Characterisation of advanced Parkinson’s disease: OBSERVE-PD observational study – results of the Swiss subgroup
DOI: https://doi.org/10.4414/smw.2021.20419
J. Carsten
Möllerab, Christian R.
Baumannc, Pierre R.
Burkhardd, Alain
Kaelin-Langefg, Irène
Küngh, Koray
Onuki, Stephan
Bohlhalterj
a Parkinson Center, Center for Neurological Rehabilitation, Zihlschlacht, Switzerland
b Faculty of Medicine, Philipps University, Marburg, Germany
c Center for Movement and Functional Neurosurgery, Department of Neurology, University Hospital Zurich, Switzerland
d Department for Clinical Neuroscience, Geneva University Hospitals (HUG), Geneva, Switzerland
e Neurocenter of Southern Switzerland, Lugano, Switzerland
f Faculty of Biomedical Sciences, Università della Svizzera Italiana, Lugano, Switzerland
g Faculty of Medicine, University of Berne, Switzerland
h AbbVie AG Switzerland, Baar, Switzerland
i AbbVie Inc., North Chicago, USA
j Neurocenter, Lucerne Kantonsspital, Lucerne, Switzerland
Summary
AIMS OF THE STUDY
Currently, the characterisation of advanced Parkinson’s disease (APD) does not follow standardised diagnostic criteria, which complicates the evaluation of ongoing care and treatment strategies, such as eligibility for device-aided treatment (DAT). Therefore, this study aimed to determine the proportion of APD and non-advanced Parkinson’s disease (non-APD) patients treated at specialised movement disorder centres in Switzerland, to compare clinical characteristics of APD versus non-APD patients and to assess eligibility for and use of DAT. Furthermore, potential differences between the Swiss and international situation should be uncovered.
METHODS
OBSERVE-PD was a cross-sectional, international, observational study including 2615 patients from 128 movement disorder centres in 18 countries. For the Swiss subgroup of the study analysed here, which included 134 patients from 5 movement disorder centres, motor and non-motor symptoms, activities of daily living and quality of life were assessed as endpoints. The correlation between physician’s judgement of APD and the Delphi criteria for APD, which were developed by an international expert group, as well as the clinical burden in APD and non-APD patients and eligibility for and use of DAT were evaluated. The results for the Swiss subgroup were subsequently compared with the international full analysis set of the OBSERVE-PD study.
RESULTS
Based on physician’s judgement, 69.4% of patients included in the Swiss study suffered from APD. A moderate correlation between physician’s judgement and the Delphi criteria for APD was observed (Κ = 0.480, 95% confidence interval 0.317–0.642). Clinical burden was higher for APD patients, as shown by worse scores for activities of daily living, motor symptom severity, dyskinesia duration/disability, duration of “off” time, non-motor symptoms and quality of life as compared with non-APD patients (p <0.0001 for all). The Swiss data for disease burden were comparable to the international findings, except that the Swiss patients showed less “off” time. Amongst APD patients eligible for DAT, the main reason for no DAT in Switzerland was patient refusal, whereas patients needing more time to decide about it was the most frequent reason in the international analysis.
CONCLUSIONS
The study shows that the burden of APD in tertiary care centres in Switzerland is comparable to the international situation. Patient refusal is the main reason for no DAT amongst eligible APD patients in such centres. The identification of standard APD classification parameters and evaluation of the reasons for no DAT are relevant for optimising treatment strategies and the transition to DAT.
Abbreviations
- APD
-
advanced Parkinson’s disease
- DAT
-
device-aided treatment
- NMSS
-
Non-motor Symptom Scale
- Non-APD
-
non-advanced Parkinson’s disease
- PD
-
Parkinson’s disease
- PDQ-8
-
8-Item Parkinson’s Disease Questionnaire
- s.c.
-
subcutaneous
- SD
-
standard deviation
- UPDRS
-
Unified Parkinson’s Disease Rating Scale
Introduction
Parkinson’s disease is an incurable neurological disorder, which gradually progresses and may eventually lead to severe disability despite therapeutic intervention [1–5].Device-aided treatment (DAT) such as continuous infusion therapies (apomorphine s.c. or intraduodenal levodopa) or stereotactic surgery with deep brain stimulation are nowadays recognised options in the advanced stages of Parkinson’s disease [2]. DAT is thereby generally provided by a multidisciplinary expert group at a specialised movement disorder centre or clinic [3].
