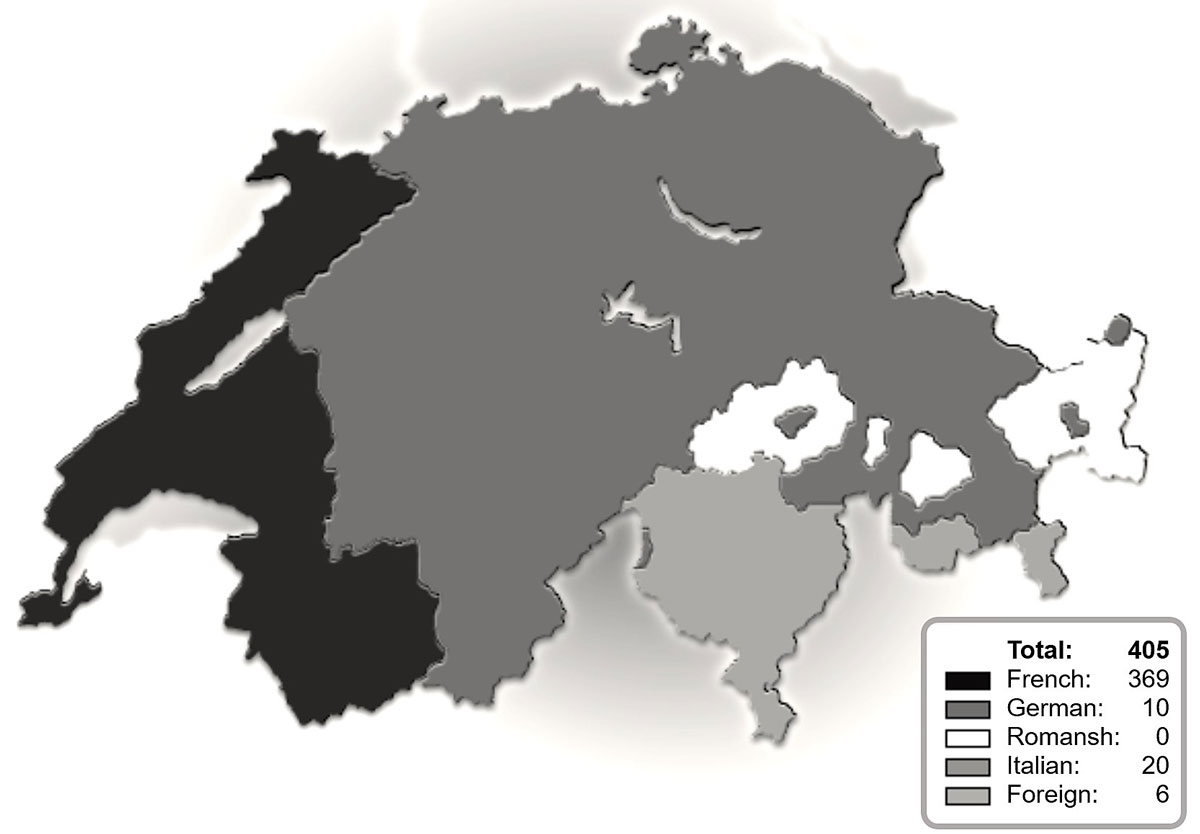
Figure 1 Numbers and origins of requests at the Swiss regional centre for trophoblastic disease.
DOI: https://doi.org/10.4414/smw.2021.20406
Gestational trophoblastic disease is characterised by lesions produced by abnormal trophoblastic proliferation that arise after abnormal fertilisation. These lesions are classified as benign (partial and complete moles) or malignant (malignant invasive mole, choriocarcinoma, atypical placental site nodule, placental site trophoblastic tumour and epithelioid trophoblastic tumour) [1]. These malignant forms are referred to collectively as gestational trophoblastic neoplasia. Gestational trophoblastic disease and neoplasia may both arise after normal pregnancies, miscarriage, ectopic pregnancies or abortion, but most gestational trophoblastic neoplasms will arise after complete moles. Due to its trophoblastic origin, with its characteristic high degree of proliferation, gestational trophoblastic neoplasms may quickly metastasise and present as advanced disease. Even in these advanced cases, treatment with curative intent is mandatory, because of the high response rate of most of these tumours to chemotherapy. Gestational trophoblastic disease is rare, with an incidence estimated to be around 150 cases per year in Switzerland [2], based on the incidence of one to three cases per 1000 pregnancies [3]. Because gestational trophoblastic diseases are rare, each country needs a reference centre to provide adequate diagnosis and treatment, as recommended by the European Society of Medical Oncology [4]. The first trophoblastic disease centre was created in 1965 in the USA, followed by the UK in 1973 [5] and France in 1999 [6]. These pioneer centres improved efficacy in diagnostic precision, optimal treatment and adequate care. Reports indicate that patients treated in specialised centres are better diagnosed and exhibit decreased mortality compared with patients not treated at specialised centres [6]. In this context, the first Swiss trophoblastic disease centre was created in 2009 in Geneva in collaboration with other public hospitals and private doctors.
