Multimodal treatment strategies for colorectal liver metastases
DOI: https://doi.org/10.4414/smw.2021.20390
Dominique Lisa
Birrera, Christoph
Tschuora, Cäcilia S.
Reinerc, Ralph
Fritschb, Thomas
Pfammatterc, Helena Garcia
Schülerd, Matea
Pavicd, Michelle
De Oliveiraa, Henrik
Petrowskya, Philip
Dutkowskia, Christian E.
Oberkoflera, Pierre-Alain
Claviena
a Department of Surgery, Swiss Hepato-pancreato-biliary and Transplantation Centre, University Hospital Zurich, Switzerland
b Department of Medical Oncology and Haematology, University Hospital Zurich, Switzerland
c Institut für Diagnostische und Interventionelle Radiologie, University Hospital Zurich, Switzerland
d Department of Radiation Oncology, University Hospital Zurich Switzerland
Summary
Colorectal cancer is the third most common cancer worldwide. Half of CRC patients develop liver metastases during the course of the disease, with a 5-year survival rate close to zero in the absence of therapy. Surgical resection remains the only possible curative option, and current guidelines recommend adjuvant chemotherapy, resulting in a 5-year survival rate exceeding 50%. Neoadjuvant systemic therapy is not indicated in cases with simple resection but should be offered to all patients with extensive bilobar disease. Personalised systemic treatment is essential to convert upfront non-resectable lesions to resectable ones. Anatomical resections, non-anatomical resections and two-stage hepatectomies can be performed though open or minimally invasive (laparoscopic or robotic) surgery.
The extent of a hepatic resection is limited by the risk of postoperative liver failure due to a too small liver remnant, inflow or outflow obstruction or insufficient biliary drainage. About 75% of patients are diagnosed with non-resectable liver metastases not amenable to a standard upfront resection. In recent years, effective therapeutic approaches have revolutionised liver surgery and new strategies have enabled the conversion of primarily non-resectable metastatic disease for resection. These strategies include oncological and surgical therapies, as well as combinations of the two. From an oncological perspective, colorectal liver metastases may be treated by systemic chemotherapy or immunotherapy, or selective intra-hepatic arterial infusion chemotherapy, depending on the extent of the disease and the mutational status. In surgery, we often apply two-stage strategies using portal vein occlusion, such as portal vein embolisation or ligation, or complex two-stage hepatectomy such as associating liver partition and portal vein ligation for staged hepatectomy. Other additive tools to reach curative resection are tumour ablations (electroporation, microwave or radiofrequency). The role of stereotactic radiation of liver metastases is not yet well defined. Modern radiation techniques, including image guidance, breath hold and gating, were only introduced for a larger patient population in recent years. Therefore, prospective studies with larger patient cohorts are still pending.
Over the last decade, liver transplantation has gained increasing attention in selective cases of non-resectable colorectal liver metastases, with promising cohort studies, but definitive recommendations must await the results of ongoing randomised controlled trials.
The optimal treatment of patients with colorectal liver metastases requires the timely association of various strategies, and all cases must be discussed at multidisciplinary team conferences. While colorectal liver metastases was a uniformly lethal condition a few decades ago, it has become amenable to curative therapies, with excellent quality of life in many scenarios. This review reports on up-to-date treatment modalities and their combinations in the treatment algorithm of colorectal liver metastases.
Introduction
In Switzerland, the age-standardised* incidence of new cases of colon cancer between the years 2012-2016 was 45.8 for men and 29.7 for women. Compared to their age group†, women with colon cancer are 9.7 times and men 15.9 times more likely to die, making it the third most common cause of cancer death in both sexes. (* The age-standardised incidence indicates the disease rate in a specific time period which would be expected in the considered population if its age structure agreed with that of the standard population. † Standardised mortality rate per 100,000 inhabitants, European standard) [1].
Worldwide, an estimated 1.4 million new colorectal cancer (CRC) cases are diagnosed every year, with 700,000 cancer deaths [2]. In 30–50% of patients with CRC the liver is the predominant site for metastases, due to its immediate drainage from the gastrointestinal tract [3–6]. Half of CRC patients are diagnosed with synchronous liver metastases [4, 7]. Without treatment the prognosis is dismal, with a 5-year survival rate of close to zero in historical data [8, 9]. Surgical resection of the metastatic tumours is the only curative treatment with a 5-year survival rate currently exceeding 50% in most series [10, 11]. Unfortunately, only 25% of patients with colorectal liver metastases (CRLM) qualify for resection at initial presentation. An increasing number of patients may benefit from down-staging strategies and eventually from a curative resection, often followed by encouraging long-term outcomes. Combinations of systemic chemotherapy and immunotherapy lead to current response rates of over 50% for down-staging [12].
The combination of multimodal surgical and non-surgical therapies offers the best chance for cure in patients with CRLM. In this review, we present up-to-date, personalised treatment options for the management of CRLM as practiced in our hepato-pancreato-biliary (HPB) centre in Zurich (fig. 1)
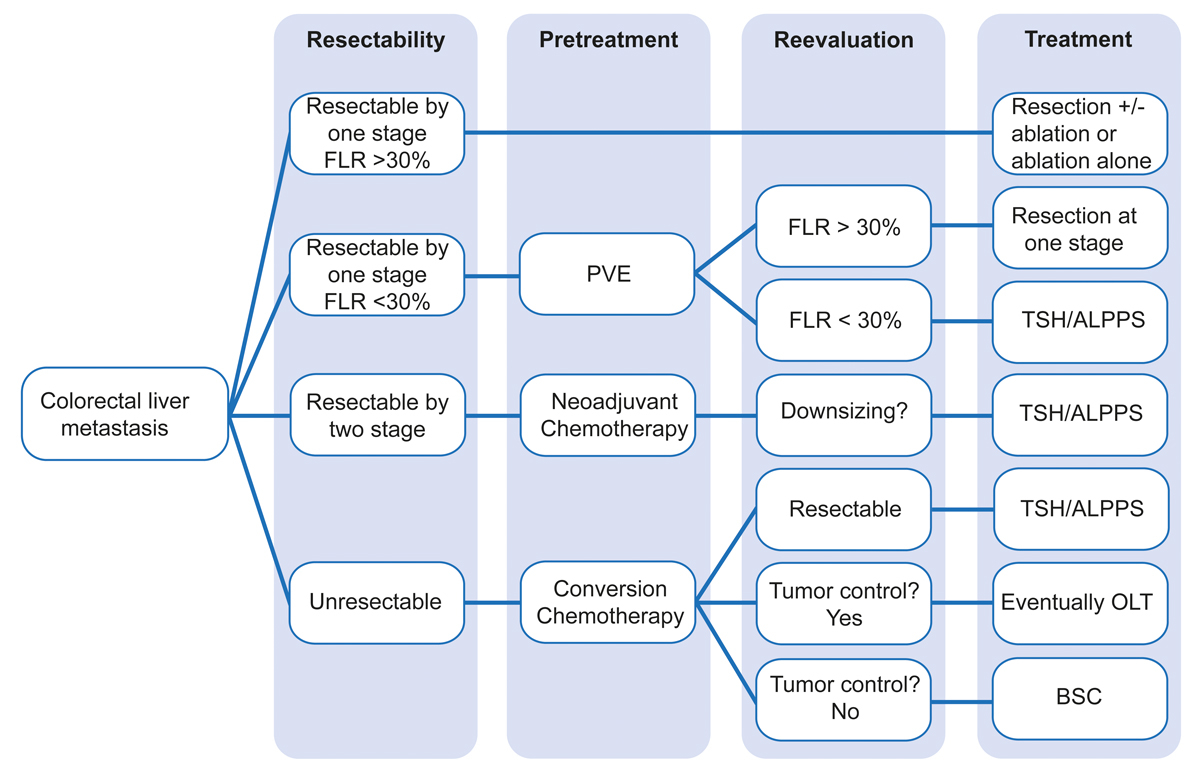
Figure 1 Flow chart of the treatment algorithm for colorectal metastases at the Swiss HPB Centre, Zurich, Switzerland.