Progression to advanced Parkinson’s disease (APD) is not well defined, even though the increased severity of cardinal Parkinson’s disease symptoms such as tremor, rigidity and akinesia, or the development of axial signs such as postural instability, gait disorders or truncal deformities, may be observed as indicators of disease progression. In the pre-levodopa era, natural progression of Parkinson’s disease was formalised by Margaret Hoehn and Melvin Yahr in their well-established scale, whereby reaching stage 3 would be considered a crucial step toward advanced disease [6]. However, the introduction of levodopa therapy had a major impact on the clinical features previously used to assess disease progression. Especially levodopa-related manifestations, such as motor fluctuations and levodopa-induced dyskinesia, are now considered more reliable indicators of progression, even though there is currently no distinct threshold above which the disease is classified as advanced [2, 4]. Therapeutic decisions, such as eligibility for DAT, which are required to optimise patient outcomes, are complicated by the lack of a clear classification system [1]. Therefore, significant efforts have been made in recent years to reach global consensus on the definition of APD as an operational tool to provide guidance on the timing of DAT initiation [1]. Currently reported percentages of patients with APD treated in a movement disorder centre vary between studies [7–11]. However, a clearer understanding of the number of APD patients within a movement disorder centre forms an important basis for efficient planning of DAT initiation [1]. In the past few years, the decision to implement DAT in individual patients has shifted towards earlier stages of Parkinson’s disease, which further complicates the definition of APD [12].
Here we report the results of the Swiss subgroup of the cross-sectional, multi-country, observational OBSERVE-PD study, which aimed at assessing the proportion of APD patients in movement disorder centres, based on physician’s judgement and the Delphi criteria for APD, the clinical burden of APD and the eligibility for and use of DAT. In addition, the data of the Swiss subgroup was compared with the results obtained from the previously published international full analysis set [3]. The study results are reported in accordance to the STROBE statement for observational studies [13].
Methods
The detailed methods of the international OBSERVE-PD study have been published previously and will be briefly described here [3].
Study setting
This observational study was conducted in a cross-sectional, non-interventional, multi-country, multicentre format in movement disorder centres/clinics in 18 active countries, including 5 in Switzerland, for which the subgroup analysis is presented here. The main eligibility criteria for participating movement disorder centres was the availability of DAT, including levodopa-carbidopa intestinal gel, continuous subcutaneous apomorphine infusion or deep brain stimulation. Patients were documented between February 2015 and January 2016. The project database was closed in June 2016, and the study results were finally reported in September 2016. To ensure that the distance of upper and lower limits of the two-sided 95% confidence interval (CI) for the primary endpoint would not exceed 2.0%, a sample size of approximately 2500 patients was chosen.
The study was approved by the local ethics committee and was performed according to International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use and Good Clinical Practice requirements and followed the principles of the Declaration of Helsinki.
Patient selection
Parkinson’s disease patients at least 18 years of age, i.e., outpatients attending routine clinical visits or inpatients at participating movement disorder centres, were eligible to participate in the study. A clinical diagnosis of Parkinson’s disease according to the assessment of the treating physician and written informed consent were further requirements. Exclusion criteria were “off” stage at any time of the visit, uncertainty around the Parkinson’s disease diagnosis and participation in another clinical study. Patients were identified consecutively and were asked to participate in the study to avoid selection bias.
Study assessments
Data were collected for each patient during a single study visit following provision of signed informed consent. As previously published, the data collected included demographics, Parkinson’s disease-related information, Parkinson’s disease treatment information and comorbidities [3]. The assessment of patient eligibility for DAT was based on the movement disorder specialist’s judgement and did not require stringent eligibility criteria. Generally, motor fluctuations refractory to oral treatment was the main criterion. Assessment of motor symptoms during the study visit was based on the Unified Parkinson’s Disease Rating Scale (UPDRS) in “on” stage (Part II – activities of daily living; Part III – motor signs; Part IV – items 32, 33, 34, 39, complications of therapy; Part V – modified Hoehn and Yahr scale). Non-motor symptoms and quality of life were assessed using the Non-motor Symptoms Scale (non-motor symptoms S) and the 8-Item Parkinson’s Disease Quality of Life Questionnaire (PDQ-8), respectively.
Endpoints
The primary endpoint of the study was the proportion of Parkinson’s disease patients with APD according to physician’s judgement. Secondary endpoints included parameters used to select the advanced stage Parkinson’s disease patients versus non-advanced Parkinson’s disease patients, the number of APD patients according to physician’s judgement eligible for invasive therapies per physician’s judgement, referral source and duration since diagnosis, and the proportion of APD patients identified by physician’s judgement compared with the proportion identified by APD criteria created by the Delphi method for each of the eleven APD criteria questions separately.
Delphi criteria for APD
The Delphi criteria were previously developed by an international expert group as a staging tool to help with the identification of patients with APD and to optimise patient care (e.g., DAT eligibility). Here, experts used the Delphi consensus methodology to identify 15 clinically relevant indicators for suspected APD, including six motor symptoms, five non-motor symptoms and four functional impairments. Furthermore, consensus was reached on seven indicators describing characteristics of APD patients eligible for DAT [1].
A predefined endpoint of this study was to compare Delphi staging with the physician’s judgement. Therefore, the patients were assessed by the investigators (movement disorder specialists) using their own judgement, followed by an assessment using eleven questions developed by the Delphi method. These questions aimed, amongst others, at assessing the level of troublesome motor fluctuations, the number of hours during the waking day in which the patient has “off” symptoms, whether the patient has “off” time at least every 3 hours, and the level of capacity in activities of daily living (table 1). Patients fulfilling any of these eleven indicators were considered to have APD. Importantly, patients with ongoing DAT were not assessed using the Delphi criteria.
