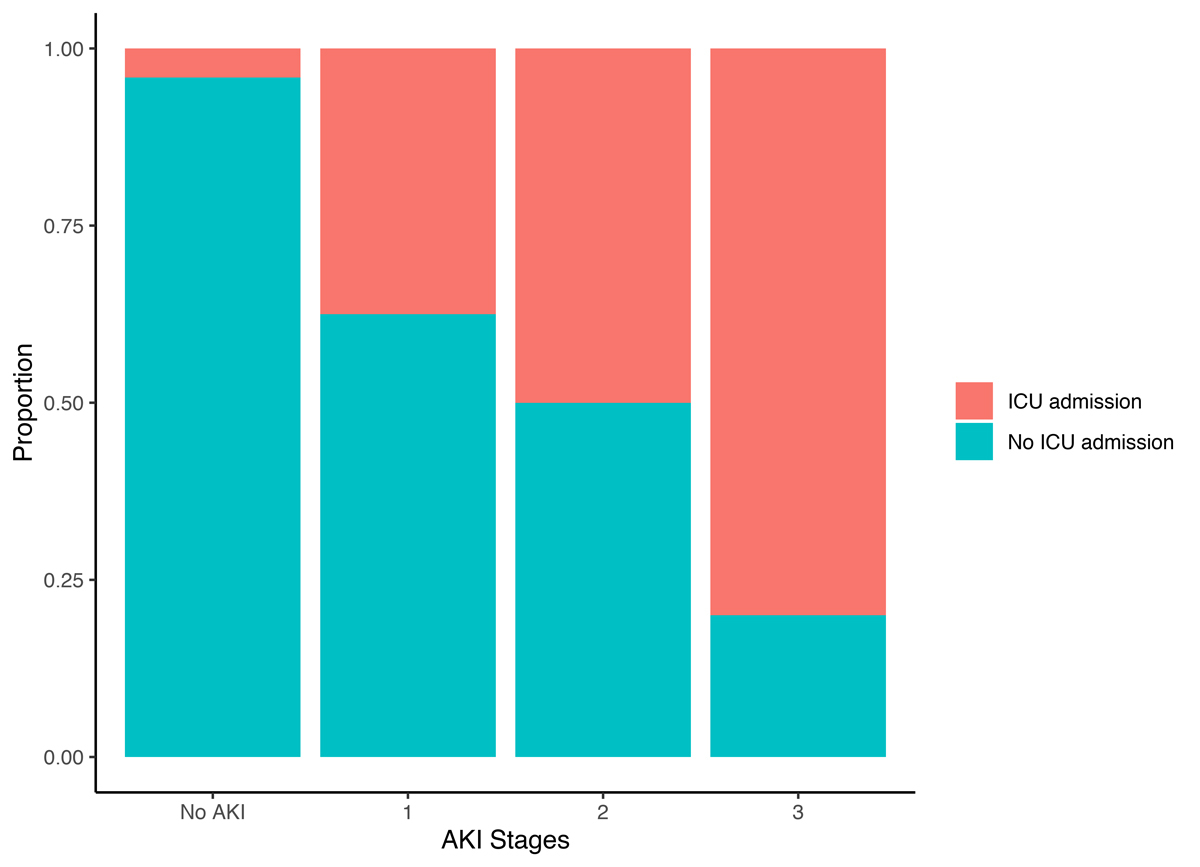
Figure 1 Proportion of patients admitted to the intensive care unit (ICU) stratified by stage of acute kidney injury (AKI).
DOI: https://doi.org/10.4414/smw.2021.20482
Since its first description in 2019 and its rapid spread around the world, the pandemic of the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has been posing a challenge for healthcare systems worldwide [1]. Although the resulting illness, coronavirus disease 2019 (COVID-19), mainly affects the lungs and can cause acute respiratory distress syndrome (ARDS) [1], other organ systems are commonly involved as well [2, 3]. Acute kidney injury (AKI) is a complication in patients with severe COVID-19, although the pathophysiology is not fully understood. As possible mechanisms of acute kidney injury, early studies proposed direct infection of the kidney with SARS-CoV2, an immune response dysregulation or acute kidney injury as a result of multi-organ failure [1, 4]. Whereas reports from China indicated a low incidence of acute kidney injury, ranging from 0.5% to 7% [1, 2], newer data suggest a much higher incidence up to 46% and indicate that AKI is associated with COVID-19 severity and outcomes [5, 6]. This difference in incidence of AKI may partly be explained by different definitions of AKI (e.g., definition of baseline creatinine), but may also be the result of diverse study populations with different proportion of severely ill patients. There is still a paucity of data about acute kidney injury (AKI) in patients with COVID-19, in particular from Europe. Also, preliminary reports mainly focused on the general presentation of patients with COVID-19 and only a few studies focused on a detailed analysis of AKI, such as timing, presentation and risk factors [5, 6].
This study aimed to report incidence, in-hospital recovery rate, risk factors and mortality of AKI associated with COVID-19 and share our experience from a tertiary hospital in Switzerland.
