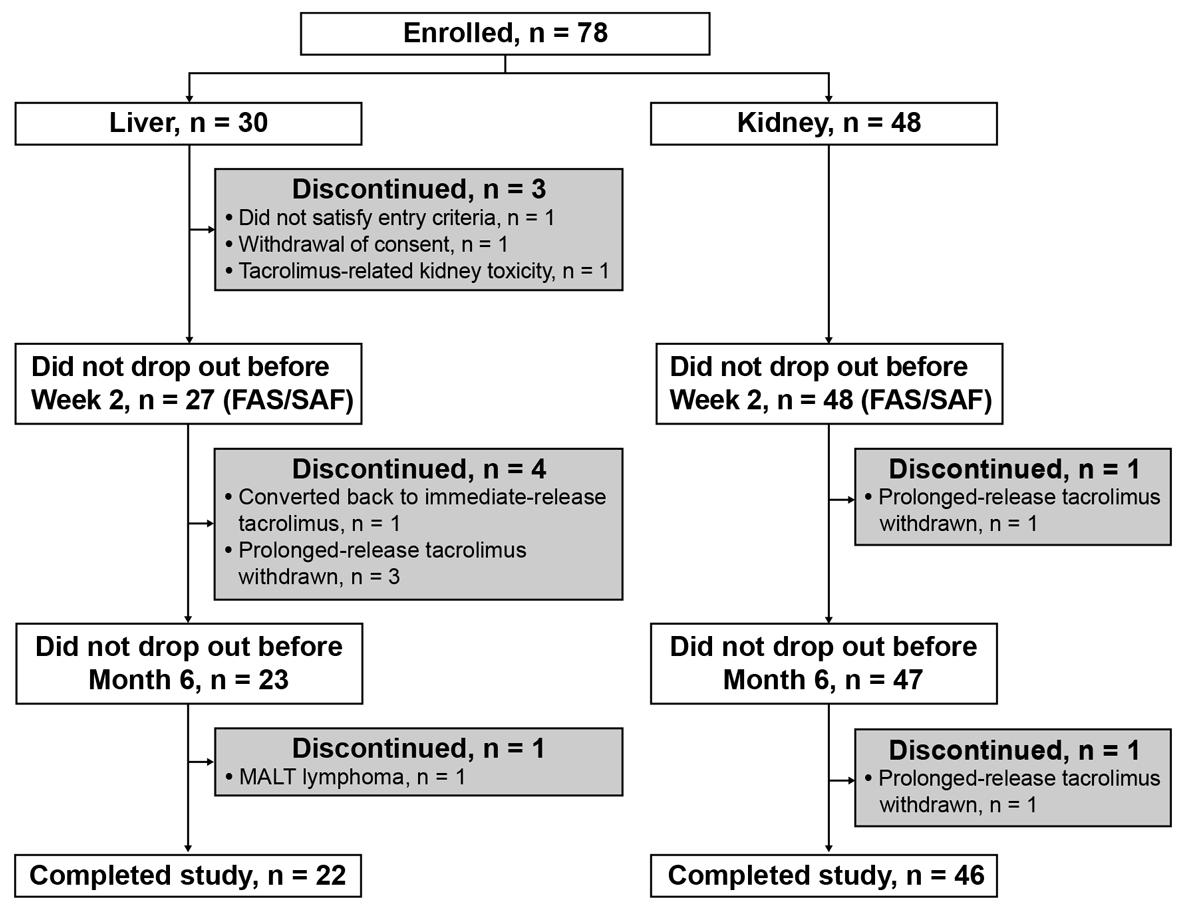
Figure 1 Flow of patients through study, stratified by organ transplanted. FAS = full analysis set; MALT = mucosa-associated lymphoid tissue; SAF = safety analysis set.
DOI: https://doi.org/10.4414/smw.2021.20453
In order to preserve long-term graft function, solid organ transplant recipients are required to adhere to immunosuppressive regimens. However, non-adherence to immunosuppressive therapy remains a concern in post-transplantation management, being reported in up to 55% and 66% of kidney and liver transplant recipients, respectively [1, 2]. In kidney transplant recipients, non-adherence has been associated with de novo donor-specific antibody development, antibody-mediated rejection and diminished graft survival [3–5]. Furthermore, increased risk for graft loss and acute rejection has also been reported in non-adherent liver transplant recipients [6]. There are many causes of non-adherence, including the need for twice-daily dosing and a large number of prescribed pills [7–9]. In addition, a high pill burden can reduce quality of life [10]. For example, in a large study of 3462 kidney and liver transplant recipients, 21% and 23% of patients, respectively, considered taking two to three doses of medication per day to represent a lifestyle restriction [10].
As tacrolimus is the mainstay of immunosuppressive regimens in kidney and liver transplantation [11, 12], increasing adherence to tacrolimus-based regimens post-transplantation is essential for optimising graft and patient outcomes. Tacrolimus is available as a twice-daily, immediate-release formulation, and as a once-daily, prolonged-release formulation. Short-term clinical outcomes are comparable with both formulations in de novo kidney and liver transplant recipients [13, 14]. However, prolonged-release tacrolimus offers a simplified regimen comprising a single, daily, morning dose [15]. As such, compared with immediate-release tacrolimus, the prolonged-release formulation has the potential to improve implementation adherence to tacrolimus-based regimens and, thereby, reduce the risk of rejection and graft loss [3–6]. Prolonged-release tacrolimus may also reduce pill burden for the patient, as compared with the twice-daily formulation.
Adherence data regarding implementation of tacrolimus following conversion from the immediate-release to the prolonged-release formulation are scarce, and at the time of this study, there were no implementation adherence data for adult patients treated with prolonged-release tacrolimus after kidney and liver transplantation in Switzerland. Furthermore, few studies have used more than one method to assess adherence, and multiple measures of implementation might permit a more comprehensive understanding of medication compliance in clinical practice. Therefore, the primary aim of this study was to evaluate various parameters of implementation adherence (including delaying, omitting, or taking extra doses) at baseline and after 12 months, in stable kidney and liver transplant recipients converted from immediate-release to prolonged-release tacrolimus-based immunosuppression in a multicentre study in Switzerland. The secondary aims were to assess tacrolimus pill burden before and after conversion, patient satisfaction with the prolonged-release formulation and clinical parameters (e.g., rejection, graft loss and renal function) associated with, and the safety of, prolonged-release tacrolimus.
This was a multicentre, non-interventional, 12-month study to investigate adherence, convenience and tolerability of prolonged-release tacrolimus (Advagraf®; Astellas Pharma Europe BV, Netherlands) in stable adult kidney and liver transplant recipients, converted from immediate-release tacrolimus (Prograf®; Astellas Pharma Ltd, Chertsey, UK) in routine clinical practice in Switzerland. The investigator made the clinical decision to define a patient as stable and include them in the study. Both formulations of tacrolimus were fully covered by healthcare insurance throughout the study. Previous adherence to medication was not an eligibility criterion. Patients were enrolled between September 2013 and June 2015 from four kidney and two liver transplant centres at five hospitals in Switzerland. The study was conducted in accordance with local ethics committees’ regulations, the Declaration of Helsinki and the International Council of Harmonisation Good Clinical Practice guidelines. Patients provided written informed consent and could withdraw from the study at any time.
Stable kidney and liver transplant patients who were aged ≥18 years and receiving immediate-release tacrolimus were eligible for inclusion in the study, if they were being converted to the prolonged-release formulation in routine practice, according to the Swiss prolonged-release tacrolimus label. There is no restriction regarding the time point post-transplantation at which patients can be converted from immediate-release to prolonged-release tacrolimus in Switzerland.
Patients were converted from immediate-release to prolonged-release tacrolimus on a 1 mg : 1 mg total-daily-dose basis, with subsequent dose adjustments permitted at the investigator’s discretion. Patients were permitted to receive concomitant medication as per routine clinical practice. Patients attended four clinic visits: at baseline (pre-conversion), and then at week 2 (± 1 week), month 6 (± 1 month) and month 12 (± 1 month) post-conversion. Patients who had their dose of prolonged-release tacrolimus adjusted early after conversion attended an additional visit, 2 weeks after the week 2 visit (week 4).
The schedule of data collection is presented in table 1.
