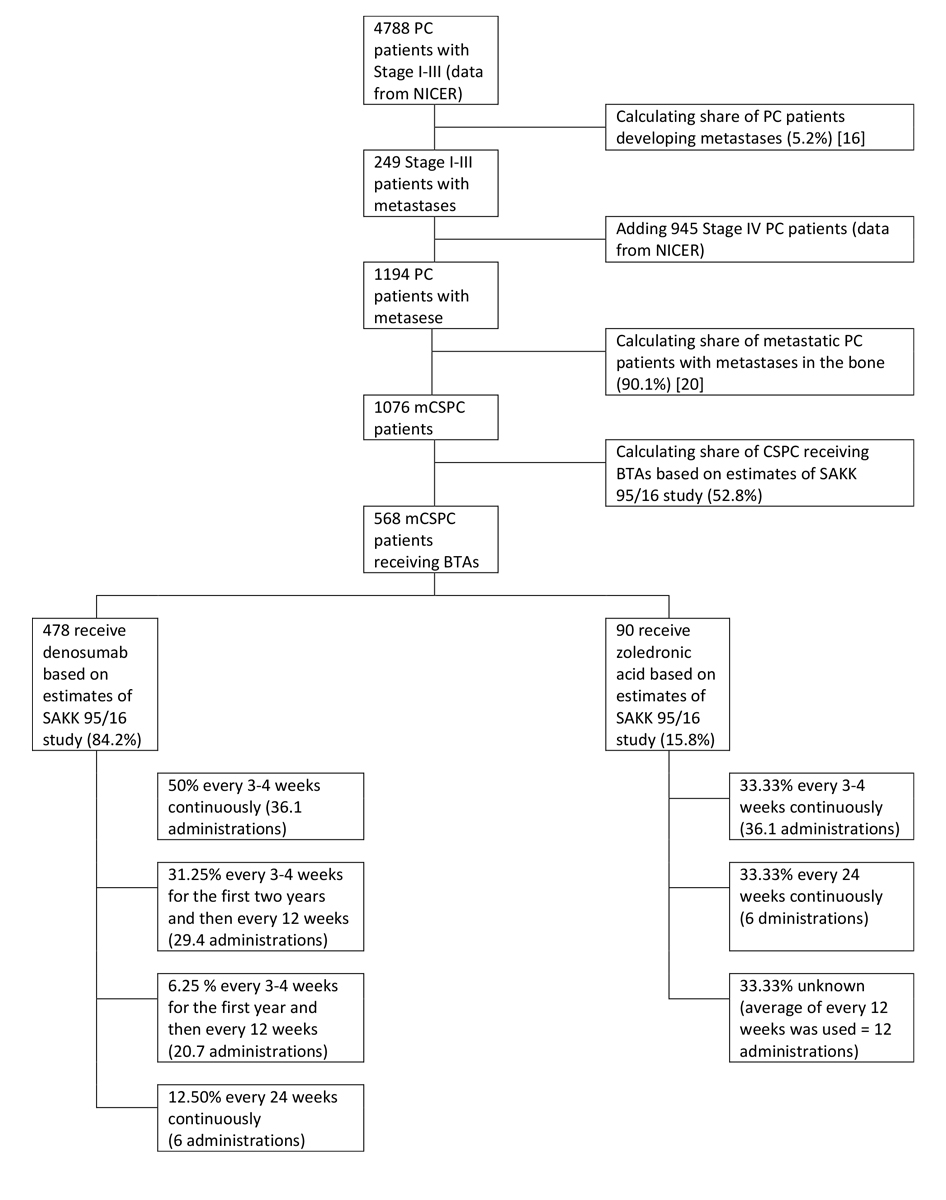
Figure 1 Estimation of the Swiss metastatic hormone-sensitive prostate cancer (mCSPC) population that received bone-targeted agents (BTAs) in 2016. PC = prostate cancer
DOI: https://doi.org/10.4414/smw.2021.20464
Skeletal-related events are a major concern for cancer patients with bone metastases [1]. They can pose a significant health burden, in the form of pathological fractures and spinal cord compression, which negatively influence emotional, functional and physical well-being [2]. Bone-targeted agents (BTAs) are used to reduce the risk of skeletal-related events as well as for cancer treatment-related bone loss induced by androgen deprivation therapy [3].
BTAs include bisphosphonates, drugs with a phosphorus-carbon-phosphorus backbone which decrease the risk of fracture by minimising bone resorption [4]. Alternatively, denosumab has emerged as a clinically effective human monoclonal antibody reducing osteoclast formation and preventing skeletal-related events caused by bone metastases from solid tumours [5]. Denosumab was originally developed for the treatment of osteoporosis, is injected subcutaneously after a calcium level check and does not require renal monitoring [6].
In a randomised phase III clinical trial in patients with metastatic castration-resistant prostate cancer (mCRPC) with bone metastases, denosumab (120 mg every 4 weeks) performed better than zoledronic acid (4 mg every 4 weeks) regarding prevention of skeletal-related events [7]. Based on this study, the European Society of Medical Oncology (ESMO) recommends the use of denosumab or bisphosphonates for mCRPC patients with bone metastases at high risk for clinically significant skeletal-related events [8]. The trial leading to approval of denosumab exclusively included patients with metastatic castration-resistant disease and not castration-sensitive disease. In the case of metastatic castration-sensitive prostate cancer (mCSPC) with bone metastases, no clinical trials have investigated the clinical benefit of denosumab [9]. In contrast, zoledronic acid has been tested in this setting in two randomised trials and did not reduce skeletal-related event risk [10, 11]. Thus, the American Society of Clinical Oncology (ASCO) and ESMO recommend that BTAs in the bone metastasis dose should not be part of the standard of care for patients with mCSPC [12, 13]. Use of BTAs is, however, indicated for patients on androgen deprivation treatment to prevent cancer treatment-induced bone loss and ultimately osteoporotic fractures. The dose in this indication is 10–13 times lower, denosumab 60 mg every 6 months or zoledronic acid 5 mg once per year.
