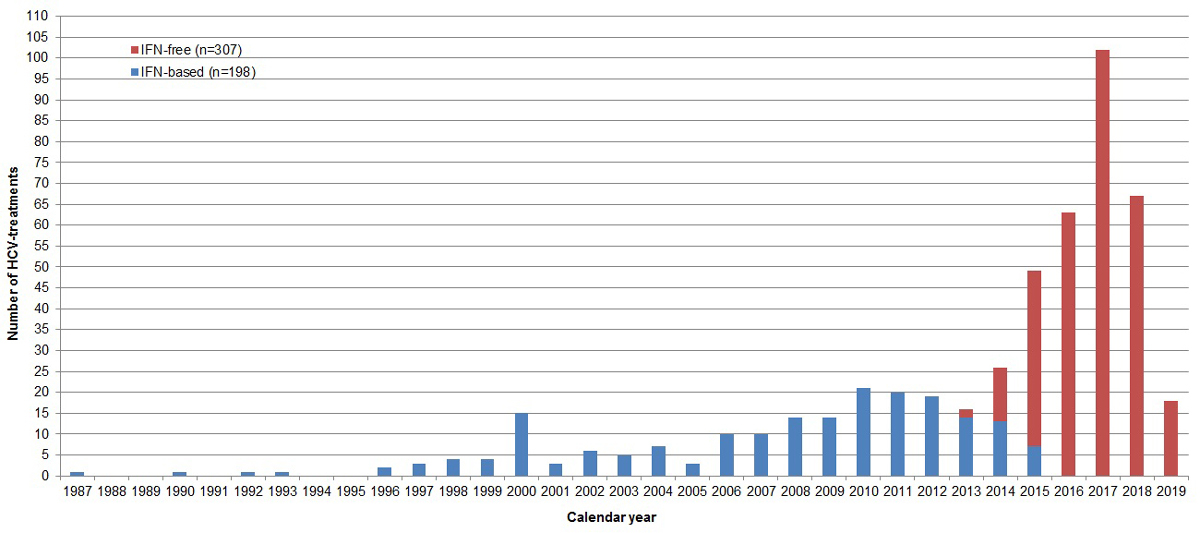
Figure 1 Number of interferon-based and interferon-free hepatitis C virus treatments per calendar year. IFN = interferon (status as of 2 September 2019, overall 942 SAMMSU patients).
DOI: https://doi.org/10.4414/smw.2021.20460
Hepatitis C is a blood-borne viral infection, which is highly prevalent among persons who inject drugs (PWID) as a result of the common use of injection material (needle, syringe, filter, spoon, water) [1]. Hepatitis C virus (HCV) transmission also occurs if snorting straws for intranasal drug use are shared [2, 3]. Although about 25% of HCV infected people spontaneously clear the virus, the remaining 75% develop chronic infection [4]. Presenting mostly with unspecific symptoms slowly appearing over years, such as fatigue, joint pain and neurocognitive disorders, hepatitis C may remain undetected for years and become a “silent killer” [5]. About 20% of chronically infected individuals develop liver cirrhosis after 20 years [6], with an annual risk of hepatocellular carcinoma of 1–5% and of hepatic decompensation of 3–6%. After an episode of decompensation, the risk of death in the following year is 15–20% [7]. In addition, hepatitis C is associated with an elevated non-liver-related mortality [8].
Globally, there are 15.6 million PWID, with 52.3% being HCV antibody positive [9]. The HCV antibody prevalence in Switzerland is 0.7% in the general population [10], 26-48% in oral opioid agonist therapy (OAT) programmes and 60–80% in heroin substitution programmes [11]. Of the 22,000–27,000 opioid addicts in Switzerland [12], about 80% are cared for in OAT programmes (oral OAT: 18,000; heroin: 1600) [13]. In about 60%, OAT is prescribed by a general practitioner (GP) [13]. About 27% of Swiss OAT patients have ongoing intravenous drug use (IDU) [14].
In view of the highly effective and well tolerated pangenotypic interferon-free direct-acting antiviral (DAA) treatments available [15–18], access to diagnosis, care and treatment has become the main challenge in chronic hepatitis C management. Early diagnosis and successful treatment of chronic hepatitis C prevents HCV-related complications and extrahepatic manifestations (individual benefit), as well as further transmission (“Treatment as Prevention; TasP)” [19] (social benefit).
Both the World Health Organization (WHO) and the Swiss Hepatitis Strategy aim at HCV elimination by the year 2030 [20, 21]. In order to reach this goal, 80% treatment uptake is necessary [20]. The “National Addiction Strategy 2017–2024” aims to reduce premature deaths due to addiction [22]. This includes prevention and treatment of infectious diseases such as hepatitis C. Since May 2017, PWID and since October 2017, all patients with chronic hepatitis C in Switzerland can be treated with DAAs irrespective of liver fibrosis stage [23, 24]. Before, reimbursement restrictions withheld DAA treatment from two thirds of patients with chronic hepatitis C, i.e., those with no or only mild fibrosis (F0/F1) [25]. Due to the high costs (CHF 30,706.20 for 8 weeks of Maviret® [glecaprevir/pibrentasvir] and CHF 30,952.20 for 12 weeks of Epclusa® [sofosbuvir/velpatasvir] http://www.spezialitätenliste.ch/ShowPreparations.aspx), only infectious disease specialists, gastroenterologists and addiction specialists with experience in HCV treatment (http://www.bag.admin.ch/sl-ref) are allowed to prescribe DAAs in Switzerland. In March 2019, the Federal Office of Public Health (FOPH) published guidelines for HCV management in drug users [26].
Since 2014, the Swiss Association for the Medical Management in Substance Users (SAMMSU)-cohort (http://www.sammsu.ch/cohort-database.html) has recruited OAT patients from eight different centres throughout Switzerland [27].
To evaluate the benefit of interferon-based and interferon-free HCV treatment in OAT patients and monitor HCV elimination, a 2-year study commissioned by the Swiss FOPH and conducted within the SAMMSU cohort was performed between 2017 and 2019. HCV treatment uptake, adherence and success, as well as reinfection rates, the effect of early versus late treatment and the efficacy of the “treatment-as-prevention” approach were analysed.
The SAMMSU cohort was approved by the ethics committees of all participating centres (leading ethical committee: St Gallen, EKSG 13/144). All participants gave written informed consent. Data are stored and analysed in an anonymised way.
The SAMMSU cohort is an open cohort, which has, since 2014, recruited current or former drug users >18 years old in all parts of Switzerland. Participants must have been in an OAT programme for at least one day. There are no exclusion criteria. During routine clinical care or on HCV action days, patients of the eight currently participating centres (Aarau, Basel, Bern, Geneva, Lausanne, Lugano, St Gallen and Zurich) are contacted by study nurses/physicians. Both centralised and decentralised OAT settings are represented, i.e., specialised OAT programmes with integrated somatic care (led by a psychiatrist, somatic physician on-site) as well as patients receiving their OAT via the general practitioner (GP).
In contrast to the Swiss Hepatitis C Cohort Study (SCCS) [28], which enrols only HCV-positive patients, and the Swiss HIV Cohort Study (SHCS) [29], which enrols only human immunodeficiency virus (HIV)-positive patients, the SAMMSU cohort also recruits both HCV- and HIV-negative individuals.
At enrolment and thereafter at yearly intervals, study nurses/physicians of each centre enter sociodemographic and medical data into an electronic questionnaire, including information about drug use / risk behaviour, comorbidities, medication, HCV treatment, diagnostic tests (e.g., liver biopsy, Fibroscan®, abdominal sonography), vaccinations and laboratory values. Every study nurse/physician has a personal password-protected account and can only edit data of her/his own centre. At first data entry, every patient gets a unique ID consisting of two letters for the centre and a five-digit consecutive number. Every centre maintains its own code list. The web-based central database was established with SecuTrial® and is maintained by the Clinical Trial Unit of the University Basel. For this study, a database extract from 2 September 2019 was used.
To evaluate the effect of the abrogation of the DAA reimbursement restrictions for PWID on HCV treatment uptake, two cross-sectional surveys at the patient level were conducted one and two years after the 1 May 2017. This also allowed three point-prevalence measurements regarding HCV antibody and HCV-RNA positivity (1 May 2017, 1 May 2018, 1 May 2019).
To evaluate if patients enrolled into the SAMMSU cohort are representative for the Swiss population of OAT patients and to describe the OAT settings on the 1 May 2017, 1 May 2018 and 1 May 2019, the SAMMSU centres received an additional centre questionnaire in May 2018 and May 2019.
The questionnaires for the cross-sectional surveys at patient and centre level were collectively developed by the study physicians and are presented in appendices 2 and 3 , respectively.
New HCV infection: first detection of HCV antibodies (first diagnosis), irrespective of the time-point of infection.
HCV reinfection: first detection of HCV-RNA after spontaneous clearance or successful treatment.
Spontaneous clearance/elimination: HCV-RNA becomes persistently undetectable without treatment.
Chronic hepatitis C: HCV-RNA persistence 6 months after new infection or reinfection.
HCV treatment uptake: proportion of patients with chronic hepatitis C ever treated.
Treatment success = sustained virological response (SVR): 12 or 24 weeks after HCV treatment no HCV-RNA detectable.
Preterm treatment stop: treatment stop before the intended treatment duration has been completed.
Adherence: assessment by the treating physician (SAMMSU cohort: adherence problems during treatment: yes/no; cross-sectional survey at the patient level: excellent/moderate/bad).
Adherence-supporting measures: directly observed therapy (DOT) ≥5×/week, weekly dispensing of the HCV medication in a pill box.
