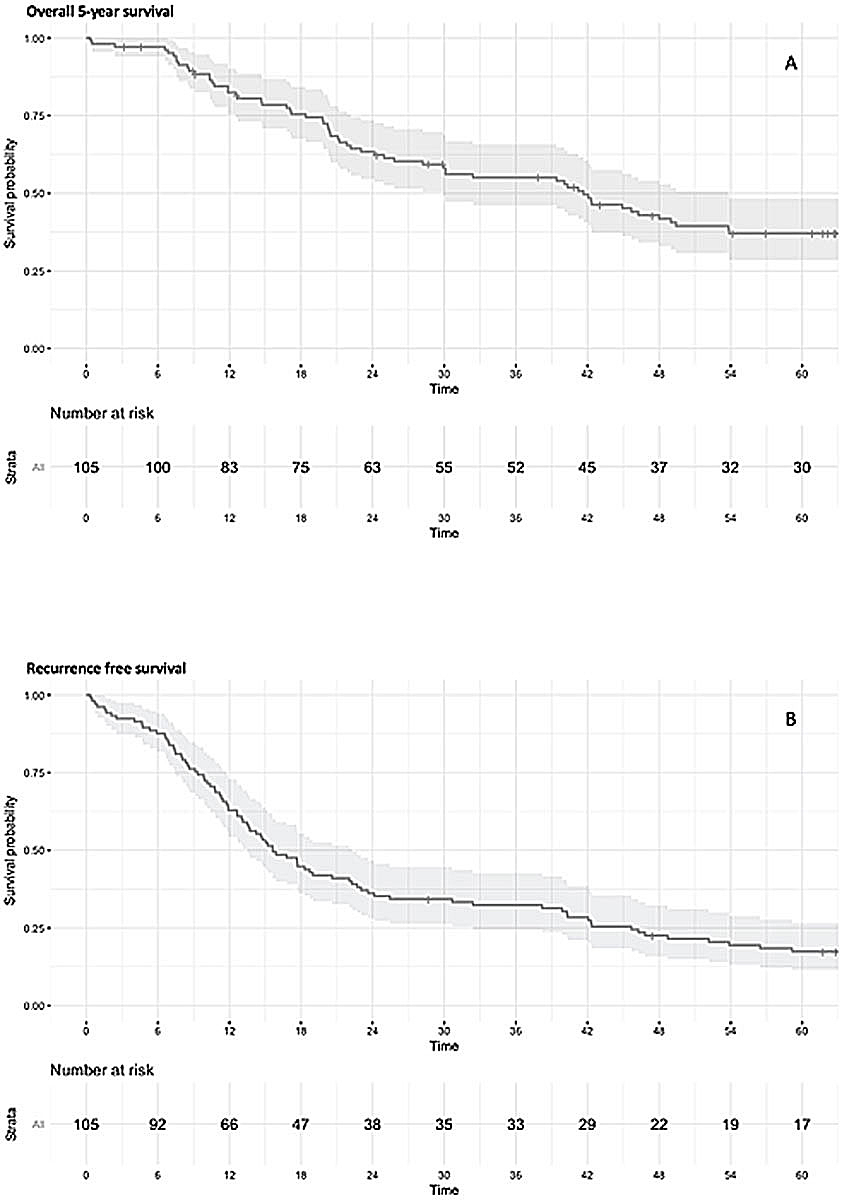
Figure 1 Kaplan Meier curves. A: Overall 5-year survival. B: Recurrence-free survival.
DOI: https://doi.org/10.4414/smw.2021.20385
confidence interval
computed tomography
endobronchial ultrasound guided fine needle aspiration
hazard ratio
the International Association for the Study of Lung Cancer
lymphovascular invasion
magnetic resonance imaging
non-small cell lung cancer
positron emission tomography
pathologically proven N2
standard deviation
tumour, node and metastasis classification
Involvement of mediastinal lymph nodes is the most significant prognostic factor in patients with non-small cell lung cancer (NSCLC) [1, 2].
Nevertheless, the N2 cohort – as defined in the 8th edition of the tumour, node and metastasis (TNM) classification for lung cancer by the International Association for the Study of Lung Cancer (IASLC) – consists of a highly heterogeneous group, ranging from ipsilateral single node involvement to bulky, multilevel mediastinal lymph node stations [3].
A stratification of N2 patients into prognostic subgroups has yet to be developed and validated.
Technical changes and extension of the lymphadenectomy have been studied for years, producing inconsistent results and mostly involving inhomogeneous lymphatic spread (pN0–pN2) [1, 4–6].
Studies analysing only the pN2 population have focused on the extent of nodal spreading, as well as on typical and atypical nodal spread according to the anatomical tumour location. The resulting implications for patient survival were inconsistent among various retrospective analyses [4, 7–9].
The broad spectrum of factors that were found to influence survival of the pN2 NSCLC cohort implies that further characterisation of this heterogeneous subgroup warrants important information, information that might accelerate the construction of future stratification schemes.
Accordingly, we examined our NSCLC pN2 cohort undergoing anatomical lung resection and lymphadenectomy between 2001 and 2010. We aimed to (i) analyse both the nodal spread according to tumour location and the possible implications of an unusual spreading pattern on survival, (ii) thoroughly characterise our pN2 cohort with special attention to preoperative staging and false negative results, postoperative complications, and (iii) identify factors influencing postoperative survival after anatomical lung resection.