Despite the lack of evidence in the mCSPC setting, denosumab at the dose of 120 mg (Xgeva®) is currently approved by Swissmedic and the European Medicines Agency (EMA) for the treatment of patients with bone metastases from solid tumours in conjunction with standard antineoplastic therapy, irrespective of castration status for prostate cancer patients [14, 15]. Similarly, zoledronic acid can be administered for the treatment of bone metastases in patients with solid tumours, including prostate cancer [16, 17].
We found widespread implementation of guideline-recommended BTA prescribing in Switzerland [17], but little is known about the prevalence and health economic consequences of BTA use for mCSPC patients. Health spending in Switzerland is among the highest in the world. The total cost of cancer per capita in Switzerland was EUR 578 in 2018, corresponding to more than EUR 4000 million [18]. To address this gap in the literature, we used data from a recent pattern of care study. The study estimated the costs of administering denosumab and zoledronic acid based on marketing approval by Swissmedic for mCSPC patients. To our knowledge, this is the first study that investigates the economic consequences of administering BTAs for bone metastases to mCSPC patients.
Information about the prevalence of BTA administration to mCSPC patients was taken from the SAKK 95/16 study [19]. This cross-sectional survey study was conducted between November 2017 and May 2018, and included 86 oncologists from 18 sites across Switzerland. Oncologists were recruited through the Swiss Group for Clinical Cancer Research (SAKK) network with the support of the Schweizerische Gesellschaft für Medizinische Onkologie (SGMO). Eligible oncologists could practice at either public hospitals or private clinics within Switzerland. These were asked about their BTA prescribing patterns for patients with solid tumours and bone metastases in a total of 417 patients, for whose treatment decisions they were personally responsible at their centre. Study details have been described elsewhere [19]. Briefly, included patients were at least 18 years old, with solid tumours and at least one bone metastasis, and were receiving routine management at the participating physician’s centre over the 3-month study period. The most common underlying solid tumour type was breast cancer (169/417, 40.5%), followed by prostate cancer (106/417, 25.4%) and lung cancer (62/417, 14.9%). Almost one third of the prostate cancer patients were castration-sensitive (36/106, 34.0%).
As the incidence of mCSPC in Switzerland is unknown, we estimated the size of the mCSPC population based on the total number of annual prostate cancer cases obtained from the Swiss National Agency for Cancer Registration (NACR) and the estimated development of bone metastases. Specifically, mCSPC patients who had either stage IV prostate cancer, or stage I–III prostate cancer with prostate-specific antigen (PSA) progression and development of metastases were considered. For this, we extracted the total number of incident prostate cancer cases in stages I to IV for the latest available year (2016) from NACR. We then multiplied the stage I–III prostate cancer patients by the probability of developing metastases (5.2%), taken from a US study, and added them to the stage IV patients [20]. Finally, we multiplied the number of metastatic prostate cancer patients by the probability (90.1%) that these metastases are present in the bone [21].
To get an estimate of how many new mCSPC patients are treated each year with BTAs, we combined the information from the survey about the proportion of mCSPC patients receiving BTAs from their oncologist with the estimated size of the Swiss mCSPC patient population.
The time to PSA progression of mCSPC patients treated with BTAs was based on a US trial, which reported a median time to PSA progression of 33.2 months (144.26 weeks) with 75th percentile of 12.1 months (52.58 weeks) in mCSPC patients on continuous treatment with abiraterone acetate and prednisone [22]. As the trial did not reach the time to measure the 25th percentile, we used the sum of the median time and the difference to the 75th percentile (33.2 months + (33.2 months – 12.1 months) = 54.3 months (235.95 weeks)) as an upper value in the sensitivity analysis.
To estimate the health-economic consequences of administering BTAs to mCSPC patients in Switzerland, we calculated the costs of administering BTAs and the costs of osteonecrosis of the jaw (ONJ) as a treatment-related bone complication for the estimated number of treated mCSPC patients. The analysis used the perspective of the healthcare system and combined information about BTA administration and reported health complications from the SAKK 95/16 cross-sectional study with above-described estimates about the mCSPC population in Switzerland. All costs were calculated in Swiss francs (CHF). The cost of administering BTAs was based on the 2020 Swiss tariffs for outpatient physician services TARMED [23]. Specifically, it included a physician visit with blood extraction, a short physical examination and the cost of calcium and albumin analyses and subcutaneous injection of Xgeva® in the case of denosumab. The resulting total costs were CHF 132.28. For zoledronic acid, the administration cost was CHF 183.77 and included a short examination by the physician with blood extraction and the cost of creatinine analysis and the intravenous infusion of zoledronic acid. Drug costs were based on 2020 public prices from the “Spezialitätenliste” [24]. In the case of denosumab, we assumed that Xgeva® was used, i.e., denosumab at a dose of 120 mg every 4 weeks, as it was the only drug with an administration interval fitting the intervals reported by the oncologists in SAKK95/16. Its public price was CHF 478.05 for one dose [24]. For zoledronic acid, there were seven different products available for treating metastases. The least expensive was Zoledronat Fresenius Onco® (CHF 129.45 for one dose of 5 ml) and the most expensive Zometa® (CHF 212.25 for one dose of 5 ml) [24]. For the analysis, we used an average price of CHF 170.35 per dose. Thus, total costs for each administration of denosumab and zoledronic acid were estimated at CHF 545.67 (CHF 478.05 + CHF 47.62) and CHF 314.16 (CHF 170.35 + CHF 143.81), respectively. To simplify the calculation, we assumed that for patients whose administration was recorded to be between 3–4 weeks, BTAs were given every 4 weeks [8].