ALPPS = associating liver partition and portal vein ligation for staged hepatectomy; TSH = classical two-stage hepatectomy; BSC = best supportive care; FLR = future liver remnant; OLT = orthotopic liver transplantation; PVE = portal vein embolisation.
Diagnostic work-up of colorectal liver metastases
Medical history with physical examination, laboratory tests including carcinoembryonic antigen, and abdomen ultrasound or a CT scan are usually available at the time of referral to the surgeon. In our hepato-pancreato-biliary centre in Zurich, Switzerland, we favour MRI of the liver with hepatobiliary contrast media (Primovist, Bayer Healthcare) to better detect liver metastases and surgically relevant liver structures [13]. MRI with hepatobiliary contrast media shows the highest sensitivity for liver metastases compared to contrast-enhanced CT or PET-CT, especially for lesions <1 cm and in patients who have undergone chemotherapy [13, 14].
If the lesions appear resectable, PET-CT is performed to detect extrahepatic disease [15]. The CT scan is crucial for detecting pulmonary metastases. Lymph node metastases and malignant disease in other organs may also be identified more easily with PET-CT [15]. With the use of additional PET-CT in patients with resectable CRLM based on CT scans, surgical management is changed in 10% of patients and the rate of futile laparotomies can be reduced [15].
Assessment of liver function
Postoperative outcomes mainly depend on the size and quality of the future liver remnant (FLR).
A preoperative assessment of the liver for volume and function is important in order to predict the risk of post-hepatectomy liver failure. Assessing and quantifying liver function before surgery is still controversial because no single physiologic marker or score alone predicts postoperative liver failure.
There are three types of liver function tests: conventional liver function tests, scoring systems and quantitative tests [14]. The most widely used conventional liver function test in clinical practice is assessment of the laboratory values, including serum bilirubin, factor V and Quick test. The Child-Pugh score, which was designed to assess the risk of death in cirrhotic patients undergoing surgery for portal hypertension, remains one of the most popular scoring systems [16, 17]. This score applies only to patients with cirrhosis. Patients with Child-Pugh score A usually have a favourable course, while Child-Pugh score C represents a contraindication for surgery. Surgery in Child-Pugh score B cirrhotic patients must be discussed case by case.
Among the quantitative tests, the indocyanine green test is correlated with liver function and the regenerative capacity of the liver [18–20]. This is a simple, non-invasive test that can be performed at the bedside. Indocyanine green test is a water-soluble dye that is injected via a peripheral venous access. It binds to proteins, and after being taken up by hepatocytes, is excreted into the bile. The plasma disappearance rate and the retention rate at 15 minutes are measured by pulse spectrophotometry [21]. Plasma disappearance rate values between 18% and 24% and a retention rate at 15 minutes <15% may be indicators for normal liver capacity and low-risk liver resection. Nevertheless, since indocyanine green testing depends on the hepatocyte uptake as well as excretion via the bile, chronic or acute liver diseases may influence indocyanine green test results, limiting their predictive value [21–26].
The LiMAx test is another method for investigating liver function at the bedside. It is based on the assessment of 13C-methacetin metabolism by the liver-specific cytochrome P450 1A2 system. 13C-methacetin is administered intravenously and the ratio of 12CO2 to the metabolised 13CO2 is measured by mask breathing. The maximum liver function capacity is defined as the maximum value of the substrate conversion normalised to body weight. A normal liver function is defined as ≥315 μg/kg/h. Values between 140 and 315 μg/kg/h show limited hepatic impairment, while 0–140 μg/kg/h means significant hepatic injury. Preoperative performance in the LiMAx test can help to determine the correct timing of hepatectomy after previous chemotherapy, surgery or portal vein manipulation, preventing post-hepatectomy liver failure [27]. However, both the indocyanine green and LiMAx tests assess only global liver function, and not the function of the FLR specifically.
Exact FLR function can, however, be assessed with a hepatobiliary iminodiacetic acid scan. This is an imaging modality used to investigate metabolic diseases of the liver, as well as excretion dynamics of the gallbladder and bile ducts. A radioactive tracer is injected, taken up by the bile-producing cells and excreted into the biliary tree, which drains into the duodenum. Imaging is obtained by a γ camera and shows liver function for the whole liver as well as for regions or parts of interest, such as the FLR [28].
Quantitative tests evaluate the metabolism and the clearance of different substrates that are mostly or entirely cleared by the liver. It is important to note that quantitative tests provide more reliable information on the preoperative liver function than on the postoperative liver function because they address one of the liver’s true processes (i.e., galactose elimination or bile acid clearance). While in patients with normal liver function the suggested limits for safe resection range from 20 to 30% FLR volume, most guidelines indicate 30% [10, 29].
Perioperative systemic chemotherapy for colorectal liver metastases
Systemic chemotherapy is an integral part of the multimodal management of CRLM. Perioperative chemotherapy targets micro-metastatic disease in order to reduce the risk of recurrence following resection. In more advanced situations, preoperative chemotherapy aims to convert upfront non-resectable CRLM to resectable ones. Modern personalised combination chemotherapy for metastatic CRC has improved substantially, with current response rates of over 50%, up from approximately 10% with a single-agent treatment prior to 2000.
Upfront resectable CRLM with a favourable risk profile must undergo upfront resection followed by adjuvant chemotherapy, particularly when the patient has not received any previous adjuvant treatment [12]. In less favourable risk constellations, such as with synchronous metastases or a short interval between primary tumour resection and systemic tumour recurrence, perioperative treatment with 6 months of FOLFOX (leucovorin, fluorouracil, oxaliplatin) or CAPOX (capecitabine, oxaliplatin) split evenly before and after surgery, based on the pivotal EPOC trial, is a widely used option [30]. Median overall survival improved from 47 months in the surgery only group to 62 months when adjuvant chemotherapy followed the surgery [31].
In patients with technically non-resectable CRLM, chemotherapy aiming to render them resectable prior to surgery is essential. Conversion treatment aims for maximum tumour shrinkage and typically involves combinations of chemotherapy doublets or triplets with monoclonal antibodies (anti-endothelial growth factor receptor [anti-EGFR], anti-vascular endothelial growth factor [anti-VEGF]). Treatment is personalised, based on molecular profile and biological criteria, most prominently primary tumour sidedness [10]. Reported success rates of conversion treatment are as high as 30% [9, 10]. The optimal duration of conversion therapy has not been established; maximum tumour shrinkage in metastatic CRC is typically achieved after 12–16 weeks of chemotherapy. In these situations, accumulation of toxicity in the liver due to prolonged application of intensified chemotherapy protocols poses a significant risk. It is important to note that modern perioperative treatment, as an essential part of multimodality treatment of CRLM, must be personalised: guided by established principles but highly adaptable to individual constellations.