Table 1 Delphi criteria for advanced Parkinson’s disease (APD) [1].
|
Delphi consensus criteria questions
|
Patient has
|
| 1. Troublesome motor fluctuations, severity level |
Moderate or severe |
| 2. “Off” time, hours/waking day |
≥2 or <2 |
| 3. Night-time sleep disturbances, severity level |
Moderate or severe |
| 4. Troublesome dyskinesia, hours/waking day |
2-3 or >3 |
| 5. Non-motor fluctuations present |
Yes |
| 6. “Off” time at least every 3 hours |
Yes |
| 7. ≥5 times daily oral levodopa dosing |
Yes |
| 8. Activities of daily living limitation, severity level |
Moderate or severe |
| 9. Falling, frequency |
Most of the time or all the time |
| 10. Dementia, severity level |
Moderate or severe |
| 11. Psychosis, severity level |
Moderate or severe |
Safety
This observational study was not designed to identify or quantify any safety aspects [3].
Statistical analysis
Statistical analyses were essentially carried out as previously described for the international full analysis set, using the SAS® package, version 9.2 (SAS Institute, Cary, NC) [3]. Below, the statistical methods applied for the Swiss subgroup analysis are described in detail. Because of the small sample size, regression analysis was not done for the Swiss subgroup.
Quantitative data were analysed by standard statistical parameters (valid n, missing n, mean, standard deviation [SD], minimum, lower quartile, median, upper quartile, and maximum) and an additional frequency distribution was supplied after appropriate grouping of the data. Qualitative data are presented through absolute and relative frequency distributions. For categorical variables associated with score values, the mean was also calculated. If not stated otherwise, the valid data per parameter excluding patients with missing values were used for calculation of percentages.
For the primary endpoint and for selected secondary endpoints, two-sided 95% confidence intervals were provided. Confidence intervals and p-values (two-sample t-test) were calculated for differences between advanced vs non-advanced Parkinson’s disease patients. Cohen’s kappa was calculated for correlation analyses.
The secondary endpoint parameters used to select the advanced stage Parkinson’s disease patients versus non-advanced Parkinson’s disease patients (demographics, disease duration since diagnosis, UPDRS, modified Hoehn and Yahr staging, quality of life, comorbidities, anti-PD medication) were evaluated based on a stratified descriptive presentation (APD patients, non-APD patients, all patients).
The full analysis set consists of all patients enrolled in the study who had a physician’s judgment on the stage of the disease. Patient data was collected in a single visit. Therefore, imputation methods for missing data, such as last-observation-carried-forward, were not applicable.
All patient characteristics (demographics, Parkinson’s disease-related data and referral history, comorbidities, anti-Parkinson’s disease medication, APD criteria, UPDRS II, III, IV, and V, NMSS, PDQ-8) are presented by the total population and stratified by APD and non-APD patients.
Results
APD vs non-APD patients
Out of 2627 documented patients, a total of 2615 were evaluable and therefore included in the full analysis set of the international study. The Swiss subgroup analysis included 134 patients from five participating movement disorder centres in Switzerland. Based on physician’s judgement, 69.4% (95% CI 61.6–77.2; 93/134 patients) of the recruited Parkinson’s disease patients suffered from APD. The mean age was 70.7 years within the APD group, and 67 years in the non-APD group (table 2). Overall, age, sex and the proportion of patients living at home were comparable between patients with APD and non-APD (table 2). However, caregiver support was required by 55.2% (48/87 patients) of APD patients, and by only 7.5% (3/40 patients) of non-APD patients (table 2). The mean time since Parkinson’s disease diagnosis was markedly longer for APD than for non-APD patients (11. 4 years [SD 6.3] vs 3.9 years [SD 3.2], table 2). Mean scores for activities of daily living, motor symptom severity, dyskinesia duration and disability, average duration of “off” time per day, non-motor symptoms and quality of life were all significantly worse in APD patients than non-APD patients (p <0.0001 for all, fig. 1). Comorbidities were observed with an overall comparable frequency in both groups (table 3). However, more APD patients experienced cognitive dysfunction, sleep disorders and fatigue as compared with non-APD patients (table 3).
Table 2 Patient Characteristics of ADP and non-ADP based on physician’s judgement.