We consider ONJ as the main treatment-related bone complication. Our survey contained two questions asking oncologists whether they stopped BTA treatment or changed the administration interval in the event of ONJ, no oncologist treating mCSPC patients stated any occurrences. Thus, the frequencies of ONJ were calculated from a phase III trial that compared denosumab with zoledronic acid in patients with metastatic prostate cancer [8]. The extension of the trial found that 12 out of 147 patients (8.2%) treated with denosumab and 7 out of 118 patients (5.9%) using zoledronic acid developed ONJ [25]. For the costs of ONJ treatment, we used estimates from a detailed US study, and adjusted for inflation to 2020 prices [26, 27]. The costs covered the pharmacological management (e.g., steroid injections, antibiotics), simple incision and drainage biopsies, dental extraction, root canal treatment, non-surgical sequestrectomy, debridement, and surgical resection and reconstruction [26]. For the conversion of the costs in US dollars (USD), we used the purchase power parity of 1.148 from the OECD [28]. The resulting median cost of treating ONJ in one prostate cancer patient used for the analysis was CHF 4299.05 (USD 3744.82) with 25th percentile CHF 2682.69 (USD 2336.84) and 75th percentile CHF 7623.88 (USD 6641.01).
The cost analysis reports the costs of one cohort of mCSPC patients over the median time to PSA progression of 33.2 months (2.78 years). Assuming a constant incidence of mCSPS over time, one can assume that each year a new cohort of the same size would start BTA treatment. Thus, in each year, there would be a cohort of patients in their first year of treatment and other cohorts of the same size in the second year and the third year of treatment. The total costs of administering BTAs in a given year can therefore be approximated by the cost of treating one cohort until PSA progression.
Because of the many uncertainties associated with the estimation of the health economic consequences of administering BTAs to mCSPC patients, univariate sensitivity analyses were used to test the robustness of the calculations. The following variables were varied:
While the first five variables were subjected to ± 30% variation, the cost of zoledronic acid used the lowest and highest Swiss product prices. Further, the 25th and 75th percentiles were used for the cost of ONJ and the time to PSA progression of the patients. A summary of the model variables used is shown in table 5 in the results section “Sensitivity analyses”.
Of the 86 oncologists participating in the survey, 20 treated 36 patients with mCSPC. Overall, 11 of these 20 oncologists (55.0%) prescribed BTAs such as denosumab and zoledronic acid. Eight (72.7%) oncologists reported initiating BTAs with all their mCSPC patients, and 3 (27.3%) administered them to some of their mCSPC patients. Table 1 summarises the demographic characteristics of these oncologists. Most of the oncologists who prescribed BTAs to at least some mCSPC patients were senior consultants (45.4%), followed by consultants (36.4%), residents (9.1%) and department heads (9.1%). None of them were private practitioners. Almost half (45.4%) had between 10 and 20 years of medical experience, 27.3% had between 5 and 10 years’ experience, 18.2% had more than 20 years’ experience, followed by 9.1% with up to 5 years’ experience. The table also shows that the vast majority worked at a cantonal hospital (72.7%).
Table 1 Characteristics of oncologists treating patients with metastatic castration-sensitive prostate cancer (n = 20) in SAKK 95/16.
| No BTAs administered (n = 9) |
BTAs administered
(n = 8) |
BTAs sometimes administered (n = 3) |
Overall
(n = 20) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Experience in years | ||||||||
| 0–5 years | 1 | (12.5) | 0 | (0.0) | 1 | (33.3) | 2 | (10.5) |
| 5–10 years | 0 | (0.0) | 3 | (37.5) | 0 | (0.0) | 3 | (15.8) |
| 10–20 years | 5 | (62.5) | 4 | (50.0) | 1 | (33.3) | 10 | (52.6) |
| >20 years | 2 | (25.0) | 1 | (12.5) | 1 | (33.3) | 4 | (21.1) |
| Expertise | ||||||||
| Resident | 1 | (11.1) | 1 | (12.5) | 0 | (0.0) | 2 | (10.0) |
| Consultant | 1 | (11.1) | 3 | (37.5) | 1 | (33.3) | 5 | (25.0) |
| Senior consultant | 2 | (22.2) | 4 | (50.0) | 1 | (33.3) | 7 | (35.0) |
| Department head | 1 | (11.1) | 0 | (0.0) | 1 | (33.3) | 2 | (10.0) |
| Practitioner | 3 | (33.3) | 0 | (0.0) | 0 | (0.0) | 3 | (15.0) |
| Not answered | 1 | (11.1) | 0 | (0.0) | 0 | (0.0) | 1 | (5.0) |
| Number of patients with bone metastases | ||||||||
| >10 | 0 | (0.0) | 1 | (12.5) | 0 | (0.0) | 1 | (5.0) |
| 10–25 | 2 | (22.2) | 1 | (12.5) | 1 | (33.3) | 4 | (20.0) |
| 26–50 | 3 | (33.3) | 4 | (50.0) | 1 | (33.3) | 8 | (40.0) |
| >50 | 3 | (33.3) | 2 | (25.0) | 1 | (33.3) | 6 | (30.0) |
| Not answered | 1 | (11.1) | 0 | (0.0) | 0 | (0.0) | 1 | (5.0) |
| Number of pat. with newly diagnosed bone metastases | ||||||||
| >10 | 0 | (0.0) | 2 | (25.0) | 0 | (0.0) | 2 | (10.0) |
| 10-25 | 6 | (66.1) | 3 | (37.5) | 2 | (66.7) | 11 | (55.0) |
| 26-50 | 2 | (22.2) | 3 | (37.5) | 0 | (0.0) | 5 | (25.0) |
| >50 | 0 | (0.0) | 0 | (0.0) | 1 | (33.3) | 1 | (5.0) |
| Not answered | 1 | (11.1) | 0 | (0.0) | 0 | (0.0) | 1 | (5.0) |
| Place of work | ||||||||
| Cantonal hospital | 5 | (55.6) | 6 | (75.0) | 2 | (66.7) | 13 | (65.0) |
| Private clinic | 1 | (11.1) | 1 | (12.5) | 0 | (0.0) | 2 | (10.0) |
| Private practice | 2 | (22.2) | 0 | (0.0) | 0 | (0.0) | 2 | (10.0) |
| Regional hospital | 0 | (0.0) | 1 | (12.5) | 1 | (33.3) | 2 | (10.0) |
| University hospital | 0 | (0.0) | 1 | (12.5) | 0 | (0.0) | 1 | (5.0) |
The table shows descriptive statistics for physicians who reported treating metastatic castration-sensitive prostate (mCSPC) cancer patients. The first group contains the nine physicians who did not treat their mCSPC patients with bone-targeted agents (BTAs). The second group contains the eight physicians who treated all their mCSPC cancer patients with BTAs. The third group shows the three physicians who treated some of their mCSPC patients with BTAs.