Table 1 Schedule of data collection.
| Data recorded |
Baseline
(pre-conversion) |
Week 2
(± 1 week) |
Week 4* |
Month 6
(± 1 month) |
Month 12
(± 1 month) |
|
|---|---|---|---|---|---|---|
| Adherence parameters | BAASIS interview questionnaire | X | X | |||
| Adherence VAS | X | X | ||||
| Investigator assessment of adherence | X | X | X | X | X | |
| Tacrolimus trough levels† | ↔ | |||||
| Other parameters | Patient satisfaction | X | ||||
| Patient baseline and demographic data | X | |||||
| Vital signs | X | X | X | X | X | |
| Laboratory assessments | X | X | X | X | X | |
| Renal function (eGFR) | X | X | X | X | X | |
| Prolonged-release tacrolimus dose† | ↔ | |||||
| Immunosuppressive medication† | ↔ | |||||
| Concomitant medication† | ↔ | |||||
| Rejection episodes† | ↔ | |||||
| Graft survival/retransplantation† | ↔ | |||||
| Dialysis dependence† | ↔ | |||||
| Investigator rating of efficacy | X | |||||
| ADRs† | ↔ | |||||
| Investigator rating of tolerability | X | |||||
ADR = adverse drug reaction; BAASIS = Basel Assessment of Adherence to Immunosuppressive Medications Scale; eGFR = estimated glomerular filtration rate; VAS = visual analogue scale * Patients who had their dose of prolonged-release tacrolimus adjusted early after conversion attended an additional visit, 2 weeks after the week 2 visit (week 4). † All available data throughout the study were collected..
In Switzerland, transplant centres differ markedly regarding implementation of measures to improve immunosuppression adherence, ranging from no measures, to patient brochures and training programmes for nurses to facilitate patient education about adherence. Indeed, some centres have implemented adherence programmes for liver and kidney transplant recipients. A factor hindering adherence is often a lack of resources for, and focus on, promoting immunosuppression adherence, including implementation of the treatment regimen.
Implementation adherence (based on item ‘B’ in the ABC taxonomy [16]) was defined as consistently taking medication at the correct time and at the correct dose in order to achieve target tacrolimus trough levels. Implementation adherence was evaluated in three ways: using the Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS©) interview questionnaire [17], investigator-rated patient adherence, and by measurement of tacrolimus trough levels. Switzerland has no easily accessible database of pharmacy refill records, and electronic monitoring is too complex and expensive to be integrated into general clinical practice. Therefore, a questionnaire-based approach to measuring adherence was the only feasible assessment method, coupled with measurement of tacrolimus trough levels as per routine clinical practice.
The BAASIS interview questionnaire was implemented at baseline (pre-conversion) and at month 12. It measures the adherence of patients to their immunosuppressive medication after kidney transplantation within the past 4 weeks, based on four domains – taking, drug-holiday (omitting several consecutive doses of medication), timing and dose alteration [17]. The questionnaire was adapted to specifically ask about tacrolimus immunosuppressive medication to determine if, and how often, patients recalled non-adherence to their medication regimen during the previous 4 weeks, under the four domains. Patients also completed the self-rated BAASIS visual analogue scale (VAS) adapted to specifically ask about tacrolimus immunosuppressive medication, which ranged from 0% (never took tacrolimus medication as prescribed) to 100% (always took tacrolimus medication as prescribed). The BAASIS interview questionnaire and VAS are standard measures of adherence, but their use could render patients more conscious of taking their medication as prescribed, with the result of increasing patient adherence.
Across all visits, investigators rated (based on their personal opinion) patients’ adherence to their tacrolimus medication over the previous 2 weeks (4 weeks for baseline visit) as “good”, “moderate” or “poor”, in response to the question “How do you rate the patient’s current adherence with regard to tacrolimus intake within the last 2 weeks?”. This assessment is unlikely to affect patient adherence to their medication regimen, unless the investigator expresses concerns to their patient regarding poor adherence.
Adherence was also identified by assessing tacrolimus trough levels; sub-therapeutic levels were predefined by the investigator on an individual patient basis and could be adjusted throughout the study at the investigator’s discretion; over-therapeutic levels were defined as tacrolimus trough levels >15 ng/ml (based on maintenance therapy ranges reported in the summary of product characteristics). All available data for tacrolimus trough levels were collected. If investigators provided patients with details regarding sub- or over-therapeutic tacrolimus trough levels as part of their standard practice, then this might impact patient medication adherence.
At baseline and at month 12, the BAASIS interview questionnaire contained, at the beginning of the form, an additional question, which asked the investigator to state how many tacrolimus capsules the patient was taking daily. Pill burden was defined as the daily number of immediate-release or prolonged-release tacrolimus capsules to be taken.
Patient satisfaction with prolonged-release tacrolimus was assessed at month 12, by three questions: (1) “How satisfied are you with the once-daily administration of prolonged-release tacrolimus?” (very satisfied, satisfied, not satisfied); (2) “Do you think it is easier to remember when to take your tacrolimus capsules by the once-daily administration of prolonged-release tacrolimus?” (yes, no); and (3) “Do you think the once-daily administration of prolonged-release tacrolimus is more convenient than a twice-daily administration?” (yes, no).
Across the visits, prolonged-release tacrolimus dose and trough levels and medication use were recorded. Renal function (estimated glomerular filtration rate [eGFR] from the Chronic Kidney Disease Epidemiology Collaboration formula) was assessed at baseline, week 2, month 6, and month 12. Rejection episodes, graft survival, retransplantation, dialysis and patient death were also reported throughout the study. Investigators rated (based on their personal opinion) the overall efficacy of prolonged-release tacrolimus at month 12 as “good”, “moderate” or “poor”, in response to the question “How do you rate the overall efficacy of prolonged-release tacrolimus?”.
Adverse drug reactions (ADRs) were assessed by clinicians and details were collected in a form, including ADR seriousness, start/end date, outcome, causal relationship with tacrolimus (probable, possible, or unassessable) and concomitant drugs. ADRs were considered serious if they resulted in death, were life-threatening or medically significant, or resulted in hospitalisation (or prolonged hospitalisation), persistent or notable disability, congenital abnormality, or birth defect. At month 12, investigators rated (based on their personal opinion) the overall tolerability of prolonged-release tacrolimus as “good”, “moderate” or “poor”, in response to the question “How do you rate the overall tolerability of prolonged-release tacrolimus?”.
Across the visits, laboratory assessments (haemoglobin, glucose, glycated haemoglobin [HbA1c], serum creatinine, urine protein and urine albumin; optional: cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides), and vital signs (systolic and diastolic blood pressure, and pulse rate) were assessed by investigators as normal, abnormal and clinically not relevant, or abnormal and clinically relevant.
The primary endpoint was the rate of non-adherence at month 12, based on being non-adherent according to at least one of three aspects: (1) non-adherence according to the BAASIS interview questionnaire at month 12 (affirmative response to any of the taking, timing or dose alteration dimensions); (2) investigator rating of adherence as “poor” in at least one assessment after baseline; (3) at least one sub- or over-therapeutic tacrolimus trough level at month 6 or 12. This composite endpoint was chosen as a strict measure of adherence that would also permit measurement of adherence using different methods. Secondary adherence variables included: overall non-adherence on the BAASIS interview questionnaire and non-adherence indicated by individual questionnaire items, at baseline and month 12; actual tacrolimus pill burden before and after conversion using the BAASIS; patient-rated adherence on the BAASIS VAS; and patient satisfaction at month 12.
Secondary clinical endpoints consisted of change from baseline to month 12 for eGFR and tacrolimus dose and trough levels, concomitant medication, clinical outcomes (rejection and graft loss) and overall clinical efficacy, as assessed by the physician at month 12. Incidence of ADRs, physician-rated tolerability, laboratory parameters and vital signs were recorded as part of the safety evaluation.
Although no formal sample size calculation was performed, it was planned to include 150 patients from four kidney and two liver transplant centres at five hospitals. However, owing to limited recruitment options, only 78 patients were enrolled in this study, of whom 75 had evaluable data. Both the full analysis set (FAS) and safety analysis set (SAF) comprised all patients who received at least one dose of study drug, and for whom any data were reported after the first dose of study drug. The FAS was used for summaries, and primary and secondary analyses of efficacy data, as well as select demographic and baseline characteristics. However, only patients in the FAS with data for all three aspects of the primary composite endpoint were evaluable for the primary endpoint. The SAF was used for all safety- and tolerability-related variables.