In this retrospective observational study, clinical data was collected on 124 consecutive NSCLC patients with a pathological N2 (stage IIIA or B) [3] undergoing anatomical lung resection at our institution between 2001 and 2010. The pathologically proven N2 (pN2) cohort accounted for 16% of all our NSCLC patients undergoing anatomical lung resection within the study period.
Data were collected from a chart review of the electronic patient database. Detailed information about lymphadenectomy was gathered from the operative and pathology reports along with other procedural and histological details.
The local research ethics review committee approved the study (KEK-ZH-No. 2016.01712).
We assessed, in a descriptive manner, baseline demographics, lymph node involvement according to anatomical location of the tumour, histological details (Elastica van Gieson staining for vascular invasion and immunohistochemical staining with anti-D2-40 antibody to detect lymphovascular invasion (LVI)), procedural details and preoperative staging.
In addition, factors influencing 5-year overall survival and recurrence-free survival were analysed.
The lymph node stations were grouped into zones according to the IASLC nodal chart, and these zones were used to calculate the positive-to-sampled lymph node ratios (station 1–4 = upper zone; stations 5 and 6 = aortopulmonary zone; station 7 = subcarinal zone; stations 8 and 9 = lower zone; stations 10 and 11 = hilar/interlobar zone; stations 12–14 = peripheral zone; see table S1 in appendix 1) [10].
The lymphadenectomy that has been standard at our institution since 1999 was published as official recommendations by the European Society of Thoracic Surgeons in 2006, and consists of the resection of at least three N1 nodes, as well as three N2 nodes from three different mediastinal stations, including the subcarinal station [2]. For a right-sided tumour, the minimum acceptable lymphadenectomy consists of the extraction of stations 2 and 4, and the subcarinal (station 7) region; for left-sided tumours, the aortopulmonary (stations 5 and 6) and the subcarinal station should always be sampled. See appendix 1 for details regarding incomplete lymphadenectomy.
For this report we applied the 8th edition of the International Association for the Study of Lung Cancer (IASLC) TNM staging guidelines [3].
Preoperative tumour staging consisted of computed tomography (CT) and/or positron emission tomography (PET-CT; available at our institution from 2001), brain magnetic resonance imaging (MRI), or PET/MRI where there was clinical suspicion or tumour of stage II or greater.
Every patient underwent bronchoscopy; depending on tumour location, this process has included an endobronchial ultrasound guided fine needle aspiration (EBUS FNA) since 2003. For patients with PET positive mediastinal lymph node stations, either mediastinoscopy or EBUS was used for nodal staging.
All cases of malignant thoracic disease are reviewed on a multidisciplinary tumour board with representatives from thoracic surgery, pulmonology, pathology, radio-oncology and oncology present. The decision for a surgical resection is based on preliminary staging results in concordance with current guidelines and represents the consensus of the institutional tumour board.
Descriptive statistics were used to summarise patients’ characteristics. Continuous variables were reported as mean and standard deviation (SD) or median and interquartile range and were compared between the two groups using two-sample independent t-tests or the Mann-Whitney U-test (non-normal data). Categorical variables were summarised as frequencies (%) and compared using Pearson’s chi-squared test or Fisher’s exact test where applicable.
After completion of the descriptive statistics, an additional variable was created for atypical lymphatic spreading patterns – according to our findings and adapted from Sun et al. [8] – and included in the analysis as ‘beyond lobe specific lymphatic nodal zone involvement
Kaplan-Meier analysis was used to compare postoperative 5-year overall survival and recurrence-free survival.
Predictors of 5-year overall survival and recurrence-free survival were determined using Cox proportional hazards regression analysis. The variables included for univariate analysis in the Cox proportional Hazards model were a priori determined after literature review [4, 8, 9, 11, 12] and based on clinical parameters:
Variables with a p-value less than 0.25 in univariable analysis were retained in the multivariable cox models. ‘Adjuvant therapy’ was forced into the multivariable models in order to control for unspecified comorbidities leading to preclusion of an adjuvant treatment (the variable was included in the multivariable models irrespective of the p-value in univariable analysis). The likelihood ratio test for the global statistical significance of each model is reported. All variables retained in the multivariable model had no more than a weak correlation (Spearman correlation coefficient r<0.39, as suggested by Evans et al.) [13]. The proportional hazard assumption was assessed by plotting Schoenfeld residuals (smoothed plots). Adjusted hazard ratios (HRs) are reported for each variable with 95% confidence intervals (CIs).
SPSS version 24 (IBM corp., Armonk, NY) and R Studio version 3.2.1. (RStudio, Inc., Boston, MA) were used for data analysis. P-values <0.05 (two- tailed) were considered statistically significant.
We excluded patients who had undergone prior anatomical resection and lymphadenectomy for a NSCLC (not pN2; n = 3), had an R1 resection (n = 9) or stage IV NSCLC due to distant metastases (n = 1 adrenal gland, n = 5 brain metastases), and one patient with a history of heart transplant and consecutive immunosuppression, leaving a total of 105 patients in the analysis. Median age at time of surgery was 62 years (interquartile range 54 to 71 years), and 56% (n = 59) of the patients were males. Neoadjuvant therapy was given in 29 (27.6%) cases. Neoadjuvant chemotherapy consisted of three to six cycles of a combination of cisplatin or carboplatin with paclitaxel, docetaxel, pemetrexed (alone or combined with bevacizumab), or gemcitabine.