This was a retrospective observational cohort study of the University Hospital of Basel. All hospitalised adult patients with a positive nasopharyngeal polymerase chain reaction (PCR) test for SARS-CoV2 between 1 February and 30 June 2020 at the University Hospital of Basel were eligible for this analysis. The hospitalisation for COVID-19 or hospitalisation during which a positive PCR test was obtained was used for this analysis. Subsequent hospitalisations were not included. By the time of this analysis, all patients had either died or had been discharged from the hospital. In reporting these results, we adhered to the STROBE reporting guidelines [7]. A checklist is provided in the appendix. The institutional review board approved this research, allowing for analysis of patient data if general consent was not withdrawn. The study adheres to the Declaration of Helsinki.
Data on patient demographics, medical history, medication and laboratory test results were collected from the electronic health records. AKI was defined according to the serum creatinine criteria of the 2012 KDIGO clinical practice guideline for acute kidney injury [8] – as an increase in serum creatinine ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days. The nadir creatinine of the hospitalisation was used as baseline creatinine. We were unable to use the urine output criteria to define AKI as it was not regularly documented. In-hospital renal recovery was defined as a discharge creatinine value less than 1.25 times baseline creatinine [9].
Chronic kidney disease was defined as a persistent estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2 during the hospitalisation, which was not presumed to be a cause of unrecovered AKI. Diabetes and history of hypertension were defined as present if documented in the final discharge letter and corresponding medication was prescribed. Chronic heart failure was defined as present if documented in the final discharge letter and an echocardiography report was available using the European Society of Cardiology Clinical Practice Guidelines definitions [10]. Coronary and peripheral artery disease were defined as present if documented in the final discharge letter.
Urine microscopy was not routinely performed, but was at the discretion of the treating physician. White blood cells and red blood cells were counted per field of view. If available, these values were used in this analysis. If no urine microscopy was performed, values from automated urinalysis were used if available. Proteinuria was estimated by dipstick analysis.
The primary outcome was the development of AKI. Secondary outcomes included need for renal replacement therapy, in-hospital renal recovery and overall mortality.
Analyses were performed using R (Version 4.0.2, R Core Team 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/). All hypothesis testing was two-tailed and an alpha level of <0.05 was considered statistically significant. Discrete variables are expressed as counts (percentage) and continuous variables as median and interquartile range (IQR). Comparisons between groups were made using Kruskal-Wallis test and Pearson’s chi-square test, as appropriate. Logistic regression was used to explore risk factors for the development of AKI. To build a multivariable regression model and given the low event rate, we studied all baseline characteristics as potential predictors of AKI using least absolute shrinkage and selection operator (LASSO) regression [11]. A mean cross-validated error within one standard error of the minimum defined the shrinkage factor lambda. Body mass index (BMI), d-dimer and lactate dehydrogenase were missing in 60%, 34% and 10% of patients, respectively, and were therefore not included in the regression model. Based on the regression analysis results, we chose a final model, forcing age into the model as a known predictor of AKI. Areas under the receiver operating characteristic (ROC) curve (AUC) were used to assess the model performance, model calibration was assessed visually by plotting predicted versus observed probabilities.
The association of in-hospital mortality and AKI was assessed using logistic regression analysis. An adjusted analysis was performed with age as a potential confounder which has been reported to be associated with in-hospital mortality in patients with COVID-19 [12]. No imputation was used to address missing values.
From 1 February to 30 June of 2020, 188 hospitalised patients with a positive PCR test for SARS-CoV2 were included in this study. Detailed baseline characteristics of the study population are summarised in table 1 . AKI occurred in 22% of patients. Patients with AKI were older, predominantly men with a history of hypertension and chronic kidney disease. Laboratory studies identified higher white blood cell count, higher C-reactive protein levels, higher lactate dehydrogenase and lower lymphocyte count in patients suffering from COVID-19-associated AKI. Preadmission medication more often included angiotensin-II receptor blockers. No differences were found regarding angiotensin converting-enzyme (ACE) inhibitors. No patient had a history of maintenance dialysis.
Table 1 Baseline characteristics.