|
Characteristics
|
APD, N = 93
|
Non-APD, N = 41
|
|
n/N (%)
|
Mean (SD)
|
n/N (%)
|
Mean (SD)
|
| Age, years |
|
70.7 (9.4) |
|
67.0 (10.5) |
| Sex, male |
62/93 (66.7) |
|
26/41 (63.4) |
|
| Living at home |
81/93 (87.1) |
|
41/41 (100) |
|
| Caregiver support, yes |
48/87† (55.2) |
|
3/40† (7.5) |
|
| Time since Parkinson’s disease diagnosis, years |
|
11.4 (6.3) |
|
3.9 (3.2) |
| Motor fluctuations present, yes*
|
72/93 (77.4) |
|
9/41 (22.0) |
|
| Duration of motor fluctuations, years*
|
|
4.6 (3.3) |
|
1.6 (0.9) |
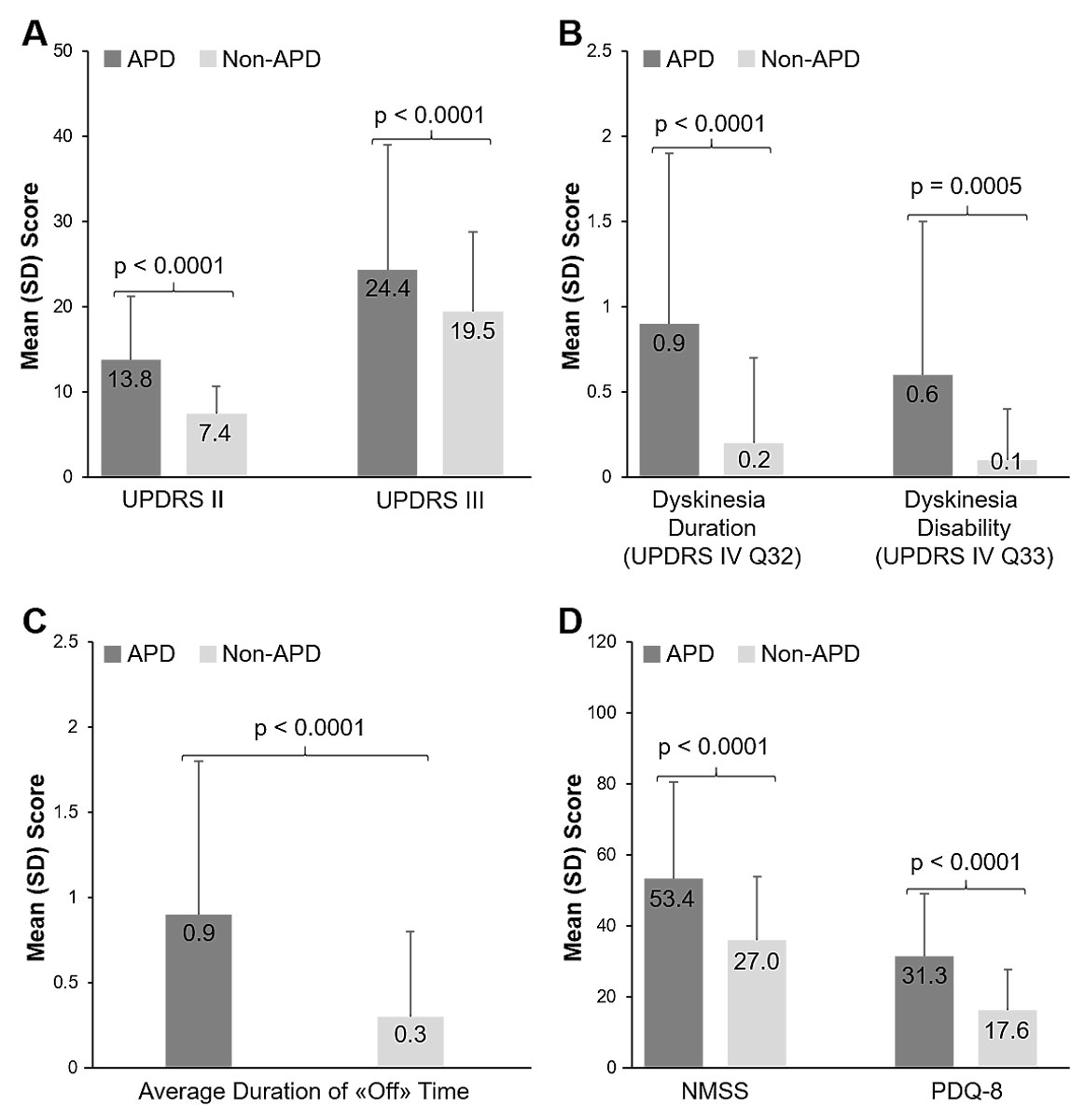
Figure 1 Clinical assessments of APD and Non-APD patients. A) Activities of daily living (UPDRS II), motor symptom severity (UPDRS III); B) Dyskinesia duration and disability (UPDRS Part IV Q32 and Q33); C) Average duration of “off” time (UPDRS Part IV Q39); D) non-motor symptom burden (NMSS) and quality of life (PDQ-8). Error bars indicate standard deviation, p-values from a paired t-test show statistical significance. APD = advanced Parkinson’s disease; non-APD = Non-advanced Parkinson’s disease; NMSS = Non-motor Symptoms Scale; PDQ-8 = Parkinson’s disease 8-item questionnaire; SD = Standard deviation; UPDRS = Unified Parkinson’s Disease Rating Scale.