Neoadjuvant radiation therapy consisted of 22 × 2 Gy to the region of the primary tumour; in one case, it consisted of prophylactic cranial irradiation (total 44 Gy). See table 1 for more information about baseline characteristics.
Table 1 Baseline characteristics.
| Overall n = 105 | |
|---|---|
| Year of surgery | |
| – 2001 | 4 (3.8) |
| – 2002 | 6 (5.7) |
| – 2003 | 8 (7.6) |
| – 2004 | 16 (15.2) |
| – 2005 | 20 (19.1) |
| – 2006 | 7 (6.7) |
| – 2007 | 11 (10.5) |
| – 2008 | 7 (6.7) |
| – 2009 | 16 (15.2) |
| – 2010 | 10 (9.5) |
| Tumour location | |
| – Upper lobe | 52 (49.5) |
| Right upper lobe | 28 (26.7) |
| Left upper lobe | 24 (22.9) |
| – Middle lobe | 10 (9.5) |
| – Lower lobe | 43 (41.0) |
| Right lower lobe | 27 (25.7) |
| Left lower lobe | 16 (15.2) |
| Right sided tumour | 65 (61.9) |
| Neoadjuvant chemotherapy | 23 (21.9) |
| Neoadjuvant chemoradio therapy | 6 (5.7) |
| Non-smokers* | 15 (14.3) |
| Active smokers | 21 (20.0) |
| Former smokers | 68 (64.8) |
| Pack years, median (interquartile range) | 40.0 (20–55) |
| Preop. FEV1 (% predicted value), median (interquartile range) | 87.0 (74–102) |
| FVCex (% predicted value), median (interquartile range) | 97.0 (83–108) |
| Carbon monoxide diffusion (% predicted value), median (interquartile range)† | 75.0 (60–90) |
EBUS = endobronchial ultrasound, preop. = preoperative, FEV1 = forced expiratory volume in one second, FVCex = forced vital capacity. Data are n (%) unless otherwise stated * The smoking status for one patient remained unknown, † Data on CO diffusion were available for only 87 patients (82.9%).
Almost half the study population was clinically staged as N0 (n = 50, 47.6%). Three patients clinically staged as cN3 prior to induction chemotherapy were downstaged in the post-induction staging PET-CT, and underwent surgical resection afterwards. Of all patients undergoing EBUS (n = 85), 29.4% were falsely deemed to be cN0 (n = 25). PET-CT had a rate of 37/90 false negative (cN0) cases, and mediastinoscopy deemed 10 out of 21 patients mistakenly as cN0 (data not displayed in tables).
Median tumour diameter was 3.5 cm (interquartile range 2.2 to 4.7 cm), and the most common pathological primary tumour stage was pT2 (n = 45, 42.9%).
Intratumoural vascular invasion was present in 33 patients (31.4%), and LVI in 30 (28.6%). Involvement of the visceral pleura (T2) [3] was reported for 39 patients (37.1%).
One third of the study group had no N1 lymph node station involved (skip N2; n = 32, 30.5%), and 47.6% had only one N2 lymph node station involved (single station N2, n = 50). The lymphadenectomy consisted of a mean of 4.1 extracted N2 stations (SD 1.3), of which 8.6 nodes (SD 6.0) were extracted. The mean ratio of positive to sampled nodal zones was 0.6 (SD 0.27). Table 2 presents additional data regarding preoperative staging and further histological analyses.
Table 2 Staging and pathological specimen.
| Overall n = 105 | ||
|---|---|---|
| Histology | ||
| – Adenocarcinoma | 70 (66.7) | |
| – Squamous cell carcinoma | 22 (21.0) | |
| – Others* | 13 (12.4) | |
| Regional lymph node involvement (cN)† | ||
| – cN1 | 16 (15.2) | |
| – cN2 | 36 (34.3) | |
| Primary tumour (pT)† | ||
| – pT1 | 27 (25.7) | |
| – pT2‡ | 45 (42.9) | |
| – pT3‡ | 18 (17.1) | |
| – pT4‡ | 15 (14.3) | |
| Stage† | ||
| – IIIA | 72 (68.6) | |
| – IIIB | 33 (31.4) | |
Data are n (%) * Large cell carcinoma or adenosquamous carcinoma † IASLC NSCLC Staging Guidelines 8th Edition [3] ‡ 10 patients with T2 only due to invasion of the visceral pleura, while 29 T2 patients had tumour diameter >3 cm and showed invasion of the visceral pleura; 5 patients with T3 due to separate tumour nodules in the same lobe; and 8 patients with T4 due to invasion of the mediastinum (n = 4), infiltration of the recurrent laryngeal nerve (n = 1), infiltration of the vena cava (n = 1) or due to separate tumour nodules in a different lobe of the ipsilateral lung (n = 2).
As listed in table 3, lobectomy was the most common anatomical resection, followed by pneumonectomy (63.8% vs 25.7%, respectively). The only re-interventions were (n = 3) chest drains in the postoperative course.