Table 2 shows the demographic characteristics of the sample of mCSPC patients covered by the survey. Of the 36 patients physicians reported on, 19 (52.8%) were treated with BTAs. Their median age of those who received BTAs was 73 years (25th percentile 80 years and 75th percentile 68 years) and most (79%) were retired. More than four fifths (84.2%) had three or more bone metastases. The most common sites of these bone metastases were vertebrae (79.0%), the hip/pelvis (63.2%) and/or ribs (42.1%). The most frequently reported co-morbidities were hypertension (47.4%), diabetes mellitus (26.3%), chronic obstructive pulmonary disease (8.6%), renal impairment (8.6%) and coronary heart disease (8.1%).
Table 2 Characteristics of metastatic hormone-sensitive prostate cancer patients in study SAKK95/16 (n = 36).
| No BTAs (n = 17) | BTAs (n = 19) | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Age (mean and SD) | 76.8 | 7.9 | 72.5 | 8.3 |
| Education | ||||
| Compulsory schooling | 6 | (35.3) | 4 | (21.1) |
| High school degree | 2 | (11.8) | 4 | (21.1) |
| University degree | 2 | (11.8) | 3 | (15.8) |
| Vocational school | 6 | (35.3) | 7 | (36.1) |
| Unknown | 1 | (5.8) | 1 | (5.9) |
| Employment status | ||||
| Retired | 16 | (94.1) | 15 | (79.0) |
| Working part-time | 1 | (5.9) | 1 | (5.2) |
| Working full time | 0 | (0.0) | 3 | (15.8) |
| Smoking status | ||||
| Never smoker | 8 | (47.0) | 12 | (63.1) |
| Ex-smoker | 7 | (41.2) | 5 | (26.3) |
| Current smoker | 1 | (5.9) | 1 | (5.3) |
| Unknown | 1 | (5.9) | 1 | (5.3) |
| Location of bone metastases | ||||
| Arm | 3 | (17.7) | 5 | (26.3) |
| Hip/pelvis | 12 | (70.6) | 12 | (63.2) |
| Leg | 5 | (29.4) | 5 | (26.3) |
| Ribs | 8 | (47.1) | 8 | (42.1) |
| Skull | 3 | (17.7) | 3 | (15.8) |
| Vertebrae | 12 | (70.6) | 15 | (79.0) |
| Current number of bone metastases | ||||
| Three or fewer | 1 | (5.9) | 3 | (15.8) |
| More than three | 16 | (94.1) | 16 | (84.2) |
| Treatments received | ||||
| Chemotherapy | 9 | (52.9) | 8 | (42.1) |
| Hormone therapy | 16 | (94.1) | 18 | (94.7) |
| Immunotherapy | 0 | (0.0) | 0 | (0.0) |
| Radiotherapy | 6 | (35.3) | 8 | (42.1) |
| Radioisotope therapy | 0 | (0.0) | 2 | (10.5) |
| Surgery | 5 | (29.4) | 6 | (31.6) |
| Targeted treatments | 0 | (0.0) | 2 | (10.5) |
| Supportive treatments | ||||
| Antidepressants | 0 | (0.0) | 4 | (21.1) |
| Antiemetics | 1 | (5.9) | 1 | (5.3) |
| Corticosteroids | 3 | (17.7) | 6 | (31.6) |
| Nonopioid analgesics | 3 | (17.7) | 8 | (42.1) |
| Opioid analgesics | 1 | (5.9) | 4 | (21.1) |
| BTA = bone-targeted agent; SD = standard deviation | ||||
Of the 19 mCSPC patients receiving BTAs, the vast majority (84.2%) received denosumab and few (15.8%) zoledronic acid. At the time of the survey, more than four fifths of the mCSPC patients (84.2%) were still receiving BTAs. In the case of denosumab, almost half (43.8%) of the 16 patients received it every 3–4 weeks, 31.3% received it every 3–4 weeks for the first two years and then once every 12 weeks, 12.5% received it every 24 weeks, 6.2% every 3–4 weeks unless there was a substantial deterioration in the patient’s performance status, and 6.2% every 3–4 weeks for the first year and then once every 12 weeks. Of the three mCSPC patients who received zoledronic acid, one received it continuously every 3–4 weeks, one every 24 weeks, and for the third there was no information about the administration available. Bone complications were reported for four patients treated with denosumab (25%); two had bone radiation (12.5%), one had a pathological fracture (6.3%), and one had another bone complication not specified further (5.3%). No complications were reported for patients treated with zoledronic acid. No BTA treatment was interrupted because of ONJ.
The most frequently mentioned reasons for administering BTAs to mCSPC patients were bone pain, high risk of bone complications, number of bone metastases, and the patient’s prior history of bone complications (table 3).