No hypotheses were tested and all analyses are presented descriptively, for the entire study population as well as by transplanted organ type. All values were included in the analyses, and missing data were not imputed; however, when a patient became dialysis-dependent, their eGFR was set to 0. No sensitivity analyses were planned or performed. Data processing, summaries and analyses were performed using SPSS Version 20 (International Business Machines Corporation, New York, United States). The 95% confidence intervals (CIs) (using the Wilson method) for proportions were calculated in the R programming language, Version 3.2.5 (R Foundation for Statistical Computing, Vienna, Austria), with use of the package “Hmisc” [18].
Overall, 78 patients were enrolled from four kidney and two liver transplant centres at five hospitals in Switzerland. Three patients dropped out before week 2; therefore, the FAS and SAF comprised 75 patients (liver transplant recipients, n = 27; kidney transplant recipients, n = 48); 68 patients (liver transplant recipients, n = 22; kidney transplant recipients, n = 46) completed the study (fig. 1). Overall, there were 75 study participants at baseline and week 2, 70 participants at month 6, and 68 participants at month 12. Ten patients discontinued the study (fig. 1). The most common reason for discontinuation was withdrawal of prolonged-release tacrolimus (n = 5). One liver transplant recipient was converted back to immediate-release tacrolimus.

Figure 1 Flow of patients through study, stratified by organ transplanted. FAS = full analysis set; MALT = mucosa-associated lymphoid tissue; SAF = safety analysis set.
Patient demographics and baseline characteristics for the FAS are presented in table 2. The mean ± standard deviation (SD) age of all patients was 53.1 ± 12.8 years (median 55.0 years; range 20.0–74.0 years) and 70.7% of patients were male. At baseline (time of conversion), the mean ± SD time since the last transplantation was 73.8 ± 73.4 months (median 42.6 months; range 0.8–293.4 months). The most common known reason for transplantation was polycystic disease in kidney transplant recipients and cirrhosis in liver transplant recipients. None of the kidney transplant recipients, but 48.1% of the liver transplant recipients, received tacrolimus monotherapy at baseline. The most common concomitant immunosuppressive medication at baseline was mycophenolate mofetil (CellCept®; Roche Registration Ltd, Welwyn Garden City, UK; 36.0% of patients), followed by mycophenolic acid (Myfortic®; Novartis Pharmaceuticals UK Ltd, Camberley, UK; 8.0%) and azathioprine (6.7%). Additionally, 20% of patients were receiving mycophenolate mofetil plus corticosteroids.
Table 2 Patient demographics and baseline characteristics, stratified by transplanted organ and overall (full analysis set).
| Characteristics |
Liver transplant
(n = 27) |
Kidney transplant
(n = 48) |
Overall
(N = 75) |
|
|---|---|---|---|---|
| Age, years | 51.1 ± 13.1 | 54.3 ± 12.5 | 53.1 ± 12.8 | |
| Male sex, n (%) | 19 (70.4) | 34 (70.8) | 53 (70.7) | |
| Caucasian race, n (%) | 27 (100.0) | 46 (95.8) | 73 (97.3) | |
| BMI, kg/m2 | 26.6 ± 5.5 | 26.2 ± 4.8 | 26.3 ± 5.0 | |
| First transplantation, n (%) | 25 (92.6) | 35 (72.9) | 60 (80.0) | |
| Time since last transplantation, months | 53.5 ± 68.1 | 85.3 ± 74.5 | 73.8 ± 73.4 | |
| Median (minimum, maximum) | 21.9 (0.8, 239.2) | 68.1 (2.3, 293.4) | 42.6 (0.8, 293.4) | |
| Concomitant diseases*, n (%) | Hypertension | 7 (25.9) | 36 (75.0) | 43 (57.3) |
| Diabetes | 7 (25.9) | 7 (14.6) | 14 (18.7) | |
| Coronary heart disease | 3 (11.1) | 10 (20.8) | 13 (17.3) | |
| Dyslipidaemia | 0 | 10 (20.8) | 10 (13.3) | |
| Viral infection | 6 (22.2) | 2 (4.2) | 8 (10.7) | |
| Osteoporosis | 1 (3.7) | 6 (12.5) | 7 (9.3) | |
| Rheumatism | 0 | 4 (8.3) | 4 (5.3) | |
| Malignancies | 0 | 3 (6.3) | 3 (4.0) | |
| Other | 14 (51.9) | 28 (58.3) | 42 (56.0) | |
| Previous rejection†, n (%) | 2 (7.4) | 13 (27.1) | 15 (20.0) | |
| Acute | 2 (7.4) | 10 (20.8) | 12 (16.0) | |
| Chronic | 0 | 5 (10.4) | 5 (6.7) | |
| Primary reason for transplantation*, n (%) | Cirrhosis | 11 (40.7) | – | – |
| Carcinoma | 6 (22.2) | – | – | |
| Sclerosing cholangitis | 1 (3.7) | – | – | |
| Other | 9 (33.3) | 16 (33.3) | – | |
| Unknown | 1 (3.7) | 1 (2.1) | – | |
| Polycystic disease | – | 16 (33.3) | – | |
| Glomerulonephritis | – | 11 (22.9) | – | |
| Diabetic nephropathy | – | 5 (10.4) | – | |
| Chronic pyelonephritis | – | 2 (4.2) | – | |
| Number of concomitant medications | 2.0 ± 1.5 | 3.6 ± 1.9 | 3.1 ± 2.0 | |
| Immunosuppression combined with immediate-release tacrolimus, n (%) | MMF | 8 (29.6) | 19 (39.6) | 27 (36.0) |
| MPA | 1 (3.7) | 5 (10.4) | 6 (8.0) | |
| Azathioprine | 0 | 5 (10.4) | 5 (6.7) | |
| Corticosteroids | 3 (11.1) | 0 | 3 (4.0) | |
| Sirolimus | 0 | 1 (2.1) | 1 (1.3) | |
| Leflunomide | 0 | 1 (2.1) | 1 (1.3) | |
| MMF + corticosteroids | 2 (7.4) | 13 (27.1) | 15 (20.0) | |
| MPA + corticosteroids | 0 | 3 (6.3) | 3 (4.0) | |
| Azathioprine + corticosteroids | 0 | 1 (2.1) | 1 (1.3) | |
| None | 13 (48.1) | 0 | 13 (17.3) | |
BMI = body mass index; MMF = mycophenolate mofetil; MPA = mycophenolic acid; SD = standard deviation Data are mean ± SD, unless otherwise stated. * More than one answer was possible. † Patients could have both acute and chronic previous rejection.
Mean tacrolimus dose remained stable over 12 months post-conversion, irrespective of organ transplanted (fig. 2A). Overall, the mean ± SD change in tacrolimus dose from baseline (immediate-release tacrolimus) to month 12 (prolonged-release tacrolimus) in the 68 patients who completed the study was −0.35 ± 1.71 mg/day. After conversion, the mean ± SD tacrolimus dose decreased by 5.1% from 3.53 ± 2.42 mg/day at baseline to 3.35 ± 2.26 mg/day at week 2 (n = 75). Moreover, 26.7% (20/75) of patients (kidney, n = 10; liver, n = 10) required dose adjustments after conversion and, therefore, attended a visit at week 4.