Molecular subtyping of metastatic CRC is evolving. At least seven clinically relevant subgroups of metastatic CRC exist in clinical routine and impact on perioperative conversion treatment [10]. CRLM from left-sided wild-type tumours (wild-type = absence of a common driver oncogene alteration such as the KRAS, NRAS and BRAF mutations or HER2/neu amplification) should always be treated with chemotherapy plus anti-EGFR antibodies, while CRLM from right-sided wild-type tumours do not appear to benefit from anti-EGFR and are treated with anti-VEGFR plus chemotherapy. Moreover, CRLM from right-sided primaries are associated with inferior prognosis and show inferior response to conversion treatment compared to CRLM from left-sided primaries. BRAF-V600E mutant metastatic CRC show aggressive biological behaviour and very poor prognosis following liver resection. The highly immunogenic dMMR/MSI-H subgroup show excellent responses to immune checkpoint inhibitors, while HER2-amplified metastatic CRCs show encouraging results with HER2 blocking agents. The largest molecular subgroup of KRAS/NRAS mutant metastatic CRC are resistant to anti-EGFR treatment and are commonly treated with a chemotherapy doublet or triplet anti-VEGF-receptor (bevacizumab) for conversion treatment.
In selected cases, locoregional chemotherapeutic treatment can be combined with systemic treatment to maximise tumour response. Hepatic artery infusion chemotherapy via a pump, combining systemic and locoregional treatment in the preoperative setting, was introduced in 1969. Continuous hepatic artery infusion chemotherapy with floxuridine in combination with systemic chemotherapy has been associated with satisfactory conversion rates in selected cases of non-resectable CRLM [29].
Surgical strategies for synchronous colorectal liver metastases
Several surgical strategies may be considered to address synchronous CRLM. (1) The classic approach is to treat the primary colon or rectum tumour first followed by the liver metastasis in a second step, in combination with variable applications of perioperative chemotherapy. This strategy remains the gold standard in patients with symptomatic primary tumours. (2) Simultaneous resection combining resection of the primary tumour and the liver metastases. This one-step procedure is feasible and has been reported with success in many articles, usually in cases with minimal liver involvement (e.g., <3 segments) [32, 33]. This method can often be done with a minimal invasive approach (laparoscopic, robotic) [33]. (3) The “liver first approach” involves resecting the CRLM upfront and is routinely associated with neoadjuvant chemotherapy [10]. It is the preferred approach at our hepato-pancreato-biliary centre in Zurich, where we often deal with major spread of the liver tumour. We often start with systemic chemotherapy over 3 months, followed by restaging and, in the case of tumour response, eventually a two-stage hepatectomy. A few weeks later, the excision of the primary colon tumour completes the complete removal of tumour disease [10, 29, 34].
Convincing data on survival advantages between colon first and liver first strategies are lacking [32]. In the context of hepatic resections, anatomical resections are defined as resections of one or more anatomic liver segments [35]. For example, a right hemi-hepatectomy involves the resection of segments V to VIII. Non-anatomical resections, also named parenchymal-sparing hepatectomies, aim to achieve oncological resections with a minimum sufficient margin while preserving as much liver parenchyma as possible. It has been suggested that a 1-mm oncological margin is sufficient in CRLM [36]. In our centre, we accept only resection with a 0.5-cm tumour-free margin, which we achieve, when necessary, with additional application of radiofrequency on the margin (e.g., Aquamantys, Medtronics) [37].
Strategies to increase the future liver remnant
A number of strategies have been developed to increase the FLR. (fig. 2, fig. 3c–h). The first human application of such a strategy, portal vein ligation of the tumour-bearing lobe during surgery, was described in 1975 [38]. Portal vein ligation was thought to induce tumour regression in the affected lobe. But portal vein occlusion of one liver lobe leads to compensatory hypertrophy of the contralateral, non-occluded FLR, which may ultimately enable surgery in cases where the FLR does not yet have the necessary volume/function to allow resection of the diseased part of the liver [38, 39].
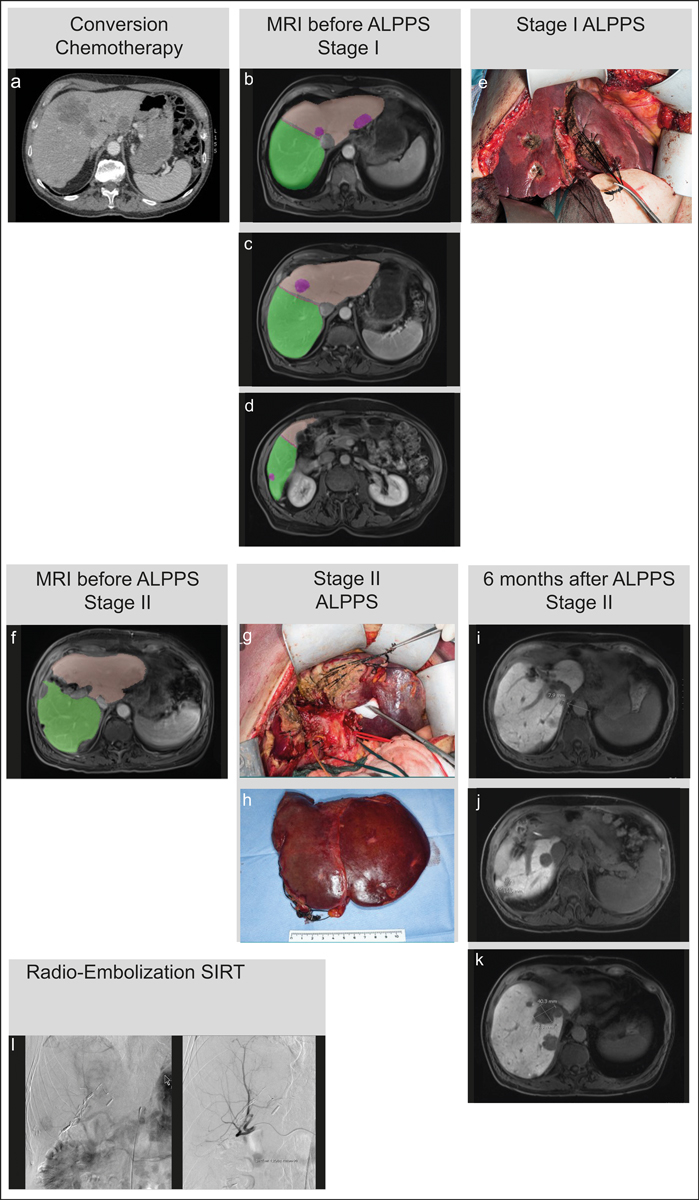
Figure 2 Treatment algorithm for an individual patient with bilobar colorectal liver metastasis at the Swiss HPB Centre, Zurich, Switzerland. (a) Start of chemotherapy. (b–d) MRI (Primovist) global reduction of all lesions. Lesion: 22.4 cm3
, FLR right liver: 58.69% / 742 cm3. (e) Intraoperative situs ALPPS Stage I. (f) MRI (Primovist) one week after Stage I: clean FLR (right lobe) with 901 cm3 and adequate compensatory overgrowth before Stage II. g) Intraoperative situs ALLPS Stage II. (h) Anatomical specimen after hepatectomy. (i–k) 6 months after surgery, CRLM reappears. (l) hypervascucularised CRLM poor in contrast with selective probing of the right hepatic artery with a 2.7F catheter. Selective internal radiation therapy (SIRT) for recurrent lesion after 7 months.