Duration of infectiousness: first intravenous drug use (IDU) as potential time-point of infection until first HCV treatment.
Liver fibrosis stage according to Fibroscan®: F0/F1 (no/mild fibrosis): ≤7.0 kPa; F2 (significant fibrosis): >7.0 kPa and ≤9.5 kPa; F3 (severe fibrosis): >9.5 kPa and ≤12.5 kPa; F4 (cirrhosis): >12.5 kPa [30, 31]
Categorical variables were compared with the chi-square and the Fisher’s exact tests. Continuous variables were analysed with the Wilcoxon rank-sum test (unpaired data). A two-sided p-value <0.05 was considered significant.
Time-to-event analyses: For the primary HCV infection rate, the observation time was from the first IDU to HCV first diagnosis (first positive HCV antibody test) or the last negative HCV antibody test for patients remaining HCV antibody negative. For the HCV re-infection rate after successful treatment, the observation time was from the end of treatment until the patients became HCV RNA positive again or the last negative HCV RNA for patients remaining HCV RNA negative.
Test-frequency analyses: Observation time was one year prior to registration until the last follow up.
All statistical analyses were performed with Stata Version 12.0.
On 1 May 2017, 1 May 2018 and 1 May 2019, 623, 757 and 900 patients, respectively, were enrolled in the SAMMSU cohort. This is a yearly increase of 22% (134 patients) and 19% (143 patients). There was no significant change in the baseline characteristics at registration over time. Of the 900 patients registered by 1 May 2019, 87% were male, the median age was 45 years at registration and 20 years at first IDU, 81% reported ever using intravenous drugs and 89% ever using intranasal drugs, 13% were HIV positive (99% ever HIV treatment) and 66% HCV antibody positive. Of the 457 patients with chronic hepatitis C, 59% had already been treated at least once at registration (table 1).
Table 1 Baseline characteristics at registration (Status: 1 May 2019).
|
Total
(n = 900) |
Aarau
(n = 367) |
Basel
(n = 107) |
Bern
(n = 29) |
Geneva
(n = 20) |
Lausanne
(n = 38) |
Lugano
(n = 117) |
St Gallen
(n = 56) |
Zurich
(n = 166) |
|
|---|---|---|---|---|---|---|---|---|---|
| Male | 77.8% (700/900) |
77.4% (284/367) |
72.9% (78/107) |
62.1% (18/29) |
80% (10/20) |
78.9% (30/38) |
79.5% (93/117) |
76.8% (43/56) |
83.1% (138/166) |
| Median age (y) at reg. (IQR) | 45.0 (37.1–50.3) |
38.7 (31.4–45.8) |
49.5 (43.6–54.2) |
46.3 (41.4–49.7) |
47 (39.3–51.1) |
47.7 (41.5–50.2) |
48 (43.7–51.8) |
49 (44.6–53.5) |
46.7 (41.5–51.7) |
| Ever IDU | 80.9% (715/884) |
75.4% (276/366) |
96.9% (94/97) |
85.7% (24/28) |
80% (16/20) |
89.5% (34/38) |
87.2% (102/117) |
92.3% (48/52) |
72.9% (121/166) |
| Median age (y) at first IDU (IQR) | 20 (18–25) (n = 696) |
20 (17–25) (n = 264) |
21 (18–25) (n = 87) |
24 (20–31) (n = 24) |
21.5 (19.5–26.5) (n = 16) |
22 (18–28) (n = 34) |
20 (17–23) (n = 102) |
21 (18–30) (n = 48) |
20 (18–26) (n = 121) |
| Median time (y) between first IDU and reg. (IQR) | 23.5 (13–30) (n = 696) |
17 (8–26) (n = 264) |
28 (20–33) (n = 87) |
19 (12–27.5) (n = 24) |
25.5 (15–33) (n = 16) |
23.5 (15–26) (n = 34) |
28 (21–34) (n = 102) |
24.5 (19–30) (n = 48) |
25 (19–29) (n = 121) |
| Ever intranasal drug use | 89.5% (784/876) |
89.0% (325/365) |
97.8% (89/91) |
66.7% (18/27) |
85% (17/20) |
71.1% (27/38) |
94.9% (111/117) |
94.2% (49/52) |
89.2% (148/166) |
| HIV positive | 12.8% (115/897) |
5.5% (20/366) |
10.3% (11/107) |
17.9% (5/28) |
10% (2/20) |
36.8% (14/38) |
4.3% (5/117) |
69.1% (38/55) |
12.0% (20/166) |
| Ever HIV-treatment* | 99.1% (113/114) |
100% (20/20) |
100% (11/11) |
100% (5/5) |
100% (2/2) |
100% (14/14) |
75% (3/4) |
100% (38/38) |
100% (20/20) |
| HCV antibody positive | 66.1% (593/897) |
48.4% (177/366) |
86.9% (93/107) |
89.3% (25/28) |
60% (12/20) |
86.8% (33/38) |
83.8% (98/117) |
87.3% (48/55) |
64.5% (107/166) |
| Ever CHC | 87.2% (457/524) |
78.5% (106/135) |
89.2% (83/93) |
87.5% (21/24) |
91.7% (11/12) |
100% (33/33) |
85.6% (83/97) |
84.4% (38/45) |
96.5% (82/85) |
| Ever HCV treatment if ever CHC | 58.9% (269/457) |
44.3% (47/106) |
71.1% (59/83) |
33.3% (7/21) |
54.5% (6/11) |
45.5% (15/33) |
72.3% (60/83) |
44.7% (17/38) |
70.7% (58/82) |
y = years; reg. = registration; IQR = interquartile range; IDU = intravenous drug use; HIV = human immunodeficiency virus; HCV = hepatitis C virus; CHC = chronic hepatitis C; HAART = highly active antiretroviral therapy * For 98 of the 115 HIV patients on 1 May 2019, the year of the first HIV treatment was available. In 13% (13/98) it was before 1996, i.e., in the pre-HAART era, and in 87% it was thereafter. At the registration visit, 96% (110/115) were currently under HIV treatment.
Of the 900 SAMMSU patients, 80.6% (725) were from the German-speaking part of Switzerland (Aarau, Basel, Bern, St Gallen, Zurich), 6.4% (58) from the French-speaking part (Geneva, Lausanne) and 13.0% (117) from the Italian-speaking part (Lugano). At 40.8%, the biggest proportion of patients came from the SAMMSU centre Aarau, followed by Zurich (18.4%), Lugano (13.0%) and Basel (11.9%). Centre-specific differences in the baseline characteristics are described in table 1.
In Aarau, 58% (367/631) of all OAT patients in the canton and 59% (75/128) of the heroin substitution programme in Brugg were enrolled into SAMMSU. In Basel, 66% (41/62) of the OAT patients of a private practice, 10% (19/199) of the ADS (Ambulanter Dienst Sucht) and 15% (22/150) of the heroin substitution programme Janus were recruited. In Bern, 12% (22/190) of the patients of the heroin substitution programme KODA (Kontrollierte Drogenabgabe) were enrolled. In Lausanne, 6% (24/415) of the POLADD (Policlinique d’Addictologie, Département de Psychiatrie du CHUV) patients and in Lugano, 13% (117/900) of the OAT patients of the canton Ticino (no heroin substitution) were recruited. In St Gallen, 48% (12/25) of the patients of the methadone substitution programme of the Infectious Diseases Outpatient Clinic of the Cantonal Hospital St Gallen, 32% (23/72) of the MSH1 (heroin substitution programme) and 4% (3/102) of the MSH2 (methadone substitution programme) were enrolled. In Zurich, 18% (159/881) of the Arud patients in Zurich take part in SAMMSU, but none of the 98 Arud patients in Horgen.
Heroin substitution programmes offer twice daily substitution 7 days/week, whereas institutions without heroin substitution are normally open only 5 days/week. The number of substitution patients ranges from 10 to 881 and the yearly fluctuation rate is 12–37%. HCV and HIV antibody rapid tests are routinely used in 5/13 institutions. So far, capillary HCV-RNA measurement on dried blood spots is established only in POLADD, Lausanne. Only 4/13 institutions offer sonography on site, whereas Fibroscan® is available in 6 institutions. In four institutions, HCV action days take place several times a year, where HCV/HIV rapid tests, capillary HCV RNA quantification (GeneXpert®), Fibroscan®, sometimes sonography, venous blood draw for hepatitis A and hepatitis B virus (HAV/HBV) serology and HAV/HBV vaccination are offered on site. On 1 May 2019, on-site prescription of DAA treatments was possible in only 7/13 institutions.
In the SAMMSU cohort, the proportion of patients with unknown HCV serostatus is <1% (table 2). However, in many institutions from which the SAMMSU patients were recruited, it was 20–25% on 1 May 2018 and decreased to 10–15% on 1 May 2019. The proportion of HCV antibody-positive patients with unknown HCV-RNA status was ≤10% in most institutions, but <5% in SAMMSU. The proportion of HCV antibody-positive patients with known HCV-RNA who had ever had chronic hepatitis C was 60–80% in the source institutions, but 80% in SAMMSU. Between 1 May 2017 and 1 May 2019, HCV treatment uptake has increased, but 80% or even 90% had not yet been achieved in all institutions.