Table 3 Reasons for administering bone-targeted agents to metastatic hormone-sensitive prostate cancer patients (n = 19).
| Reason | Most important | Second most important | Third most important | Not mentioned | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| 1 | Bone pain | 7 | (36.8) | 1 | (5.3) | 3 | (15.8) | 8 | (42.1) |
| 2 | High risk of bone complications | 4 | (21.1) | 5 | (26.3) | 2 | (10.5) | 8 | (42.1) |
| 3 | Number of bone metastases | 3 | (15.8) | 3 | (15.8) | 1 | (5.2) | 12 | (63.2) |
| 4 | Prior history of bone complications | 2 | (10.5) | 0 | (0.0) | 0 | (0.0) | 17 | (89.5) |
| 5 | Long life expectancy | 1 | (5.3) | 2 | (10.5) | 7 | (36.8) | 9 | (43.4) |
| 6 | Good performance status | 1 | (5.3) | 3 | (15.8) | 1 | (5.3) | 14 | (73.7) |
| 7 | Location of bone metastases | 0 | (0.0) | 3 | (15.8) | 3 | (15.8) | 13 | (68.4) |
| 8 | Patient’s request | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 19 | (100.0) |
NACR estimated that there were 6120 new prostate cancer cases in 2016. This number was based on extrapolated data from nine Swiss population-based cancer registries covering 64.9% of the Swiss population. In the estimation, almost four fifths (n = 4788, 78.3%) were in stages I–III, 15.5% were in stage IV (n = 945) and for 6.3% (n = 386) no information about the stage was available.
Multiplying the number of stage I–III prostate cancer patients with the probability of developing bone metastases and adding the stage IV patients led to an estimate of 1076 new mCSPC patients (17.58%) for the year 2016.
Combining the information from the survey about the frequency of BTA administration with estimates of the Swiss mCSPC patient population, we estimated that 568 new mCSPC patients (52.78% of 1076) may have been treated with BTAs in 2016 (see fig. 1). Furthermore, the estimates about the BTA of choice based on our study suggest that 478 patients may have received denosumab and 90 patients zoledronic acid.

Figure 1 Estimation of the Swiss metastatic hormone-sensitive prostate cancer (mCSPC) population that received bone-targeted agents (BTAs) in 2016. PC = prostate cancer
Table 4 shows an estimated cost of CHF 7,843,301 for administering denosumab and CHF 535,840 for zoledronic acid over an estimated time to PSA progression of 33.2 months for 568 mCSPC patients (84.2% denosumab and 15.8% zoledronic acid), given the observed administration intervals from the oncologist survey. The resulting total cost estimate of this practice in the base case is CHF 8,379,141 over the median time of PSA progression of mCSPC patients or over 1 year, assuming constant incidence over time. Because of the more frequent prescription, higher price and higher probability of treatment-related consequences, more than 93% of this total cost can be attributed to denosumab. Administering denosumab every 4 weeks over 33.2 months costs CHF 11,006 per patient (CHF 3967 per year). In contrast, administering zoledronic acid at the same interval and over the same time costs CHF 4253 (CHF 1533 per year). Costs of ONJs are marginal. For both denosumab and zoledronic acid, the most expensive components are the cost of administering BTAs and the cost of the drug. For both drugs, these costs account for more than 96% of their total costs.
Table 4 Estimated total cost of BTA use for a cohort of 568 mCSPC patients.
| Denosumab (n = 478)* | Zoledronic acid (n = 90)* | |||
|---|---|---|---|---|
| (%)† | CHF | (%)† | CHF | |
| Cost of continuous BTA administration‡ | ||||
| Every 4 weeks (36.1 administrations) | (50.0%) | (33.33%) | ||
| – Drug cost | CHF 4,085,811 | CHF 181,819 | ||
| – Administration cost | CHF 1,130,574 | CHF 196,143 | ||
| – Sum of drug and administration costs | CHF 5,216,385 | CHF 377,962 | ||
| Every 4 weeks for the first 2 years than once every 12 weeks (29.4 administrations) | (31.25%) | (0.0%) | ||
| – Drug cost | CHF 2,078,521 | CHF 0 | ||
| – Administration cost | CHF 575,142 | CHF 0 | ||
| – Sum of drug and administration costs | CHF 2,653,663 | CHF 0 | ||
| Every 4 weeks for the first year than once every 12 weeks (20.7 administrations) | (6.25%) | (0.0%) | ||
| – Drug cost | CHF 292,973 | CHF 0 | ||
| – Administration cost | CHF 81,068 | CHF 0 | ||
| – Sum of drug and administration costs | CHF 374,041 | CHF 0 | ||
| Every 12 weeks (12 administrations) | (0.0%) | (33.33%) | ||
| – Drug cost | CHF 0 | CHF 60,606 | ||
| – Administration cost | CHF 0 | CHF 65,381 | ||
| – Sum of drug and administration costs | CHF 0 | CHF 125,987 | ||
| Every 24 weeks (6 administrations) | (12.5%) | (33.33%) | ||
| – Drug cost | CHF 170,242 | CHF 30,303 | ||
| – Administration cost | CHF 47,107 | CHF 32,690 | ||
| – Sum of drug and administration costs | CHF 217,349 | CHF 62,994 | ||
| Total drug and administration costs | CHF 8’461’439 | CHF 566’943 | ||
| Cost of ONJ§ | CHF 167,084 | CHF 22,542 | ||
| TOTAL COSTS | CHF 8,628,524 | CHF 589,485 | ||
BTA = bone-targeted agent; mCSPC = metastatic castration-sensitive prostate cancer; ONJ = osteonecrosis of the jaw The table shows the total cost of administering BTAs to 568 mCSPC patients over 33.2 months (144.26 weeks). * Indicates the number of mCSPC patients receiving the respective BTA, estimated from the oncologist survey. † Indicates the proportion of patients in the specific administration interval, according to the oncologist survey. ‡ Calculated for median time to PSA progression of 33.2 months (144.26 weeks) using administration costs of CHF 132.29 for denosumab and CHF 183.77 for zoledronic acid and the prices of CHF 478.05 for denosumab (Xgeva®) and CHF 170.35 for zoledronic acid. § Costs for treatment-related complications were based on the probability of ONJ and its costs. Note that while the probabilities differ between denosumab (8.2%) and zoledronic acid (5.9%), they are independent of the administration interval.