Table 3 Neuropsychiatric comorbidities of ADP and non-ADP based on physician’s judgement
|
APD, N = 93
|
Non-APD, N = 41
|
|
n/N (%)
|
n/N (%)
|
|
Comorbidities, yes
|
76/93 (81.7) |
34/41 (82.9) |
|
Comorbidity type
|
|
|
| Depression |
20/93 (21.5) |
9/41 (22.0) |
| Cognitive dysfunction |
30/93 (32.3) |
6/41 (14.6) |
| Sleep disorders |
18/93 (19.4) |
6/41 (14.6) |
| Fatigue |
13/93 (14.0) |
3/41 (7.3) |
Delphi criteria and modified Hoehn and Yahr staging for APD
The correlation between physician’s judgement of APD and the Delphi consensus-based criteria for APD was moderate (Cohen’s kappa, Κ = 0.480, 95% CI 0.317–0.642). The highest correlations were reached for question 1, troublesome motor fluctuations (moderate/severe vs mild, Κ = 0.288, 95% CI 0.188–0.388), question 7, minimum of five oral levodopa dosages per day (yes vs no, Κ = 0.251, 95% CI 0.130–0.372), and question 8, level of limitation of capacity for activities of daily living (moderate/severe vs mild, Κ = 0.414, 95% CI 0.289–0.539), whereas Cohen’s kappa for question 2, hours of “off” symptoms during waking day (≥2 vs <2, Κ = 0.144, 95% CI 0.059–0.299), question 5, non-motor symptom fluctuations (yes vs no, Κ = 0.169, 95% CI 0.061–0.279), and question 6, “off” time at least every 3 hours (yes vs no, Κ = 0.112, 95% CI 0.029–0.197), remained low. Mean Hoehn and Yahr stage during “on” time was 2.9 (SD 0.8) for APD and 2.0 (SD 0.5) for non-APD patients, and all patients with a Hoehn and Yahr stage of 4 or greater were classed as APD according to physician’s judgement.
Eligibility, initiation and status of DAT
Of the 93 APD patients, 66.7% (62/93 patients) were considered eligible for DAT and of these, 61.3% (38/62 patients) were currently on DAT (table 4). Neurosurgical treatment (deep brain stimulation) was most frequently used (81.6%, 31/38 patients), followed by levodopa-carbidopa intestinal gel (15.8%, 6/38 patients) and apomorphine s.c. infusions (2.6%, 1/38 patients) (table 4). Of the eligible APD patients, 17.7% (11/62 patients) had decided to start DAT procedures at this visit, with the majority planning to have neurosurgical treatments (deep brain stimulation, 63.6%, 7/11 patients), followed by 27.3% (3/11 patients) and 9.1% (1/11 patients) with planned levodopa-carbidopa intestinal gel or apomorphine s.c. infusions, respectively (table 4). Across all patients with APD according to physician’s judgement, 47.3% (44/93 patients) were neither receiving nor planned for DAT, and of the APD patients eligible for DAT, 21.0% (13/62 patients) were not currently receiving DAT. Reasons for not introducing DAT were mainly patient refusal (8/13 eligible APD patients, 61.5%), motor function-related issues (2/13 patients, 15.4%) and other reasons not specified in the questionnaire (3 of 13 patients, 23.1%). Of the five non-APD patients classed as eligible for DAT, one patient (20%) was receiving deep brain stimulation, whereas the remaining four (80%) were not on DAT (table 4).
Table 4 Eligibility for device-aided treatment (DAT), type of DAT and reasons for no DAT.
|
APD, N = 93
|
Non-APD, N = 41
|
|
n/N (%)
|
n/N (%)
|
|
Eligible for DAT options
|
62/93 (66.7) |
5/41 (12.2) |
|
Status of DAT for eligible patients
|
|
|
| Ongoing |
38/62 (61.3) |
1/5 (20) |
| Decided at visit to start |
11/62 (17.7) |
0/5 (0) |
| No |
13/62 (21) |
4/5 (80) |
| Missing |
0/62 (0) |
0/5 (0) |
|
Type of DAT in group with ongoing DAT
|
|
|
| DBS |
31/38 (81.6) |
1/5 (20) |
| LCIG |
6/38 (15.8) |
|
| Apomorphine s.c. infusion |
1/38 (2.6) |
|
|
Type of DAT in group with decision to start DAT
|
|
|
| DBS |
7/11 (63.6) |
|
| LCIG |
3/11 (27.3) |
|
| Apomorphine s.c. infusion |
1/11 (9.1) |
|
|
Reason for no DAT
|
|
|
| Patient refusal |
8/13 (61.5) |
0/4 (0) |
| Patient needs more time to decide |
0/13 (0) |
1/4 (25) |
| Motor function related issues |
2/13 (15.4) |
0/4 (0) |
| Other |
3/13 (23.1) |
3/4 (75) |
Swiss subgroup vs international full analysis set
The data obtained for the Swiss subgroup were compared with the international full analysis set (including the Swiss subgroup). Overall, 69.4% (95% CI 61.6–77.2%; 93/134 patients) of the Swiss patients suffered from APD according to physician’s judgement, whereas 51.3% (95% CI 49.4–53.2%; 1343/2615 patients) of the full analysis set were classed as APD patients. In Switzerland, all patients presenting with Hoehn and Yahr stage 4 or 5 were considered to have APD (22/134 patients, 16.4%). In comparison, within the full analysis set a Hoehn and Yahr stage of 4 or 5 was identified for 299 of 2614 patients (11.4%), 18 of whom were nonetheless included in the non-APD group (6%).