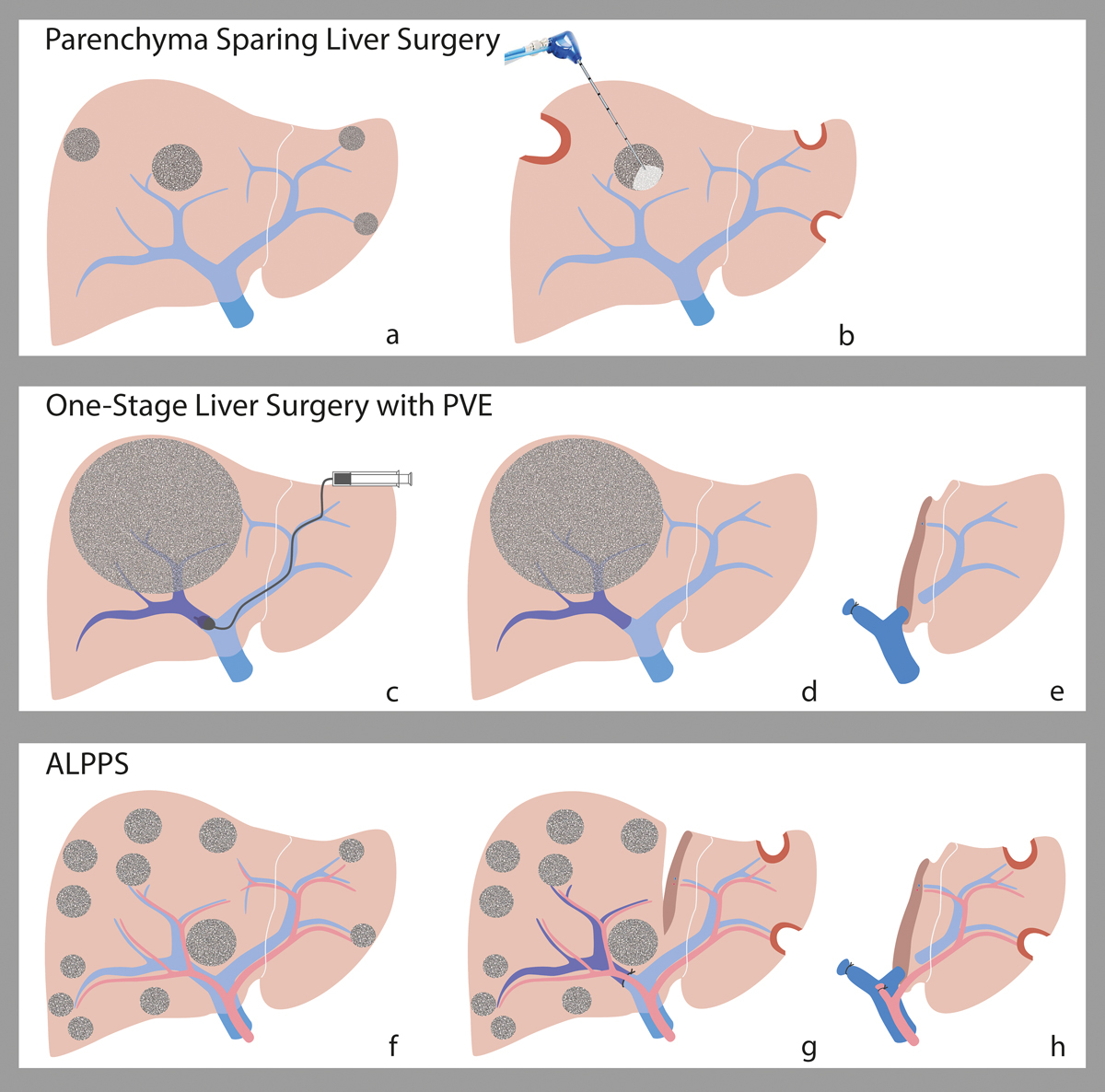
Figure 3 Strategies to increase the future liver remnant. (a) Parenchyma with bilobar CRLM before wedge resections (b) After wedge resections of small peripheral CRLM (c) One-stage liver surgery with portal vein embolisation on the lobe to be removed. (d) Hypertrophy of the FLR. (e) Removal of the embolised right lobe. (f) Bilobar CRLM before surgery g) Open ligation on the right portal vein branch and cleaning of the FLR. (h) Removal of the deportalised lobe. Illustration: Carol De Simio-Hilton.
In a classical two-stage procedure, the portal branch to the diseased liver lobe is ligated intra-operatively and the resection can be performed at a later stage, when the FLR has reached its necessary volume. Nevertheless, this might take several weeks and the waiting time cannot be justified for certain tumour growth patterns. Therefore, a concept termed associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was introduced by a group from Regensburg, Germany in 2007. ALPPS is a combination of portal vein ligation and in-situ liver parenchyma transection performed during the first stage. Initial experience suggested that ALPPS leads to faster liver regeneration (7–10 days) compared to portal vein embolisation or ligation (4–12 weeks) (fig. 2, fig. 3f–h) [38]. The mechanisms behind this enhanced stimulation and accelerated regenerative response of the liver after parenchymal liver transection combined with portal vein ligation are currently being investigated extensively [40]. Advances in patient selection and in modalities to assess liver function, as well as modifications to the ALPPS technique, have led to a significant decrease in morbidity and mortality in patients undergoing ALPPS [41–44]. Morbidity and mortality for ALPPS are currently comparable with conventional major hepatectomy procedures [45].
Laparoscopic and robotic liver surgery for colorectal liver metastases
Minimally invasive resection of CRLM with a laparoscopic or robotic approach is gaining increasing acceptance, supported by proven short-term postoperative benefits [46] and reported equal oncologic outcomes compared to open surgery. The first randomised controlled trial for open vs laparoscopic liver surgery, from Norway (COMET trial), showed that laparoscopic liver resection for CRLM is associated with significantly fewer postoperative complications compared to open surgery [47]. Moreover, a subgroup analysis of patients undergoing laparoscopic resection of CRLM located in the postero-superior segments (difficult access by any approach) revealed a shorter hospital stay and similar perioperative outcomes compared to open resection [48]. Data accumulated into a large cohort of patients from expert centres showed that laparoscopic liver resection is cost-effective [46, 47, 49, 50]. The ORANGE II PLUS trial, currently recruiting patients, will be the first randomised, multicentre study to compare open vs. laparoscopic hemi-hepatectomy. At our hepato-pancreato-biliary centre, we currently favour robot-assisted liver resection, particularly for tumours located in the difficult right posterior liver segments. Robotic surgery offers a better degree of freedom in moving the instruments, better vision and better precision [51]. Exponential growth of the use of minimally invasive liver surgery in the past few years mandates the continuous development of necessary guidelines.
Local therapies for non-resectable liver metastases
Ablative therapies
Radiofrequency and microwave ablation are the most frequent ablative thermal modalities (fig. 4). These techniques allow CT- or ultrasound-guided localisation and precise open or percutaneous destruction of central liver tumours close to main structures not accessible for surgery. Microwave ablation induces more coagulation necrosis in a shorter time than radiofrequency ablation. Microwave ablation is nowadays used more frequently as it is more effective when treating a small number of tumours (<5) of limited size (<3 cm), showing low rates of post-interventional complications (1–3%) and excellent local tumour control [52]. However, long-term oncological results for microwave ablation fail to show superiority over surgical resection for small tumours, and have clearly worse outcomes (66% local recurrence rate) for CRLM larger than 3 cm [53]. An ongoing randomised controlled trial from the COLLISION trial group may answer the question of whether or not ablation is inferior to surgery for lesions ≤3 cm. Ablation could be a promising alternative, with reduced morbidity and mortality, a shorter hospital stay and economic benefits. Unfortunately, the applicability of radiofrequency and microwave ablation for tumours close to large vessels is limited due to the “heat sink effect” (fig. 4).