The univariate sensitivity analyses in table 5 show the impact on the results of varying uncertain parameters. The largest impacts were seen when we varied the time to PSA progression, the percentage of mCSPC patients receiving BTAs and the number of mCSPC patients. Compared with the base case, total costs would increase by more than 50% (CHF 12,599,460 vs CHF 8,379,141) when the time to PSA progression and the duration of the BTA treatment of mCSPC patients was assumed to be 54.3 months instead of 33.2 months. The assumption that the percentage of mCSPC patients is 30% higher than the base case (22.86% instead of 17.58%) increases total cost by CHF 2,516,602 to CHF 10’895’743. The table also shows that while variations in the cost and proportional use of denosumab were also influential, the probability and cost of ONJ had little impact.
Table 5 Sensitivity analysis for total costs, performed by varying certain model parameters.
| Base case value | Min | Max | Result min compared with base case | Result max compared with base case | |
|---|---|---|---|---|---|
| Time to PSA progression in weeks | 144.26 | 52.58 | 235.95 | −CHF 4,219,858 | CHF 4,220,319 |
| Percentage of mCSPC patients | 17.58% | 12.31% | 22.86% | −CHF 2,511,836 | CHF 2,516,602 |
| Percentage of BTA administration | 52.78% | 36.94% | 68.61% | −CHF 2,514,695 | CHF 2,513,107 |
| Cost of denosumab | CHF 478.05 | CHF 334.64 | CHF 621.47 | −CHF 1,807,263 | CHF 1,807,389 |
| Share of denosumab | 84.21% | 58.95% | 100.00% | −CHF 1,495,503 | CHF 934,837 |
| Cost of denosumab administration | CHF 132.28 | CHF 92.60 | CHF 171.96 | −CHF 500,050 | CHF 500,050 |
| Cost of ONJ | CHF 4299.05 | CHF 2682.69 | CHF 7623.88 | −CHF 64,808 | CHF 133,308 |
| Cost of zoledronic acid administration | CHF 183.77 | CHF 128.64 | CHF 238.90 | −CHF 80,230 | CHF 80,230 |
| Cost of zoledronic acid | CHF 170.35 | CHF 129.45 | CHF 212.25 | −CHF 59,522 | CHF 60,977 |
| Percentage of ONJ with denosumab | 8.20% | 5.74% | 10.66% | −CHF 45,564 | CHF 45,564 |
| Percentage of ONJ with zoledronic acid | 5.90% | 4.13% | 7.67% | −CHF 6147 | CHF 6147 |
BTA = bone-targeted agent; mCSPC = metastatic castration-sensitive prostate cancer; max = maximum; min = minimum; ONJ = osteonecrosis of the jaw; PSA = prostate specific antigen Note: the table shows the difference in total costs of administering BTAs compared to the base case of CHF 8,379,141.
This study provides insights into the economic consequences of administering BTAs to mCSPC patients. Using the example of Switzerland, we found that BTA use in mCSPC patients is frequent, as more than half of the mCSPC patients in our survey received BTAs from their treating oncologist. Importantly, international guidelines from ESMO and ASCO do not recommend the use of BTAs in this subgroup of prostate cancer patients since zoledronic acid has been shown to have no benefit and added toxicity in two randomised trials and denosumab has never been evaluated in this setting [12, 13]. Despite this fact, the marketing approval for denosumab issued by Swissmedic does not exclude the treatment of mCSPC in conjunction with standard antineoplastic treatment [14]. A letter of Swiss opinion leaders was sent to Swissmedic in 2012, highlighting the lack of clinical data to support the approval of denosumab for prevention of skeletal-related events in mCSPC, but this letter did not lead to a change in the approval text [29].
This non-recommended treatment leads to estimated costs to the Swiss healthcare system of more than CHF 8.3 million over the time to PSA progression of 33.2 months in mCSPC patients or over 1 year. Almost all of these costs can be attributed to drug costs and administration costs. The health economic burden of this practice at the European level may also be quite substantial, as the administration of BTAs to mCSPC patients is also approved by the EMA [15]. Even more importantly, these drugs can produce relevant side effects, such as hypersensitivity reactions, musculoskeletal events and symptomatic hypocalcaemia [30]. Furthermore, ONJ is of substantial concern as it often leads to painful oral surgical procedures and relevant impairment of quality of life. Also, it is a side effect whose frequency increases with cumulative doses [31, 32]. In addition, administering BTAs in the mCSPC setting, before PSA progression under castration therapy and thus before a treatment phase where the benefit is really shown, makes the decision for how long to continue with the treatment at the time-point of castration-resistance more difficult. Note that in this study, we used a median time to PSA progression of 33.2 months, according to a trial including only patients with high risk mCSPC [22]. A more recent study allowing patients to be included irrespective of their risk situation found a much better survival outcome, with more than 60% of the patients being PSA progression-free at 3 years [33]. Although median progression-free survival from this trial has not been reported yet, this suggests that the true economic consequences may be substantially higher than in our estimation. The impact on the budget estimates – more than 8 million every year – is significant. Although only 0.2% of the cancer budget is being essentially wasted by this non-evidence-based, contraindicated and potentially harmful pharmacological intervention, this accounts for only one subtype of one cancer diagnosis.
With the nowadays growing armamentarium of systemic treatment options for mCSPC, patients, even though in a metastatic setting, have good long-term outcomes lasting for many years. In the absence of evidence supporting BTA use for mCSPC patients and knowing the aforementioned risks, side effects and substantial costs of this non-recommended practice in times of constrained resources, the licensing of BTAs in Switzerland and Europe should be revisited.
To our knowledge, this is the first study that investigated the patterns of care and economic consequences of administering BTAs to mCSPC patients in a real-world setting. We note several strengths and limitations of the study, which influence the interpretation of the findings. The strength of this study lies in the physician sample of the oncologist survey, which featured oncologists from several hospitals in the French-, German- and Italian-speaking parts of Switzerland, enhancing the generalisability of the findings.