| Variable |
Overall
(n = 188) |
No AKI
(n = 147) |
AKI
(n = 41) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 62 (48–73) | 59 (46–72) | 67 (60–77) | 0.002 |
| Male (%) | 115 (61) | 83 (56) | 32 (78) | 0.018 |
| Medical history | ||||
| CKD (%) | 28 (15) | 13 (9) | 15 (37) | <0.001 |
| Hypertension (%) | 86 (46) | 59 (40) | 27 (66) | 0.004 |
| Diabetes (%) | 35 (19) | 25 (17) | 10 (24) | 0.363 |
| Chronic heart failure (%) | 12 (6) | 7 (5) | 5 (12) | 0.139 |
| COPD/asthma (%) | 23 (12) | 17 (12) | 6 (15) | 0.595 |
| Active cancer | 14 (7) | 12 (8) | 2 (5) | 0.738 |
| Coronary artery disease (%) | 27 (14) | 18 (12) | 9 (22) | 0.133 |
| Peripheral artery disease | 5 (3) | 2 (1) | 3 (7) | 0.070 |
| BMI (kg/m2)* | 28.0 (24.8–31.0) | 27.0 (24.0–30.2) | 30.5 (28.5–33.2) | 0.020 |
| Laboratory results on admission | ||||
| Haemoglobin (g/l) | 135 (124–147) | 136.0 (125–147) | 132 (120–145) | 0.551 |
| White blood cell count (109/l) | 6.1 (4.5–8.3) | 6.0 (4.3–7.6) | 7.7 (5.3–10.1) | 0.004 |
| Lymphocyte count (109/l) | 1.0 (0.7–1.4) | 1.1 (0.8–1.4) | 0.6 (0.4–1.1) | <0.001 |
| Platelet count (109/l) | 210 (159–277) | 215 (172–277) | 178 (136–276) | 0.120 |
| C-reactive protein (mg/l) | 43 (16–103) | 35 (12–72) | 104 (48–163) | <0.001 |
| D-dimer (mg/l)† | 0.7 (0.4–1.6) | 0.6 (0.4–1.2) | 1.2 (0.5–4.2) | 0.012 |
| Lactate dehydrogenase (U/l) | 295 (222–408) | 265 (208–360) | 395 (283–485) | <0.001 |
| Creatinine kinase (U/l) | 97 (60–198) | 85 (53–139) | 212 (130–434) | <0.001 |
| Sodium (mmol/l) | 136 (133–139) | 136 (133–139) | 136 (133–139) | 0.634 |
| Potassium (mmol/l) | 3.9 (3.6–4.2) | 3.9 (3.6–4.1) | 4.1 (3.8–4.8) | 0.009 |
| Serum creatinine (mmol/l) | 78 (63–98) | 74 (61–89) | 121 (78–201) | <0.001 |
| Blood urea nitrogen (mmol/l) | 5.2 (3.8–7.3) | 4.7 (3.6–6.1) | 8.7 (5.7–19.3) | <0.001 |
| Preadmission medication | ||||
| Angiotensin converting-enzyme inhibitor (%) | 29 (15) | 23 (16) | 6 (15) | 1.000 |
| Angiotensin-II receptor blocker (%) | 47 (25) | 30 (20) | 17 (41) | 0.008 |
| Loop diuretic (%) | 17 (9) | 13 (9) | 4 (10) | 0.767 |
| Thiazide (%) | 22 (12) | 14 (10) | 8 (20) | 0.098 |
| Diuretics other (%) | 3 (2) | 0 (0) | 3 (7) | 0.010 |
| Hospitalisation | ||||
| Length of stay (days) | 6 (4–10) | 6 (4–8) | 15 (7–25) | <0.001 |
AKI = acute kidney injury; BMI = body mass index; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease Values are number (percentage) or median (interquartile range); p-values comparing no-AKI with AKI were calculated using a Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variables. * Missing in 60%, † missing in 34%
Of all patients admitted, 41 (22%) developed AKI and of these, 11 (27%) required renal replacement therapy. AKI developed after a median of 9 days (IQR 5–12) after occurrence of the first symptoms and a median of 1 day (IQR 0–5) after hospital admission. Renal replacement therapy was initiated after a median of 14 days (IQR 8.5–16.5) after first symptoms and 5 days (IQR 1–7) after hospital admission. AKI stages were stage 1 in 39%, stage 2 in 24% and stage 3 in 37%. Of the 29 (15%) patients who were admitted to the intensive care unit (ICU), 23 (79%) developed AKI. The higher the AKI stage, the more patients were admitted to the ICU within this group. (fig. 1) The peak AKI stages in patients admitted to the ICU were stage 1 in 26%, stage 2 in 22% and stage 3 in 52%. Patients who required renal replacement therapy were all treated in the ICU for a median of 7 days (IQR 2–17).

Figure 1 Proportion of patients admitted to the intensive care unit (ICU) stratified by stage of acute kidney injury (AKI).
Urine microscopy was available in 16 (39%) and automated urine analysis in 11 (27%) patients within 24 hours before or 48 hours after the development of AKI. Microscopic evaluation predominantly (65%) indicated acute tubular injury with granular and muddy brown casts. Of 27 patients with AKI and urine analysis, 48% had haematuria, 56% had leucocyturia visually or by automated urinalysis and 96% had proteinuria measured by dipstick analysis.
Renal recovery at discharge was observed in 61% of all AKI episodes and 80% of AKI episodes not requiring renal replacement therapy. Of the 11 patients requiring renal replacement therapy, 5 (45%) patients died and 1 patient (9%) was still in need of dialysis at discharge. Renal recovery rates stratified by AKI stage were 88% for stage I, 80% for stage II and 20% for stage III.
Univariable analysis showed that age, male sex, history of chronic kidney disease, history of hypertension, higher white blood cell count, higher C-reactive protein levels, higher creatinine kinase, higher potassium levels, preadmission medication with angiotensin-II receptor or ACE inhibitors and diuretics were associated with the development of AKI. (table 2) The regression model was performed on 178 cases, 10 cases (1 patient with AKI) were omitted due to missing values. (supplementary table S2 in the appendix). Independent predictors of AKI were age (adjusted odds ratio [aOR] 1.04, 95% confidence interval [CI] 1.01–1.08; p = 0.024), history of chronic kidney disease (aOR 3.47, 95% CI 1.16–10.49; p = 0.026), C-reactive protein levels (aOR 1.09, 95% CI 1.03–1.16; p=0.002) and creatinine kinase (aOR 1.03, 95% CI 1.01–1.06; p = 0.002). The AUC for the model was 0.76 (95% CI 0.69–0.84). More details on the regression model performance are provided in the appendix.