Table 3 Procedural details and hospital stay.
| Overall n = 105 | |
|---|---|
| Lobectomy | 67 (63.8) |
| Bilobectomy | 11 (10.5) |
| Pneumonectomy | 27 (25.7) |
| Intrapericardial removal | 11 (10.5) |
| Re-intervention postop. | 3 (2.9) |
| Died within 5 years | 61 (58.1) |
Data are n (%)
Adjuvant therapy was administered in 60 cases (57.1%), which consisted of chemotherapy in 40, radiation therapy in 15, and combined chemo-radiation in 5 patients. Adjuvant chemotherapy consisted of three to four cycles of a combination of cisplatin or carboplatin with gemcitabine, vinorelbine or pemetrexed.
Adjuvant radiation therapy consisted of a total of 49–65 Gy mediastinal applied in 25 to 30 sessions.
A total of 29 patients underwent surgery only; for 19 patients, adjuvant treatment was not recommended due to comorbidities (n = 3 other malignancies, n = 16 diabetes mellitus with end organ damage, coronary heart disease, kidney failure, or any other significant comorbidity). Two patients refused the postoperative recommended therapy, and for eight patients the reason for preclusion of adjuvant therapy could not be clarified retrospectively.
Table 4 displays the involved N2 stations according to anatomical tumour location. Lymph node stations 2 and 4 were more commonly involved in NSCLC from the right upper and middle lobe than from the right lower lobe. (station 2: right upper vs right lower lobe, p = 0.001 and middle vs right lower lobe, p = 0.038; station 4: right upper vs right lower lobe, p <0.001 and middle vs right lower lobe, p = 0.056), whereas tumours in the right upper lobe showed significantly less involvement of stations 7 and 8 compared with right lower lobe tumours (station 7 p <0.001, station 8 p = 0.004). Left sided tumours in the upper lobe had significantly more involvement of station 5 compared to lower lobe tumours (p = 0.009). Involved and sampled nodal zones are listed according to anatomical tumour location in table S1 in appendix 1.
Table 4 Mediastinal lymph node involvement.
| Tumour location | Involved mediastinal lymph node station | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Station 1 | Station 2 | Station 3 | Station 4 | Station 5 | Station 6 | Station 7 | Station 8 | Station 9 | |
| Right upper lobe n = 28 |
0 | 18 (64.3) | 11 (39.3) | 22 (78.6) | - | - | 7 (25) | 0 | 0 |
| Middle lobe n = 10 |
1 (10) | 6 (60) | 3 (30) | 7 (70) | - | - | 6 (60) | 3 (30) | 0 |
| p-value upper vs middle lobe | * | * | * | * | - | - | * | ** | - |
| Right lower lobe n = 27 |
1 (3.7) | 5 (18.5) | 6 (22.2) | 8 (29.6) | 20 (74.1) | 7 (25.9) | 0 | ||
| p-value upper vs lower lobe | * | ** | * | ** | ** | ** | - | ||
| p-value middle vs lower lobe | * | ** | * | * | * | * | - | ||
| Left upper lobe n = 24 |
0 | 0 | 1 (4.2) | 6 (25.0) | 18 (75.0) | 6 (25.0) | 7 (29.2) | 5 (20.8) | 0 |
| Left lower lobe n = 16 |
0 | 0 | 1 (6.3) | 3 (18.8) | 5 (31.3) | 2 (12.5) | 9 (56.3) | 2 (12.5) | 2 (12.5) |
| p-value upper vs lower lobe | - | - | * | * | ** | * | * | * | * |
| Data are n (%) unless otherwise stated, * p-value >0.05, ** p-value ≤0.05 | |||||||||
In summary, right upper lobe tumours with lower and subcarinal station involvement – for right lower lobe tumours upper zone affection, and for left lower lobe tumours the AP zone – were identified as atypical patterns. According to this data and adapted from Sun et al. [8], the variable ‘beyond lobe specific lymphatic nodal zone involvement’ was created.
Two outcomes of interest were defined, 5-year overall survival and recurrence-free survival, which consisted of the time from surgery to either recurrence of the disease or death. Figures 1A and B display 5-year overall survival and recurrence-free survival up to 5 years after surgery. Median follow-up time was 32.4 months (interquartile range 14.7 to 62.7 months). Overall, 5-year overall survival of the pN2 cohort was 41.9%, and median time to recurrence or death was 15.6 months (interquartile range 9.2 to 44.0).

Figure 1 Kaplan Meier curves. A: Overall 5-year survival. B: Recurrence-free survival.
The uni- and multivariate analyses for 5-year overall survival are displayed in table 5. A total of 61 patients died within 5 years of surgery. Neoadjuvant therapy, as well as clinical N0/1 versus clinical N2/3 status, did not emerge as prognostic factors for 5-year overall survival within the pN2 cohort in univariable analysis. Histological subtype, intratumoural vascular invasion, and LVI, were included in the multivariable model. The variable ‘age at surgery’ violated the proportional hazard (PH) assumption, so an interaction term with time (age at surgery*time) was included in the multivariable model. Finally, adjuvant therapy was forced into the multivariable model, which fulfilled the proportional hazard assumption. See figure S1 in appendix 1 for the smoothed Schoenfeld residual plots of all covariates in the multivariable models for confirmation of proportional hazards. LVI was the only independent prognostic factor for 5-year overall survival (HR 2.10, CI 1.16–3.80; p = 0.015) when controlled for adjuvant therapy.
Table 5 Uni- and multivariable Cox regression analysis of 5-year overall survival.