The present study also has several limitations. First, the small sample of mCSPC patients included may not be representative and therefore limit the generalisability. Second, prescription patterns were reported by the physician and not reviewed. Third, the survey did not collect information about the drug names but rather the compounds. For denosumab it was assumed that Xgeva® was administered, and not Prolia®, as the administration intervals of the latter do not fit those reported in the survey. Fourth, we estimated the mCSPC patient population through prostate cancer stages to be around 17.6% of all prostate cancer patients. Note that this estimate is in line with US studies, which suggest that around 14.1% of prostate cancer patients have a radical prostatectomy and then develop metastases afterwards [34, 35]. Finally, while oncologists were asked about patient-related reasons for engaging in the practice of prescribing BTAs to mCSPC patients, it is unclear whether oncologists were aware of the current international guidelines. In future studies it would be important to analyse whether BTA prescription decisions could be explained by physician's intrinsic determinants (such as knowledge, attitudes and individual characteristics), or external factors (at individual levels, e.g., patient requests, time constraints and financial incentives, and also at institutional levels, e.g., organisational culture such as shared values and behaviours among employees).
BTAs are not recommended for mCSPC patients owing to the lack of clinical data supporting the benefit of these drugs for this population. This study found that, irrespective of this, they are frequently administered by oncologists in Switzerland. This practice leads to substantial healthcare costs and also increases the risk of side effects due to added toxicity.
We thank NACR for the data on the total number of annual PC patients and Rosaria Tino (Kantonsspital Graubünden) and Andrea Furrer (SAKK) for information about the costs of administering denosumab and zoledronic acid.
This study was sponsored by Amgen. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Roger von Moos has participated in advisory boards with Amgen, GlaxoSmithKline, Novartis and Roche. Beat Thürlimann holds stock of Novartis and Roche and received consultation fees from Amgen and Roche. Richard Cathomas has participated in advisory boards for Amgen, Astellas, AstraZeneca, Bayer, BMS, Janssen, MSD, Novartis, Pfizer and Roche, and has received speaker honoraria from Astellas, BMS, Debiopharm and Janssen. Silke Gillessen received honoraria from Janssen Cilag and has a consulting or advisory role for Astellas, Amgen, Roche, Pfizer, AAA International, Janssen, Innocrin, Sanofi, Bayer, Orion Pharma, Clovis Oncology, Menarini Silicon Biosystems, Tolero Pharmaceuticals and MSD. Ursina Zürrer-Härdi received travel support from Gilead, Lilly, Pfizer, Bayer, Celgene and MSD. Sandro Anchisi has participated in advisory boards for Janssen-Lilly, Lilly and Novartis. Matthias Schwenkglenks has received research funding from Amgen, MSD and Novartis, has received honoraria from Pfizer, and has participated in advisory boards for Amgen and BMS. Michael Mark received advisory fees from BMD, MSD, AstraZeneca, Roche, Takeda and institutional research grants from AstraZeneca and Amgen. The other authors report that they have no conflict of interest in relation to this article.
1 So A , Chin J , Fleshner N , Saad F . Management of skeletal-related events in patients with advanced prostate cancer and bone metastases: Incorporating new agents into clinical practice. Can Urol Assoc J. 2012;6(6):465–70. doi:.https://doi.org/10.5489/cuaj.117
2 Weinfurt KP , Li Y , Castel LD , Saad F , Timbie JW , Glendenning GA , et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16(4):579–84. doi:.https://doi.org/10.1093/annonc/mdi122
3 Wang Z , Qiao D , Lu Y , Curtis D , Wen X , Yao Y , et al. Systematic literature review and network meta-analysis comparing bone-targeted agents for the prevention of skeletal-related events in cancer patients with bone metastasis. Oncologist. 2015;20(4):440–9. doi:.https://doi.org/10.1634/theoncologist.2014-0328
4 Yee AJ , Raje NS . Denosumab, a RANK ligand inhibitor, for the management of bone loss in cancer patients. Clin Interv Aging. 2012;7:331–8.
5 Smith MR , Saad F , Egerdie B , Szwedowski M , Tammela TL , Ke C , et al. Effects of denosumab on bone mineral density in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2009;182(6):2670–6. doi:.https://doi.org/10.1016/j.juro.2009.08.048
6 Body JJ . Denosumab for the management of bone disease in patients with solid tumors. Expert Rev Anticancer Ther. 2012;12(3):307–22. doi:.https://doi.org/10.1586/era.11.204
7 Fizazi K , Carducci M , Smith M , Damião R , Brown J , Karsh L , et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. doi:.https://doi.org/10.1016/S0140-6736(10)62344-6
8 Parker C , Castro E , Fizazi K , Heidenreich A , Ost P , Procopio G , et al.; ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–34. doi:.https://doi.org/10.1016/j.annonc.2020.06.011
9 Hegemann M , Bedke J , Stenzl A , Todenhöfer T . Denosumab treatment in the management of patients with advanced prostate cancer: clinical evidence and experience. Ther Adv Urol. 2017;9(3-4):81–8. doi:.https://doi.org/10.1177/1756287216686018
10 James ND , Sydes MR , Clarke NW , Mason MD , Dearnaley DP , Spears MR , et al.; STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–77. doi:.https://doi.org/10.1016/S0140-6736(15)01037-5
11 Smith MR , Halabi S , Ryan CJ , Hussain A , Vogelzang N , Stadler W , et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143–50. doi:.https://doi.org/10.1200/JCO.2013.51.6500
12 Saylor PJ , Rumble RB , Tagawa S , Eastham JA , Finelli A , Reddy PS , et al. Bone Health and Bone-Targeted Therapies for Prostate Cancer: ASCO Endorsement of a Cancer Care Ontario Guideline. J Clin Oncol. 2020;38(15):1736–43. doi:.https://doi.org/10.1200/JCO.19.03148
13 Horwich A , Hugosson J , de Reijke T , Wiegel T , Fizazi K , Kataja V , et al.; Panel Members; European Society for Medical Oncology. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24(5):1141–62. doi:.https://doi.org/10.1093/annonc/mds624
14Swissmedic Journal. 12/2011. Available at: https://www.swissmedic.ch/dam/swissmedic/de/dokumente/stab/journal/swissmedic_journal122011.pdf.download.pdf/swissmedic_journal122011.pdf [cited 2020 June 15]
15European Medicines Agency. Xgeva (denosumab) summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/xgeva-epar-product-information_en.pdf [cited 2020 September 2]
16Swissmedic Journal. 2/2012. Available at: https://www.swissmedic.ch/dam/swissmedic/de/dokumente/stab/journal/swissmedic_journal022012.pdf.download.pdf/swissmedic_journal022012.pdf [cited 2020 June 15]
17European Medicines Agency. Zometa (zoledronic acid) summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/zometa-epar-product-information_en.pdf [cited 2020 September 2].
