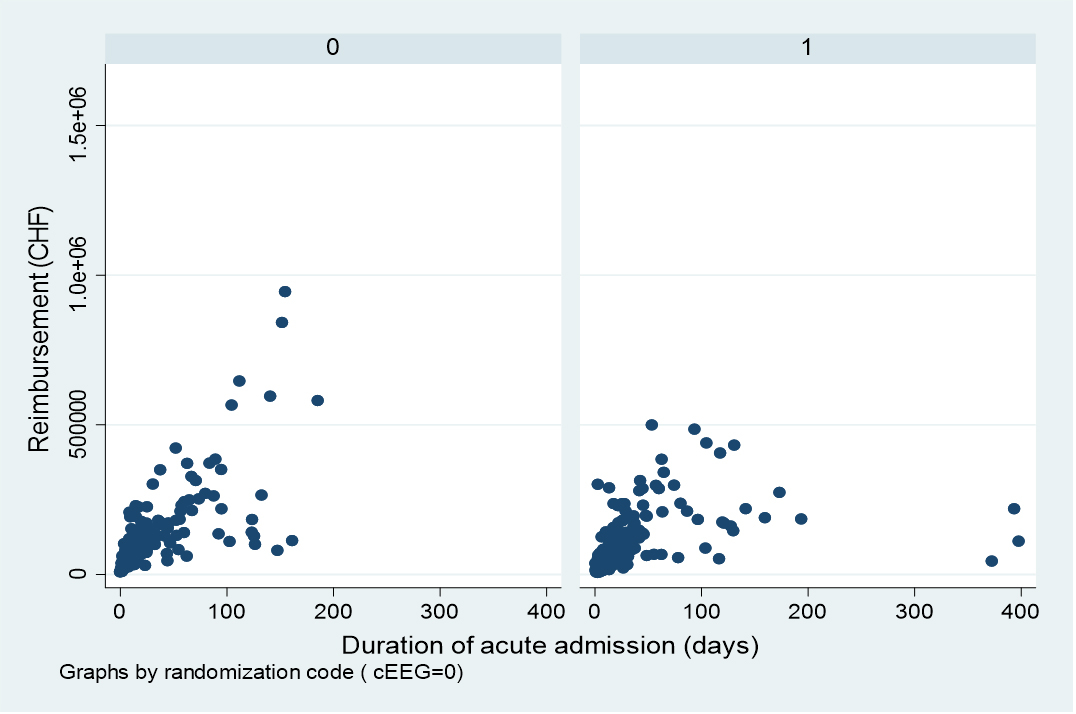
Figure 1 correlation of reimbursement and duration of acute hospitalisation stratified for cEEG (left; rho 0.817, p <0.001) and rEEG (right; rho 0.729, p <0.001).
DOI: https://doi.org/10.4414/smw.2021.20477
Continuous electroencephalographic monitoring (cEEG, typically lasting several hours to a few days) is increasingly used to assess brain function and identify epileptiform activity in patients with unexplained consciousness impairment in intensive care units (ICUs). In fact, cEEG is more sensitive for seizure or status epilepticus detection than routine EEG (rEEG, typically lasting 20–30 minutes) [1–3]. Several international guidelines strongly recommend its use in critically ill patients [4, 5]. However, cEEG is resource- and time-consuming compared with rEEG; it requires longer review times, constant machine availability and surveillance by skilled personnel [3, 6]. Therefore, its widespread clinical adoption is hampered in many hospitals, including most European centres [7].
In addition to these logistics issues, in the USA cEEG is related to higher hospitalisation charges compared with rEEG [6, 8, 9]; that hospital reimbursement system nevertheless differs from those of other settings, since different professional fees are applied to EEG procedures. Several European countries including Switzerland [10, 11], Australia, Canada and Japan [12] use diagnosis-related groups (DRGs), whose purpose is to classify patients according to demographics, principal and secondary diagnoses, co-morbidities and complications; procedures performed play a relatively minor role. Based on this information, patients will belong to a certain DRG [13] associated with a specific reimbursement estimated on similar cases [11]. DRGs are also used in the USA, but underlying charges related to equipment, technologists and physicians increase the overall cost of care [14], inducing higher prices [1, 9].