Table 2 Univariable and multivariable logistic regression analysis.
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Age, years | 1.04 | 1.02–1.07 | 0.002 | 1.04 | 1.01–1.08 | 0.024 |
| Male gender | 2.42 | 1.11–5.76 | 0.033 | |||
| CKD | 5.77 | 2.46–13.82 | <0.001 | 3.47 | 1.16–10.49 | 0.026 |
| Hypertension | 2.89 | 1.41–6.15 | 0.005 | |||
| Diabetes | 1.58 | 0.66–3.60 | 0.284 | |||
| Chronic heart failure | 3.14 | 0.86–11.03 | 0.071 | |||
| COPD/asthma | 1.09 | 0.34–3.01 | 0.876 | |||
| Active cancer | 0.75 | 0.11–3.08 | 0.726 | |||
| Coronary artery disease | 2.07 | 0.81–5.00 | 0.114 | |||
| Peripheral artery disease | 3.58 | 0.42–30.63 | 0.210 | |||
| Haemoglobin (g/l)* | 1.00 | 0.98–1.02 | 0.718 | |||
| White blood cell count (109/l)* | 1.12 | 1.03–1.23 | 0.008 | |||
| Lymphocyte count (109/l)* | 0.98 | 0.69–1.25 | 0.879 | |||
| Platelet count (109/l)* | 1.00 | 0.99–1.00 | 0.267 | |||
| C-reactive protein (mg/l)† | 1.12 | 1.07–1.19 | <0.001 | 1.09 | 1.03–1.16 | 0.002 |
| Creatinine kinase (U/l)† | 1.04 | 1.02–1.06 | <0.001 | 1.03 | 1.01–1.06 | 0.002 |
| Sodium (mmol/l)* | 1.01 | 0.94–1.09 | 0.732 | |||
| Potassium (mmol/l)* | 2.56 | 1.42–4.81 | 0.002 | |||
| ARB | 2.40 | 1.12–5.08 | <0.001 | |||
| ACE-I | 1.04 | 0.36–2.6 | 0.936 | |||
| ACE-I or ARB | 2.15 | 1.06–4.43 | 0.035 | |||
| Diuretics | 2.32 | 1.05–5.02 | 0.034 | |||
ACE-I = angiotensin converting-enzyme inhibitors; aOR = adjusted odds ratio; ARB = angiotensin-II receptor blockers; CI = confidence interval; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; OR = odds ratio The multivariable covariates were chosen using LASSO Regression. * per 1-unit increase, † per 10-unit increase
Overall, 10% of patients died during hospitalisation. In-hospital mortality was significantly higher in patients with AKI than patients with no AKI (5% vs 27%; p <0.001). An increase in stage of AKI corresponded with an increase in in-hospital mortality (fig. 2). The highest mortality was documented in patients with stage III and patients requiring renal replacement therapy. AKI was associated with in-hospital death (OR 7.33, 95% CI 2.67–21.43; p <0.001), also after adjustment for age (aOR 5.79, 95% CI 1.98–18.18; p = 0.002). Table 3 summarises key differences from other studies investigating AKI in patients with COVID-19.
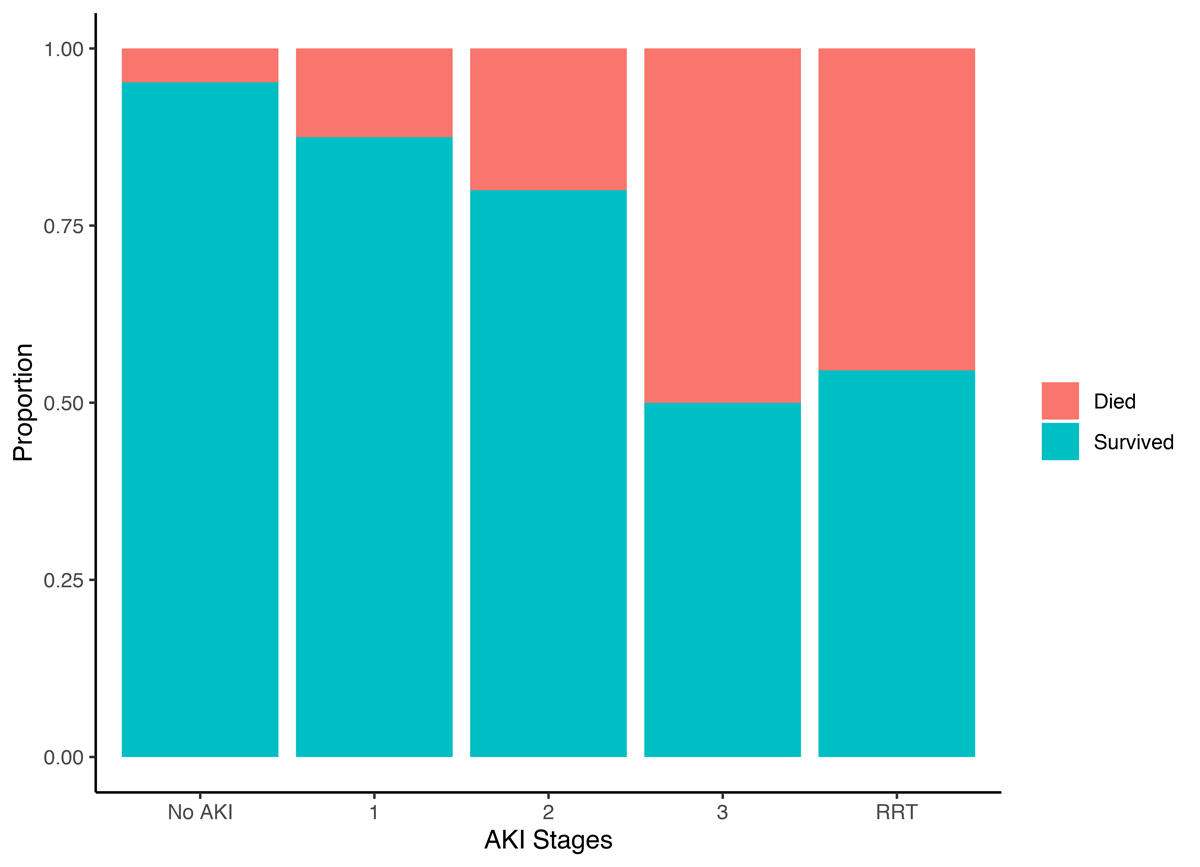
Figure 2 Mortality according to stage of acute kidney injury (AKI).
Table 3 Data on acute kidney injury in patients with COVID-19 from different analyses.
| Analysis | Study population n | Age | AKI Incidence | Need for RRT | In-hospital Mortality |
|---|---|---|---|---|---|
| Cheng et al [13] China | 1392 | 65 (50–71) | 7% | NR | 14% |
| Xheng et al [14] China | 555 | 52 (36–64) | 6% | NR | 5% |
| Hirsch et al [5] USA | 5449 | 64 (52–75) | 36.6% | 14.3% | 16.3% |
| Chan et al [6] USA | 3993 | 64 (56–78) | 46% | 19% | 27% |
| Russo et al [15] Italy | 777 | 70 (16)† | 22.6% | 12% | 35%* |
| Our analysis | 188 | 62 (48–73) | 22% | 6% | 10% |