18 Hofmarcher T , Lindgren P , Wilking N , Jönsson B . The cost of cancer in Europe 2018. Eur J Cancer. 2020;129:41–9. doi:.https://doi.org/10.1016/j.ejca.2020.01.011
19 Mark M , Thürlimann B , Ribi K , Schär C , Dietrich D , Cathomas R , et al. Patterns of care for patients with metastatic bone disease in solid tumors: A cross-sectional study from Switzerland (SAKK 95/16). J Bone Oncol. 2020;21:100273. doi:.https://doi.org/10.1016/j.jbo.2019.100273
20 Pound CR , Partin AW , Eisenberger MA , Chan DW , Pearson JD , Walsh PC . Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7. doi:.https://doi.org/10.1001/jama.281.17.1591
21 Bubendorf L , Schöpfer A , Wagner U , Sauter G , Moch H , Willi N , et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–83. doi:.https://doi.org/10.1053/hp.2000.6698
22 Fizazi K , Tran N , Fein L , Matsubara N , Rodriguez-Antolin A , Alekseev BY , et al.; LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–60. doi:.https://doi.org/10.1056/NEJMoa1704174
23Tarmed Suisse Online browser. Tarmed list 2020. Available at: https://www.tarmed-browser.ch [cited 2020 July 14]
24Swiss Federal Office of Public Health. Specialty List 2019; https://www.spezialitätenliste.ch/ [cited 2020 June 15]
25 Fizazi K , Brown JE , Carducci M , Shore ND , Sieber P , Kueppers F , et al. Denosumab in patients with metastatic prostate cancer previously treated with denosumab or zoledronic acid: 2-year open-label extension phase results from the pivotal phase 3 study. Ann Oncol. 2012;23:ix309. doi:.https://doi.org/10.1016/S0923-7534(20)33497-9
26 Najm MS , Solomon DH , Woo SB , Treister NS . Resource utilization in cancer patients with bisphosphonate-associated osteonecrosis of the jaw. Oral Dis. 2014;20(1):94–9. doi:.https://doi.org/10.1111/odi.12080
27US Bureau Of Labor Statistics. CPI Inflation calculator. Available at: https://www.bls.gov/data/inflation_calculator.htm [cited 2020 July 10]
28Organisation for Economic Co-operation and Development. Purchasing power parities (PPP). Available at: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [cited 2020 September 14]
29Rothermund C, Gillessen S, Cerny T. Zulassung Denosumab (XGEVA). Received by Schnetzer J, 2012 June 17.
30 Yee AJ , Raje NS . Denosumab, a RANK ligand inhibitor, for the management of bone loss in cancer patients. Clin Interv Aging. 2012;7:331–8. doi:.https://doi.org/10.2147/CIA.S14566
31 Miksad RA , Lai KC , Dodson TB , Woo SB , Treister NS , Akinyemi O , et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist. 2011;16(1):121–32. doi:.https://doi.org/10.1634/theoncologist.2010-0183
32 Nicolatou-Galitis O , Schiødt M , Mendes RA , Ripamonti C , Hope S , Drudge-Coates L , et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117–35. doi:.https://doi.org/10.1016/j.oooo.2018.09.008
33 Davis ID , Martin AJ , Stockler MR , Begbie S , Chi KN , Chowdhury S , et al.; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31. doi:.https://doi.org/10.1056/NEJMoa1903835
34 Antonarakis ES , Feng Z , Trock BJ , Humphreys EB , Carducci MA , Partin AW , et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109(1):32–9. doi:.https://doi.org/10.1111/j.1464-410X.2011.10422.x
35 Moul JW , Wu H , Sun L , McLeod DG , Amling C , Lance R , et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: an overview of the Department of Defense Center for Prostate Disease Research national database. Surgery. 2002;132(2):213–9. doi:.https://doi.org/10.1067/msy.2002.125315
Shared first authorship
This study was sponsored by Amgen. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Roger von Moos has participated in advisory boards with Amgen, GlaxoSmithKline, Novartis and Roche. Beat Thürlimann holds stock of Novartis and Roche and received consultation fees from Amgen and Roche. Richard Cathomas has participated in advisory boards for Amgen, Astellas, AstraZeneca, Bayer, BMS, Janssen, MSD, Novartis, Pfizer and Roche, and has received speaker honoraria from Astellas, BMS, Debiopharm and Janssen. Silke Gillessen received honoraria from Janssen Cilag and has a consulting or advisory role for Astellas, Amgen, Roche, Pfizer, AAA International, Janssen, Innocrin, Sanofi, Bayer, Orion Pharma, Clovis Oncology, Menarini Silicon Biosystems, Tolero Pharmaceuticals and MSD. Ursina Zürrer-Härdi received travel support from Gilead, Lilly, Pfizer, Bayer, Celgene and MSD. Sandro Anchisi has participated in advisory boards for Janssen-Lilly, Lilly and Novartis. Matthias Schwenkglenks has received research funding from Amgen, MSD and Novartis, has received honoraria from Pfizer, and has participated in advisory boards for Amgen and BMS. Michael Mark received advisory fees from BMD, MSD, AstraZeneca, Roche, Takeda and institutional research grants from AstraZeneca and Amgen. The other authors report that they have no conflict of interest in relation to this article.