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Exp(β) | Lower CI | Upper CI | p-value | Exp(β) | Lower CI | Upper CI | p-value | |
| Age (at surgery) | 1.01 | 0.99 | 1.04 | 0.23 | ||||
| Age*time (interaction term) | 0.99 | 0.98 | 0.99 | <0.001 | 1.01 | 1.00 | 1.02 | 0.21 |
| Histological subtype | 0.24 | 0.57 | ||||||
| – Adenocarcinoma | Ref | – | – | – | Ref | – | – | – |
| – Squamous carcinoma | 1.38 | 0.76 | 2.50 | 0.29 | 1.30 | 0.68 | 2.49 | 0.44 |
| – Other | 0.63 | 0.27 | 1.49 | 0.30 | 0.65 | 0.26 | 1.60 | 0.35 |
| Anatomical location (lung lobe and side) | 0.35 | |||||||
| – RUL | Ref | – | – | |||||
| – ML | 0.88 | 0.32 | 2.43 | 0.81 | ||||
| – RLL | 1.28 | 0.65 | 2.52 | 0.47 | ||||
| – LUL | 0.86 | 0.39 | 1.87 | 0.70 | ||||
| – LLL | 1.93 | 0.88 | 4.20 | 0.10 | ||||
| Skip N2 (pN2 but no N1 stations involved) | 0.80 | 0.46 | 1.39 | 0.43 | ||||
| Single station N2 | 1.16 | 0.70 | 1.92 | 0.55 | ||||
| Beyond lobe N2 (according to analysed lymph node spread pattern, see table S1) | 0.72 | 0.40 | 1.31 | 0.26 | ||||
| No. of positive N2 zones | 0.61 | |||||||
| – 1 | Ref | – | – | – | ||||
| – 2 | 1.01 | 0.57 | 1.79 | 0.98 | ||||
| – 3 | 0.64 | 0.20 | 2.07 | 0.46 | ||||
| Ratio of positive to sampled N2 zones | 1.55 | 0.50 | 4.87 | 0.45 | ||||
| Lymphovascular invasion | 2.03 | 1.21 | 3.43 | 0.010 | 2.10 | 1.16 | 3.80 | 0.015 |
| Intratumoural vascular invasion | 1.69 | 1.01 | 2.85 | 0.053 | 1.23 | 0.66 | 2.28 | 0.52 |
| Extranodal growth | 0.94 | 0.51 | 1.87 | 0.93 | ||||
| Neoadjuvant therapy | 1.18 | 0.68 | 2.05 | 0.56 | ||||
| Adjuvant therapy* | 0.80 | 0.48 | 1.32 | 0.38 | 1.24 | 0.43 | 1.52 | 0.51 |
| pT1–4 | 0.62 | |||||||
| – 1 | Ref | – | – | |||||
| – 2 | 0.70 | 0.38 | 1.30 | 0.25 | ||||
| – 3 | 0.75 | 0.35 | 1.63 | 0.47 | ||||
| – 4 | 1.02 | 0.47 | 2.21 | 0.96 | ||||
| cN 0/1 vs 2/3 | 1.14 | 0.133 | 1.96 | 0.34 | ||||
CI = confidence interval; LLL = left lower lobe; LUL = left upper lobe; ML = middle lobe; RLL = right lower lobe; RUL = right upper lobe * Adjuvant therapy was forced into the multivariable model. Likelihood ratio test = 13.58 on 6 df, p = 0.03464
The uni- and multivariate Cox model for recurrence-free survival is displayed in Table 6. There were 90 cases of recurrence or death within the follow-up period. Skip N2, beyond lobe-specific lymphatic nodal zone involvement, intratumoural vascular invasion and LVI, were included in the multivariable model. Adjuvant therapy was again forced into the model. All variables included in the multivariable model fulfilled the proportional hazard assumption (see figure S2 for the smoothed Schoenfeld residual plots of each covariate in the multivariable model). LVI was the only independent prognostic factor for recurrence-free survival in our study cohort with an HR 1.68 (CI 1.00–2.80; p = 0.049) when controlled for adjuvant therapy.
Table 6 Uni-and multivariable cox regression analysis of recurrence-free survival.