AKI = acute kidney injury; NR = not reported; RRT = renal replacement therapy Values are median and (interquartile range) or mean (standard deviation)†. AKI was defined according to KDIGO criteria. * Mortality after a mean follow-up of 35 ± 22 days. Comparability is limited by the small sample size in our analysis.
In this retrospective analysis, we investigated the incidence, presentation, recovery rates and risk factors of AKI associated with COVID-19. We report six major findings. First, in our centre, 22% of patients with COVID-19 experienced an episode of acute kidney injury during hospitalisation. Second, AKI occurs more often in patients in need of intensive care and the severity of AKI seems to correlate with severity of the disease. Third, in-hospital renal recovery was achieved in 61% of all AKI episodes, 80% of AKI episodes not requiring renal replacement therapy, but only 20% of stage III AKI. Fourth, our analysis indicated proteinuria and acute tubular injury to be common manifestations of AKI. Fifth, traditional risk factors such as age and history of chronic kidney disease, as well as C-reactive protein and creatine kinase levels were identified as independent predictors of AKI. Sixth, we found AKI to be strongly associated with in-hospital mortality.
These results extend and corroborate findings of previous studies establishing the important role of kidney involvement in patients hospitalised with COVID-19.
Substantial differences in AKI rates have been reported from within and especially between different countries. Studies from China report an AKI incidence of 0.5–7%, whereas studies from the United States report an incidence rate between 36.5% and 46% [2, 5, 6, 13]. The incidence of AKI observed in our study was 22%, which was in between these two estimates. There are several possible reasons for the different incidence rates, as discussed previously [13]. First, our study population had higher rates of traditional risk factors for AKI, such as hypertension and chronic kidney disease, compared with analyses from China.
Also, different approaches were used for the definition of baseline creatinine values, with some studies using the median serum creatinine values of the entire hospitalisation or imputing values, possibly resulting in different AKI rates [5, 6, 16]. We decided to use the nadir creatinine as baseline creatinine since preadmission baseline values were not available for all patients. This might have contributed to a lower AKI rate. Lastly, higher BMI has been associated with the development and severity of AKI, including in patients with COVID-19. Given the known national differences in obesity, this might also contribute to different incidences [17–19].
In our study, the incidence of AKI was higher in patients in need of intensive care treatment. This finding is in line with previous studies indicating that severe AKI occurs in patients with critical COVID-19 illness [5, 14, 15]. This might also imply that acute tubular injury in the setting of multiorgan failure is a predominant manifestation of severe AKI. In line with this hypothesis we found tubular injury to be the main pathophysiology in urine analysis. Similar results have been found in a study looking specifically at urinary sediments in patients with COVID-19 [20].