Table 2 HCV cascade on 1 May 2017, 1 May 2018 and 1 May 2019 (cohort data).
|
1 May 2017
(n = 623) |
1 May 2018
(n = 757) |
1 May 2019
(n = 900) |
|
|---|---|---|---|
| HCV antibody status known | 99.7% (621/623) |
99.6% (754/757) |
99.7% (897/900) |
| HCV antibody positive | 66.5% (413/621) |
69.1% (521/754) |
68.6% (615/897) |
| HCV-RNA known if HCV antibody positive | 93.2% (385/413) |
95.6% (498/521) |
96.4% (593/615) |
| Currently HCV-RNA positive if HCV antibody positive and HCV-RNA known | 36.1% (139/385) |
24.3% (121/498) |
19.1% (113/593) |
| Ever CHC | 77.7% (321/413) |
80.4% (419/521) |
79.7% (490/615) |
| Ever HCV treatment if ever CHC | 61.7% (198/321) |
74.5% (312/419) |
79.8% (391/490) |
| Ever SVR if ever HCV treatment | 91.9% (182/198) |
92.6% (289/312) |
89.8% (351/391) |
| Ever SVR or last HCV-RNA neg. if ever HCV treatment* | 93.9% (186/198) |
95.8% (299/312) |
95.7% (374/391) |
HCV = hepatitis C virus; CHC = chronic hepatitis C; SVR = sustained virological response * For some patients receiving HCV treatment, no check for SVR has taken place (lost to follow-up between end of treatment and SVR check) and for many of the only recently treated patients, the time for SVR check has not been reached yet. In these cases, the last available HCV-RNA measurement was used as a surrogate for HCV treatment success, even if it was the first HCV-RNA measurement under treatment or the check at the end of treatment.
Patients already HCV antibody positive at registration were about 8 years older (47 vs 39 years), their first IDU was longer ago (25 vs 13 years) and the proportion who had ever used intravenous drugs was almost twice as high (96% vs 52%) (table 3). Besides, the proportions with ongoing IDU (27% vs 17%) and ever using cocaine and benzodiazepines were higher in HCV antibody-positive patients. HIV positivity (18% vs 2%) and ever having a syringe abscess (17% vs 3%) were more frequent. Overall, HCV antibody-positive patients were longer exposed, had more frequent and more risky injections and had benefitted less from harm reduction (OAT, needle syringe programmes).
Table 3 Baseline characteristics of HCV antibody-positive and -negative patients (cohort data).
|
HCV antibody positive at reg.
(n = 613) |
HCV antibody negative at reg. (n = 326) | p-value | OR (95% CI) | |
|---|---|---|---|---|
| Male | 77.2% (473/613) |
79.4% (259/326) |
0.421 | 0.87 (0.63–1.21) |
| Median age (y) at registration (IQR) | 46.9 (41.4–51.5) |
38.5 (31–47.2) |
<0.001 | |
| Ever IDU | 95.7% (575/601) |
51.9% (167/322) |
<0.001 | 20.53 (13.09–32.18) |
| Ongoing IDU if ever IDU | 27.5% (158/575) |
16.8% (28/167) |
0.005 | 1.88 (1.21–2.94) |
| Median age (y) at first IDU (IQR) | 20 (17–25) (n = 559) |
22 (19–27) (n = 160) |
<0.001 | |
| Median time (y) between first IDU and registration (IQR) | 25 (17–30) (n = 559) |
13 (4.5–23) (n = 160) |
<0.001 | |
| Ever intranasal drug use | 91.2% (541/593) |
87.0% (280/322) |
0.042 | 1.56 (1.01–2.40) |
| Ongoing intranasal drug use if ever intranasal drug use | 29.9% (162/541) |
28.2% (79/280) |
0.606 | 1.09 (0.79–1.50) |
| HIV positive | 18.1% (111/613) |
1.8% (6/326) |
<0.001 | 11.79 (5.13–27.13) |
| Ever heroin | 99.0% (596/602) |
96.3% (312/324) |
0.004 | 3.82 (1.42–10.28) |
| Ever cocaine | 95.0% (569/599) |
89.1% (287/322) |
0.001 | 2.31 (1.39–3.84) |
| Ever benzodiazepine | 72.5% (430/593) |
61.7% (195/316) |
0.001 | 1.64 (1.23–2.19) |
| Ever cannabis | 92.8% (555/598) |
89.4% (286/320) |
0.074 | 1.52 (0.96–2.46) |
| Ever syringe abscess | 18.6% (95/510) |
3.4% (7/204) |
<0.001 | 6.44 (2.94–14.1) |
| Year of first IDU: | (n = 559) | (n = 160) | ||
| 1970–1979 | 7.3% (41) | 0.6% (1) | 0.129 | 4.28 (0.55–33.17) |
| 1980–1989 | 36.0% (201) | 13.1% (21) | 1.0 (ref.) | |
| 1990–1999 | 32.6% (182) | 26.3% (42) | 0.005 | 0.45 (0.26–0.80) |
| 2000–2009 | 18.1% (101) | 28.8% (46) | <0.001 | 0.23 (0.13–0.42) |
| 2010–2019 | 6.1% (34) | 31.3% (50) | <0.001 | 0.07 (0.03–0.15) |
HCV = hepatitis C virus, reg. = registration, OR = odds ratio, CI = confidence interval, y = years, IQR = interquartile range, IDU = intravenous drug use, HIV = human immunodeficiency virus, ref. = reference
By 2 September 2019, 505 HCV treatments were documented in the SAMMSU cohort (405 first, 78 second, 17 third, 4 fourth and 1 fifth HCV treatments). Thirty-nine percent (198) were interferon-based and 61% (307) interferon-free (16 still ongoing). Up to 2012, HCV was treated exclusively with interferon-based treatments (maximum 21 patients/year) and since 2016 with exclusively interferon-free treatment (102 patients in 2017; fig. 1).

Figure 1 Number of interferon-based and interferon-free hepatitis C virus treatments per calendar year. IFN = interferon (status as of 2 September 2019, overall 942 SAMMSU patients).
Treatment success increased 1.7-fold (95% CI 1.5–2.0) from 56.6% (112/198; 95% CI 49.6–63.3%; interferon-based) to 97.4% (261/268; 95% CI 94.7–98.7%; interferon-free; p <0.001; fig. 2). Prior non-response to interferon (onetime or repeated) reduced the SVR rate for another interferon-based, but not for interferon-free, treatment (fig. 2).

Figure 2 Sustained virological response (SVR) rate after interferon-based and interferon-free HCV treatment, if outcome known (overall and according to the number of prior HCV treatments). HCV = hepatitis C virus; IFN = interferon. * Once in combination with telaprevir and once in combination with sofosbuvir (status as of 2 September 2019, overall 942 SAMMSU patients).
For 60% (242) of the 405 first HCV treatments, a Fibroscan® result was available before treatment (20 [8.3%] interferon-based and 222 [91.7%] interferon-free treatments). Median time between Fibroscan® and start of the first HCV treatment was 90 days (IQR 42–231). With interferon-free treatment, the SVR rate was ≥97% irrespective of fibrosis stage: F0/F1 97.6% (81/83), F2 100% (33/33), F3 97.1% (33/34), F4 97.5% (39/40).
By 2 September 2019, there were 373 HCV treatments with documented SVR in 362 patients. In 2017, when DAA reimbursement restrictions were abrogated in Switzerland, 87 SVRs were achieved, as many as in the entire interferon era (86 SVRs between 1987 and 2012). The proportion with “SVR after first treatment” among all SVRs per year increased from 68.8% (33/48) in 2015 to 88.9% (48/54) in 2018 (p = 0.012).
Compared with the interferon era, the proportion with preterm treatment stop decreased in the interferon-free era, from 17.2% (34/198, 95% CI 12.6–23.0%) to 0.7% (2/307, 95% CI 0.2–2.3%; p <0.001) and the proportion with adherence problems from 8.6% (17/198, 95% CI 5.4–13.3%) to 2.3% (7/307, 95% CI 1.1–4.6%; p = 0.001; fig. 3).
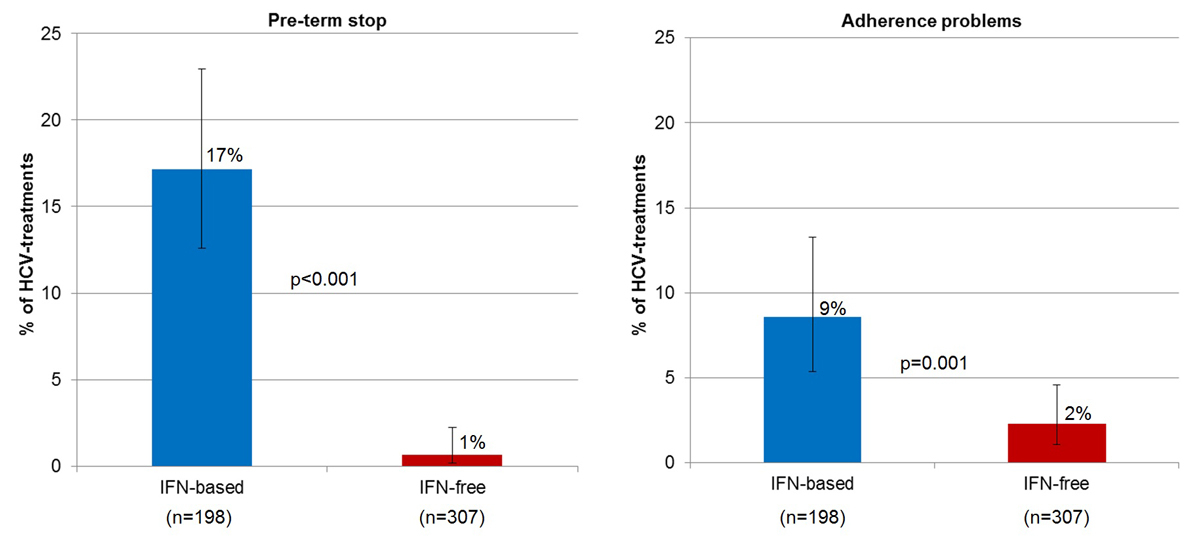
Figure 3 Proportion of interferon-based and interferon-free HCV treatments with pre-term stop and adherence problems, respectively. HCV = hepatitis C virus; IFN = interferon (status as of 2 September 2019, overall 942 SAMMSU patients).