In the context of optimisation of hospital resources, it is important to assess the value of healthcare delivery concerning the consequences on patient care and prognosis [15]. Impact of cEEG versus rEEG on clinical outcome has been recently assessed in a multicentre Swiss randomised controlled trial in adults with acute consciousness impairment [16]. The primary purpose of the present study was to analyse the impact of these procedures on hospitalisation reimbursement. In Switzerland, professional fees according to specific in-hospital procedures are not foreseen, and the DRG-based hospital reimbursement is paid by the patient’s insurance and a contribution of the state (canton).
We used data from the Continuous EEG Randomized Trial in Adults (CERTA, NCT03129438), conducted in four large Swiss hospitals (Centre Hospitalier Universitaire Vaudois in Lausanne, Inselspital Bern, Universitätsspital Basel, and Hôpital de Sion, the only non-university centre) between April 2018 and September 2019; 366 patients were analysed, exceeding the required sample size [16]. Of them, 30.1% had hypoxic-ischaemic encephalopathy, 23.8% intracranial haemorrhage, 13.4% brain trauma. Patients aged ≥18 years, presenting an alteration of consciousness of any aetiology with a Glasgow Coma Scale score ≤11 or a Full Outline of UnResponsiveness (FOUR) score ≤12, and needing an EEG either to exclude seizures or status epilepticus, or to evaluate prognosis, were randomised – upon investigator availability – 1:1 to cEEG (30–48 hours) or two rEEGs (20–30 minutes each, within 30–48 hours) [3, 16]. Several demographic and clinical variables were recorded prospectively; the detailed protocol may be found in a link from the principal publication [16].
For the present study, we considered demographics and the three most frequent aetiologies of consciousness impairment in the CERTA study, and retrieved information about the reimbursement for each participant due to acute hospitalisation, in each participating institution; this was routinely calculated according to the Swiss DRGs. We also explored different variables, such as occurrence of any EEG-triggered changes in clinical patient management (anti-seizure medications introduced or stopped, anti-seizure medications increased or decreased, brain imaging procedure order) during 60 hours following the start of the first EEG [16], mortality at 6 months, seizure or status epilepticus detection rate, duration of hospitalisation, and reimbursement related to the two EEG procedures.
In addition to crude reimbursement comparisons (in CHF) between the EEG intervention groups, we explored correlations with clinical variables. Mann-Whitney U-tests or chi-square tests were used as appropriate. Spearman’s rank coefficients were applied to assess correlations between reimbursement and continuous variables (age, duration of acute hospital stay). Tests with a p-value <0.05 were considered statistically significant. A stepwise multivariate linear regression was applied to identify variables independently related to reimbursement among those that resulted statistically significant in univariate analyses, including also EEG intervention in view of the existing literature. Effect size was estimated using partial eta squares. Calculations were made using Stata 16 (College Station, TX).
This study was approved by the CER-VD ethics commission and was conducted in accordance with the Helsinki declaration. Recruited patients (or their parents or guardians) gave their written informed consent.
A total of 366 critically ill patients were analysed, of whom 184 underwent cEEG and 182 rEEG. There were 123 (33.6%) women, with a mean age of 63.8 years (standard deviation [SD] 15.0); 177 (48.4%) patients died. Distribution of the major aetiologies (anoxic-ischaemic encephalopathy, intracranial haemorrhage, brain trauma) was not different between recruiting sites (p = 0.163, χ2).
Median acute hospitalisation reimbursement seemed somewhat higher in patients undergoing cEEG (CHF 89,631, interquartile range [IQR] 45,635–159,994) than those randomised to rEEG (CHF 73,017, IQR 43,031–158’565); however, this difference was not significant (p = 0.432, U-test). Table 1 shows further analyses for the global cohort, stratified by EEG intervention. In univariate analyses of the whole cohort, increasing amounts strongly correlated with lack of hypoxic-ischaemic encephalopathy, intracranial haemorrhage, lack of changes in clinical management within 60 hours, no detection of seizure/status epilepticus, and survival at 6 months, as well as younger age (rho –0.177, p <0.001, Spearman), and longer acute hospitalisation (rho 0.77, p <0.001, Spearman); figure 1 shows the distribution stratified by EEG intervention. Conversely, no association was found between reimbursement and gender, hospital, or brain trauma (table 1). Stratification by EEG intervention also did not reveal any significant difference.