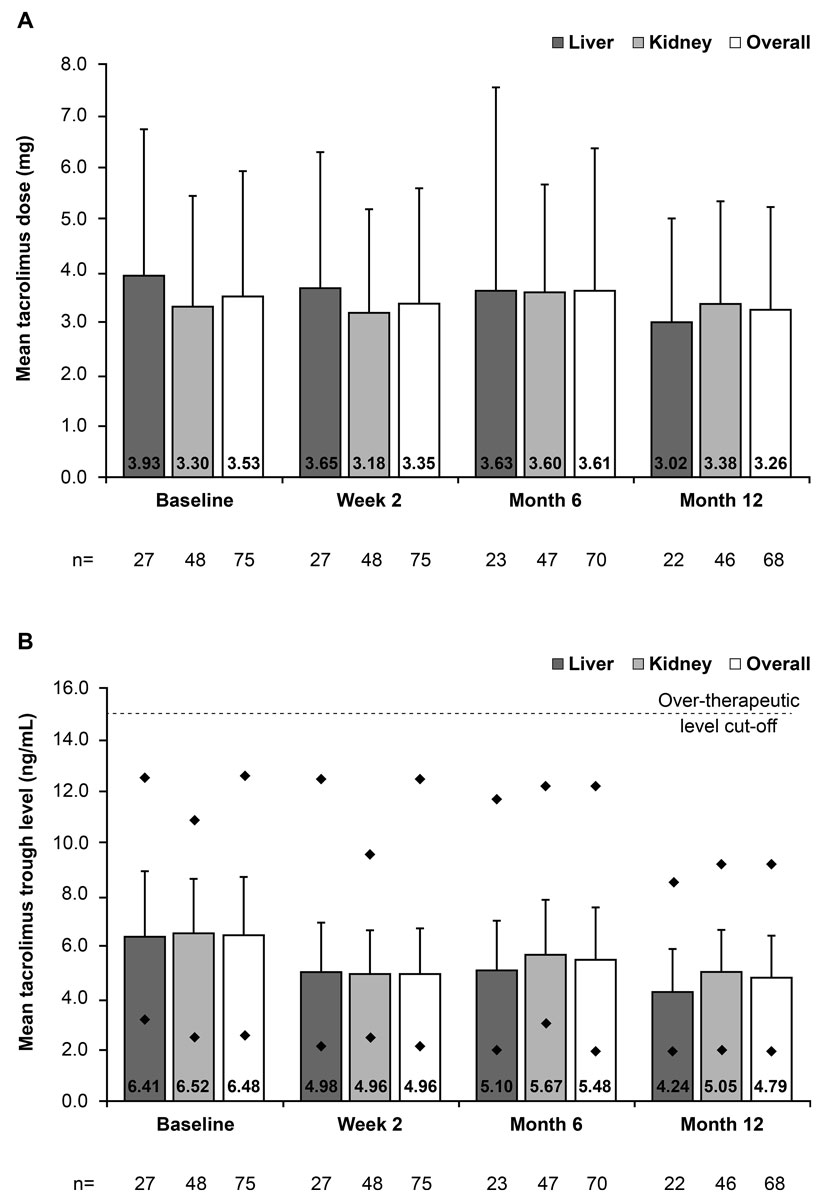
Figure 2 Mean (A) daily tacrolimus dose and (B) tacrolimus trough levels before and after conversion from immediate-release to prolonged-release tacrolimus, stratified by transplanted organ and overall (full-analysis set). Vertical lines represent the standard deviation. In figure 2B, diamonds represent the minimum and maximum tacrolimus trough level recorded at each visit.
Overall, the mean ± SD tacrolimus trough levels decreased by 23.5% between baseline and week 2 (from 6.48 ± 2.23 to 4.96 ± 1.75 ng/ml). The mean ± SD tacrolimus trough level was 4.79 ± 1.65 ng/ml at month 12, indicating a decrease from baseline of −1.54 ng/ml in the 68 patients who completed the study (fig. 2B). A similar pattern was observed when data were stratified by transplanted organ type. Overall across visits (excluding week 4), the maximum tacrolimus trough levels ranged between 9.2 ng/ml and 12.6 ng/ml, which was lower than the over-therapeutic threshold (>15 ng/ml) for defining non-adherence. The minimum tacrolimus trough levels ranged between 2.0 and 2.6 ng/ml (fig. 2B).
Concomitant medication use at baseline and subsequent study visits were similar (data not shown). The most frequent (>20%) classes of concomitant medications at all study visits for the SAF were as follows: antihypertensives (68.0%), drugs for acid-related disorders (38.7%), antithrombotic agents (26.7%), mineral supplements (25.3%), lipid modifying agents (24.0%), drugs used in diabetes (22.7%) and vitamins (22.7%).
Of 68 patients, 24 had missing data for at least one component of the primary endpoint; therefore, data for the primary composite endpoint were evaluable for 44 patients. Overall, 81.8% (36/44; 95% CI 68.0–90.5%) of patients were considered non-adherent in relation to the primary composite endpoint.
According to the BAASIS interview questionnaire, 28.3% (17/60; 95% CI 18.5–40.8%) of patients were non-adherent at month 12. Only 2.9% (2/68) of patients were given an adherence rating of “poor” by investigators (one kidney transplant recipient at week 2 [and week 4], and one liver transplant recipient at week 2). These two patients were adherent according to the BAASIS interview questionnaire and their tacrolimus trough level measurements. Overall, 50 patients had at least one valid tacrolimus trough level at months 6 or 12. Tacrolimus trough levels outside of the pre-defined therapeutic range were the largest contributor to the primary composite of non-adherence, and were detected in 62.0% (31/50) of patients. No patients had over-therapeutic tacrolimus trough levels.
Baseline data for the BAASIS interview questionnaire were available for 27 liver and 48 kidney transplant recipients (overall, n = 75). Of the patients who completed the study (liver, n = 22; kidney, n = 46), data were missing for four liver and four kidney transplant recipients at month 12. Therefore, non-adherence rates at month 12 were calculated for 18 liver and 42 kidney transplant recipients (overall, n = 60). Overall non-adherence, according to the BAASIS, was similar at baseline and month 12 (30.7 vs 28.3%, respectively), as was non-adherence in kidney transplant recipients (25.0 vs 26.2%, respectively). However, in liver transplant recipients, there was a decrease in the non-adherence rate from 40.7% at baseline (immediate-release tacrolimus), to 33.3% at month 12 (prolonged-release tacrolimus). The highest non-adherence rates were found for the timing dimension of the BAASIS interview questionnaire (29.3% and 25.0% at baseline and month 12, respectively, for the overall population). Most commonly, patients changed the timing of their tacrolimus intake once (13.3 vs 11.7% of patients at baseline and month 12, respectively) or two to three times (13.3 vs 10.0%, respectively) in the 4 weeks preceding administration of the questionnaire.
According to the BAASIS interview questionnaire, 12.0% of patients at baseline (immediate-release tacrolimus) and 10.0% of patients at month 12 (prolonged-release tacrolimus) omitted taking tacrolimus capsules within the past 4 weeks. Most commonly, patients omitted taking medication once (6.7% of patients at both baseline and month 12) or twice (5.3 vs 1.7% at baseline and month 12, respectively) within this time. When asked at baseline and month 12, no patients had omitted taking tacrolimus more than twice during the previous 4 weeks, except for one kidney transplant recipient at month 12, who omitted prolonged-release tacrolimus intake more than four times.
Data from the BAASIS interview questionnaire indicated that no patient had taken a drug holiday in the previous 4 weeks, except for one liver transplant recipient at month 12 who omitted prolonged-release tacrolimus intake twice in succession. No patient altered their dose without their doctor’s instruction to do so, or stopped taking their medication in the 4 weeks preceding administration of the questionnaire. Self-assessed adherence using the VAS was similar at baseline and month 12 (96.7% and 98.3%, respectively).
At baseline, investigators gave 89.3% (67/75), 9.3% (7/75) and 1.3% (1/75) of patients an adherence rating of “good”, “moderate”, or “poor”, respectively. Of the 68 patients who completed the study, 98.5% (67/68) were given an investigator rating of “good” at month 12, and adherence for one patient (1.5%) was rated “moderate”. For liver transplant recipients, adherence was rated “good”, except for 7.4% (2/27) of patients at baseline and 4.5% (1/22) at month 12, who were given an adherence rating of “moderate”. In kidney transplant recipients at baseline, investigators gave 87.5% (42/48), 10.4% (5/48) and 2.1% (1/48) of patients an adherence rating of “good”, “moderate”, or “poor”, respectively; adherence for all patients was rated “good” at month 12 (n = 46 with available data).
Overall, 66.7% (40/60) of patients experienced a reduction from baseline to month 12 in tacrolimus pill burden, following conversion from immediate-release to prolonged-release tacrolimus. The median daily number of tacrolimus capsules decreased in kidney recipients (from 3.0 to 2.0) and liver recipients (from 4.0 to 2.0) (table 3; mean data are presented in fig. 3).
Table 3 Daily intake of tacrolimus (number of capsules) at baseline (immediate-release tacrolimus) and month 12 (prolonged-release tacrolimus), and the proportion of patients reporting a change in tacrolimus pill burden between baseline and month 12, stratified by transplanted organ and overall (full analysis set).
| Parameter | Liver transplant | Kidney transplant | Overall | ||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 27) | Month 12 (n = 22)* | Baseline (n = 48) | Month 12 (n = 46)* | Baseline (n = 75) | Month 12 (n = 68)* | ||
| Median (range) number of tacrolimus capsules | 4.0 (2.0–8.0) |
2.0 (1.0–3.0) |
3.0 (1.5–8.0) |
2.0 (1.0–4.0) |
3.0 (1.5–8.0) |
2.0 (1.0–4.0) |
|
| n = 22* | n = 46* | n = 68* | |||||
| Change in tacrolimus pill burden at month 12 versus baseline, n (%) | Less | 12 (66.7) | 28 (66.7) | 40 (66.7) | |||
| Equal | 5 (27.8) | 8 (19.0) | 13 (21.7) | ||||
| More | 1 (5.6) | 6 (14.3) | 7 (11.7) | ||||
* Of the total study population (n = 68), data were missing for four liver and four kidney transplant recipients, and percentages were calculated based on patients with available data (liver, n = 18; kidney, n = 42; overall, n = 60).