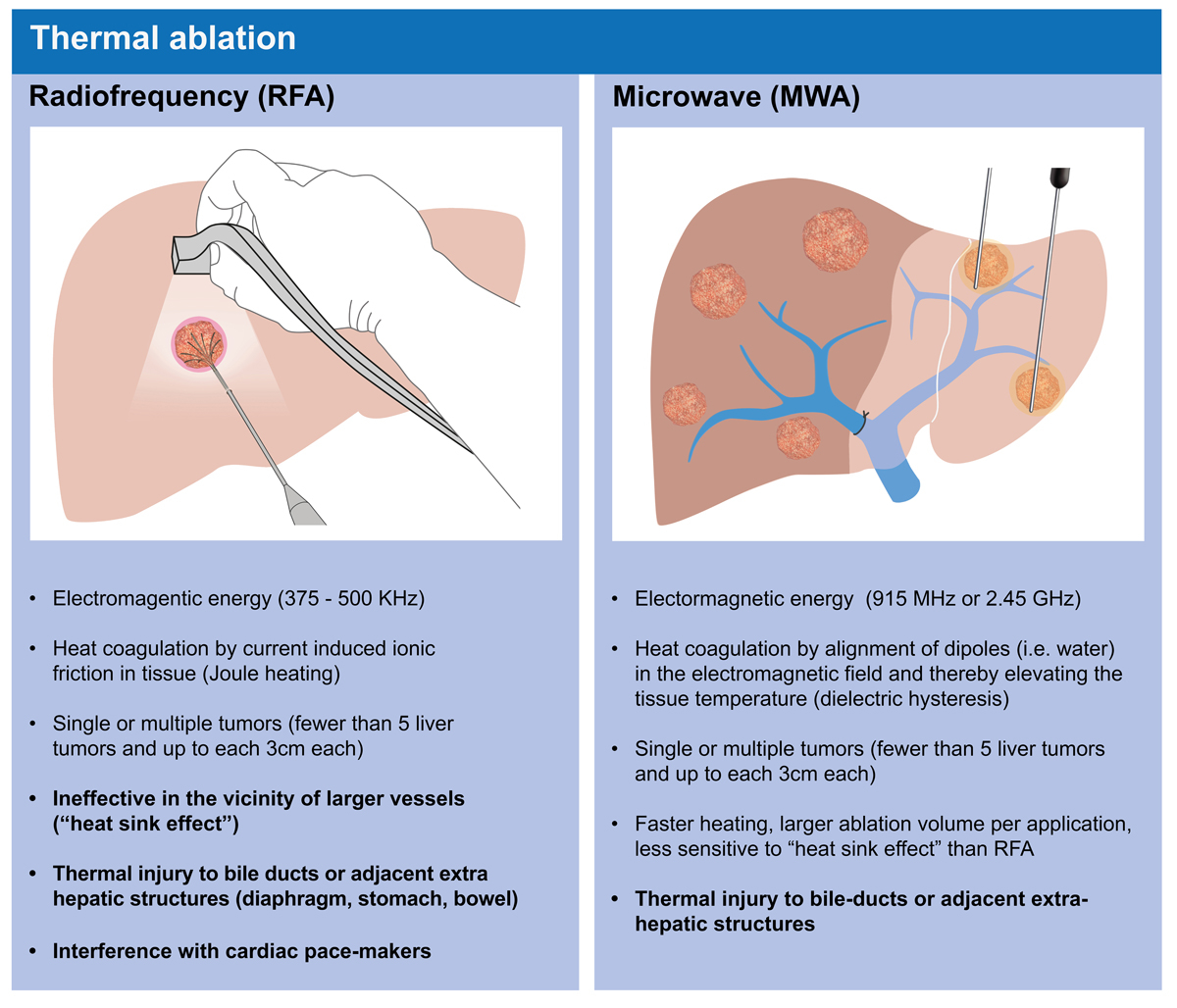
Figure 4 Radiofrequency (RFA) and microwave ablation (MWA) are the most frequently used ablative thermal modalities. Illustration: Carol De Simio-Hilton.
Irreversible electroporation is a newer, non-thermal ablative option to eliminate lesions involving important vascular or biliary structures. Irreversible electroporation does not affect the extracellular matrix and the integrity of the biliary and vascular structures is preserved, enabling cellular recolonisation. However, the high-voltage current pulses used generate excitation of skeletal and cardiac muscle. General anaesthesia with neuromuscular blockade is therefore necessary during the procedure, and cardiac arrhythmia is an absolute contraindication for irreversible electroporation. The exact placement of multiple “soft” probes is challenging and consistently time-consuming [54].
Stereotactic external radiotherapy / stereotactic body radiotherapy
Historically, external beam radiotherapy has played a minor role in the treatment of CRLM because of the low radiation tolerance of the liver [55]. Technological advances in the past few decades have enabled the conformal delivery of high radiation doses to a defined lesion whilst sparing functional liver tissue. Stereotactic body radiotherapy, a form of high-precision radiotherapy in which ablative radiation doses are delivered non-invasively in 1 to 10 ambulatory treatment sessions, was shown to be efficacious, with 2-year local control rates ranging from 59% to 84%, while having a favourable toxicity profile [56, 57]. Notably, most patients offered stereotactic body radiotherapy had serious comorbidities and were considered medically unfit for major liver surgery or had non-resectable metastases. When evaluating the optimal treatment modality for local therapy of non-resectable liver metastases, tumour size is an important factor: subgroup results report higher local control for stereotactic body radiotherapy than for radiofrequency ablation in metastases >2 cm, but not for those ≤2 cm [58]. Accordingly, the European Society for Medical Oncology consensus guidelines propose a “toolbox” concept [12], where stereotactic body radiotherapy should only be considered for patients with a contraindication for or who refuse an invasive procedure, as well as for patients with large liver metastases or metastases located at central biliary or vascular structures. The optimal integration of stereotactic body radiotherapy into modern multimodal treatment concepts remains to be defined. The recent introduction of MRI-guided radiotherapy into clinical routine, allowing for direct visualisation of the tumour and healthy tissues before each session and live-tracking of the tumour during treatment, may foster better integration of stereotactic body radiotherapy into management of liver metastases [59].
Selective internal radiation therapy
Selective internal radiation therapy is an internal, localised radiotherapy using Yttrium-90 (90Y, β-particles), which has a short radiation range (1 cm) and a half-life of <3 days (see fig. 1 for illustration). The radioactive isotope is infused into either resin or glass particles. Using an angiography targeting the branch of the hepatic artery supplying the tumour, selective administration of the radioactive isotope is achieved by infusion of 90Y microspheres through the selectively placed catheter. Complete response after selective internal radiation therapy is rarely achieved for CRLM, but stable disease is seen in many patients [60, 61]. High pre-treatment tumour volume has been shown to be an independent predictor of inferior overall survival after selective internal radiation therapy. A recently published joint analysis [62] of three studies with 549 patients assigned to FOLFOX alone and 554 patients assigned FOLFOX plus selective internal radiation therapy [62] revealed that local selective internal radiation therapy significantly reduced the incidence of radiological progression within the liver, but that selective internal radiation therapy did not have an influence on progression-free survival or median overall survival (23 vs 23 months). Additionally, toxicity was significantly increased in the selective internal radiation therapy group. Therefore, routine integration of selective internal radiation therapy into FOLFOX-based first-line chemotherapy cannot be recommended for metastatic CRC at this time.
Liver transplantation for non-resectable colorectal liver metastases
A possible therapy for end-stage liver diseases and selected primary or metastatic liver tumours is an orthotopic liver transplantation. However, strict criteria must be met to secure a reasonable outcome after orthotopic liver transplantation. Life-long immunosuppression may enhance the risk of tumour recurrence, with poorer outcomes [63]. The first seven human orthotopic liver transplants were performed for liver malignancies in the years 1963 and 1964. However, only two of these were for CRLM [64]. From 1985 to 1994, the overall survival after orthotopic liver transplantation reached 52–65%. Due to an increasing shortage of organs, indications for orthotopic liver transplantation because of liver malignancies had to be strongly justified, leading to more restrictive indications in many guidelines [63, 65, 66].