From 2016 onwards (interferon-free era), there were 201 HCV treatments in the cross-sectional data from seven centres. In 97.9% (183/187) adherence was classified as “excellent”, in 1.6% (3) as “moderate” and in 0.5% (1) as “bad”. Overall, the SVR rate was 97.7% (171/175). All three patients with only moderate adherence achieved SVR. In less than half of the treatments (45.1%, 78/173), DOT was used to support adherence. One centre (Lugano) used virtually no adherence-supporting measures (DOT and weekly pill box in only 2.7%, 1/37) without a negative effect on adherence (excellent in 100%, 35/35) and treatment success (97.1%, 34/35 SVR).
Although cirrhotic patients can be treated with success rates similar to those of patients with earlier stages of fibrosis with interferon-free treatments (see above), the time-point of treatment matters.
In the past, diagnosis and treatment of hepatitis C were delayed (fig. 4). The median time between the first IDU (surrogate for the time of infection) and the first diagnosis of HCV was 9 years (IQR 4–18; n = 539), and the median time between the first IDU and the first HCV treatment 22 years (IQR 13–29; n = 378) – the time in which the patients were infectious and could develop cirrhosis.

Figure 4 Number of patients with first IDU, first diagnosis of HCV, first time positive for HCV-RNA and first HCV treatment per calendar year. IDU = intravenous drug use; HCV = hepatitis C virus. Data at registration and follow-up (status as of 2 September 2019, overall 942 SAMMSU patients).
For 226 patients, the year of first IDU was known and a Fibroscan® result available prior to the first HCV treatment (HCV therapy start years 2007–2019). The median duration of infectiousness in patients already cirrhotic at the start of their first HCV treatment was 30 years (IQR 23–33; n = 51). In patients treated at earlier liver fibrosis stages (F0–F3), the median duration of infectiousness was 25 years (IQR 16–31; n = 175; p = 0.020; fig. 5).

Figure 5 Duration of infectiousness according to fibrosis stage at first HCV treatment. Patients with Fibroscan® measurement before the first HCV treatment and year of first intravenous drug use known (n = 226, start year of first HCV treatment 2007–2019) (status as of 2 September 2019, overall 942 SAMMSU patients). HCV = hepatitis C virus. Liver fibrosis stage according to Fibroscan®: F0/F1 (no/mild fibrosis) ≤7.0 kPa; F2 (significant fibrosis) >7.0 kPa and ≤9.5 kPa; F3 (severe fibrosis) >9.5 kPa and ≤12.5 kPa; F4 (cirrhosis) >12.5 kPa.
Between 2015 and 2018, the proportion of patients already cirrhotic at first HCV treatment declined from 50% (15/30) to 13% (7/54; p <0.001) and the proportion of patients with no or mild fibrosis at first HCV treatment increased from 0% (0/30) to 61% (33/54; p <0.001; fig. 6).
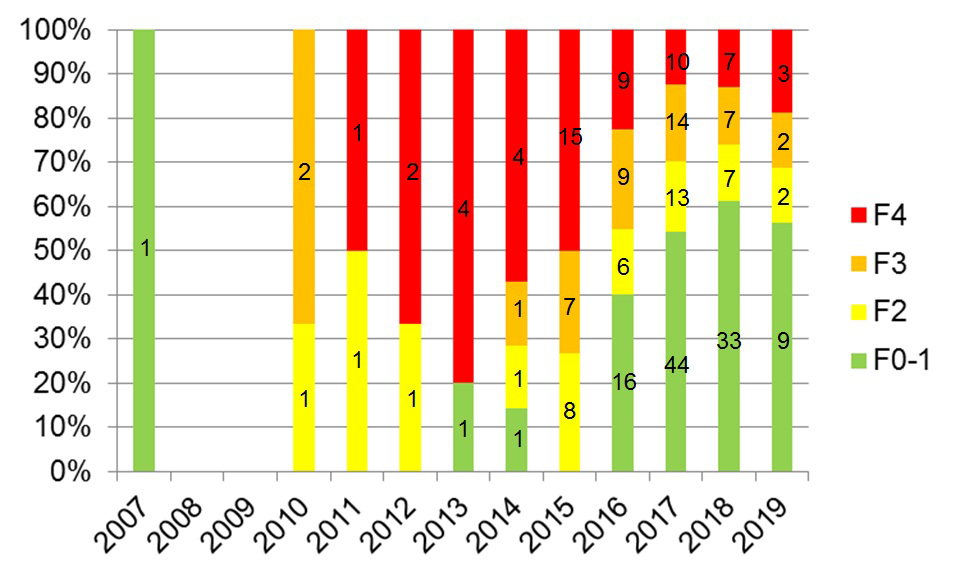
Figure 6 Distribution of fibrosis stage at first HCV treatment according to calendar year. Two hundred and forty-two first HCV treatments with Fibroscan® measurement before treatment (start year of first HCV treatment 2007–2019) (status as of 2 September 2019, overall 942 SAMMSU patients). Liver fibrosis stage according to Fibroscan®: F0/F1 (no/mild fibrosis) ≤7.0 kPa; F2 (significant fibrosis) >7.0 kPa and ≤9.5 kPa; F3 (severe fibrosis) >9.5 kPa and ≤12.5 kPa; F4 (cirrhosis) >12.5 kPa
At enrolment into the SAMMSU cohort, 95.7% (112/117) of the HIV patients were under HIV treatment, with 94.0% (109/116) having undetectable HIV-RNA (<50 cop/ml, i.e., untransmittable). At the reference dates 1 May 2017, 1 May 2018 and 1 May 2019, the HIV-RNA prevalence (≥50 cop/ml) among the HIV positives was 6.7% (6/90), 5.7% (6/105) and 6.1% (7/114, 95% CI 3.0–12.1%), respectively. Among all SAMMSU cohort patients (i.e., all HIV positives and negatives), the HIV-RNA prevalence was 1.0% (6/623), 0.8% (6/757) and 0.8% (7/900, 95% CI 0.4–1.6%) on the respective reference dates. Until 2002/2003, up to eight patients yearly were newly diagnosed with HIV, whereas in the eight years after 2011, there was only one HIV first diagnosis, in 2015 (fig. 7).

Figure 7 Number of first diagnoses of HIV and HCV per calendar year. Data at registration (status as of 2 September 2019, overall 942 SAMMSU patients). HIV = human immunodeficiency virus; HCV = hepatitis C virus
Between 1 May 2017 and 1 May 2019, HCV treatment uptake increased from 61.7% (198/321) to 79.8% (391/490; table 2). In parallel, the HCV-RNA prevalence decreased from 36.1% (139/385) to 19.1% (113/593; 95% CI 16.1–22.4%) among the HCV antibody-positive patients and from 22.3% (139/623) to 12.6% (113/900, 95% CI 10.6–14.9%) among all SAMMSU cohort patients (i.e., all HCV positives and negatives).
This development can also be seen in the cross-sectional data of seven SAMMSU centres (fig. 8): the higher the increase in treatment uptake the higher the decrease in HCV-RNA prevalence among the HCV antibody-positive patients. On the 1 May 2019, some centres had already achieved >90% treatment uptake, which was associated with a reduction of HCV-RNA prevalence to <10%. On the 1 May 2019, the SAMMSU centre Aarau, with a high proportion of patients cared for in a decentralised setting, had a markedly lower rate (79.4%, 104/131; 95% CI 71.7–85.4%) than the other five centres taken together (93.1%, 241/259; 95% CI 89.3–95.6%; p <0.001; supplementary table S1 in appendix 1.

Figure 8 hepatitis C virus (HCV) treatment uptake and HCV-RNA prevalence in seven of eight SAMMSU centres (cross-sectional data). HCV treatment uptake = proportion of chronically HCV infected patients ever treated; HCV RNA prevalence = proportion of HCV antibody positive patients who were HCV RNA positive.
To calculate the rate of HCV first diagnosis, the year of first IDU was available for 663 patients (total observation time 8801.8 years, median observation time 11.8 years, IQR 5.5–20.1, range 0.4–45.9). Between 1970 and 2019, there were 518 HCV first diagnoses, resulting in an HCV first diagnosis rate of 5.89 per 100 person-years (95% CI 5.40–6.41) and a cumulative HCV infection rate of 78.1% (95% CI 74.8–81.1%).
To calculate the reinfection rate after successful treatment, information on 280 patients with a total observation time of 739.4 years was available (median observation time 1.1 years, IQR 0.4–3.2, range 0.003–29.1). From 1988 to 2019, there were 20 diagnoses of HCV reinfection after successful treatment, resulting in a reinfection rate of 2.70 per 100 person-years (95% CI 1.75–4.19) and a cumulative HCV reinfection rate of 7.1 per 100 treated patients (95% CI 4.1–10.8).