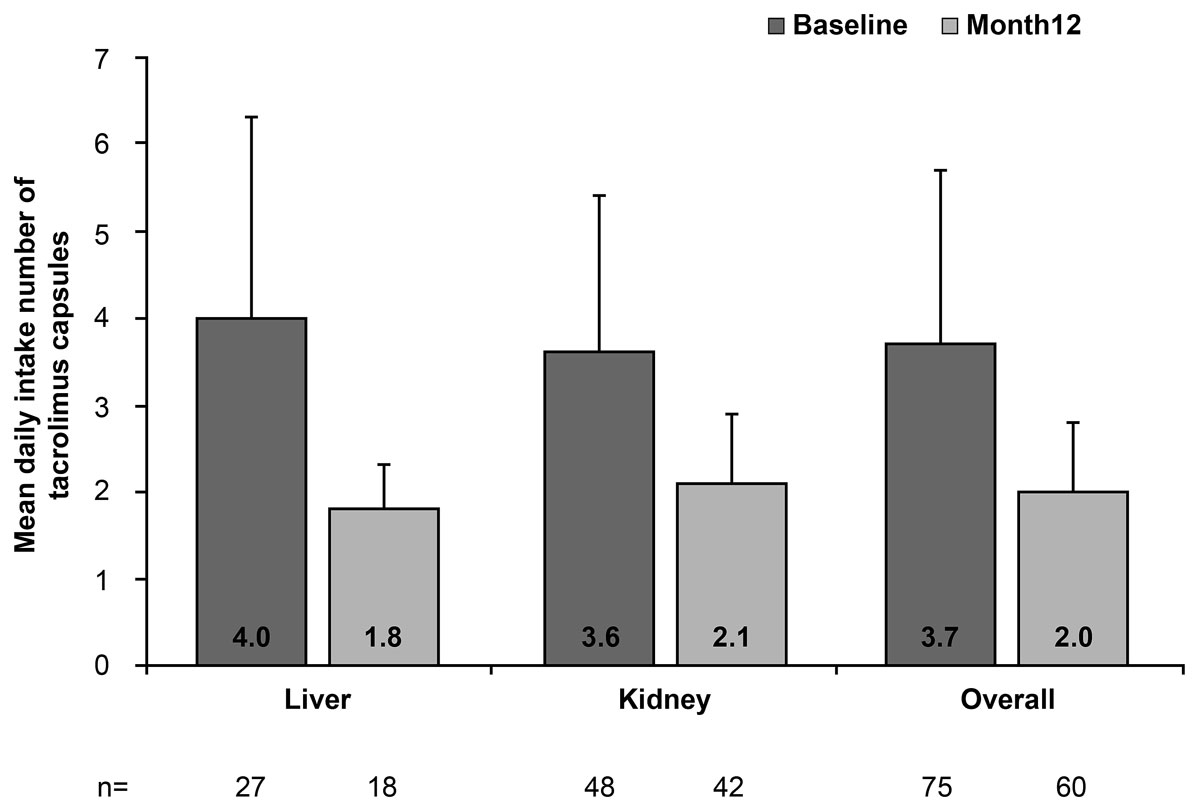
Figure 3 Mean daily intake number of tacrolimus capsules, stratified by transplanted organ and overall (full-analysis set). Vertical lines represent the standard deviation.
All patients were “very satisfied” or “satisfied” with their prolonged-release tacrolimus-based regimen 12 months after conversion from immediate-release tacrolimus (70.3% and 29.7%, respectively) (table 4). Overall, most patients perceived the prolonged-release tacrolimus formulation to be easier to remember to take (75.0% of patients) and more convenient (85.9%), compared with the immediate-release tacrolimus formulation. Furthermore, nearly all (94.7%) liver transplant recipients found the prolonged-release tacrolimus formulation to be easier to remember and more convenient to take, compared with 66.7% and 82.2%, respectively, of kidney transplant recipients (table 4).
Table 4 Patients’ satisfaction with once-daily, prolonged-release tacrolimus administration 12 months after conversion from immediate-release tacrolimus, stratified by transplanted organ and overall (full analysis set).
|
Liver transplant
(n = 27)* |
Kidney transplant
(n = 48)* |
Overall
(n = 75)* |
||
|---|---|---|---|---|
| Satisfied with once-daily administration | Very satisfied | 11 (57.9) | 34 (75.6) | 45 (70.3) |
| Satisfied | 8 (42.1) | 11 (24.4) | 19 (29.7) | |
| Not satisfied | 0 | 0 | 0 | |
| Easier to remember | Yes | 18 (94.7) | 30 (66.7) | 48 (75.0) |
| No | 1 (5.3) | 15 (33.3) | 16 (25.0) | |
| More convenient | Yes | 18 (94.7) | 37 (82.2) | 55 (85.9) |
| No | 1 (5.3) | 8 (17.8) | 9 (14.1) | |
Data are n (%). Patient satisfaction was recorded at month 12 or, in the case of early discontinuation from the study, at the time of discontinuation. * Of the total study population (n = 75), data were missing for eight liver and three kidney transplant recipients, and percentages were calculated based on patients with available data (liver, n = 19; kidney, n = 45; overall, n = 64).
Overall, mean eGFR remained stable over 12 months after conversion, irrespective of organ transplanted. Two kidney transplant recipients became dialysis-dependent during the study. When eGFR was set to 0 for these two patients, mean ± SD eGFR at baseline, week 2, month 6 and month 12 in the overall study population was 65.1 ± 26.4, 64.3 ± 25.2, 61.9 ± 25.2 and 61.8 ± 24.2 ml/min/1.73 m2, respectively. In the overall population, the mean ± SD change in eGFR from baseline to month 12 was −1.8 ± 13.1 ml/min/1.73 m2.
In liver transplant recipients, mean ± SD eGFR at baseline, week 2, month 6 and month 12 was 80.4 ± 24.0, 76.6 ± 25.1, 76.8 ± 22.3 and 74.7 ± 17.1 ml/min/1.73 m2, respectively, and the mean ± SD change in eGFR from baseline to month 12 was −5.5 ± 19.3 ml/min/1.73 m2. In kidney transplant recipients, when eGFR was set to 0 for the two patients who became dialysis-dependent during the study, mean ± SD eGFR at baseline, week 2, month 6 and month 12 was 56.5 ± 23.9, 57.0 ± 22.5, 55.0 ± 23.7 and 55.8 ± 24.8 ml/min/1.73 m2, respectively. The mean ± SD change in eGFR from baseline to month 12 was −0.01 ± 8.5 ml/min/1.73 m2.
The overall incidence of rejection, graft failure and dialysis was low, as events occurred in only two kidney transplant recipients. One kidney transplant recipient experienced an acute rejection episode, with subsequent graft loss and dialysis dependence. This patient was on dialysis at months 6 and 12. Another kidney transplant recipient became temporarily dialysis-dependent approximately 11 months after converting from immediate-release to prolonged-release tacrolimus, and was on dialysis at month 12. For both patients, the responsible investigators considered that the events of rejection, dialysis dependence and graft failure were unrelated to prolonged-release tacrolimus. There were no deaths due to graft failure or rejection episodes. Indeed, at month 12, investigators considered that the efficacy of prolonged-release tacrolimus was “very good” or “good” (65.7% and 34.3%, respectively).
Twenty ADRs were reported in 15 patients (20.0%) and are summarised in table 5; the most frequently reported system organ class was “infections and infestations” (9.3% of patients; all infections). Overall, 17 ADRs were assessed as serious, and two (extranodal marginal zone B-cell lymphoma [mucosa-associated lymphoid tissue type] and drug intolerance) led to study discontinuation in two liver transplant recipients.