Activities of daily living (UPDRS II), motor symptom severity (UPDRS III), non-motor symptom severity (NMSS) and quality of life (PQD-8) scores appeared similar in the Swiss APD and full analysis set APD groups and were significantly higher compared with the non-APD groups (fig. 2). The rate of no dyskinesia in APD patients was 45.2% (42/93 patients) in Switzerland and 37.4% (501/1338 patients) in the full analysis set (fig. 2). In the Swiss subgroup, roughly 40% of the patients with APD according to physician’s judgement had no “off” time (38.7%, 36/93 patients) or “off” time for only 1–25% of the day (39.8%, 37/93 patients). In contrast, in the full analysis set only 20.3% (271/1336 patients) of patients with APD according to physician’s judgement had no “off” time, and 43.9% (587/1336 patients) had “off” time for 1–25% of the day. Compared with the Swiss subgroup, more patients in the full analysis set had “off” time for 26-50% of the day (Swiss 16.1%, 15/93 patients; full analysis set 27.1%, 362/1336 patients; fig. 2). In Switzerland patient refusal was the major reason for no DAT amongst patients with APD according to physician’s judgement (Swiss 61.5%, 8/13 patients; full analysis set 28.3%, 94/332 patients), whereas the main reason for no DAT in the full analysis set was that the patient needed more time for a decision. None of the APD patients and only one non-APD patient in the Swiss subgroup were not on DAT because they needed more time for a decision. Even though deep brain stimulation was the most widely used DAT in the full analysis set and Swiss subgroup, it was more frequently used in Switzerland (Swiss 82.1%, 32/38 patients; full analysis set 57.4%, 229/399 patients), whereas levodopa-carbidopa intestinal gel (Swiss 15.4%, 6/39 patients; full analysis set 38.3%, 153/399 patients) and apomorphine s.c. infusions (Swiss 2.6%, 1/39 patients; full analysis set 8.3%, 33/399 patients) were used less compared with the full analysis set.
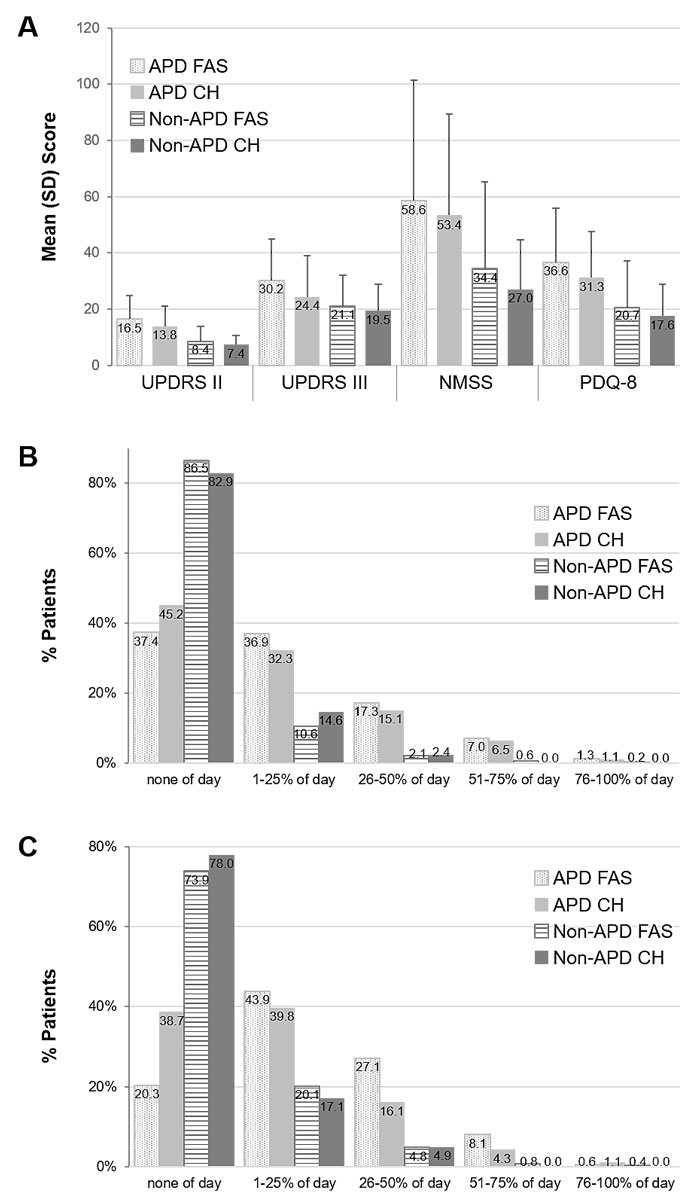
Figure 2 Comparison of the international (FAS) data with the Swiss subgroup. A) Activities of daily living (UPDRS II), motor symptom severity (UPDRS III), non-motor symptom burden (NMSS) and quality of life (PDQ-8); B) Dyskinesia duration (UPDRS Part IV Q32, categories); C) Average duration of “off” time (UPDRS Part IV Q39, categories). Error bars indicate standard deviation (SD). APD FAS = advanced Parkinson’s disease full analysis set; APD CH = advanced Parkinson’s disease Swiss subgroup; non-APD = non-advanced Parkinson’s disease FAS; non-APD CH = non-advanced Parkinson’s disease Swiss subgroup; NMSS = Non-motor Symptoms Scale; PDQ-8 = 8-Item Parkinson’s Disease Questionnaire; UPDRS = Unified Parkinson’s Disease Rating Scale
Discussion
The criteria for APD are not clearly defined and largely depend on the physician’s personal judgement. Frequently used classification systems are mainly based on motor symptom severity and do not provide a full patient profile [3]. Defining distinct disease stages may help to improve treatment strategies to provide the best possible care to the patients [1].