Seventeen reinfections were observed in 88 patients receiving interferon-based treatment during a total observation time of 545.3 years, and 3 reinfections were observed in 192 patients on interferon-free treatment during a total observation time of 194.2 years. Thus, the reinfection rate after successful interferon-based and interferon-free HCV treatment was comparable, at 3.12 (95% CI 1.94–5.01) versus 1.55 (95% CI 0.50–4.79) per 100 person-years (p = 0.253), but the cumulative reinfection rate was higher after interferon-based treatment at 19.3 (95% CI 12.4–28.8) versus 1.6 (95% CI 0.5–4.5) per 100 treated patients. The large difference between these cumulative reinfection rates is explained by the time of observation, since the median observation time was 5.06 years (IQR 2.63–8.07, range 0.025–29.07) after interferon-based treatment, but only 0.57 years (IQR 0.26–1.56, range 0.003–4.33) after interferon-free treatment.
In the cross-sectional data, 35 reinfections were documented in 33 patients. One third (12) were after spontaneous clearance and two thirds (23) after successful treatment (17 after interferon-based, 6 after interferon-free treatment; fig. 9). In 46% (16/35), the reason for reinfection was unknown. If known, it was unsafe IDU in 95% (18/19) and in one case an HCV positive partner.

Figure 9 Number of reinfections after spontaneous clearance and successful treatment (interferon-based and interferon-free) per calendar year (cross-sectional data): 35 reinfections in 33 patients. IFN = interferon; SC = spontaneous clearance; SVR = sustained virological response
In the 11 patients with a first reinfection after spontaneous clearance, the median time since HCV first diagnosis was 11.3 years (IQR 3.3–13.9). Four patients cleared the virus spontaneously, whereas seven developed chronic hepatitis C, of whom four have already been treated successfully.
In the 22 patients with a first reinfection after successful treatment, the median time since the end of treatment was 3.3 years (IQR 1.1–5.1), i.e., 4.2 years (IQR 2.3–5.3) for interferon-based treatment (n = 17) and 0.9 years (IQR 0.9–1.1) for interferon-free treatment (n = 5). Outcome after reinfection was not available for two patients. Among the remaining patients, 95% (19/20) developed chronic hepatitis C, of whom 15 have already been retreated successfully.
Two patients experienced repeated reinfections (Patient 1: chronic hepatitis C → treatment → 1st reinfection → spontaneous clearance → 2nd reinfection → chronic hepatitis C → successful treatment; Patient 2: chronic hepatitis C → treatment → 1st reinfection → chronic hepatitis C → treatment → 2nd reinfection → chronic hepatitis C → successful treatment).
The median HCV antibody test frequency in HCV antibody-negative patients (n = 89) with a median follow-up time of 3.4 years (IQR 2.9–4.3) was every 1.9 years (IQR 1.4–2.9). In patients after spontaneous clearance (n = 30) with a median follow-up time of 3.2 years (IQR 2.3–4.3), the median HCV-RNA test frequency was every 1.8 years (IQR 1.1–3.3), and in patients after successful treatment (n = 7 3) with median follow-uptime of 3.4 years (IQR 2.3–4), every 1.4 years (IQR 0.8–2.2), i.e., slightly higher than after spontaneous clearance (p = 0.062).
With interferon-free DAA treatment, HCV treatment success in OAT patients increased to almost 100%, irrespective of cirrhosis or prior non-response to interferon. With shorter and better tolerated treatments, adherence was excellent (even in the absence of adherence-supporting measures) and preterm stops became rare. Between 1 May 2017 and 1 May 2019, HCV treatment uptake could be increased to 80%, resulting in a reduction of HCV RNA prevalence to <20% among the HCV antibody positive patients, who represent two thirds of the SAMMSU cohort. Since DAA reimbursement restrictions were abrogated in Switzerland in 2017, patients are treated at earlier fibrosis stages, which results in a shorter duration of infectiousness. At 2.7/100 person-years, the reinfection rate after successful treatment was low. The number of HCV first diagnoses per year decreased from >20 up to 2015 to <10 in 2017 and 2018. However, HCV transmission is still ongoing, whereas HIV transmission has been virtually stopped with universal ART.
Prejudices that PWID are less adherent, have more side effects and less treatment success in the case of HCV treatment persist stubbornly [32], although they were already refuted in the era of interferon-based treatment [33–36]. In our study, with interferon-free DAA treatment, adherence problems and preterm stops declined to 2% and 1%, respectively, irrespective of DOT [37]. In the C-EDGE CO-STAR study (elbasvir/grazoprevir in OAT patients with and without ongoing drug use), similarly high adherence was observed [38]: 96% completed treatment and >97% had an adherence >95%. Drug use at baseline and during HCV treatment did not negatively influence adherence and treatment success. In contrast, in the SIMPLIFY study (sofosbuvir/velpatasvir in patients with IDU in the past 6 months) [39], low adherence (<90%) was observed in one third of the participants. It was associated with recent or ongoing injection of stimulants (cocaine and/or other amphetamines), but did not negatively affect treatment outcome [40]. Remarkably, SVR rates were not worse, even when at least seven consecutive doses were missed [41]. Thus, 100% adherence is not necessary to prevent resistance and consequent treatment failure.
In our study with interferon-free DAA treatment, the SVR rate in those with known outcome was 97%, irrespective of cirrhosis or prior non-response to interferon. In the German Hepatitis C Registry, OAT patients achieved a comparably high per protocol SVR rate, which was not different from that in non-OAT patients (96% vs 95%, p = 0.464) [42]. However, OAT patients had a higher rate of loss to follow-up between the end of treatment and SVR (10% vs 4%, p <0.001), which might be explained by a change of the healthcare setting, difficult venous access and presumed cure given the high treatment efficacy [43]. Since 78% of relapses post-treatment occur within 4 weeks, SVR4 can predict SVR12 with a positive predictive value of 98% and a negative predictive value of 100% [44]. In settings with a high yearly fluctuation rate (up to 37% in our study), SVR4 determination might reduce the proportion of completed HCV treatments with unknown outcome (8%, 23/291, of interferon-free treatments in our study).
The risk of reinfection and the high costs of retreatment are often mentioned as a reason to withhold HCV therapy from people with ongoing IDU. In our study (OAT patients, one quarter to one third with ongoing IDU), the overall HCV reinfection rate was low (2.7/100 person-years), with no difference between interferon-based and interferon-free treatment (3.1 vs 1.6/100 person-years, p = 0.253). A recent meta-analysis (17 studies with interferon-based, 19 studies with DAA treatment) showed a comparable HCV reinfection rate of 3.8/100 person-years (95% CI 2.5–5.8) among OAT patients. It was markedly lower in OAT patients with no recent drug use than in people recently injecting drugs (1.4 vs 6.2/100 person-years) [45]. As in our study, reinfection rates were comparable following interferon-based and DAA treatment (5.4 vs 3.9/100 person-years). Interestingly, the German hepatitis C cohort (GECCO) reported an overall reinfection rate of 1.9/100 person-years (95% CI 1.4–2.5) since 2014 [46]. Reinfection was less frequent in PWID than in men who have sex with men (1.1 vs 9.0/100 person-years).
Mathematical models suggest an increased HCV reinfection incidence in the initial phase of treatment scale-up [14]. However, with decreasing HCV-RNA prevalence, reinfection incidence will decrease again. Actually, more HCV reinfections were diagnosed in recent years in the SAMMSU cohort, but >80% were after spontaneous clearance or interferon-based treatment. Thus, this might partly be explained by increased HCV-RNA testing as a result of better treatment options (detection bias). Reinfection incidence and probability of spontaneous clearance can be underestimated if HCV-RNA testing frequency is too low [47]. Spontaneous clearance of primary HCV infection occurs in about 25%, but spontaneous clearance is more frequent in reinfections after spontaneous clearance (83%) [48]. In our study, 95% of the first reinfections after successful treatment became chronic, compared with only 64% after spontaneous clearance. Anyway, reinfections can be treated as successfully as primary infections. To shorten the duration of infectiousness, chronic hepatitis C can be diagnosed early, i.e., if HCV-RNA decreases <2 log U/ml 4 weeks after diagnosis (85% negative predictive value for spontaneous clearance) [49–51].
Since 95% of reinfections with a known cause were due to unsafe IDU, patient information, sufficient OAT dosing [52] and needle-syringe distribution coverage are important [53].
Mathematical models have shown that a substantial reduction of HCV-RNA prevalence cannot be achieved with OAT and needle-syringe programmes alone, but treatment uptake must be increased [54]. According to the WHO, 80% treatment uptake is necessary to succeed in HCV elimination by 2030 [20]. In our study, 80% treatment uptake has been achieved with interferon-free DAA treatment and was associated with a reduction of HCV-RNA prevalence to 19% among HCV antibody-positive patients and to 13% in the total SAMMSU cohort. Although the number of HCV first diagnoses per year has declined both in our study and according to FOPH data [55], HCV transmission is still ongoing, whereas HIV transmission has virtually been stopped with universal antiretroviral therapy [56].
For HIV, the UNAIDS 90-90-90-target (90% diagnosed, 90% treated, 90% virologically suppressed) [57] has been achieved in the SAMMSU cohort: 99.7% were tested, 96% of the HIV-positive patients were under antiretroviral therapy, of whom 97% were virologically suppressed. This resulted in an HIV-RNA prevalence of 6% among the HIV positives and <1.0% in the total SAMMSU cohort.
A reduction in HCV-RNA prevalence to 1% in the SAMMSU cohort would require 98% HCV treatment uptake (100% treatment success, 50% ever had chronic hepatitis C). Besides, HCV is ~10 times more infectious than HIV through blood-to-blood contact [58] and SAMMSU cohort patients may be part of injection networks [59] with higher HCV-RNA prevalence. Contact tracing may help to identify individuals not yet engaged in health care and harm reduction [60]. In a model study, a “treat your friends” strategy was more effective than random treatment [59].