Orthotopic liver transplantation was abandoned for many types of tumours, including CRLM, in the 1990s due to a very poor 5-year survival rate of only 12–18% [66–68]. Since then, the oncologic management of CRLM has evolved dramatically, with the availability of new immunosuppressive agents such as mTOR (mammalian target of rapamycin) inhibitors conferring antineoplastic effects [67–69]. These advances encouraged a Norwegian group to re-introduce orthotopic liver transplantation indication in selected cases of CRLM. Such a study was possible in Norway because the average waiting time for a cadaveric graft is <1 month. The surfeit of donor livers has stimulated a study investigating the potential role of orthotopic liver transplantation in long-term survival for non-resectable CRLM [68]. The 5-year survival rate was surprisingly high, at 60% (21 patients). Nevertheless, recurrence was seen in 19 out of 21 patients after a median of 6 months. Recurrent tumours were treated with surgery or local ablation. After a median of 27 months, 33% of patients were tumour free. In this study, first-line chemotherapy for non-resectable CRLM demonstrated a 5-year survival rate of 9%, while patients undergoing orthotopic liver transplantation had a 5-year survival rate of 56%. Disease-free survival did not differ between these two groups. Despite these spectacular results in Norway, widespread use of orthotopic liver transplantation in other countries is currently compromised due to donor shortages. At the University Hospital Zurich, we do offer orthotopic liver transplantation for selected patients, mostly those with tumours that have been well-controlled by chemotherapy for at least 6 months. Due to the lack of available cadaveric grafts, we preferentially offer living donor liver transplantation.
Conclusion
In recent years, the management of CRLM has seen several advances, including novel imaging and molecular modalities which can be used to adjust treatment more precisely and in a more personalised manner, and which are routinely discussed at our weekly multidisciplinary team meetings. Different strategies for the resection of CRLM, like the classic, simultaneous and liver first approaches, are established for different, well-defined scenarios [10]. In particular cases, we have opted for aggressive approaches, sometimes combining two-stage procedures like ALPPS to enable curative resection in patients with very small, intra-arterial chemotherapy, and eventually orthotopic liver transplantation.
In a rapidly evolving field, different regimens of systemic and local chemotherapy, including different antibodies depending on the mutational status, are used to convert non-resectable hepatic metastatic disease into a resectable situation, providing a chance of cure. Additive tools such as thermal and non-thermal ablative procedures are essential for individually tailoring CRLM treatment options, especially when surgical interventions alone are limited due to proximity of tumours to central vascular or biliary structures. The combination of ablation and surgery is often chosen when aiming for a parenchyma-sparing procedure with respect to the disease course and liver tissue quality. Stereotactic radiation treatment of CRLM offers a non-invasive treatment in tumours greater than 3 cm in size, even in high-risk situations or sick patients, with acceptable results and limited impact on quality of life.
Because many modern, individually tailored multimodal concepts require a strong and demanding adherence of patients to long-term therapies, doctors must lead comprehensive, interdisciplinary conversations with patients and their families in order to make adequate decisions in the fight against CRLM.
Acknowledgements
The authors would like to thank
Carol De Simio-Hilton for her wonderful illustrations.
References
1
https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/krankheiten/krebs/spezifische.html July, 20th, 2020.
2
Torre
LA
,
Bray
F
,
Siegel
RL
,
Ferlay
J
,
Lortet-Tieulent
J
,
Jemal
A
. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. Published online February 06, 2015. doi:.https://doi.org/10.3322/caac.21262
3
Poston
GJ
,
Adam
R
,
Alberts
S
,
Curley
S
,
Figueras
J
,
Haller
D
, et al.
OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23(28):7125–34. Published online September 30, 2005. doi:.https://doi.org/10.1200/JCO.2005.08.722
4
Slesser
AA
,
Simillis
C
,
Goldin
R
,
Brown
G
,
Mudan
S
,
Tekkis
PP
. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol. 2013;22(1):36–47. Published online December 21, 2012. doi:.https://doi.org/10.1016/j.suronc.2012.11.002
5
Hackl
C
,
Neumann
P
,
Gerken
M
,
Loss
M
,
Klinkhammer-Schalke
M
,
Schlitt
HJ
. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14(1):810. doi:.https://doi.org/10.1186/1471-2407-14-810
6
Manfredi
S
,
Lepage
C
,
Hatem
C
,
Coatmeur
O
,
Faivre
J
,
Bouvier
AM
. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9. doi:.https://doi.org/10.1097/01.sla.0000217629.94941.cf
7
Nordlinger
B
,
Sorbye
H
,
Glimelius
B
,
Poston
GJ
,
Schlag
PM
,
Rougier
P
, et al.; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–16. doi:.https://doi.org/10.1016/S0140-6736(08)60455-9
8
Wagner
JS
,
Adson
MA
,
Van Heerden
JA
,
Adson
MH
,
Ilstrup
DM
. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg. 1984;199(5):502–8. doi:.https://doi.org/10.1097/00000658-198405000-00002
9
Lehmann
K
,
Rickenbacher
A
,
Weber
A
,
Pestalozzi
BC
,
Clavien
PA
. Chemotherapy before liver resection of colorectal metastases: friend or foe?
Ann Surg. 2012;255(2):237–47. Published online November 02, 2011. doi:.https://doi.org/10.1097/SLA.0b013e3182356236
10
Petrowsky
H
,
Fritsch
R
,
Guckenberger
M
,
De Oliveira
ML
,
Dutkowski
P
,
Clavien
PA
. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17(12):755–72; Epub ahead of print. doi:.https://doi.org/10.1038/s41575-020-0314-8
11
Nordlinger
B
,
Sorbye
H
,
Glimelius
B
,
Poston
GJ
,
Schlag
PM
,
Rougier
P
, et al.; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15. doi:.https://doi.org/10.1016/S1470-2045(13)70447-9
12
Van Cutsem
E
,
Cervantes
A
,
Adam
R
,
Sobrero
A
,
Van Krieken
JH
,
Aderka
D
, et al.
ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422. Published online July 07, 2016. doi:.https://doi.org/10.1093/annonc/mdw235
13
Niekel
MC
,
Bipat
S
,
Stoker
J
. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257(3):674–84. Published online September 11, 2010. doi:.https://doi.org/10.1148/radiol.10100729
14Chu QD, Volmer CM, Zibari GB, Orloff SL, Williams M, Gimenez ME, eds. Hepato-Pancreato-Biliary and Transplant Surgery: Practical Management of Dilemmas. AHPBA: Beaux Books; 2018.
15
Moulton
CA
,
Gu
CS
,
Law
CH
,
Tandan
VR
,
Hart
R
,
Quan
D
, et al.
Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA. 2014;311(18):1863–9. Published online May 16, 2014. doi:.https://doi.org/10.1001/jama.2014.3740
16
Pugh
RN
,
Murray-Lyon
IM
,
Dawson
JL
,
Pietroni
MC
,
Williams
R
. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. Published online August 01, 1973. doi:.https://doi.org/10.1002/bjs.1800600817
17Child CG (ed.) The Liver and Portal Hypertension. Philadelphia and London: W. B. Saunders Co. Ltd; 1964.
18
Zipprich
A
,
Kuss
O
,
Rogowski
S
,
Kleber
G
,
Lotterer
E
,
Seufferlein
T
, et al.
Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut. 2010;59(7):963–8. Published online June 29, 2010. doi:.https://doi.org/10.1136/gut.2010.208595
19
Stauber
RE
,
Wagner
D
,
Stadlbauer
V
,
Palma
S
,
Gurakuqi
G
,
Kniepeiss
D
, et al.