Here we analysed the data of the Swiss subgroup of the international OBSERVE-PD study and compared these country-specific results with the previously published international data [3]. The Swiss dataset thereby provides insight into how APD and DAT eligibility is recognised in Switzerland. The proportion of patients with APD according to physician’s judgement was higher in Switzerland than in the international full analysis set (69.4% vs. 51.3%). Similarly, Fasano et al. reported a wide variation in the proportion of APD patients between the different countries included in the international study, which highlights the differences between healthcare systems, clinics and possibly treatment availability [3].
In Switzerland, the correlation between physician’s judgement of APD and the Delphi criteria was highest for motor fluctuations (question 1), number of oral levodopa doses per day (question 7) and limitations of activities of daily living (question 8). The overall APD correlation was slightly higher than for question 8 alone (overall 0.48, question 8 0.414), but remained moderate, as was also observed in the international study population [3].
Validated clinical measures can help to recognise APD not adequately controlled by oral medication, which in turn can lead to earlier intervention and improved results [14]. The Hoehn and Yahr staging system is frequently used for this purpose, but has several limitations. The system emphasises specific aspects of Parkinson’s disease, including postural instability and mobility problems, whilst neglecting non-motor symptoms and levodopa-associated complications, including motor fluctuations and dyskinesia [15]. Within the Swiss subgroup, the mean Hoehn and Yahr stage was higher in APD (2.9) than in non-APD (2.0) patients, and in contrast to the full analysis set, all Swiss patients with Hoehn and Yahr stage ≥4 were considered to have APD, which provides some support for this staging system for the definition of advanced disease. However, the finding that Swiss patients had a shorter duration of “off” than the full analysis set may indicate that, compared with other aspects of Parkinson’s disease, gait disturbance and postural stability could have a greater influence on physician’s judgement of APD in Switzerland, whereas motor fluctuations had less of an impact, when compared with the full analysis set [3]. In the Swiss subgroup there was descriptively somewhat less dyskinesia and only a low correlation between physician’s judgement of APD and questions 1, 2 and 6 (troublesome motor fluctuations, hours with “off” symptoms during the waking day and “off” time at least every 3 hours) of the Delphi criteria.
Within the group with APD according to physician’s judgement, 66.7% of the patients were considered eligible for DAT, which is comparable to the 66% in the full analysis set [3]. However, 12.2% of non-APD patients in Switzerland were also DAT-eligible, which is in line with what was found in the international cohort (10%) [3].
Most eligible APD patients in the Swiss subgroup were already receiving DAT or initiation of DAT was planned. Interestingly, the number of DAT-eligible patients already receiving DAT was markedly higher in Switzerland than in the international study population. This finding may reflect easy accessibility of relevant information for Parkinson’s disease patients and the quality of service offered to Parkinson’s disease patients in Switzerland, where access to all therapeutic options is usually available without lengthy waiting times or financial restrictions. The number of eligible patients who were not planning to initiate DAT was substantially lower in the Swiss cohort at 21.0% compared with 37.7% in the international study population [3]. In Switzerland, the main reason for no DAT was patient refusal. This contrasts with the international full analysis set, where the most frequent reason for not initiating DAT was that the patient needed more time to decide [3]. In this regard, patient education might be useful to overcome potential negative prejudices against DAT and patient motivation for DAT should be investigated in a separate study.
The previously published limitations for the international OBSERVE-PD study also apply to the Swiss subgroup [3].The sample size of the Swiss subgroup was small and the study was not designed to determine the percentage of APD patients (prevalence). Patients were recruited from movement disorder centres, which possibly resulted in increased prevalence of patients with more advanced Parkinson’s disease. Also, there was no assessment of the proportion of patients with APD who were not referred to a specialised centre and therefore did not have access DAT. Not all movement disorder centres in Switzerland participated in the study. The movement disorder centres included were selected on the basis of their willingness to participate and the availability of DAT, which most likely led to a bias towards a disproportionate representation of DAT. Thus, data on the therapeutic decision-making in DAT-eligible APD patients treated outside these centres is not available, constituting a significant inclusion bias. The participating investigators were trained for the study and thereby received information about the Delphi criteria, which might have influenced their understanding and judgement, thereby introducing a bias. An additional limitation is the post hoc nature of the analysis and that the data were obtained from only one single independent patient visit. There was no follow-up to determine if DAT-eligible patients not currently on DAT eventually initiated DAT. In addition, DAT-ineligible patients were not followed, and it was not determined if their DAT-eligibility status changed after the study concluded.