Treating chronic hepatitis C in OAT patients needs an extra effort. Since they frequently have other priorities and OAT programmes are often exclusively led by psychiatrists, first of all, they must be made aware of the better HCV treatment options available. Referral to gastroenterologists / infectious disease specialists for DAA prescription is often unrewarding because these patients often have difficulty keeping appointments. Additionally, difficult venous access after long-term intravenous drug use complicates diagnosis. Thus, DAA reimbursement irrespective of liver fibrosis stage does not automatically result in increased HCV treatment uptake. Awareness campaigns, HCV treatment on-site and capillary blood HCV antibody [25] and RNA testing [61–64] helped to remove barriers to diagnosis and treatment.
How to bring HCV treatment to OAT patients differs between centralised and decentralised settings. In decentralised settings with a low case-load, capillary HCV-RNA quantification with the dried blood spot method [65] might be an alternative to Xpert® HCV Viral Load Fingerstick point-of-care testing [61–64]. Similarly in such settings, the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) score [APRI = (AST/upper limit of normal of AST)/platelet count (G/l) × 100] can replace Fibroscan® for non-invasive exclusion of liver cirrhosis. Both an APRI score <1.0 [66] and Fibroscan® ≤12.5 kPa [30] have a negative predictive value of ~95% at a liver cirrhosis prevalence of 15% and 25%, respectively. In our study, chronic hepatitis C patients starting their first HCV treatment in 2018 had a cirrhosis prevalence of 13%.
FOPH guidelines [26] recommend yearly HCV screening of patients at risk (OAT, ongoing drug use [injecting and non-injecting]), which has not been achieved yet in the SAMMSU cohort. In Switzerland, new HCV diagnoses must be reported to the cantonal physician, who also has to review all OAT prescriptions every 1–2 years. Documentation of HCV status for each OAT patient and yearly HCV screening reminders sent to the OAT prescriber/provider (GP/pharmacy) within the already existing platform www.substitution-online.ch could facilitate implementation of the FOPH guidelines and monitoring of HCV elimination.
Unlike in Australia [67] and France [68], in Switzerland GPs cannot prescribe DAA. The HepCare-project, initiated by the Swiss Hepatitis Strategy in spring 2019, allows HCV treatment on the site of OAT provision (GP/psychiatrist). In a randomised controlled trial in Australia, treatment uptake was higher in the GP than the hospital setting (75% vs 35%), whereas treatment success was comparable [43]. Increased retention in care halved the average cost of treatment initiation [69].
With almost 1000 patients in different OAT settings throughout Switzerland, the SAMMSU cohort is a useful tool for monitoring HCV elimination. Patients from German- and Italian-speaking regions of Switzerland are overrepresented (81% vs 63% and 13% vs 8%, respectively), whereas patients from French-speaking Switzerland are underrepresented (6% vs 23%) [70]. Rhaeto-Romanic-speaking Switzerland (0.5%) is not represented. Besides, patients cared for in decentralised OAT settings (OAT via GP or psychiatrist and pharmacy) are underrepresented, not least because their recruitment and follow up is more difficult. Since HCV management is better in centralised than decentralised settings [25], and better inside than outside the cohort [71], HCV screening and treatment uptake in the SAMMSU cohort is probably higher than in the general Swiss OAT population.
Owing to a lack of manpower, in most centres only 5–20% of the patients are enrolled into the SAMMSU cohort. Patients willing to be tested and treated are more likely to be enrolled (enrolment bias), leading to an overrepresentation of HCV antibody-positive patients, patients with chronic hepatitis C and treated patients. HCV antibody-positive patients are older, the proportion with ever and ongoing IDU is higher, their first IDU is longer ago and a higher proportion are HIV positive. In contrast, unstable patients who are only a short time at one institution are less likely to be enrolled. Thus, adherence might be overestimated.
So far, there are only a few reinfections after DAA treatment and the follow-up time is still quite short. A longer observation period is necessary to estimate the reinfection rate after DAA treatment more reliably.
To counteract the delay of data entry resulting from only yearly follow-up in the SAMMSU cohort, two cross-sectional surveys 1 May 2018 and 1 May 2019 (1 and 2 years after the abrogation of DAA reimbursement restrictions for PWID) were performed. Since the study physician and/or the institution changed, the SAMMSU centre Geneva could not contribute any cross-sectional data, and Lausanne could only provide data in the first survey.
In the SAMMSU-cohort, 91% (445) of the 490 patients who had ever had chronic hepatitis C had a Fibroscan®, but only 27% (131) a liver biopsy (often many years ago).
With nearly 100% DAA treatment success and a low reinfection rate, treatment uptake directly translates into a reduction of HCV-RNA prevalence. Eighty percent treatment uptake is feasible in OAT patients, and adherence and treatment success are not worse than in other populations. Duration of infectiousness and thus HCV transmission can be reduced by early detection and treatment of chronic hepatitis C.
Table S1 HCV treatment uptake and HCV-RNA prevalence in seven of eight SAMMSU centres
| 1 May 2017 | 1 May 2018 | 1 May 2019 | |
|---|---|---|---|
| Aarau (n = 367) | |||
| Treatment uptake* | 49.1% (57/116) | 65.6% (80/121) | 79.4% (104/131) |
| HCV-RNA prevalence† | 48.3% (73/151) | 34.0% (54/159) | 20.2% (35/173) |
| Basel (n = 82) | |||
| Treatment uptake* | 29.7% (19/64) | 69.4% (43/62) | 93.7% (59/63) |
| HCV-RNA prevalence† | 66.7% (48/72) | 31.9% (23/72) | 8.3% (6/72) |
| Bern (n = 27) | |||
| Treatment uptake* | 42.1% (8/19) | 63.2% (12/19) | 84.2% (16/19) |
| HCV-RNA prevalence† | 50% (12/24) | 41.7% (10/24) | 21.7% (5/23) |
| Lausanne (n = 33) ‡ | |||
| Treatment uptake* | 60.7% (17/28) | 75% (21/28) | |
| HCV-RNA prevalence† | 43.3% (13/30) | 33.3% (10/30) | |
| Lugano (n = 111) | |||
| Treatment uptake* | 74.0% (57/77) | 92.2% (71/77) | 96.1% (73/76) |
| HCV-RNA prevalence† | 27.2% (25/92) | 8.8% (8/91) | 4.6% (4/87) |
| St Gallen (n = 46) | |||
| Treatment uptake* | 54.8% (17/31) | 93.3% (28/30) | 92.9% (26/28) |
| HCV-RNA prevalence† | 41.0% (16/39) | 7.7% (3/39) | 5.6% (2/36) |
| Zurich (n = 159) | |||
| Treatment uptake* | 79.2% (42/53) | 90.4% (66/73) | 91.8% (67/73) |
| HCV-RNA prevalence† | 21.1% (15/71) | 10.1% (10/99) | 8.0% (8/100) |
| Total (n = 825) | |||
| Treatment uptake* | 55.9% (217/388) | 78.3% (321/410) | 88.5% (345/390) |
| HCV-RNA prevalence† | 42.2% (202/479) | 23.0% (118/514) | 12.2% (60/491) |
| Total without Aarau (n = 458) | |||
| Treatment uptake* | 58.8% (160/272) | 83.4% (241/289) | 93.1% (241/259) |
| HCV-RNA prevalence† | 39.3% (129/328) | 18.0% (64/355) | 7.9% (25/318) |
HCV = hepatitis C virus * In the case of chronic hepatitis C (currently HCV RNA positive or currently HCV RNA negative and ever treated); † among the HCV antibody positive patients; ‡ no data for 1 May 2019 due to site interruption
Appendices 2 and 3 are available in the PDF version of the article.
We thank Dr Mathieu Rougement for implementing the SAMMSU-cohort in Geneva (Hopitaux Universitaires de Génève [HUG]).
The present study was commissioned and financed by the Federal Office of Public Health (FOPH) (Contract number: 17.010967).
SAMMSU has been financially supported by Infodrog (on behalf of the FOPH), pharma industry (BMS, AbbVie, Gilead, Merck, Roche), SSAM (Swiss Society for Addiction Medicine), SEVHep (Swiss Experts in Viral Hepatitis), University Hospital Bern, Cantonal Hospital St. Gallen, Cantonal Hospital Aarau, Arud Zentrum für Suchtmedizin, Zfs Zentrum für Suchtmedizin Basel, University Hospital Zurich, Hopitaux Universitaires de Génève (HUG).
PB received project, travel and research grants as well as speaker honoraria from Abbvie, Gilead and MSD. EC is Gilead Swiss Grant recipient and expert honorarium recipient from Abbvie and Gilead. AB received project grants from Abbvie and Gilead. All other authors declared no conflict of interest.
1 Richard JL , Schaetti C , Basler S , Mäusezahl M . The epidemiology of hepatitis C in Switzerland: trends in notifications, 1988-2015. Swiss Med Wkly. 2018;148:w14619.