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Exp(β) | Lower CI | Upper CI | p-value | Exp(β) | Lower CI | Upper CI | p-value | |
| Age (at surgery) | 0.99 | 0.98 | 1.01 | 0.52 | ||||
| Histological subtype | 0.31 | |||||||
| – Adenocarcinoma | Ref | – | – | – | ||||
| – Squamous carcinoma | 0.85 | 0.50 | 1.43 | 0.53 | ||||
| – Other | 0.62 | 0.32 | 1.20 | 0.15 | ||||
| Anatomical location (lung lobe and side) | 0.52 | |||||||
| – RUL | Ref | – | – | – | ||||
| – ML | 1.03 | 0.46 | 2.30 | 0.94 | ||||
| – RLL | 1.30 | 0.74 | 2.27 | 0.36 | ||||
| – LUL | 0.81 | 0.14 | 1.49 | 0.50 | ||||
| – LLL | 1.34 | 0.69 | 2.60 | 0.38 | ||||
| Skip N2 (pN2 but no N1 stations involved) | 0.62 | 0.39 | 0.99 | 0.037 | 0.74 | 0.44 | 1.23 | 0.25 |
| Single station N2 | 0.85 | 0.56 | 1.28 | 0.43 | ||||
| Beyond lobe N2 (according to analysed lymph node spread pattern, see tableS1) | 1.43 | 0.91 | 2.24 | 0.13 | 1.14 | 0.69 | 1.86 | 0.61 |
| No. of positive N2 zones | 0.61 | |||||||
| – 1 | Ref | – | – | – | ||||
| – 2 | 1.28 | 0.79 | 2.06 | 0.32 | ||||
| – 3 | 1.15 | 0.52 | 2.52 | 0.73 | ||||
| Ratio of positive to sampled N2 zones | 1.78 | 0.60 | 5.31 | 0.30 | ||||
| Lymphovascular invasion | 2.08 | 1.32 | 3.29 | 0.003 | 1.68 | 1.00 | 2.80 | 0.049 |
| Intratumoural vascular invasion | 1.51 | 0.97 | 2.36 | 0.078 | 1.27 | 0.78 | 2.06 | 0.32 |
| Extranodal growth | 0.98 | 0.57 | 1.68 | 0.94 | ||||
| Neoadjuvant therapy | 1.05 | 0.59 | 1.53 | 0.83 | ||||
| Adjuvant therapy* | 1.16 | 0.76 | 1.76 | 0.50 | 1.06 | 0.68 | 1.64 | 0.79 |
| pT1–4 | 0.87 | |||||||
| – 1 | Ref | – | – | – | ||||
| – 2 | 0.82 | 0.49 | 1.35 | 0.43 | ||||
| – 3 | 0.84 | 0.44 | 1.58 | 0.58 | ||||
| – 4 | 0.81 | 0.41 | 1.61 | 0.55 | ||||
| cN 0/1 vs 2/3 | 0.96 | 0.77 | 1.20 | 0.72 | ||||
CI = confidence interval; LLL = left lower lobe; LUL = left upper lobe; ML = middle lobe; RLL = right lower lobe; RUL = right upper lobe * Adjuvant therapy was forced into the multivariable model. Likelihood ratio test = 11.99 on 5 df, p = 0.03493
With regard to the importance of lymphatic spread for the prognosis of NSCLC patients, lymphadenectomy and its impact on survival have been widely studied [14, 15], including nodal spread according to tumour location [5, 7, 16] and possible implications of an unusual spreading pattern on survival [7, 8], as well as the selection of cases where only partial lymphadenectomy might be acceptable [14, 15]. The 8th edition of the TNM classification recommends collecting further data, including the number of involved lymphatic stations or nodes [17]. This should permit further evaluation of subgroups within the pN2 population (skip N2, single station N2 and multiple station N2, as well as subdivision of N1 into single N1 and multiple station N1).
Baseline characteristics in our cohort confirmed known patterns within the pN2 NSCLC population, with right upper lobe as most common location [15, 16], and a propensity for metastases in the superior mediastinal zone.
Five-year overall survival rate was excellent: 41.9%, compared to 20.8% in the cohort from Sun et al. (all patients R0 resection, median survival time 31 months) [8], 29.9% reported by Kawasaki et al. [9], and 23% in the study population from Andre et al. [12]. The latter two studies did not exclude R1 or R2 resections; the study population from Andre et al. consisted of 23% incomplete resections, and the data collected was from 1989–1996. Yoo et al. reported a comparable 5-year overall survival rate of 37.7%, with a study period from 1997–2004 and inclusion of R0 resection only [4]. The reported 5-year overall survival rate was 36% for pN2 patients in the study population used by Asamura et al. for determining the validity of the current N descriptors in the TNM staging guidelines [17].
Our study cohort consisted of pathologically proven N2 NSCLC patients operated on at a single institution by certified thoracic surgeons, following a lymphadenectomy protocol valid for the whole department and including patients with complete tumour resection (R0) only. Therefore, our cohort consisted of a considerably homogenous population. Our resulting definition of beyond lobe-specific lymphatic spread accords well with the findings from much larger cohorts, like the results of Sun et al., who have published the largest pN2 NSCLC cohort so far (654 patients) [8].
However, as previously reported, independent predictors of 5-year overall survival or recurrence-free survival could not be confirmed in our cohort: skip N2 [5], single station N2 [4, 5, 12, 14, 15], ratio of positive to sampled N2 zones [6] and lymph node metastasis beyond lobe-specific lymphatic spread did not emerge as independent prognostic factors when controlled for adjuvant therapy. Neither did pT stage, which correlated significantly with tumour size (Spearman’s r = 0.605, p <0.001). Both tumour size and T stage have previously been reported as independent predictors of 5-year overall survival or recurrence-free survival [18–20].
Previous authors did not control for correlation between the variables in their multivariable cox model, and even the evaluation of proportional hazards is rarely reported (see figs S1 and S2: ‘Assessment of the proportional hazard assumption by plotting Schoenfeld residuals (smoothed plots)’). We strictly adhered to current statistical reporting guidelines and included the likelihood ratio test for each multivariable model [21, 22].
Interestingly, neither neoadjuvant therapy nor clinical N0/1 versus clinical N2/3 status emerged as prognostic factors for 5-year overall survival or recurrence-free survival within the pN2 cohort in univariable analyses; also, 37/90 pN2 patients undergoing a staging PET-CT scan were interpreted as cN0. These results have to be interpreted with caution. Of all patients with clinical N2 – or even N3 – status, we only included the ones who remained pN2 after induction treatment. The pN2 cohort consists of cN0 patients who were not expected to have positive lymph nodes according to preoperative staging, as well as cN3 patients who were downstaged after induction treatment and remained pN2 in the pathological workup. Patients with cN2 or cN3, and those with tumour regression after induction therapy to pN1 or pN0, were not included in the present study. Therefore, no conclusion regarding the general influence of neoadjuvant treatment can drawn, and no comparisons with regard to the number of correctly staged patients with PET-CT should be made from the presented data.