Table 5 ADRs grouped by system organ class and preferred term (safety analysis set).
| System organ class | Preferred term | Incidence (n)* | Organ transplanted | ADR serious | Determined causality of ADR |
|---|---|---|---|---|---|
| Gastrointestinal disorders | 2.7% (2) | ||||
| Diarrhoea | 2.7% (2) | Kidney | No | Possible | |
| General disorders and administration site conditions | 1.3% (1) | ||||
| Drug intolerance | 1.3% (1) | Liver | No | Possible | |
| Infections and infestations | 9.3% (7) | ||||
| Pneumonia | 2.7% (2) | Kidney | Yes | Possible | |
| Respiratory tract infection | 2.7% (2) | Kidney | Yes | Possible | |
| BK virus infection | 1.3% (1) | Kidney | Yes | Possible | |
| CMV enterocolitis | 1.3% (1) | Kidney | Yes | Possible | |
| CMV infection | 1.3% (1) | Kidney | Yes | Possible | |
| CMV viraemia | 1.3% (1) | Kidney | Yes | Possible | |
| Metabolism and nutrition disorders | 1.3% (1) | ||||
| Gout | 1.3% (1) | Kidney | Yes | Possible | |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 5.3% (4) | ||||
| Squamous cell carcinoma of the skin | 2.7% (2) | Kidney | Yes | Possible | |
| B-cell lymphoma | 1.3% (1) | Kidney | Yes | Possible | |
| Extranodal marginal zone B-cell lymphoma (MALT type) recurrent | 1.3% (1) | Liver | Yes | Possible | |
| Oesophageal carcinoma | 1.3% (1) | Kidney | Yes | Could not be assessed | |
| Nervous system disorders | 1.3% (1) | ||||
| Hypoaesthesia | 1.3% (1) | Liver | Yes | Possible | |
| Paraesthesia | 1.3% (1) | Liver | Yes | Possible | |
| Respiratory, thoracic and mediastinal disorders | 1.3% (1) | ||||
| Dyspnoea | 1.3% (1) | Liver | Yes | Possible |
ADR = adverse drug reaction; CMV = cytomegalovirus; MALT = mucosa-associated lymphoid tissue; Note: patients could experience more than one ADR within a system organ class. * Incidences were calculated based on the safety analysis set (n = 75) and several events in the same patient falling into the same system organ class or preferred term were only counted once for the respective class or term.
For most patients, investigators rated the tolerability of prolonged-release tacrolimus at month 12 as “very good” or “good” (80.6% and 16.4%, respectively).
No new safety concerns were detected by evaluating clinical laboratory parameters and vital signs, and few abnormal, clinically relevant measurements were detected during the study. In the overall population, across study visits (excluding week 4), the mean ± SD haemoglobin level was between 127.6 ± 16.8 and 135.7 ± 15.3 g/l, and the mean ± SD glucose level was between 6.07 ± 1.73 and 6.37 ± 2.16 mmol/l. Mean ± SD HbA1c increased from 5.64 ± 0.75% pre-conversion to 6.72 ± 5.98% at week 2 post-conversion, but was similar to pre-conversion levels by month 12 (5.77 ± 0.96%). Mean ± SD serum creatinine ranged between 117.7 ± 43.3 and 123.7 ± 58.2 µmol/l, mean urine protein / urine creatinine was between 19.9 ± 23.8 and 28.8 ± 52.2 mg/mmol and mean ± SD urine albumin / urine creatinine was between 2.9 ± 3.5 and 5.3 ± 8.8 mg/mmol. For the optional tests, mean ± SD total cholesterol across visits was between 4.32 ± 1.22 and 4.49 ± 1.18 mmol/l, mean ± SD low-density lipoprotein was between 2.49 ± 1.06 and 2.62 ± 1.05 mmol/l, mean ± SD high-density lipoprotein was between 1.32 ± 0.58 and 1.66 ± 1.08 mmol/l and the mean ± SD triglyceride level was between 1.22 ± 0.46 and 1.56 ± 0.86 mmol/l.
Systolic and diastolic blood pressure remained stable during the study, as did pulse rate. In the overall population, across study visits (excluding week 4), the mean ± SD systolic blood pressure was between 130.4 ± 17.7 and 134.1 ± 17.0 mm Hg and the mean ± SD diastolic blood pressure was between 80.0 ± 12.2 and 81.0 ± 10.5 mm Hg.
Few studies have assessed adherence with prolonged-release tacrolimus in a real-world setting. This study provides the first implementation adherence data from adult, stable kidney and liver transplant recipients converted from immediate-release to prolonged-release tacrolimus as part of routine clinical practice in Switzerland. Furthermore, it is one of few published studies that used a three-part composite endpoint for measuring adherence. The study showed that a high proportion of patients were non-adherent for the primary composite endpoint, and that this was predominantly driven by sub-therapeutic tacrolimus trough levels. Tacrolimus adherence rates were similar at baseline and 12 months after conversion from the immediate-release to the prolonged-release formulation. However, patients experienced a reduction in tacrolimus pill burden between baseline and month 12 compared with the immediate-release formulation, and prolonged-release tacrolimus was associated with a convenience benefit.
The overall implementation non-adherence rate at month 12, as detected by the BAASIS, was 28.3%, which is within the range of published non-adherence rates following kidney and liver transplantation assessed with a variety of measurement methods and definitions [1, 2, 6, 19–25]. However, compared with previous reports of non-adherence rates (e.g., 30.9% reported by Beckebaum et al. [BAASIS] and 18.5% cited in the ADMIRAD study [electronic monitoring] [1, 7]), a higher proportion of patients (81.8%) were non-adherent for the primary composite endpoint in this study, following conversion from immediate-release to prolonged-release tacrolimus. This disparity is likely to be due to the strict definition of non-adherence applied here, which included sub-therapeutic tacrolimus trough levels – the largest contributor to the primary composite of non-adherence. A recently-published 18-month study, conducted in 153 kidney transplant recipients in Germany, used a composite endpoint similar to the one in our study, defining non-adherence as at least one of: (1) an affirmative response to any of the taking, timing or dose alteration dimensions on the BAASIS interview questionnaire at month 18; (2) investigator rating of adherence as “poor” at month 18; (3) at least one sub- or over-therapeutic tacrolimus trough level throughout the observation period [26]. The authors concluded that this definition of non-adherence might be too stringent [26]. However, the purpose of promoting immunosuppression adherence is to ensure adequate exposure to tacrolimus, particularly as tacrolimus is a drug with a narrow therapeutic index [27] and exposure to the drug is associated with transplant outcomes [28]. Therefore, we suggest that it is meaningful to consider sub- or over-therapeutic tacrolimus trough levels when assessing adherence in clinical studies. Whereas implementing a single sub-therapeutic tacrolimus trough level cut-off for all patients might have altered the primary outcome of this study, use of sub-therapeutic levels predefined by the investigator on an individual patient basis is more aligned with real-world clinical practice.
Implementation non-adherence rates were lower with investigator rating than with patient-rated non-adherence using the BAASIS interview questionnaire. Indeed, the two patients (2.9%) who were judged by investigators to be non-adherent, were adherent according to the BAASIS questionnaire. Discrepancy between physician and patient reporting of non-adherence was also recorded in the French PREDICT study of 370 kidney and liver transplant recipients [29]. Physicians considered their patients to be adherent with their immunosuppressive regimen in 61.6% of cases (using a VAS, where good adherence was defined as a score >median), whereas the patients considered themselves to be only moderately or poorly adherent (based on a validated six-item patient self-reported questionnaire) [29]. Therefore, when designing adherence studies, it is important to consider that adherence measurements can vary depending on the detection method used and whether data are reported by the patient or clinician. The BAASIS interview questionnaire and investigator rating of adherence are subjective and the questionnaire may be associated with recall bias. In this regard, a composite endpoint, such as that used in our study, may be a useful option.
Using the BAASIS adapted to capture adherence behaviour specific to tacrolimus, similar non-adherence rates were observed before and after conversion from immediate-release to prolonged-release tacrolimus for the overall population. This is contrary to what might be expected after simplification of the dosing regimen and with the convenience benefit associated with once-daily tacrolimus administration. Indeed, conversion from immediate-release to prolonged-release tacrolimus has previously been associated with improved medication adherence, assessed using a variety of measures of adherence to immunosuppressive regimens and to tacrolimus specifically [1, 7, 30, 31]. Of note, a numerical increase in adherence by the BAASIS was found in liver transplant recipients between baseline and month 12. This may be because almost half of the liver transplant patients were receiving tacrolimus monotherapy at baseline. Therefore, these patients were likely to be taking once-daily tacrolimus as the only immunosuppressive agent during follow-up, which would result in a simplified once-daily dosing regimen compared with immediate-release tacrolimus-based therapy. However, patient numbers are too small to draw conclusions.