2 Aaron S , McMahon JM , Milano D , Torres L , Clatts M , Tortu S , et al. Intranasal transmission of hepatitis C virus: virological and clinical evidence. Clin Infect Dis. 2008;47(7):931–4. doi:.https://doi.org/10.1086/591699
3 Fernandez N , Towers CV , Wolfe L , Hennessy MD , Weitz B , Porter S . Sharing of Snorting Straws and Hepatitis C Virus Infection in Pregnant Women. Obstet Gynecol. 2016;128(2):234–7. doi:.https://doi.org/10.1097/AOG.0000000000001507
4 Grebely J , Page K , Sacks-Davis R , van der Loeff MS , Rice TM , Bruneau J , et al.; InC3 Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1):109–20. doi:.https://doi.org/10.1002/hep.26639
5 Edlin BR . Perspective: test and treat this silent killer. Nature. 2011;474(7350):S18–9. doi:.https://doi.org/10.1038/474S18a
6 Thein HH , Yi Q , Dore GJ , Krahn MD . Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31. doi:.https://doi.org/10.1002/hep.22375
7 Westbrook RH , Dusheiko G . Natural history of hepatitis C. J Hepatol. 2014;61(1, Suppl):S58–68. doi:.https://doi.org/10.1016/j.jhep.2014.07.012
8 Backus LI , Belperio PS , Shahoumian TA , Mole LA . Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology. 2018;68(3):827–38. doi:.https://doi.org/10.1002/hep.29811
9 Degenhardt L , Peacock A , Colledge S , Leung J , Grebely J , Vickerman P , et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–207. doi:.https://doi.org/10.1016/S2214-109X(17)30375-3
10Zahnd C, Brezzi M, Bertisch B, Giudici F, Keiser O. Analyse de Situation des Hépatites B et C en Suisse. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=2ahUKEwiC_KLc0YLnAhXDb1AKHWdQAW0QFjABegQIBxAC&url=https%3A%2F%2Fwww.bag.admin.ch%2Fdam%2Fbag%2Fde%2Fdokumente%2Fmt%2Fforschungsberichte%2Fsituationsanalyse-hepatitis-bericht.pdf.download.pdf%2Fsituationsanalyse-hepatitis-bericht-de.pdf&usg=AOvVaw03m8hsizPqQkqZgnEfZfY_
11Cominetti F, Simonson T, Dubois-Arber F, Gervasoni JP, Schaub M, Monnat M. Analyse der Hepatitis-C-Situation bei den drogenkonsumierenden Personen in der Schweiz. Lausanne: Institut universitaire de médecine sociale et préventive; 2015. (Raisons de santé 234b) Available from: https://www.iumsp.ch/Publications/pdf/rds234b_de.pdf
12Bundesamt für Gesundheit (BAG). Substitutionsgestützte Behandlungen bei Opioidabhängigkeit. Revision July 2013. Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/npp/drogen/sucht/hegebe/substitutionsgestuetzte-behandlungen-bei-opioid-abhaengigkeit.pdf.download.pdf/BAG_Brosch_SGB_d(5)_def.pdf [accessed: 2020 March 15].
13Bundesamt für Gesundheit (BAG). Substitutionsgestützte Behandlungen bei Opioidabhängigkeit (1 July 2019). Available from: https://www.bag.admin.ch/bag/de/home/gesund-leben/sucht-und-gesundheit/suchtberatung-therapie/substitutionsgestuetzte-behandlung.html
14 Bruggmann P , Blach S , Deltenre P , Fehr J , Kouyos R , Lavanchy D , et al. Hepatitis C virus dynamics among intravenous drug users suggest that an annual treatment uptake above 10% would eliminate the disease by 2030. Swiss Med Wkly. 2017;147:w14543.
15 Lee R , Kottilil S , Wilson E . Sofosbuvir/velpatasvir: a pangenotypic drug to simplify HCV therapy. Hepatol Int. 2017;11(2):161–70. doi:.https://doi.org/10.1007/s12072-016-9776-8
16 Puoti M , Foster GR , Wang S , Mutimer D , Gane E , Moreno C , et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: An integrated analysis of HCV genotype 1-6 patients without cirrhosis. J Hepatol. 2018;69(2):293–300. doi:.https://doi.org/10.1016/j.jhep.2018.03.007
17 Brown RS, Jr , Buti M , Rodrigues L , Chulanov V , Chuang WL , Aguilar H , et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol. 2020;72(3):441–9. doi:.https://doi.org/10.1016/j.jhep.2019.10.020
18 Zeuzem S . Hepatitis-C-Therapie: State of the Art 2018 [Treatment of Hepatitis C: State of the Art 2018]. Dtsch Med Wochenschr. 2018;143(24):1784–8. Article in German. doi:.https://doi.org/10.1055/a-0591-5916
19 Hickman M , De Angelis D , Vickerman P , Hutchinson S , Martin NK . Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence. Curr Opin Infect Dis. 2015;28(6):576–82. doi:.https://doi.org/10.1097/QCO.0000000000000216
20World Health Organization. Combating hepatitis B and C to reach elimination by 2030 - Advocacy brief (May 2016). Available from: http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf [accessed: 2020 Jan 14]
21Swiss Hepatitis Strategy 2014-2030. Available from: https://www.hepatitis-schweiz.ch/files/Dokumente/PDF/Process_Paper_14_02_2019.pdf [accessed: 2020 Jan 14]
22Bundesrat 2015. Nationale Strategie Sucht 2017-2024. https://www.bag.admin.ch/dam/bag/de/dokumente/npp/strategie-sucht/strategiedokumente/stategie-sucht.pdf.download.pdf/Nationale%20Strategie%20Sucht.pdf [accessed: 2020 Mar 15]
23Bundesamt für Gesundheit (BAG). BAG erweitert Vergütung von Medikamenten gegen Hepatitis C (27 Apr 17). Available from: https://www.bag.admin.ch/bag/de/home/das-bag/aktuell/medienmitteilungen.msg-id-66508.html
24Bundesamt für Gesundheit (BAG). Hepatitis C: Uneingeschränkte Vergütung der neuen Arzneimittel für alle Betroffenen (25 Sept 2017). Available from: https://www.bag.admin.ch/bag/de/home/das-bag/aktuell/medienmitteilungen.msg-id-68158.html
25 Bregenzer A , Conen A , Knuchel J , Friedl A , Eigenmann F , Näf M , et al. Management of hepatitis C in decentralised versus centralised drug substitution programmes and minimally invasive point-of-care tests to close gaps in the HCV cascade. Swiss Med Wkly. 2017;147:w14544.
26Bundesamt für Gesundheit (BAG). Hepatitis C bei Drogenkonsumierenden: Richtlinien mit settingspezifischen Factsheets (Mar 2019). Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/infektionskrankheiten/hepatitis-c/richtlinien-hepatitis-c-drogen.pdf.download.pdf/richtlinien-hepatitis-c-drogen-de.pdf [accessed: 2020 Jan 14]
27 Bregenzer A , Bruggmann P , Castro E , Moriggia A , Rothen M , Thurnheer MC , et al. Schweizer OAT-Programme auf ihrem Weg zur HCV-Elimination – Die SAMMSU-Kohorte. Suchtmedizin. 2019;21(2):75–90.
28 Prasad L , Spicher VM , Zwahlen M , Rickenbach M , Helbling B , Negro F ; Swiss Hepatitis C Cohort Study Group. Cohort Profile: the Swiss Hepatitis C Cohort Study (SCCS). Int J Epidemiol. 2007;36(4):731–7. doi:.https://doi.org/10.1093/ije/dym096
29 Schoeni-Affolter F , Ledergerber B , Rickenbach M , Rudin C , Günthard HF , Telenti A , et al.; Swiss HIV Cohort Study. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39(5):1179–89. doi:.https://doi.org/10.1093/ije/dyp321
30 Castéra L , Vergniol J , Foucher J , Le Bail B , Chanteloup E , Haaser M , et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. doi:.https://doi.org/10.1053/j.gastro.2004.11.018
31 Castera L , Forns X , Alberti A . Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–47. doi:.https://doi.org/10.1016/j.jhep.2008.02.008
32 Asher AK , Portillo CJ , Cooper BA , Dawson-Rose C , Vlahov D , Page KA . Clinicians’ Views of Hepatitis C Virus Treatment Candidacy With Direct-Acting Antiviral Regimens for People Who Inject Drugs. Subst Use Misuse. 2016;51(9):1218–23. doi:.https://doi.org/10.3109/10826084.2016.1161054
33 Backmund M , Meyer K , Von Zielonka M , Eichenlaub D . Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34(1):188–93. doi:.https://doi.org/10.1053/jhep.2001.25882
34 Belfiori B , Ciliegi P , Chiodera A , Bacosi D , Tosti A , Baldelli F , et al. Peginterferon plus Ribavirin for chronic hepatitis C in opiate addicts on methadone/buprenorphine maintenance therapy. Dig Liver Dis. 2009;41(4):303–7. doi:.https://doi.org/10.1016/j.dld.2008.08.009
35 Grebely J , deVlaming S , Duncan F , Viljoen M , Conway B . Current approaches to HCV infection in current and former injection drug users. J Addict Dis. 2008;27(2):25–35. doi:.https://doi.org/10.1300/J069v27n02_04
36 Bruggmann P , Falcato L , Dober S , Helbling B , Keiser O , Negro F , et al.; Swiss Hepatitis C Cohort Study. Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15(10):747–52. doi:.https://doi.org/10.1111/j.1365-2893.2008.01010.x
37 McDermott CL , Lockhart CM , Devine B . Outpatient directly observed therapy for hepatitis C among people who use drugs: a systematic review and meta-analysis. J Virus Erad. 2018;4(2):118–22. doi:.https://doi.org/10.1016/S2055-6640(20)30255-7
38 Dore GJ , Altice F , Litwin AH , Dalgard O , Gane EJ , Shibolet O , et al.; C-EDGE CO-STAR Study Group. Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med. 2016;165(9):625–34. doi:.https://doi.org/10.7326/M16-0816
39 Grebely J , Dalgard O , Conway B , Cunningham EB , Bruggmann P , Hajarizadeh B , et al.; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153–61. doi:.https://doi.org/10.1016/S2468-1253(17)30404-1