Evaluation of indocyanine green clearance and model for end-stage liver disease for estimation of short-term prognosis in decompensated cirrhosis. Liver Int. 2009;29(10):1516–20. doi:.https://doi.org/10.1111/j.1478-3231.2009.02104.x
20
Lisotti
A
,
Azzaroli
F
,
Buonfiglioli
F
,
Montagnani
M
,
Cecinato
P
,
Turco
L
, et al.
Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology. 2014;59(2):643–50. Published online September 17, 2013. doi:.https://doi.org/10.1002/hep.26700
21
de Liguori Carino
N
,
O’Reilly
DA
,
Dajani
K
,
Ghaneh
P
,
Poston
GJ
,
Wu
AV
. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol. 2009;35(9):957–62. doi:.https://doi.org/10.1016/j.ejso.2009.02.003
22
Ray
S
,
Mehta
NN
,
Golhar
A
,
Nundy
S
. Post hepatectomy liver failure - A comprehensive review of current concepts and controversies. Ann Med Surg (Lond). 2018;34(34):4–10. doi:.https://doi.org/10.1016/j.amsu.2018.08.012
23
Ge
PL
,
Du
SD
,
Mao
YL
. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat Dis Int. 2014;13(4):361–70. Published online August 08, 2014. doi:.https://doi.org/10.1016/S1499-3872(14)60267-8
24
Hoekstra
LT
,
de Graaf
W
,
Nibourg
GA
,
Heger
M
,
Bennink
RJ
,
Stieger
B
, et al.
Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257(1):27–36. Published online July 28, 2012. doi:.https://doi.org/10.1097/SLA.0b013e31825d5d47
25
Mizuguchi
T
,
Kawamoto
M
,
Meguro
M
,
Hui
TT
,
Hirata
K
. Preoperative liver function assessments to estimate the prognosis and safety of liver resections. Surg Today. 2014;44(1):1–10. Published online March 12, 2013. doi:.https://doi.org/10.1007/s00595-013-0534-4
26
Du
ZG
,
Li
B
,
Wei
YG
,
Yin
J
,
Feng
X
,
Chen
X
. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10(3):265–9. Published online June 15, 2011. doi:.https://doi.org/10.1016/S1499-3872(11)60044-1
27
Lock
JF
,
Westphal
T
,
Rubin
T
,
Malinowski
M
,
Schulz
A
,
Jara
M
, et al.
LiMAx Test Improves Diagnosis of Chemotherapy-Associated Liver Injury Before Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2017;24(9):2447–55. Published online May 19, 2017. doi:.https://doi.org/10.1245/s10434-017-5887-2
28
Bennink
RJ
,
Dinant
S
,
Erdogan
D
,
Heijnen
BH
,
Straatsburg
IH
,
van Vliet
AK
, et al.
Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med. 2004;45(6):965–71.
29
Clavien
PA
,
Petrowsky
H
,
DeOliveira
ML
,
Graf
R
. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545–59. Published online April 13, 2007. doi:.https://doi.org/10.1056/NEJMra065156
30
Nordlinger
B
,
Guiguet
M
,
Vaillant
JC
,
Balladur
P
,
Boudjema
K
,
Bachellier
P
, et al.; Association Française de Chirurgie. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77(7):1254–62. Published online April 01, 1996. doi:.https://doi.org/10.1002/(SICI)1097-0142(19960401)77:7<1254::AID-CNCR5>3.0.CO;2-I
31
Mitry
E
,
Fields
AL
,
Bleiberg
H
,
Labianca
R
,
Portier
G
,
Tu
D
, et al.
Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26(30):4906–11. Published online September 17, 2008. doi:.https://doi.org/10.1200/JCO.2008.17.3781
32
Baltatzis
M
,
Chan
AK
,
Jegatheeswaran
S
,
Mason
JM
,
Siriwardena
AK
. Colorectal cancer with synchronous hepatic metastases: Systematic review of reports comparing synchronous surgery with sequential bowel-first or liver-first approaches. Eur J Surg Oncol. 2016;42(2):159–65. doi:.https://doi.org/10.1016/j.ejso.2015.11.002
33
de Haas
RJ
,
Adam
R
,
Wicherts
DA
,
Azoulay
D
,
Bismuth
H
,
Vibert
E
, et al.
Comparison of simultaneous or delayed liver surgery for limited synchronous colorectal metastases. Br J Surg. 2010;97(8):1279–89. Published online June 26, 2010. doi:.https://doi.org/10.1002/bjs.7106
34
Mentha
G
,
Majno
PE
,
Andres
A
,
Rubbia-Brandt
L
,
Morel
P
,
Roth
AD
. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93(7):872–8. Published online May 04, 2006. doi:.https://doi.org/10.1002/bjs.5346
35
Zorzi
D
,
Mullen
JT
,
Abdalla
EK
,
Pawlik
TM
,
Andres
A
,
Muratore
A
, et al.
Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10(1):86–94. Published online December 22, 2005. doi:.https://doi.org/10.1016/j.gassur.2005.07.022
36
Hamady
ZZ
,
Lodge
JP
,
Welsh
FK
,
Toogood
GJ
,
White
A
,
John
T
, et al.
One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259(3):543–8. Published online June 05, 2013. doi:.https://doi.org/10.1097/SLA.0b013e3182902b6e
37
Hammond
JS
,
Muirhead
W
,
Zaitoun
AM
,
Cameron
IC
,
Lobo
DN
. Comparison of liver parenchymal ablation and tissue necrosis in a cadaveric bovine model using the Harmonic Scalpel, the LigaSure, the Cavitron Ultrasonic Surgical Aspirator and the Aquamantys devices. HPB (Oxford). 2012;14(12):828–32. doi:.https://doi.org/10.1111/j.1477-2574.2012.00547.x
38
Eshmuminov
D
,
Raptis
DA
,
Linecker
M
,
Wirsching
A
,
Lesurtel
M
,
Clavien
PA
. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg. 2016;103(13):1768–82. Published online September 17, 2016. doi:.https://doi.org/10.1002/bjs.10290
39
de Santibañes
E
,
Clavien
PA
. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255(3):415–7. Published online February 15, 2012. doi:.https://doi.org/10.1097/SLA.0b013e318248577d
40
Langiewicz
M
,
Schlegel
A
,
Saponara
E
,
Linecker
M
,
Borger
P
,
Graf
R
, et al.
Hedgehog pathway mediates early acceleration of liver regeneration induced by a novel two-staged hepatectomy in mice. J Hepatol. 2017;66(3):560–70. Published online October 25, 2016. doi:.https://doi.org/10.1016/j.jhep.2016.10.014
41
Petrowsky
H
,
Györi
G
,
de Oliveira
M
,
Lesurtel
M
,
Clavien
PA
. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261(4):e90–2. Published online February 24, 2015. doi:.https://doi.org/10.1097/SLA.0000000000001087
42
Moris
D
,
Ronnekleiv-Kelly
S
,
Kostakis
ID
,
Tsilimigras
DI
,
Beal
EW
,
Papalampros
A
, et al.
Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J Surg. 2018;42(3):806–15. Published online August 12, 2017. doi:.https://doi.org/10.1007/s00268-017-4181-6
43
Lau
WY
,
Lai
EC
. Modifications of ALPPS - from complex to more complex or from complex to less complex operations. Hepatobiliary Pancreat Dis Int. 2017;16(4):346–52. Published online August 22, 2017. doi:.https://doi.org/10.1016/S1499-3872(17)60034-1
44
Linecker
M
,
Stavrou
GA
,
Oldhafer
KJ
,
Jenner
RM
,
Seifert
B
,
Lurje
G
, et al.
The ALPPS Risk Score: Avoiding Futile Use of ALPPS. Ann Surg. 2016;264(5):763–71. Published online October 18, 2016. doi:.https://doi.org/10.1097/SLA.0000000000001914
45
Eshmuminov
D
,
Tschuor
C
,
Raptis
DA
,
Boss
A
,
Wurnig
MC
,
Sergeant
G
, et al.
