123–9. doi:.https://doi.org/10.1074/jbc.M110675200
28
Bingisser
RM
,
Tilbrook
PA
,
Holt
PG
,
Kees
UR
. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160(12):5729–34.
29
Rivoltini
L
,
Carrabba
M
,
Huber
V
,
Castelli
C
,
Novellino
L
,
Dalerba
P
, et al.
Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002;188(1):97–113. doi:.https://doi.org/10.1034/j.1600-065X.2002.18809.x
30
Kusmartsev
S
,
Nefedova
Y
,
Yoder
D
,
Gabrilovich
DI
. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172(2):989–99. doi:.https://doi.org/10.4049/jimmunol.172.2.989
31
Schmielau
J
,
Finn
OJ
. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61(12):4756–60.
32
Sauer
H
,
Wartenberg
M
,
Hescheler
J
. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11(4):173–86. doi:.https://doi.org/10.1159/000047804
33
Cobbs
CS
,
Whisenhunt
TR
,
Wesemann
DR
,
Harkins
LE
,
Van Meir
EG
,
Samanta
M
. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 2003;63(24):8670–3.
34
Nagaraj
S
,
Gupta
K
,
Pisarev
V
,
Kinarsky
L
,
Sherman
S
,
Kang
L
, et al.
Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828–35. doi:.https://doi.org/10.1038/nm1609
35
Movahedi
K
,
Guilliams
M
,
Van den Bossche
J
,
Van den Bergh
R
,
Gysemans
C
,
Beschin
A
, et al.
Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–44. doi:.https://doi.org/10.1182/blood-2007-07-099226
36
Diaz-Montero
CM
,
Salem
ML
,
Nishimura
MI
,
Garrett-Mayer
E
,
Cole
DJ
,
Montero
AJ
. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi:.https://doi.org/10.1007/s00262-008-0523-4
37
Trovato
R
,
Canè
S
,
Petrova
V
,
Sartoris
S
,
Ugel
S
,
De Sanctis
F
. The Engagement Between MDSCs and Metastases: Partners in Crime. Front Oncol. 2020;10:165. doi:.https://doi.org/10.3389/fonc.2020.00165
38
Calcinotto
A
,
Spataro
C
,
Zagato
E
,
Di Mitri
D
,
Gil
V
,
Crespo
M
, et al.
IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363–9. doi:.https://doi.org/10.1038/s41586-018-0266-0
39
Pernicová
Z
,
Slabáková
E
,
Kharaishvili
G
,
Bouchal
J
,
Král
M
,
Kunická
Z
, et al.
Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia. 2011;13(6):526–36. doi:.https://doi.org/10.1593/neo.11182
40
Westin
P
,
Stattin
P
,
Damber
JE
,
Bergh
A
. Castration therapy rapidly induces apoptosis in a minority and decreases cell proliferation in a majority of human prostatic tumors. Am J Pathol. 1995;146(6):1368–75.
41
Seruga
B
,
Ocana
A
,
Tannock
IF
. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(1):12–23. doi:.https://doi.org/10.1038/nrclinonc.2010.136
42
Grasso
CS
,
Wu
YM
,
Robinson
DR
,
Cao
X
,
Dhanasekaran
SM
,
Khan
AP
, et al.
The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–43. doi:.https://doi.org/10.1038/nature11125
43
Tran
C
,
Ouk
S
,
Clegg
NJ
,
Chen
Y
,
Watson
PA
,
Arora
V
, et al.
Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–90. doi:.https://doi.org/10.1126/science.1168175
44
Attard
G
,
Reid
AH
,
Yap
TA
,
Raynaud
F
,
Dowsett
M
,
Settatree
S
, et al.
Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–71. doi:.https://doi.org/10.1200/JCO.2007.15.9749
45
Garcia
AJ
,
Ruscetti
M
,
Arenzana
TL
,
Tran
LM
,
Bianci-Frias
D
,
Sybert
E
, et al.
Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol Cell Biol. 2014;34(11):2017–28. doi:.https://doi.org/10.1128/MCB.00090-14
46
Di Mitri
D
,
Toso
A
,
Chen
JJ
,
Sarti
M
,
Pinton
S
,
Jost
TR
, et al.
Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515(7525):134–7. doi:.https://doi.org/10.1038/nature13638
47
Wang
G
,
Lu
X
,
Dey
P
,
Deng
P
,
Wu
CC
,
Jiang
S
, et al.
Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016;6(1):80–95. doi:.https://doi.org/10.1158/2159-8290.CD-15-0224
48
Bezzi
M
,
Seitzer
N
,
Ishikawa
T
,
Reschke
M
,
Chen
M
,
Wang
G
, et al.
Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat Med. 2018;24(2):165–75. doi:.https://doi.org/10.1038/nm.4463
49
Siegel
RL
,
Miller
KD
,
Jemal
A
. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:.https://doi.org/10.3322/caac.21590
50
Bray
F
,
Ferlay
J
,
Soerjomataram
I
,
Siegel
RL
,
Torre
LA
,
Jemal
A
. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:.https://doi.org/10.3322/caac.21492
51
Vargo-Gogola
T
,
Rosen
JM
. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7(9):659–72. doi:.https://doi.org/10.1038/nrc2193
52
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi:.https://doi.org/10.1038/nature11412
53
Parker
JS
,
Mullins
M
,
Cheang
MC
,
Leung
S
,
Voduc
D
,
Vickery
T
, et al.
Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–7. doi:.https://doi.org/10.1200/JCO.2008.18.1370
54
Foulkes
WD
,
Smith
IE
,
Reis-Filho
JS
. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. doi:.https://doi.org/10.1056/NEJMra1001389
55
Cortazar
P
,
Zhang
L
,
Untch
M
,
Mehta
K
,
Costantino
JP
,
Wolmark
N
, et al.
Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. Published online February 14, 2014. doi:.https://doi.org/10.1016/S0140-6736(13)62422-8
56
Yu
J
,
Wang
Y
,
Yan
F
,
Li
H
,
Ren
X
. Response to comment on “Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer”. J Immunol. 2013;190(11):5341–2. doi:.https://doi.org/10.4049/jimmunol.1390024
57
Huang
B
,
Pan
PY
,
Li
Q
,
Sato
AI
,
Levy
DE
,
Bromberg
J
, et al.
Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–31. doi:.https://doi.org/10.1158/0008-5472.CAN-05-1299
58
Kaplan
RN
,
Riba
RD
,
Zacharoulis
S
,
Bramley
AH
,
Vincent
L
,
Costa
C
, et al.
VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. doi:.https://doi.org/10.1038/nature04186
59
Szczerba
BM
,
Castro-Giner
F
,
Vetter
M
,
Krol
I
,
Gkountela
S
,
Landin
J
, et al.
Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566(7745):553–7. doi:.https://doi.org/10.1038/s41586-019-0915-y
60
Templeton
AJ
,
McNamara
MG
,
Šeruga
B
,
Vera-Badillo
FE
,
Aneja
P
,
Ocaña
A
, et al.
Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:.https://doi.org/10.1093/jnci/dju124
61
Gentles
AJ
,
Newman
AM
,
Liu
CL
,
Bratman
SV
,
Feng
W
,
Kim
D
, et al.
The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45. doi:.https://doi.org/10.1038/nm.3909
62
Bosiljcic
M
,
Cederberg
RA
,
Hamilton
MJ
,
LePard
NE
,
Harbourne
BT
,
Collier
JL
, et al.
Targeting myeloid-derived suppressor cells in combination with primary mammary tumor resection reduces metastatic growth in the lungs. Breast Cancer Res. 2019;21(1):103. doi:.https://doi.org/10.1186/s13058-019-1189-x
63
Danilin
S
,
Merkel
AR
,
Johnson
JR
,
Johnson
RW
,
Edwards
JR
,
Sterling
JA
. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. OncoImmunology. 2012;1(9):1484–94. doi:.https://doi.org/10.4161/onci.21990
64
Ma
X
,
Wang
M
,
Yin
T
,
Zhao
Y
,
Wei
X
. Myeloid-Derived Suppressor Cells Promote Metastasis in Breast Cancer After the Stress of Operative Removal of the Primary Cancer. Front Oncol. 2019;9:855. doi:.https://doi.org/10.3389/fonc.2019.00855
65
Siegel
RL
,
Miller
KD
,
Jemal
A
. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:.https://doi.org/10.3322/caac.21332
66
Baert
T
,
Vankerckhoven
A
,
Riva
M
,
Van Hoylandt
A
,
Thirion
G
,
Holger
G
, et al.
Myeloid Derived Suppressor Cells: Key Drivers of Immunosuppression in Ovarian Cancer. Front Immunol. 2019;10:1273. doi:.https://doi.org/10.3389/fimmu.2019.01273
67
Horikawa
N
,
Abiko
K
,
Matsumura
N
,
Baba
T
,
Hamanishi
J
,
Yamaguchi
K
, et al.