Table 1 Reimbursement and variables of interests (for the whole cohort, and stratified by EEG intervention group). Numbers represent medians (and interquartile range). Bold numbers are significant (Mann-Whitney U-tests).
| Variable | Acute hospitalisation reimbursement (CHF) | p-value | Continuous EEG | Routine EEG | p-value |
|---|---|---|---|---|---|
| Hospital of recruitment | |||||
| CHUV (289) | 88,303.64 (46,148–168,184) |
0.164 | 91,212 (46,714–162,952) | 84,691 (46,045–171,972) | 0.895 |
| Others (77) | 73,964.75 (39,786–130,988) |
84,294 (41,003–148,945) | 64,117 (35,588–107,865) | 0.116 | |
| Gender | |||||
| Female (123) | 72,069 (42,254–148,388) |
0.322 | 86,731 (44043–148,388) | 70,908 (40,782–146,598) | 0.460 |
| Male (243) | 87,437 (45,702 - 165,717) |
92,649 (46,034–165,716) | 82,518 (45,702–158,564) | 0.650 | |
| Hypoxic-ischaemic encephalopathy | |||||
| Yes (112) | 56,467 (30,536–125,633) |
< 0.001 | 53,188 (30,210–111,904) | 59,057 (30,915–135,033) | 0.624 |
| No (254) | 97,264 (54,068–172,433) |
106,790 (60,058–181,368) | 87,511 (52,391–171,574) | 0.112 | |
| Intracranial haemorrhage | |||||
| Yes (87) | 108,968 (62,548–172,433) | 0.012 | 98,306 (64,918–168,184) | 115,005 (61,379–176,922) | 0.304 |
| No (279) | 80,863 (38,221–153,038) |
85,159 (43,602–155,959) | 69,779 (35,427–146,641) | 0.646 | |
| Brain trauma | |||||
| Yes (49) | 87,666 60,210–146,418 |
0.380 | 86,412 (59,026–156,613) | 100,467 (60,210–138,770) | 0.737 |
| No (317) | 83,429 43,260–162,221 |
92,650 (43,772 162,758) | 72,069 (42,932–162,221) | 0.554 | |
| Diagnosis of seizures/status epilepticus | |||||
| Yes (37) | 55,456 (30,216–119,899) |
0.006 | 55,456 (30,901–100,831) | 51,385 (20,077–156,310) | 0.824 |
| No (329) | 87,437 (46,046–168,184) |
100,473 (47,133–174,866) | 75,251 (45,182–158,565) | 0.167 | |
| Changes in clinical management within 60 hours of intervention | |||||
| Yes (145) | 67,092 (35,427–141,569) |
0.002 | 67,974 (33,846–144,853) | 63,726 (37,578–134,401) | 0.813 |
| No (221) | 92,138 (52,930–171,972) |
101,050 (61,728–174,866) | 88,288 (46,148–162,221) | 0.246 | |
| Outcome at 6 months | |||||
| Death (177) | 65,161 (36,560–129,293) |
<0.001 | 68,049 (34,521–129,203) | 62,716 (37,578–133,486) | 0.851 |
| Survival (189) | 108,968 (54,068–181,312) |
112,205 (61,728–183,950) | 100,041 (51,328–175,412) | 0.461 |
CHUV = Centre Hospitalier Universitaire Vaudois; EEG = electroencephalography

Figure 1 correlation of reimbursement and duration of acute hospitalisation stratified for cEEG (left; rho 0.817, p <0.001) and rEEG (right; rho 0.729, p <0.001).
Median acute hospitalisation was longer in surviving patients than those who were deceased (41 days, IQR 14–53 vs 10 days, IQR 5–23; p <0.001) and patients undergoing rEEG (19 days, IQR 9–37 vs 16 days, IQR 7 – 42; p = 0.012); it was shorter in those having changes in clinical management (12 days, IQR –31 vs 20 days, IQR 10–45; p <0.001), who had hypoxic-ischaemic encephalopathy (9 days, IQR 4–24 vs 22 days IQR 11–43; p <0.001), or had seizures or status epilepticus detected (9 days, IQR 5–27 vs 19 days, IQR 9–42; p = 0.003; all U-tests). Patients had higher mortality if having clinical management changes (62.8% vs 38.9%, p <0.001), if they had anoxic-ischaemic encephalopathy (60.7% vs 42.9%, p = 0.002), or if seizures/status epilepticus were detected (81.1% vs 44.7%, p <0.001), whereas presence of intracranial haemorrhage was not significant (p = 0.317; all χ2), as the EEG intervention [16].