Over half of patients reported a reduction in tacrolimus pill burden following conversion from immediate-release to prolonged-release tacrolimus. As a high pill burden can reduce quality of life [10], reducing pill burden with prolonged-release versus immediate-release tacrolimus is likely to benefit the patient, and may have contributed to the high levels of satisfaction the patients felt with the prolonged-release tacrolimus-based regimen. Indeed, patient satisfaction was greater in the liver than in the kidney transplant recipients, possibly because over 40% of liver transplant patients were taking tacrolimus monotherapy at baseline. Most patients also perceived the prolonged-release tacrolimus formulation to be more convenient and easier to remember to take, compared with the immediate-release tacrolimus formulation, which is consistent with previous conversion studies in kidney transplant recipients [26, 30]. Such findings are expected, as patients prefer to reduce dosing frequency, ideally to once-daily dosing [32, 33], and remove the evening dose of medication [10]. Conversion from immediate-release to prolonged-release tacrolimus may, therefore, provide convenience benefits for the patient in clinical practice.
Changes in mean tacrolimus trough levels generally reflected changes in mean tacrolimus dose. In line with a mean dose reduction of 5.1% observed between baseline and week 2, mean tacrolimus trough levels also decreased, albeit to a greater extent – by approximately 20%. A decrease in mean tacrolimus trough levels after conversion from immediate-release to prolonged-release tacrolimus has been previously reported [1, 31, 33]. For example, Dumortier et al. reported a tacrolimus trough level decrease in liver transplant recipients of approximately 20% (6.1 ng/ml before conversion vs 4.9 ng/ml after conversion) [34]. Sukkha et al. also recently reported a 23% reduction in tacrolimus trough level after conversion from immediate-release to prolonged-release tacrolimus early post-transplantation in de novo kidney transplant recipients [35]. Importantly, the decrease in tacrolimus trough levels was seemingly not associated with deleterious outcomes as, in line with previous reports, conversion from immediate-release to prolonged-release tacrolimus was associated with good graft and patient survival [36, 37]. Furthermore, renal function (eGFR) remained stable after conversion from immediate-release to prolonged-release tacrolimus, irrespective of organ transplanted, which is in line with previous reports [33, 34, 36]. In addition, no new safety signals were detected during the study, and the incidence and type of reported ADRs were as expected.
This study was associated with the limitations typical of non-interventional multicentre studies, such as potential bias due to patient selection being driven by unknown circumstances, and different practices between centres. However, the collection of ‘real-world’ data is of great relevance to inform conversion from immediate-release to prolonged-release tacrolimus in routine clinical practice. The study was not designed to evaluate trough tacrolimus levels, as measurement methods varied between centres, and the therapeutic target levels were investigator-defined. This may have impacted non-adherence for the primary composite endpoint. Additionally, it may be preferable to define non-adherence on the basis of several, rather than single, sub-therapeutic or over-therapeutic tacrolimus trough levels. A further limitation of using tacrolimus trough levels as a marker of non-adherence in this study was that no comparative trough level data were available for the 12 months before patients converted from immediate-release to prolonged-release tacrolimus. Tacrolimus trough levels were also collected and assayed based on individual centre protocol, with resulting variability in measurements; however, as tacrolimus trough levels were individualised to the patient, this was unlikely to impact the study findings. We acknowledge that the wording of patient satisfaction questions (2) and (3) may have been leading and, therefore, introduced bias. Tacrolimus pill burden and number of daily tacrolimus doses was reduced following conversion from immediate-release to prolonged-release tacrolimus, but this may not be true for overall pill burden and the number of daily doses. The number of pills associated with other immunosuppressive medications and concomitant medication was not collected. Recruitment was also slow and, therefore, fewer patients were included than initially intended. Additionally, due to the nature of the study, data were missing for some patients, which further reduced the number of patients available for analysis and may have impacted the robustness of the results.
As the results obtained in this observational study are based on a small number of adult stable transplant patients converted from immediate-release to prolonged-release tacrolimus, the generalisability of the findings herein may be limited. Nevertheless, the findings may be transferrable to other kidney and liver transplant patients treated in Switzerland and other similar European countries. Furthermore, the overall non-adherence rate detected by the BAASIS interview questionnaire is likely relevant to other patient populations, as are the patient convenience aspects of once-daily administration of tacrolimus.
This study provides the first implementation adherence data for adult, stable kidney and liver transplant recipients converted from immediate-release to prolonged-release tacrolimus in routine clinical practice in Switzerland. Sub-therapeutic tacrolimus trough levels were the largest contributor to non-adherence in this study, compared with non-adherence by the BAASIS interview questionnaire or investigator rating. Although non-adherence rates before and after conversion were similar, prolonged-release tacrolimus was associated with good patient satisfaction and reduced tacrolimus pill burden. Furthermore, prolonged-release tacrolimus was efficacious, and no new safety signals were detected.
Researchers may request access to anonymised participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
This study was sponsored by Astellas Pharma AG Switzerland. Daniella T Draper, PhD, CMPP, and Kirstie Park, BSc, for Cello Health MedErgy, assisted in drafting the initial version of the manuscript under the direction of the authors and provided editorial support throughout its development. The Basel Assessment of Adherence Scale for Immunosuppressives (BAASIS) was used in this study under a licence agreement between Astellas Pharma AG and the licence holder, the University of Basel.
This study was sponsored by Astellas Pharma, Inc. Two authors of this manuscript are employees of the sponsor, and fulfilled all authorship criteria. The sponsor reviewed the manuscript and analysed the data, but authors retained control over the data to be included in the publication, and the final decision to submit the manuscript. Editorial support was funded by Astellas Pharma, Inc.
All authors report non-financial support from Astellas during the conduct of the study. In addition, SG is an employee of Astellas, and MS is a former employee of Astellas. NG reports personal fees from Astellas, during the conduct of the study. RPW reports personal fees from Astellas, during the conduct of the study, and personal fees from Novartis, Otsuka and Vifor, outside of the submitted work.