Anti-VEGF therapy resistance in ovarian cancer is caused by GM-CSF-induced myeloid-derived suppressor cell recruitment. Br J Cancer. 2020;122(6):778–88. doi:.https://doi.org/10.1038/s41416-019-0725-x
68
Alfaro
C
,
Teijeira
A
,
Oñate
C
,
Pérez
G
,
Sanmamed
MF
,
Andueza
MP
, et al.
Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res. 2016;22(15):3924–36. doi:.https://doi.org/10.1158/1078-0432.CCR-15-2463
69
Alfaro
C
,
Sanmamed
MF
,
Rodríguez-Ruiz
ME
,
Teijeira
Á
,
Oñate
C
,
González
Á
, et al.
Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. doi:.https://doi.org/10.1016/j.ctrv.2017.08.004
70
Obermajer
N
,
Muthuswamy
R
,
Odunsi
K
,
Edwards
RP
,
Kalinski
P
. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71(24):7463–70. doi:.https://doi.org/10.1158/0008-5472.CAN-11-2449
71
Tartour
E
,
Pere
H
,
Maillere
B
,
Terme
M
,
Merillon
N
,
Taieb
J
, et al.
Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30(1):83–95. doi:.https://doi.org/10.1007/s10555-011-9281-4
72
Cui
TX
,
Kryczek
I
,
Zhao
L
,
Zhao
E
,
Kuick
R
,
Roh
MH
, et al.
Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39(3):611–21. doi:.https://doi.org/10.1016/j.immuni.2013.08.025
73
Kodumudi
KN
,
Woan
K
,
Gilvary
DL
,
Sahakian
E
,
Wei
S
,
Djeu
JY
. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–94. doi:.https://doi.org/10.1158/1078-0432.CCR-10-0733
74
Vincent
J
,
Mignot
G
,
Chalmin
F
,
Ladoire
S
,
Bruchard
M
,
Chevriaux
A
, et al.
5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–61. doi:.https://doi.org/10.1158/0008-5472.CAN-09-3690
75
Sumida
K
,
Wakita
D
,
Narita
Y
,
Masuko
K
,
Terada
S
,
Watanabe
K
, et al.
Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol. 2012;42(8):2060–72. doi:.https://doi.org/10.1002/eji.201142335
76
Crittenden
MR
,
Savage
T
,
Cottam
B
,
Bahjat
KS
,
Redmond
WL
,
Bambina
S
, et al.
The peripheral myeloid expansion driven by murine cancer progression is reversed by radiation therapy of the tumor. PLoS One. 2013;8(7):e69527. doi:.https://doi.org/10.1371/journal.pone.0069527
77
Mace
TA
,
Ameen
Z
,
Collins
A
,
Wojcik
S
,
Mair
M
,
Young
GS
, et al.
Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73(10):3007–18. doi:.https://doi.org/10.1158/0008-5472.CAN-12-4601
78
Toso
A
,
Revandkar
A
,
Di Mitri
D
,
Guccini
I
,
Proietti
M
,
Sarti
M
, et al.
Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014;9(1):75–89. doi:.https://doi.org/10.1016/j.celrep.2014.08.044
79
Zhao
D
,
Pan
C
,
Sun
J
,
Gilbert
C
,
Drews-Elger
K
,
Azzam
DJ
, et al.
VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene. 2015;34(24):3107–19. doi:.https://doi.org/10.1038/onc.2014.257
80
Lu
X
,
Horner
JW
,
Paul
E
,
Shang
X
,
Troncoso
P
,
Deng
P
, et al.
Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. 2017;543(7647):728–32. doi:.https://doi.org/10.1038/nature21676
81
Steele
CW
,
Karim
SA
,
Leach
JDG
,
Bailey
P
,
Upstill-Goddard
R
,
Rishi
L
, et al.
CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016;29(6):832–45. doi:.https://doi.org/10.1016/j.ccell.2016.04.014
82
Kaneider
NC
,
Agarwal
A
,
Leger
AJ
,
Kuliopulos
A
. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11(6):661–5. doi:.https://doi.org/10.1038/nm1245
83
Jamieson
T
,
Clarke
M
,
Steele
CW
,
Samuel
MS
,
Neumann
J
,
Jung
A
, et al.
Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122(9):3127–44. doi:.https://doi.org/10.1172/JCI61067
84
Nicholls
DJ
,
Wiley
K
,
Dainty
I
,
MacIntosh
F
,
Phillips
C
,
Gaw
A
, et al.
Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther. 2015;353(2):340–50. doi:.https://doi.org/10.1124/jpet.114.221358