Rapid liver volume increase induced by associating liver partition with portal vein ligation for staged hepatectomy (ALPPS): Is it edema, steatosis, or true proliferation?
Surgery. 2017;161(6):1549–52. Published online April 16, 2017. doi:.https://doi.org/10.1016/j.surg.2017.01.005
46
Wakabayashi
G
,
Cherqui
D
,
Geller
DA
,
Buell
JF
,
Kaneko
H
,
Han
HS
, et al.
Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261(4):619–29. Published online March 06, 2015. doi:.https://doi.org/10.1097/sla.0000000000001184
47
Fretland
AA
,
Dagenborg
VJ
,
Bjørnelv
GMW
,
Kazaryan
AM
,
Kristiansen
R
,
Fagerland
MW
, et al.
Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267(2):199–207. Published online June 29, 2017. doi:.https://doi.org/10.1097/SLA.0000000000002353
48
Aghayan
DL
,
Fretland
AA
,
Kazaryan
AM
,
Sahakyan
MA
,
Dagenborg
VJ
,
Bjørnbeth
BA
, et al.
Laparoscopic versus open liver resection in the posterosuperior segments: a sub-group analysis from the OSLO-COMET randomized controlled trial. HPB (Oxford). 2019;21(11):1485–90. Published online April 10, 2019. doi:.https://doi.org/10.1016/j.hpb.2019.03.358
49
Clavien
PA
,
Barkun
J
. Consensus conference on laparoscopic liver resection: a jury-based evaluation. Ann Surg. 2015;261(4):630–1. Published online March 06, 2015. doi:.https://doi.org/10.1097/SLA.0000000000001183
50
Abu Hilal
M
,
Aldrighetti
L
,
Dagher
I
,
Edwin
B
,
Troisi
RI
,
Alikhanov
R
, et al.
The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268(1):11–8. Published online October 25, 2017. doi:.https://doi.org/10.1097/SLA.0000000000002524
51
Fruscione
M
,
Pickens
R
,
Baker
EH
,
Cochran
A
,
Khan
A
,
Ocuin
L
, et al.
Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB (Oxford). 2019;21(7):906–11. Published online January 09, 2019. doi:.https://doi.org/10.1016/j.hpb.2018.11.011
52
Seror
O
. Ablative therapies: Advantages and disadvantages of radiofrequency, cryotherapy, microwave and electroporation methods, or how to choose the right method for an individual patient?
Diagn Interv Imaging. 2015;96(6):617–24. Published online May 20, 2015. doi:.https://doi.org/10.1016/j.diii.2015.04.007
53
Loveman
E
,
Jones
J
,
Clegg
AJ
,
Picot
J
,
Colquitt
JL
,
Mendes
D
, et al.
The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: systematic review and economic evaluation. Health Technol Assess. 2014;18(7):vii–viii, 1–283. doi:.https://doi.org/10.3310/hta18070
54
Coletti
L
,
Battaglia
V
,
De Simone
P
,
Turturici
L
,
Bartolozzi
C
,
Filipponi
F
. Safety and feasibility of electrochemotherapy in patients with unresectable colorectal liver metastases: A pilot study. Int J Surg. 2017;44:26–32. doi:.https://doi.org/10.1016/j.ijsu.2017.06.033
55
Dawson
LA
,
Normolle
D
,
Balter
JM
,
McGinn
CJ
,
Lawrence
TS
,
Ten Haken
RK
. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–21. Published online July 04, 2002. doi:.https://doi.org/10.1016/S0360-3016(02)02846-8
56
Petrelli
F
,
Comito
T
,
Barni
S
,
Pancera
G
,
Scorsetti
M
,
Ghidini
A
; SBRT for CRC liver metastases. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol. 2018;129(3):427–34. Published online July 13, 2018. doi:.https://doi.org/10.1016/j.radonc.2018.06.035
57
Lee
J
,
Shin
IS
,
Yoon
WS
,
Koom
WS
,
Rim
CH
. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: Meta-analyses and a systematic review. Radiother Oncol. 2020;145:63–70. Published online January 11, 2020. doi:.https://doi.org/10.1016/j.radonc.2019.12.004
58
Jackson
WC
,
Tao
Y
,
Mendiratta-Lala
M
,
Bazzi
L
,
Wahl
DR
,
Schipper
MJ
, et al.
Comparison of Stereotactic Body Radiation Therapy and Radiofrequency Ablation in the Treatment of Intrahepatic Metastases. Int J Radiat Oncol Biol Phys. 2018;100(4):950–8. doi:.https://doi.org/10.1016/j.ijrobp.2017.12.014
59
Witt
JS
,
Rosenberg
SA
,
Bassetti
MF
. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21(2):e74–82. Published online February 03, 2020. doi:.https://doi.org/10.1016/S1470-2045(20)30034-6
60
Vilgrain
V
,
Pereira
H
,
Assenat
E
,
Guiu
B
,
Ilonca
AD
,
Pageaux
GP
, et al.; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–36. Published online November 07, 2017. doi:.https://doi.org/10.1016/S1470-2045(17)30683-6
61
Morse
MA
,
Hanks
BA
,
Suhocki
P
,
Doan
PL
,
Liu
EA
,
Frost
P
, et al.
Improved time to progression for transarterial chemoembolization compared with transarterial embolization for patients with unresectable hepatocellular carcinoma. Clin Colorectal Cancer. 2012;11(3):185–90. Published online January 28, 2012. doi:.https://doi.org/10.1016/j.clcc.2011.11.003
62
Wasan
HS
,
Gibbs
P
,
Sharma
NK
,
Taieb
J
,
Heinemann
V
,
Ricke
J
, et al.; FOXFIRE trial investigators; SIRFLOX trial investigators; FOXFIRE-Global trial investigators. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18(9):1159–71. doi:.https://doi.org/10.1016/S1470-2045(17)30457-6
63
Dutkowski
P
,
De Rougemont
O
,
Müllhaupt
B
,
Clavien
PA
. Current and future trends in liver transplantation in Europe. Gastroenterology. 2010;138(3):802–9.e4. doi:.https://doi.org/10.1053/j.gastro.2010.01.030
64
Starzl
TE
. The saga of liver replacement, with particular reference to the reciprocal influence of liver and kidney transplantation (1955-1967). J Am Coll Surg. 2002;195(5):587–610. doi:.https://doi.org/10.1016/S1072-7515(02)01498-9
65
Gores
GJ
. Liver transplantation for malignant disease. Gastroenterol Clin North Am. 1993;22(2):285–99. Published online June 01, 1993.
66
Penn
I
. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110(4):726–34, discussion 734–5. Published online October 01, 1991.
67
Moris
D
,
Tsilimigras
DI
,
Chakedis
J
,
Beal
EW
,
Felekouras
E
,
Vernadakis
S
, et al.
Liver transplantation for unresectable colorectal liver metastases: A systematic review. J Surg Oncol. 2017;116(3):288–97. doi:.https://doi.org/10.1002/jso.24671
68
Hagness
M
,
Foss
A
,
Line
PD
,
Scholz
T
,
Jørgensen
PF
,
Fosby
B
, et al.
Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257(5):800–6. Published online January 31, 2013. doi:.https://doi.org/10.1097/SLA.0b013e3182823957
69
Toso
C
,
Pinto Marques
H
,
Andres
A
,
Castro Sousa
F
,
Adam
R
,
Kalil
A
, et al.; Compagnons Hépato-Biliaires Group. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl. 2017;23(8):1073–6. doi:.https://doi.org/10.1002/lt.24791