1 Beckebaum S , Iacob S , Sweid D , Sotiropoulos GC , Saner F , Kaiser G , et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011;24(7):666–75. doi:.https://doi.org/10.1111/j.1432-2277.2011.01254.x
2 Vasquez EM , Tanzi M , Benedetti E , Pollak R . Medication noncompliance after kidney transplantation. Am J Health Syst Pharm. 2003;60(3):266–9. doi:.https://doi.org/10.1093/ajhp/60.3.266
3 Sellarés J , de Freitas DG , Mengel M , Reeve J , Einecke G , Sis B , et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–99. doi:.https://doi.org/10.1111/j.1600-6143.2011.03840.x
4 Wiebe C , Gibson IW , Blydt-Hansen TD , Pochinco D , Birk PE , Ho J , et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15(11):2921–30. doi:.https://doi.org/10.1111/ajt.13347
5 Gaynor JJ , Ciancio G , Guerra G , Sageshima J , Hanson L , Roth D , et al. Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up: a single-center observational study. Transplantation. 2014;97(9):925–33. doi:.https://doi.org/10.1097/01.TP.0000438199.76531.4a
6 Lieber SR , Volk ML . Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824–34. doi:.https://doi.org/10.1007/s10620-012-2412-0
7 Kuypers DRJ , Peeters PC , Sennesael JJ , Kianda MN , Vrijens B , Kristanto P , et al.; ADMIRAD Study Team. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333–40. doi:.https://doi.org/10.1097/TP.0b013e3182725532
8 Chapman JR . Compliance: the patient, the doctor, and the medication? Transplantation. 2004;77(5):782–6. doi:.https://doi.org/10.1097/01.TP.0000110411.23547.D4
9 Neuberger JM , Bechstein WO , Kuypers DR , Burra P , Citterio F , De Geest S , et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) group. Transplantation. 2017;101(4S, Suppl 2):S1–56. doi:.https://doi.org/10.1097/TP.0000000000001651
10 Morales JM , Varo E , Lázaro P . Immunosuppressant treatment adherence, barriers to adherence and quality of life in renal and liver transplant recipients in Spain. Clin Transplant. 2012;26(2):369–76. doi:.https://doi.org/10.1111/j.1399-0012.2011.01544.x
11 Hart A , Smith JM , Skeans MA , Gustafson SK , Wilk AR , Castro S , et al. OPTN/SRTR 2018 Annual Data Report: Kidney. Am J Transplant. 2020;20(Suppl s1):20–130. doi:.https://doi.org/10.1111/ajt.15672
12 Kwong A , Kim WR , Lake JR , Smith JM , Schladt DP , Skeans MA , et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20(Suppl s1):193–299. doi:.https://doi.org/10.1111/ajt.15674
13 Trunečka P , Boillot O , Seehofer D , Pinna AD , Fischer L , Ericzon B-G , et al.; Tacrolimus Prolonged Release Liver Study Group. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010;10(10):2313–23. doi:.https://doi.org/10.1111/j.1600-6143.2010.03255.x
14 Albano L , Banas B , Klempnauer JL , Glyda M , Viklicky O , Kamar N ; Optimising immunoSuppression After Kidney transplantation with ADVAGRAF Study Group. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation. 2013;96(10):897–903. doi:.https://doi.org/10.1097/TP.0b013e3182a203bd
15Astellas Pharma Europe Ltd. Summary of Product Characteristics: Advagraf 0.5mg, 1mg, 3mg and 5mg prolonged-release hard capsules. [Internet]. 2019 [cited 2019 Sep 19]. Available from: https://www.medicines.org.uk/emc/product/345/smpc
16 De Geest S , Zullig LL , Dunbar-Jacob J , Helmy R , Hughes DA , Wilson IB , et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169(1):30–5. doi:.https://doi.org/10.7326/M18-0543
17 Dobbels F , Berben L , De Geest S , Drent G , Lennerling A , Whittaker C , et al.; Transplant360 Task Force. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation. 2010;90(2):205–19. doi:.https://doi.org/10.1097/TP.0b013e3181e346cd
18Harrell FE, Dupont C, et al. Hmisc: Harrell miscellaneous. R package version 3.16-0. [Internet]. 2015. Available from: http://cran.microsoft.com/snapshot/2015-07-13/web/packages/Hmisc/index.html
19 Vlaminck H , Maes B , Evers G , Verbeke G , Lerut E , Van Damme B , et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004;4(9):1509–13. doi:.https://doi.org/10.1111/j.1600-6143.2004.00537.x
20 Butler JA , Roderick P , Mullee M , Mason JC , Peveler RC . Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769–76. doi:.https://doi.org/10.1097/01.TP.0000110408.83054.88
21 Rodrigue JR , Nelson DR , Hanto DW , Reed AI , Curry MP . Patient-reported immunosuppression nonadherence 6 to 24 months after liver transplant: association with pretransplant psychosocial factors and perceptions of health status change. Prog Transplant. 2013;23(4):319–28. doi:.https://doi.org/10.7182/pit2013501
22 Dew MA , DiMartini AFA , De Vito Dabbs A , Myaskovsky L , Steel J , Unruh M , et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–73. doi:.https://doi.org/10.1097/01.tp.0000258599.65257.a6
23 Chisholm-Burns M , Pinsky B , Parker G , Johnson P , Arcona S , Buzinec P , et al. Factors related to immunosuppressant medication adherence in renal transplant recipients. Clin Transplant. 2012;26(5):706–13. doi:.https://doi.org/10.1111/j.1399-0012.2011.01589.x
24 Tielen M , van Exel J , Laging M , Beck DK , Khemai R , van Gelder T , et al. Attitudes to medication after kidney transplantation and their association with medication adherence and graft survival: a 2-year follow-up study. J Transplant. 2014;2014:675301. doi:.https://doi.org/10.1155/2014/675301
25 Schmid-Mohler G , Thut MP , Wüthrich RP , Denhaerynck K , De Geest S . Non-adherence to immunosuppressive medication in renal transplant recipients within the scope of the Integrative Model of Behavioral Prediction: a cross-sectional study. Clin Transplant. 2010;24(2):213–22. doi:.https://doi.org/10.1111/j.1399-0012.2009.01056.x
26 Lehner LJ , Reinke P , Hörstrup JH , Rath T , Suwelack B , Krämer BK , et al. Evaluation of adherence and tolerability of prolonged-release tacrolimus (Advagraf™) in kidney transplant patients in Germany: a multicenter, noninterventional study. Clin Transplant. 2018;32(1):13142. doi:.https://doi.org/10.1111/ctr.13142
27 Tanzi MG , Undre N , Keirns J , Fitzsimmons WE , Brown M , First MR . Pharmacokinetics of prolonged-release tacrolimus and implications for use in solid organ transplant recipients. Clin Transplant. 2016;30(8):901–11. doi:.https://doi.org/10.1111/ctr.12763
28 Undre NA , van Hooff J , Christiaans M , Vanrenterghem Y , Donck J , Heeman U , et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc. 1999;31(1–2):296–8. doi:.https://doi.org/10.1016/S0041-1345(98)01633-9
29 Dharancy S , Giral M , Tetaz R , Fatras M , Dubel L , Pageaux G-P . Adherence with immunosuppressive treatment after transplantation: results from the French trial PREDICT. Clin Transplant. 2012;26(3):E293–9. doi:.https://doi.org/10.1111/j.1399-0012.2012.01652.x
30 van Boekel GAJ , Kerkhofs CHH , Hilbrands LB . Treatment satisfaction in renal transplant patients taking tacrolimus once daily. Clin Ther. 2013;35(11):1821–9.e1. doi:.https://doi.org/10.1016/j.clinthera.2013.09.014
31 Sabbatini M , Garofalo G , Borrelli S , Vitale S , Torino M , Capone D , et al. Efficacy of a reduced pill burden on therapeutic adherence to calcineurin inhibitors in renal transplant recipients: an observational study. Patient Prefer Adherence. 2014;8:73–81. doi:.https://doi.org/10.2147/PPA.S54922
32 Ichimaru N , Kakuta Y , Abe T , Okumi M , Imamura R , Isaka Y , et al. Treatment adherence in renal transplant recipients: a questionnaire survey on immunosuppressants. Transplant Proc. 2008;40(5):1362–5. doi:.https://doi.org/10.1016/j.transproceed.2008.02.083
33 Guirado L , Cantarell C , Franco A , Huertas EG , Fructuoso AS , Fernández A , et al.; GREAT Study Group. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant. 2011;11(9):1965–71. doi:.https://doi.org/10.1111/j.1600-6143.2011.03571.x
34 Dumortier J , Guillaud O , Boillot O . Conversion from twice daily tacrolimus to once daily tacrolimus in long-term stable liver transplant recipients: a single-center experience with 394 patients. Liver Transpl. 2013;19(5):529–33. doi:.https://doi.org/10.1002/lt.23638
35 Sukkha S , Chindavijak B , Montakantikul P , Ingsathit A , Nosoongnoen W , Sumethkul V . Trough level from twice daily to once daily tacrolimus in early conversion kidney transplant recipients: a prospective study. Int J Clin Pharm. 2017;39(6):1298–303. doi:.https://doi.org/10.1007/s11096-017-0549-9
36 Guirado L , Burgos D , Cantarell C , Fernández A , Franco A , Gentil MA , et al. Medium-term renal function in a large cohort of stable kidney transplant recipients converted from twice-daily to once-daily tacrolimus. Transplant Direct. 2015;1(7):e24. doi:.https://doi.org/10.1097/TXD.0000000000000536
37 Kim SH , Lee SD , Kim YK , Park S-J . Conversion of twice-daily to once-daily tacrolimus is safe in stable adult living donor liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2015;14(4):374–9. doi:.https://doi.org/10.1016/S1499-3872(15)60378-2
All authors analysed and interpreted the data, revised the article critically for important intellectual content, and approved the final version for submission. All authors, except SG, NG and MS, collected data. SG coordinated data collection and analysis and wrote the clinical study report, NG conducted the statistical analyses, and MS contributed to the concept and design of the study.
This study was sponsored by Astellas Pharma, Inc. Two authors of this manuscript are employees of the sponsor, and fulfilled all authorship criteria. The sponsor reviewed the manuscript and analysed the data, but authors retained control over the data to be included in the publication, and the final decision to submit the manuscript. Editorial support was funded by Astellas Pharma, Inc.
All authors report non-financial support from Astellas during the conduct of the study. In addition, SG is an employee of Astellas, and MS is a former employee of Astellas. NG reports personal fees from Astellas, during the conduct of the study. RPW reports personal fees from Astellas, during the conduct of the study, and personal fees from Novartis, Otsuka and Vifor, outside of the submitted work.