Most of the AKI episodes, in particular mild AKI, are reversible. One third of patients with AKI did not recover kidney function at discharge. However, we did not have information on follow-up creatinine values. Some patients have been discharged to rehabilitation facilities and their kidney function might not have fully improved at the time of discharge from the hospital. Therefore, studies with longer follow up and serial creatinine values are needed to investigate long-term renal morbidity after AKI in patients with COVID-19.
Our finding of higher C-reactive protein levels in patients with AKI and C-reactive protein levels as predictors of AKI might indicate a contribution of the frequently discussed “cytokine storm” as a cause of AKI. Cheng et al. reported that C-reactive protein levels are elevated in patients with AKI. However, the impact of a systemic inflammatory disease in COVID-19 has been challenged recently. An analysis looking at interleukin-6 levels as a marker for systemic inflammation found that they were not significantly higher in COVID-19 patients than those typically reported in ARDS [21]. Cytokines have been found to be much higher in patients treated with chimeric antigen receptor T-cell therapy, cytokine release syndrome or sepsis [22]. Lastly, early results from the COVACTA trial investigating the effect of tocilizumab, a monoclonal antibody targeting interleukin-6 activity, indicate no benefit of a blockade of interleukin-6 [23].
Another possible mechanism is a direct cytopathic effect of the virus using the ACE2 receptor for host cell entry [24]. Whether therapy with ACE inhibitors or angiotensin receptor blockers might have an impact on susceptibility of the virus is a subject of ongoing debate. Interestingly, in our analysis patients with AKI were more often treated with angiotensin receptor blockers and in univariable analysis preadmission treatment with ACE or angiotensin receptor blockers were associated with the development of AKI. However, this finding was not significant in multivariable regression and might also be explained by the high percentage of patients with hypertension. Also, the first randomised controlled study assessing the safety of ACE inhibitors and angiotensin receptor blockers found no benefit on mortality in patients who suspended the medication during the infection [25]. Therefore, a change of expression of ACE receptors in the kidneys and resulting change of AKI incidence in patients with ACE inhibitor or angiotensin receptor blocker therapy seems unlikely. However, treatment with these drugs could still have an impact on patients as a modulator of kidney perfusion.
Lastly, in our analysis, creatinine kinase levels were a predictor of AKI. Although rhabdomyolysis has been described as a possible pathway of development of acute kidney injury in patients with COVID-19, given the low creatinine kinase levels in our analysis it seems unlikely to be a cause [18, 26]. Whether this corresponds to a viral myositis or occurred in the setting of haemodynamic instability remains unclear since we did not analyse laboratory samples drawn during hospitalisation.
Our study sample was comparable to a recently published study of COVID-19 patients in Switzerland regarding age, sex and comorbidities, and we believe it is representative of the Swiss population [27].
Some limitations merit consideration when interpreting the findings of this study. First, owing to the observational and retrospective character of this analysis, no causal inference can be derived. Although we carefully adjusted our statistical model, we cannot eliminate the potential for residual confounding. Second, we did not have any information about the urine output for the diagnosis of acute kidney injury. Third, no follow-up was performed to better understand long-term morbidity and mortality of acute kidney injury in patients with COVID-19. Fourth, because of the small study size, we cannot exclude a minor effect of contributing factors or an effect in a subset of patients. Fifth, due to the ongoing change in medical treatment options and management of COVID-19 patients, we were unable to analyse the association of different drugs and the occurrence of and recovery from AKI. Lastly, we did not assess ethnicity for this analysis. However, since the vast majority of patients treated at the University of Basel are Caucasians, we do not believe that this would have changed the main results.
In conclusion, AKI is common in hospitalised patients with COVID-19, is mostly reversible in mild cases but has poor in-hospital recovery in advanced AKI stages. It is more often seen in patients with severe COVID-19 illness and correlates with the severity of the disease and is likely induced by acute tubular injury.
We would like to thank the hospital staff for their efforts during this pandemic, and thank all patients involved in this study.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Huang C , Wang Y , Li X , Ren L , Zhao J , Hu Y , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:.https://doi.org/10.1016/S0140-6736(20)30183-5
2 Guan WJ , Ni ZY , Hu Y , Liang WH , Ou CQ , He JX , et al.; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi:.https://doi.org/10.1056/NEJMoa2002032
3 Cheng Y , Luo R , Wang K , Zhang M , Wang Z , Dong L , et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38. doi:.https://doi.org/10.1016/j.kint.2020.03.005
4 Diao B , Wang C , Wang R , Feng Z , Tan Y , Wang H , et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020:2020.03.04.20031120.
5 Hirsch JS , Ng JH , Ross DW , Sharma P , Shah HH , Barnett RL , et al.; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18. doi:.https://doi.org/10.1016/j.kint.2020.05.006
6 Chan L , Chaudhary K , Saha A , Chauhan K , Vaid A , Zhao S , et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–60. doi:.https://doi.org/10.2215/CJN.12360720
7 Vandenbroucke JP , von Elm E , Altman DG , Gøtzsche PC , Mulrow CD , Pocock SJ , et al.; STROBE initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163-94. doi:.https://doi.org/10.7326/0003-4819-147-8-200710160-00010-w1
8 Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. 2012;2:1–138.