In order to assess the relative interplay of the different variables of interest regarding reimbursement, we performed a multivariate linear regression including including EEG intervention and variables that resulted significant in univariate analyses (table 1). Aetiology, change in clinical management and mortality were not significant, whereas duration of acute hospitalisation duration (t = 10.74, p <0.001), cEEG (t = 2.35, p = 0.019) and no detection of seizures/status epilepticus (t = 2.10, p = 0.036) were independently related to higher reimbursement. In particular, EEG intervention was significant only when considering hospitalisation length, and effect size estimation (partial eta squares) showed the strong contribution of hospitalisation duration (0.261), compared with cEEG (0.015) and seizure/status epilepticus detection (0.012).
This study in a Swiss DRG billing environment shows that cEEG has a higher reimbursement than rEEG in critically ill adults; this is, however, not directly evident and can be identified only after considering duration of acute hospitalisation, which in turn represents by far the main cost determinant.
To our knowledge, no European study has addressed this question to date, and this represents the first analysis based on a randomised controlled trial. It is intriguing to observe that EEG length did not correlate with reimbursement in univariable analysis, and thus at first glance may not generate any direct financial incentive to perform cEEG recordings; its role appeared only together with hospitalisation duration (and, to a lesser extent, no detection of seizures / status epilepticus). This illustrates the really complex interplay between these variables: as ictal events are more frequently identified in patients undergoing cEEG [16], these two variables have opposite influences on reimbursement, which is mostly determined by hospitalisation length. While patients undergoing cEEG tend to have shorter hospitalisations (tending to lower costs, see below), adjusting for hospitalisation length probably unravels the cEEG contribution. In turn, seizure/status epilepticus detection likely acts as a mortality surrogate (and thus shorter hospitalisation and lower costs). Our results, generated by a DRG system apparently similar to those used in several other settings, including many European countries, where a specific procedure’s cost is “diluted” into the diagnostic group, differ from US studies, where the cEEG billing system leads to higher charges that are readily recognisable, without need for adjustments [9, 15].
Higher mortality was related to shorter hospitalisation, therefore inducing lower reimbursements. This applied to patients with anoxic-ischaemic encephalopathy, or in whom seizures / status epilepticus were detected, or clinical management was modified.
Our results should be interpreted in the context of some limitations. Firstly, the relatively low number of patients in each group, particularly after stratification for EEG intervention, might reduce statistical power (the CERTA study was designed to detect a difference in survival between EEG intervention groups, not this secondary endpoint). Secondly, our data were generated in Switzerland; nevertheless, several other countries use DRGs and we believe that our findings may apply to them as well. Third, this study was not designed to estimate the specific costs generated by the detection (or not) of seizures/status epilepticus. On the other hand, one of the study strengths is the multicentre randomised design to assign cEEG or rEEG. Also, since DRGs are used as a national billing system in Switzerland, internal validity should be preserved; indeed, no significant difference was found across recruiting centres. Application of multivariable analyses allowed a relatively refined understanding of the different parameters related to the final bill. Furthermore, inclusion of patients in university and non-university hospitals supports generalisability of our results.
In a European DRG-based billing system applied to critically ill adults, reimbursement depends mostly on duration of acute hospital stay, whereas use of cEEG and lack of seizures / status epilepticus detection also play a role. This differs from the USA billing system, where the impact of cEEG on hospitalisation charges appears more direct.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The Swiss National Science Foundation (grant 320030_169379) supported this study.
JWL: contract work for Bioserenity, Teladoc; co-founder of Soterya Inc; research funding: NIH (site PI for R01 NS062092). The other authors declare no conflict of interest.