40 Cunningham EB , Amin J , Feld JJ , Bruneau J , Dalgard O , Powis J , et al.; SIMPLIFY study group. Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: The SIMPLIFY study. Int J Drug Policy. 2018;62:14–23. doi:.https://doi.org/10.1016/j.drugpo.2018.08.013
41 Cunningham EB , Hajarizadeh B , Amin J , Litwin AH , Gane E , Cooper C , et al.; SIMPLIFY and D3FEAT study groups. Adherence to Once-daily and Twice-daily Direct-acting Antiviral Therapy for Hepatitis C Infection Among People With Recent Injection Drug Use or Current Opioid Agonist Therapy. Clin Infect Dis. 2020;71(7):e115–24. doi:.https://doi.org/10.1093/cid/ciz1089
42 Christensen S , Buggisch P , Mauss S , Böker KHW , Schott E , Klinker H , et al. Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: Still a concern in clinical practice? Addiction. 2018;113(5):868–82. doi:.https://doi.org/10.1111/add.14128
43 Wade AJ , Doyle JS , Gane E , Stedman C , Draper B , Iser D , et al. Outcomes of treatment for hepatitis C in primary care compared to hospital-based care: a randomized controlled trial in people who inject drugs. Clin Infect Dis. 2020;70(9):1900–6. doi:.https://doi.org/10.1093/cid/ciz546
44 Yoshida EM , Sulkowski MS , Gane EJ , Herring RW, Jr , Ratziu V , Ding X , et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61(1):41–5. doi:.https://doi.org/10.1002/hep.27366
45 Hajarizadeh B , Cunningham EB , Valerio H , Martinello M , Law M , Janjua NZ , et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J Hepatol. 2020;72(4):643–57. doi:.https://doi.org/10.1016/j.jhep.2019.11.012
46 Ingiliz P , Wehmeyer MH , Boesecke C , Schulze Zur Wiesch J , Schewe K , Lutz T , et al.; European AIDS Treatment Network (NEAT) Study Group; German Hepatitis C Cohort (GECCO) Study Group. Reinfection With the Hepatitis C Virus in Men Who Have Sex With Men After Successful Treatment With Direct-acting Antivirals in Germany: Current Incidence Rates, Compared With Rates During the Interferon Era. Clin Infect Dis. 2020;71(5):1248–54. doi:.https://doi.org/10.1093/cid/ciz949
47 Vickerman P , Grebely J , Dore GJ , Sacks-Davis R , Page K , Thomas DL , et al., International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3). The more you look, the more you find: effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design. J Infect Dis. 2012;205(9):1342–50. doi:.https://doi.org/10.1093/infdis/jis213
48 Osburn WO , Fisher BE , Dowd KA , Urban G , Liu L , Ray SC , et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138(1):315–24. doi:.https://doi.org/10.1053/j.gastro.2009.09.017
49Vogel M, Page E, Matthews G, et al. The use of Week 4 HCV-RNA after Acute HCV Infection to Predict Chronic HCV (Poster). 17th Conference on Retroviruses and Opportunistic Infections 2010 (CROI)
50European AIDS Clinical Society. EACS Guidelines Version 10.0 - November 2019 https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf
51 Martinello M , Hajarizadeh B , Grebely J , Dore GJ , Matthews GV . Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat Rev Gastroenterol Hepatol. 2018;15(7):412–24. doi:.https://doi.org/10.1038/s41575-018-0026-5
52 Artenie AA , Minoyan N , Jacka B , Høj S , Jutras-Aswad D , Roy É , et al. Opioid agonist treatment dosage and patient-perceived dosage adequacy, and risk of hepatitis C infection among people who inject drugs. CMAJ. 2019;191(17):E462–8. doi:.https://doi.org/10.1503/cmaj.181506
53 Colledge S , Leung J , Larney S , Peacock A , Grebely J , Hickman M , et al. Frequency of injecting among people who inject drugs: A systematic review and meta-analysis. Int J Drug Policy. 2020;76:102619. doi:.https://doi.org/10.1016/j.drugpo.2019.102619
54 Martin NK , Vickerman P , Grebely J , Hellard M , Hutchinson SJ , Lima VD , et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. doi:.https://doi.org/10.1002/hep.26431
55Bundesamt für Gesundheit (BAG). Hepatitis C in der Schweiz, epidemiologische Situation 2015–2018. Bull BAG 2019;45:12–24; Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/mt/infektionskrankheiten/hepatitis-c/hepatitis-c-epidemiologie-2015-18.pdf.download.pdf/hepatitis-c-epidemiologie-2015-18-de.pdf [accessed: 2020 Nov 30]
56 Saag MS , Benson CA , Gandhi RT , Hoy JF , Landovitz RJ , Mugavero MJ , et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–96. doi:.https://doi.org/10.1001/jama.2018.8431
57UNAIDS. 90–90–90—An ambitious treatment target to help end the AIDS epidemic. 2014. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf [accessed: 2020 Mar 10]
58 Coutinho RA . HIV and hepatitis C among injecting drug users. BMJ. 1998;317(7156):424–5. doi:.https://doi.org/10.1136/bmj.317.7156.424
59 Hellard M , Rolls DA , Sacks-Davis R , Robins G , Pattison P , Higgs P , et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–70. doi:.https://doi.org/10.1002/hep.27403
60 Katzman C , Mateu-Gelabert P , Kapadia SN , Eckhardt BJ . Contact tracing for hepatitis C: The case for novel screening strategies as we strive for viral elimination. Int J Drug Policy. 2019;72:33–9. doi:.https://doi.org/10.1016/j.drugpo.2019.04.003
61 Lamoury FMJ , Bajis S , Hajarizadeh B , Marshall AD , Martinello M , Ivanova E , et al.; LiveRLife Study Group. Evaluation of the Xpert HCV Viral Load Finger-Stick Point-of-Care Assay. J Infect Dis. 2018;217(12):1889–96. doi:.https://doi.org/10.1093/infdis/jiy114
62 Cooper C . Rapid HCV RNA testing: removing the final obstacle to elimination. Lancet Gastroenterol Hepatol. 2017;2(7):468–9. doi:.https://doi.org/10.1016/S2468-1253(17)30086-9
63 Bregenzer A , Warmann N , Ottiger C , Fux CA . Rapid point-of-care HCV RNA quantification in capillary whole blood for diagnosing chronic HCV infection, monitoring treatment and detecting reinfection. Swiss Med Wkly. 2019;149:w20137. doi:.https://doi.org/10.4414/smw.2019.20137
64 Bajis S , Maher L , Treloar C , Hajarizadeh B , Lamoury FMJ , Mowat Y , et al.; LiveRLife Study Group. Acceptability and preferences of point-of-care finger-stick whole-blood and venepuncture hepatitis C virus testing among people who inject drugs in Australia. Int J Drug Policy. 2018;61:23–30. doi:.https://doi.org/10.1016/j.drugpo.2018.08.011
65 Wlassow M , Poiteau L , Roudot-Thoraval F , Rosa I , Soulier A , Hézode C , et al. The new Xpert HCV viral load real-time PCR assay accurately quantifies hepatitis C virus RNA in serum and whole-blood specimens. J Clin Virol. 2019;117:80–4. doi:.https://doi.org/10.1016/j.jcv.2019.06.007
66 Shaheen AA , Myers RP . Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46(3):912–21. doi:.https://doi.org/10.1002/hep.21835
67The Kirby Institute. Monitoring hepatitis C treatment uptake in Australia (Issue 10). The Kirby Institute, UNSW Sydney, NSW, Australia, June 2019, Available from: https://kirby.unsw.edu.au/report/monitoring-hepatitis-c-treatment-uptake-australia-issue-10-june-2019 [accessed: 2020 Dec 01]
68 Loustaud-Ratti V , Debette-Gratien M , Carrier P . European Association for the Study of the Liver and French hepatitis C recent guidelines: The paradigm shift. World J Hepatol. 2018;10(10):639–44. doi:.https://doi.org/10.4254/wjh.v10.i10.639
69 Palmer AY , Wade AJ , Draper B , Howell J , Doyle JS , Petrie D , et al. A cost-effectiveness analysis of primary versus hospital-based specialist care for direct acting antiviral hepatitis C treatment. Int J Drug Policy. 2020;76:102633. doi:.https://doi.org/10.1016/j.drugpo.2019.102633
70Bundesamt für Statistik. Hauptsprache der ständigen Wohnbevölkerung 2017; https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/sprachen-religionen/sprachen.html (published: 29 Jan 2019)
71 Schürch S , Fux CA , Dehler S , Conen A , Knuchel J , Friedl A , et al. Management of hepatitis C in opioid agonist therapy patients of the Swiss canton Aargau within and outside the cohort study. Swiss Med Wkly. 2020;150:w20317. doi:.https://doi.org/10.4414/smw.2020.20317
The present study was commissioned and financed by the Federal Office of Public Health (FOPH) (Contract number: 17.010967).
SAMMSU has been financially supported by Infodrog (on behalf of the FOPH), pharma industry (BMS, AbbVie, Gilead, Merck, Roche), SSAM (Swiss Society for Addiction Medicine), SEVHep (Swiss Experts in Viral Hepatitis), University Hospital Bern, Cantonal Hospital St. Gallen, Cantonal Hospital Aarau, Arud Zentrum für Suchtmedizin, Zfs Zentrum für Suchtmedizin Basel, University Hospital Zurich, Hopitaux Universitaires de Génève (HUG).
PB received project, travel and research grants as well as speaker honoraria from Abbvie, Gilead and MSD. EC is Gilead Swiss Grant recipient and expert honorarium recipient from Abbvie and Gilead. AB received project grants from Abbvie and Gilead. All other authors declared no conflict of interest.