9 Long TE , Helgadottir S , Helgason D , Sigurdsson GH , Gudbjartsson T , Palsson R , et al. Postoperative Acute Kidney Injury: Focus on Renal Recovery Definitions, Kidney Disease Progression and Survival. Am J Nephrol. 2019;49(3):175–85. doi:.https://doi.org/10.1159/000496611
10 Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JGF , Coats AJS , et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
11 Pavlou M , Ambler G , Seaman SR , Guttmann O , Elliott P , King M , et al. How to develop a more accurate risk prediction model when there are few events. BMJ. 2015;351:h3868. doi:.https://doi.org/10.1136/bmj.h3868
12 Zhou F , Yu T , Du R , Fan G , Liu Y , Liu Z , et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi:.https://doi.org/10.1016/S0140-6736(20)30566-3
13 Cheng Y , Luo R , Wang X , Wang K , Zhang N , Zhang M , et al. The Incidence, Risk Factors, and Prognosis of Acute Kidney Injury in Adult Patients with Coronavirus Disease 2019. Clin J Am Soc Nephrol. 2020;15(10):1394–402. doi:.https://doi.org/10.2215/CJN.04650420
14 Zheng X , Yang H , Li X , Li H , Xu L , Yu Q , et al. Prevalence of Kidney Injury and Associations with Critical Illness and Death in Patients with COVID-19. Clin J Am Soc Nephrol. 2020;15(11):1549–56. doi:.https://doi.org/10.2215/CJN.04780420
15 Russo E , Esposito P , Taramasso L , Magnasco L , Saio M , Briano F , et al.; GECOVID working group. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2020. doi:.https://doi.org/10.1007/s40620-020-00875-1
16 Ng JH , Hirsch JS , Hazzan A , Wanchoo R , Shah HH , Malieckal DA , et al. Outcomes Among Patients Hospitalized With COVID-19 and Acute Kidney Injury. Am J Kidney Dis. 2021;77(2):204–215.e1. doi:.https://doi.org/10.1053/j.ajkd.2020.09.002
17 Ng M , Fleming T , Robinson M , Thomson B , Graetz N , Margono C , et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi:.https://doi.org/10.1016/S0140-6736(14)60460-8
18 Mohamed MMB , Lukitsch I , Torres-Ortiz AE , Walker JB , Varghese V , Hernandez-Arroyo CF , et al. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney360. 2020;1(7):614–22. doi:.https://doi.org/10.34067/KID.0002652020
19 Beretta A . Obesity, inflammation and COVID-19. Swiss Med Wkly. 2020;150:w20349.
20 Hernandez-Arroyo CF , Varghese V , Mohamed MMB , Velez JCQ . Urinary Sediment Microscopy in Acute Kidney Injury Associated with COVID-19. Kidney360. 2020;1(8):819–23. doi:.https://doi.org/10.34067/KID.0003352020
21 Sinha P , Matthay MA , Calfee CS . Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152–4. doi:.https://doi.org/10.1001/jamainternmed.2020.3313
22 Nadim MK , Forni LG , Mehta RL , Connor MJ, Jr , Liu KD , Ostermann M , et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020. doi:.https://doi.org/10.1038/s41581-020-00356-5
23 Furlow B . COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2020;2(10):e592. doi:.https://doi.org/10.1016/S2665-9913(20)30313-1
24 Li W , Moore MJ , Vasilieva N , Sui J , Wong SK , Berne MA , et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–4. doi:.https://doi.org/10.1038/nature02145
25Lopes R. Continuing versus suspending ACE inhibitors and ARBs: Impact of adverse outcomes in hospitalized patients with COVID-19--The BRACE CORONA Trial. ESC Congress 2020. [Accessed 2020 Oct 16] Available from: https://www.escardio.org/Congresses-&-Events/ESC-Congress/Congress-resources/Congress-news/hot-line-can-aceis-and-arbs-be-safely-continued-in-patients-hospitalised-with-covid-19-results-from-the-brace-corona-trial.