1 Ney JP , van der Goes DN , Nuwer MR , Nelson L , Eccher MA . Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005-2009. Neurology. 2013;81(23):2002–8. doi:.https://doi.org/10.1212/01.wnl.0000436948.93399.2a
2 Hilkman DM , van Mook WN , van Kranen-Mastenbroek VH . Continuous electroencephalographic-monitoring in the ICU: an overview of current strengths and future challenges. Curr Opin Anaesthesiol. 2017;30(2):192–9. doi:.https://doi.org/10.1097/ACO.0000000000000443
3 Rossetti AO , Schindler K , Alvarez V , Sutter R , Novy J , Oddo M , et al. Does Continuous Video-EEG in Patients With Altered Consciousness Improve Patient Outcome? Current Evidence and Randomized Controlled Trial Design. J Clin Neurophysiol. 2018;35(5):359–64. doi:.https://doi.org/10.1097/WNP.0000000000000467
4 Hirsch LJ , LaRoche SM , Gaspard N , Gerard E , Svoronos A , Herman ST , et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27. doi:.https://doi.org/10.1097/WNP.0b013e3182784729
5 Claassen J , Taccone FS , Horn P , Holtkamp M , Stocchetti N , Oddo M ; Neurointensive Care Section of the European Society of Intensive Care Medicine. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39(8):1337–51. doi:.https://doi.org/10.1007/s00134-013-2938-4
6 Hill CE , Blank LJ , Thibault D , Davis KA , Dahodwala N , Litt B , et al. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology. 2019;92(1):e9–18. doi:.https://doi.org/10.1212/WNL.0000000000006689
7 Hilkman DMW , van Mook WNKA , Mess WH , van Kranen-Mastenbroek VHJM . The Use of Continuous EEG Monitoring in Intensive Care Units in The Netherlands: A National Survey. Neurocrit Care. 2018;29(2):195–202. doi:.https://doi.org/10.1007/s12028-018-0525-9
8 Singh J , Britton J , Alwaki A , Singh P . After-Hours EEG: Relative Value of Emergent Routine Versus Prolonged EEG Recordings. J Clin Neurophysiol. 2019;36(1):32–5. doi:.https://doi.org/10.1097/WNP.0000000000000529
9 Abend NS , Topjian AA , Williams S . How much does it cost to identify a critically ill child experiencing electrographic seizures? J Clin Neurophysiol. 2015;32(3):257–64. doi:.https://doi.org/10.1097/WNP.0000000000000170
10 Busse R , Geissler A , Aaviksoo A , Cots F , Häkkinen U , Kobel C , et al. Diagnosis related groups in Europe: moving towards transparency, efficiency, and quality in hospitals? BMJ. 2013;346(jun07 3):f3197. doi:.https://doi.org/10.1136/bmj.f3197
11 Scheller-Kreinsen D , Quentin W , Busse R . DRG-based hospital payment systems and technological innovation in 12 European countries. Value Health. 2011;14(8):1166–72. doi:.https://doi.org/10.1016/j.jval.2011.07.001
12 Wild V , Pfister E , Biller-Andorno N . Les DRG: l’éthique contre l’économie? Bull ASSM. 2009;09(1):1–5.
13 Mathauer I , Wittenbecher F . Hospital payment systems based on diagnosis-related groups: experiences in low- and middle-income countries. Bull World Health Organ. 2013;91(10):746–56A. doi:.https://doi.org/10.2471/BLT.12.115931
14Centers for Medicare & Medicaid Services. Medicaid. Feb 2020 [June 2020]; Available from: https://www.medicaid.gov/medicaid/index.html.
15 Crepeau AZ , Fugate JE , Mandrekar J , White RD , Wijdicks EF , Rabinstein AA , et al. Value analysis of continuous EEG in patients during therapeutic hypothermia after cardiac arrest. Resuscitation. 2014;85(6):785–9. doi:.https://doi.org/10.1016/j.resuscitation.2014.01.019
16 Rossetti AO , Schindler K , Sutter R , Rüegg S , Zubler F , Novy J , et al. Continuous vs Routine Electroencephalogram in Critically Ill Adults With Altered Consciousness and No Recent Seizure. JAMA Neurol. 2020;77(10):1225–32. doi:.https://doi.org/10.1001/jamaneurol.2020.2264
In summary:
VU: designed study, analysed data, drafted paper; JN: revised paper; KS: designed study, revised paper; SR: designed study, revised paper; VA: designed study, revised paper; FZ: revised paper; MO: revised paper; JWL: revised paper; AOR: designed study, analysed data, drafted paper.
The Swiss National Science Foundation (grant 320030_169379) supported this study.
JWL: contract work for Bioserenity, Teladoc; co-founder of Soterya Inc; research funding: NIH (site PI for R01 NS062092). The other authors declare no conflict of interest.