Conclusions
In this study, the situation regarding ADP and related DAT in Swiss tertiary care centres was assessed and compared with the international situation. There was a moderate correlation between physician’s judgement and the Delphi criteria for APD, which had been defined as a tool to improve APD recognition by highlighting quantifiable criteria. This emphasises the need for creating awareness of specific symptoms to allow patients and physicians to prepare for the changing medical needs as the disease progresses. Furthermore, evaluation of the proportion of APD patients at movement disorder centres forms the basis for efficient DAT planning. Overall, the burden of APD in tertiary care centres in Switzerland was found to be comparable to the international situation, and patient refusal was identified as the main reason for no DAT amongst eligible APD patients – an observation that underlines the importance of patient education. Altogether, these findings help practicing physicians to better classify APD patients and potentially lower their burden of disease, by providing adequate treatment according to clinical features.
References
1
Antonini
A
,
Stoessl
AJ
,
Kleinman
LS
,
Skalicky
AM
,
Marshall
TS
,
Sail
KR
, et al.
Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–73. doi:.https://doi.org/10.1080/03007995.2018.1502165
2
Clarke
CE
,
Worth
P
,
Grosset
D
,
Stewart
D
. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(10):728–41. doi:.https://doi.org/10.1016/j.parkreldis.2009.09.005
3
Fasano
A
,
Fung
VSC
,
Lopiano
L
,
Elibol
B
,
Smolentseva
IG
,
Seppi
K
, et al.
Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019;19(1):50–50. doi:.https://doi.org/10.1186/s12883-019-1276-8
4
Kulisevsky
J
,
Luquin
MR
,
Arbelo
JM
,
Burguera
JA
,
Carrillo
F
,
Castro
A
, et al.
[Advanced Parkinson’s disease: clinical characteristics and treatment (part 1)]. Neurologia. 2013;28(8):503–21. doi:.https://doi.org/10.1016/j.nrl.2013.05.001
5
von Campenhausen
S
,
Bornschein
B
,
Wick
R
,
Bötzel
K
,
Sampaio
C
,
Poewe
W
, et al.
Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473–90. doi:.https://doi.org/10.1016/j.euroneuro.2005.04.007
6
Hoehn
MM
,
Yahr
MD
. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi:.https://doi.org/10.1212/WNL.17.5.427
7
Bach
JP
,
Riedel
O
,
Klotsche
J
,
Spottke
A
,
Dodel
R
,
Wittchen
HU
. Impact of complications and comorbidities on treatment costs and health-related quality of life of patients with Parkinson’s disease. J Neurol Sci. 2012;314(1-2):41–7. doi:.https://doi.org/10.1016/j.jns.2011.11.002
8
Barone
P
,
Antonini
A
,
Colosimo
C
,
Marconi
R
,
Morgante
L
,
Avarello
TP
, et al.; PRIAMO study group. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–9. doi:.https://doi.org/10.1002/mds.22643
9
Benito-León
J
,
Bermejo-Pareja
F
,
Morales-González
JM
,
Porta-Etessam
J
,
Trincado
R
,
Vega
S
, et al.; Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62(5):734–41. doi:.https://doi.org/10.1212/01.WNL.0000113727.73153.68
10
Hely
MA
,
Morris
JG
,
Traficante
R
,
Reid
WG
,
O’Sullivan
DJ
,
Williamson
PM
. The sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry. 1999;67(3):300–7. doi:.https://doi.org/10.1136/jnnp.67.3.300
11
Muangpaisan
W
,
Mathews
A
,
Hori
H
,
Seidel
D
. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J Med Assoc Thai. 2011;94(6):749–55.
12
Schuepbach
WMM
,
Rau
J
,
Knudsen
K
,
Volkmann
J
,
Krack
P
,
Timmermann
L
, et al.; EARLYSTIM Study Group. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–22. doi:.https://doi.org/10.1056/NEJMoa1205158
13
von Elm
E
,
Altman
DG
,
Egger
M
,
Pocock
SJ
,
Gøtzsche
PC
,
Vandenbroucke
JP
; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. doi:.https://doi.org/10.1016/j.ijsu.2014.07.013
14Odin P, et al. Clinical indicators of advanced Parkinson’s Disease: Evaluating diagnostic properties from retrospective analysis of multi-country, cross-sectional observational study. Presented at the 2018 International Congress of Parkinson’s Disease and Movement Disorders, 2018.
15
Goetz
CG
,
Poewe
W
,
Rascol
O
,
Sampaio
C
,
Stebbins
GT
,
Counsell
C
, et al.; Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–8. doi:.https://doi.org/10.1002/mds.20213