26 Taxbro K , Kahlow H , Wulcan H , Fornarve A . Rhabdomyolysis and acute kidney injury in severe COVID-19 infection. BMJ Case Rep. 2020;13(9):e237616. doi:.https://doi.org/10.1136/bcr-2020-237616
27 Pellaud C , Grandmaison G , Pham Huu Thien HP , Baumberger M , Carrel G , Ksouri H , et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area - a retrospective cohort study. Swiss Med Wkly. 2020;150:w20314.doi:.https://doi.org/10.4414/smw.2020.20314
| Table S1: STROBE Statement Checklist. | |||
| Item no. | Recommendation | Provided on page* | |
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 2 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 3 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 3 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 4 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 4 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | 4 |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | NA | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 5 |
| Data sources/ measurement | 8 | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 4-5 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 5-6 |
| Study size | 10 | Explain how the study size was arrived at | 4 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 5-6 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 5-6 |
| (b) Describe any methods used to examine subgroups and interactions | NA | ||
| (c) Explain how missing data were addressed | 6 | ||
| (d) If applicable, explain how loss to follow-up was addressed | NA | ||
| (e) Describe any sensitivity analyses | NA | ||
| Results | |||
| Participants | 13 | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 6 |
| (b) Give reasons for non-participation at each stage | 4 | ||
| (c) Consider use of a flow diagram | NA | ||
| Descriptive data | 14 | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | https://doi.org/10.4414/smw.2020.20314 |
| (b) Indicate number of participants with missing data for each variable of interest | S2 | ||
| (c) Summarise follow-up time (eg, average and total amount) | NA | ||
| Outcome data | 15 | Report numbers of outcome events or summary measures over time | 6-8 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Table 2 |
| (b) Report category boundaries when continuous variables were categorized | NA | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | NA | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | NA |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 9 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 12 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 9-12 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 13 |
| * Page numbers refer to the submitted manuscript | |||
| Table S2: Missing values. | |
| Variable | Number of missing values (n = 188) |
| Age | 0 |
| Male | 0 |
| CKD | 0 |
| Hypertension | 0 |
| Diabetes | 0 |
| Chronic heart failure | 0 |
| COPD/asthma | 0 |
| Active cancer | 0 |
| Coronary artery disease | 0 |
| Peripheral artery disease | 0 |
| BMI | 112 |
| Haemoglobin | 2 |
| White blood cell count | 2 |
| Lymphocyte count | 8 |
| Platelet count | 2 |
| C-reactive protein | 2 |
| D-dimer | 63 |
| Lactate dehydrogenase | 19 |
| Creatinine kinase | 5 |
| Sodium | 2 |
| Potassium | 2 |
| Serum creatinine | 0 |
| Blood urea nitrogen | 5 |
| Angiotensin converting-enzyme inhibitor | 0 |
| Angiotensin II receptor blocker | 0 |
| Loop diuretic | 0 |
| Thiazide | 0 |
| Diuretics other | 0 |
| Length of stay | 0 |
| BMI = body mass index; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease | |
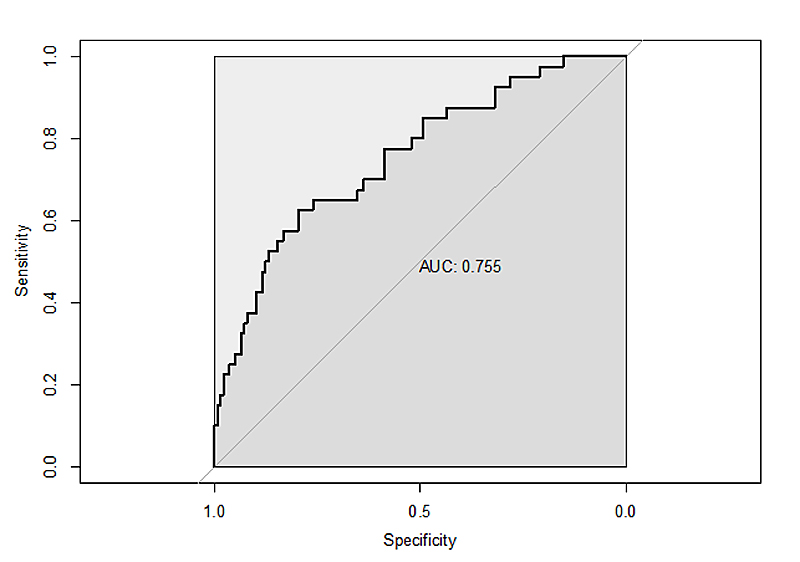
Figure S1 Receiver operator characteristics (ROC) curve for the prediction model for acute kidney injury in patients with COVID-19.
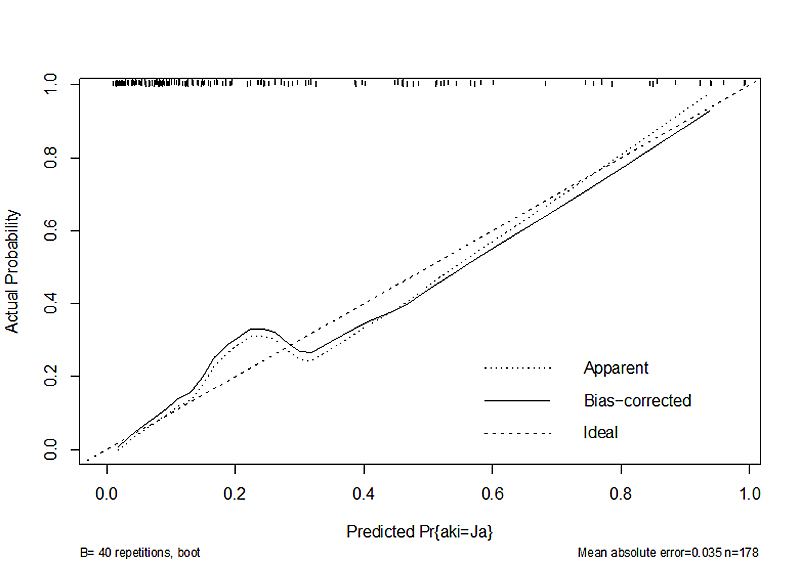
Figure S2 Calibration plot for the prediction of acute kidney injury. The calibration function of the rms package was used for this plot. Bootstrapping using 40 repetitions were used to get bias corrected estimates of the predicted versus the actual probability. An underprediction was observed at lower predicted probabilities.