LVI emerged as the only independent predictor for 5-year overall survival and recurrence-free survival when controlled for adjuvant therapy in our cohort. Matsumura et al. examined a cohort of over 1000 patients for lymphatic permeation after resection of a NSCLC, and reported an incidence of 12% intratumoural, and 9% extratumoural, lymphatic permeation. However, except for patients who underwent neoadjuvant treatment, all tumour stages were included in this cohort. The authors concluded that lymphatic permeation should be evaluated after resection of a NSCLC due to its adverse prognostic impact [23].
In a meta-analysis of the effect of LVI, Wang et al. reported an overall incidence of 32.1% in the tumour samples from the 53 included studies examining over 18,000 patients with all stages of NSCLC. Additionally, only 6 of the included studies analysed LVI by immunohistochemistry, and a subanalysis of these studies could not confirm a significantly increased risk for recurrence in the resulting LVI population [24]. Due to the single centre setting, our study population is considerably smaller; with regard to the lymphadenectomy protocol and limited time frame of data collection, however, it is the most homogenous collectible sample within the pN2 NSCLC population.
Higgins et al. reported the presence of lymphovascular space invasion as an adverse prognostic factor of long-term survival in a cohort of 1559 NSCLC patients [11]. LVI was also independently associated with the presence of regional lymph node involvement, and strongly associated with an increased risk of developing distant metastases. The article was further discussed by R. Rami-Porta, who outlined the discrepancy between a general agreement on the adverse prognostic effects of LVI [25] and the lack of clear guidelines for the indication of adjuvant therapy [26]. Our study extends these results to a stage III population and confirms the adverse prognostic effect of LVI detected by immunohistochemistry, thereby revealing another subgroup within the pN2 population with worse prognosis regarding 5-year overall survival and recurrence-free survival.
Our department will continue to refine the data on nodal spread, lymphadenectomy, and – especially – LVI (including the quantification of LVI), in order to further examine this subgroup of NSCLC patients.
Our study suffers from a small sample size, which could inadvertently lead to a type II error regarding the influence of some variables on survival. Any pN2 cohort from a single institution will be relatively small. As mentioned above, however, collecting data within a single institution following a lymphadenectomy protocol valid for the whole department, and limiting the time frame of this collection, makes our sample considerably homogenous for a pN2 NSCLC cohort. As previously posited, this might limit generalisability to other pN2 cohorts, potentially explaining why some of the previously reported variables that characterised subgroups within the pN2 population did not emerge as independent predictors in our cohort. The survival analyses were meticulously documented, however, and fitted very cautiously with a priori determination of the tested variables, and the likelihood ratio test of each model was reported.
Lymphovascular invasion was identified as an independent prognostic factor for 5-year overall survival and recurrence-free survival when controlled for effects of adjuvant therapy in our pathologically proven N2 NSCLC cohort. However, previously reported variables characterising subgroups within the pN2 population – such as lymph node ratio or number of involved nodal zones – did not emerge as independent predictors, which suggests our results are limited in their generalisability.
The appendix files can be found in the PDF version of this article.
No financial support and no potential conflict of interest relevant to this article was reported
1 Saji H , Tsuboi M , Shimada Y , Kato Y , Yoshida K , Nomura M , et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest. 2013;143(6):1618–25. doi:.https://doi.org/10.1378/chest.12-0750
2 Lardinois D , De Leyn P , Van Schil P , Porta RR , Waller D , Passlick B , et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. 2006;30(5):787–92. doi:.https://doi.org/10.1016/j.ejcts.2006.08.008
3 Goldstraw P , Chansky K , Crowley J , Rami-Porta R , Asamura H , Eberhardt WE , et al.; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39–51. doi:.https://doi.org/10.1016/j.jtho.2015.09.009
4 Yoo C , Yoon S , Lee DH , Park SI , Kim DK , Kim YH , et al. Prognostic Significance of the Number of Metastatic pN2 Lymph Nodes in Stage IIIA-N2 Non-Small-Cell Lung Cancer After Curative Resection. Clin Lung Cancer. 2015;16(6):e203–12. doi:.https://doi.org/10.1016/j.cllc.2015.04.004
5 Riquet M , Rivera C , Pricopi C , Arame A , Mordant P , Foucault C , et al. Is the lymphatic drainage of lung cancer lobe-specific? A surgical appraisal. Eur J Cardiothorac Surg. 2015;47(3):543–9. doi:.https://doi.org/10.1093/ejcts/ezu226
6 Ohtaki Y , Shimizu K , Kaira K , Nagashima T , Obayashi K , Nakazawa S , et al. Risk factors associated with recurrence of surgically resected node-positive non-small cell lung cancer. Surg Today. 2016;46(10):1196–208. doi:.https://doi.org/10.1007/s00595-015-1301-5
7 Shimada Y , Saji H , Kakihana M , Honda H , Usuda J , Kajiwara N , et al. Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non-small cell lung cancer. World J Surg. 2012;36(12):2865–71. doi:.https://doi.org/10.1007/s00268-012-1743-5
8 Sun Y , Gao W , Zheng H , Jiang G , Chen C , Zhang L . Mediastinal lymph-nodes metastasis beyond the lobe-specific: an independent risk factor toward worse prognoses. Ann Thorac Cardiovasc Surg. 2014;20(4):284–91. doi:.https://doi.org/10.5761/atcs.oa.13-00028
9 Kawasaki K , Sato Y , Suzuki Y , Saito H , Nomura Y , Yoshida Y . Prognostic Factors for Surgically Resected N2 Non-small Cell Lung Cancer. Ann Thorac Cardiovasc Surg. 2015;21(3):217–22. doi:.https://doi.org/10.5761/atcs.oa.14-00218
10 Rusch VW , Asamura H , Watanabe H , Giroux DJ , Rami-Porta R , Goldstraw P ; Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(5):568–77. doi:.https://doi.org/10.1097/JTO.0b013e3181a0d82e
11 Higgins KA , Chino JP , Ready N , D’Amico TA , Berry MF , Sporn T , et al. Lymphovascular invasion in non-small-cell lung cancer: implications for staging and adjuvant therapy. J Thorac Oncol. 2012;7(7):1141–7. doi:.https://doi.org/10.1097/JTO.0b013e3182519a42
12 Andre F , Grunenwald D , Pignon JP , Dujon A , Pujol JL , Brichon PY , et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18(16):2981–9. doi:.https://doi.org/10.1200/JCO.2000.18.16.2981
13 Douketis JD , Spyropoulos AC , Kaatz S , Becker RC , Caprini JA , Dunn AS , et al.; BRIDGE Investigators. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373(9):823–33. doi:.https://doi.org/10.1056/NEJMoa1501035
14 Wu Y , Huang ZF , Wang SY , Yang XN , Ou W . A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer. 2002;36(1):1–6. doi:.https://doi.org/10.1016/S0169-5002(01)00445-7
15 Darling GE , Allen MS , Decker PA , Ballman K , Malthaner RA , Inculet RI , et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141(3):662–70. doi:.https://doi.org/10.1016/j.jtcvs.2010.11.008
16 Cerfolio RJ , Bryant AS . Distribution and likelihood of lymph node metastasis based on the lobar location of nonsmall-cell lung cancer. Ann Thorac Surg. 2006;81(6):1969–73, discussion 1973. doi:.https://doi.org/10.1016/j.athoracsur.2005.12.067
17 Asamura H , Chansky K , Crowley J , Goldstraw P , Rusch VW , Vansteenkiste JF , et al.; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015;10(12):1675–84. doi:.https://doi.org/10.1097/JTO.0000000000000678
18 Chen CY , Wu BR , Chen CH , Cheng WC , Chen WC , Liao WC , et al. Prognostic Value of Tumor Size in Resected Stage IIIA-N2 Non-Small-Cell Lung Cancer. J Clin Med. 2020;9(5):1307. doi:.https://doi.org/10.3390/jcm9051307
19 Liao Y , Wang X , Zhong P , Yin G , Fan X , Huang C . A nomogram for the prediction of overall survival in patients with stage II and III non-small cell lung cancer using a population-based study. Oncol Lett. 2019;18(6):5905–16. doi:.https://doi.org/10.3892/ol.2019.10977
20 Castello A , Toschi L , Rossi S , Finocchiaro G , Grizzi F , Mazziotti E , et al. Predictive and Prognostic Role of Metabolic Response in Patients With Stage III NSCLC Treated With Neoadjuvant Chemotherapy. Clin Lung Cancer. 2020;21(1):28–36. doi:.https://doi.org/10.1016/j.cllc.2019.07.004
21 Wu R , Glen P , Ramsay T , Martel G . Reporting quality of statistical methods in surgical observational studies: protocol for systematic review. Syst Rev. 2014;3(1):70. doi:.https://doi.org/10.1186/2046-4053-3-70
22 Hickey GL , Dunning J , Seifert B , Sodeck G , Carr MJ , Burger HU , et al.; EJCTS and ICVTS Editorial Committees. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg. 2015;48(2):180–93. doi:.https://doi.org/10.1093/ejcts/ezv168
23 Matsumura Y , Hishida T , Shimada Y , Ishii G , Aokage K , Yoshida J , et al. Impact of extratumoral lymphatic permeation on postoperative survival of non-small-cell lung cancer patients. J Thorac Oncol. 2014;9(3):337–44. doi:.https://doi.org/10.1097/JTO.0000000000000073
24 Wang J , Wang B , Zhao W , Guo Y , Chen H , Chu H , et al. Clinical significance and role of lymphatic vessel invasion as a major prognostic implication in non-small cell lung cancer: a meta-analysis. PLoS One. 2012;7(12):e52704. doi:.https://doi.org/10.1371/journal.pone.0052704
25 Salvati F . Lymphovascular invasion in non-small-cell lung cancer. J Thorac Oncol. 2013;8(1):e8–9. doi:.https://doi.org/10.1097/JTO.0b013e3182797de5
26 Rami-Porta R . Adjuvant therapy for resected non-small-cell lung cancer with lymphovascular invasion. J Thorac Oncol. 2013;8(1):e9–10. doi:.https://doi.org/10.1097/JTO.0b013e3182797f3b
No financial support and no potential conflict of interest relevant to this article was reported