The objectives of this study were to report the activity of the first regional Swiss trophoblastic disease centre during the past 10 years and to analyse the outcome of gestational trophoblastic disease.
This was a retrospective study from a database that includes the files of all patients registered at our trophoblastic disease centre since its opening in 2009. The cases were referred from Switzerland, Belgium and France. We analysed all patients registered at the Swiss trophoblastic disease centre from January 2009 to January 2019. To be registered at the centre, written informed consent from the patient is required. The trophoblastic disease centre reviews all histological specimens and provides clinical guidelines for treatment and follow-up of gestational trophoblastic disease and neoplasia. All cases were supervised by a certified onco-gynaecologist. Most of the patients were followed up or treated in their own region by their physician.
During the study period, we progressively changed the recommended duration of follow-up of certain gestational trophoblastic diseases and neoplasms, in line with international recommendations [4, 7].
The clinical features analysed were age, nature of previous gestation, the international prognostic International Federation of Gynecology and Obstetrics (FIGO) score and FIGO stages (in the case of gestational trophoblastic neoplasia), human chorionic gonadotropin (hCG) follow-up, treatment and outcome.
Gestational trophoblastic neoplasms were identified by histology or by the persistence of hCG as defined by the European Organization for Treatment of Trophoblastic Diseases (EOTTD) guidelines [8]. Staging and prognostic scores were based on the scoring system of the World Health Organization (WHO) and FIGO, as reported by FIGO [8, 9]. According to the FIGO scoring system, a score of 6 or less was defined as low risk, and a score of 7 or more as high risk. In our analysis, we decided to further separate the low risk category in two groups — a score equal to or less than 4 and a score of 5 to 6 — because of the increased risk of resistance in this latter subgroup [10, 11].
Chemotherapy treatment was based on the FIGO score, WHO staging and response to initial treatment. Surgical treatment was based on histology (presence of atypical placental site nodule, placental site trophoblastic tumour or epithelioid trophoblastic tumour), FIGO score, desire for future fertility and previous response to chemotherapy. Whenever possible, three courses of consolidation chemotherapy were administered. During treatment, serum hCG was measured every 2 weeks at a local laboratory. Proposed follow-up for low-grade gestational trophoblastic neoplasia was 1 year and for high-grade neoplasms was 2 years. Follow-up included monthly serum hCG measured at a laboratory chosen by the patient and her physician; ideally the same laboratory was used throughout treatment to avoid differences in sensitivities of the assays available on the market. In the event of resistance to initial treatment, as defined by the EOTTD [7], treatment was changed either to another single-agent therapy or to multi-agent therapy, depending on the newly calculated FIGO score.
Histological slides were reviewed by the pathologists of our centre. In 2010, genetic analysis of microsatellites based on quantitative fluorescence polymerase chain reaction (QF-PCR) testing was added to the review of histological slides. We considered the reviewed cases as being upgraded if the prognosis was worse than that of the original pathology, for example from partial to complete hydatidiform mole, or from complete hydatidiform mole to invasive hydatidiform mole or another gestational trophoblastic neoplasm. Similar logic was used to downgrade the final diagnosis.
We calculated descriptive statistics for the demographics of patients with gestational trophoblastic disease. Partial and complete hydatidiform moles were compared with the chi-square test and Fischer’s exact test for proportions and Student’s t-test for means. Type I error rates were set at 0.05. We performed all analyses with Stata/SE version 14.2.
The study was conducted at Geneva University Hospitals in accordance with Good Clinical Practice (Declaration of Helsinki 2002). Written informed consent was signed by each patient for clinical cases to be registered at the centre. The original consent form is available upon request. This study was approved by the local Human Research Ethics Committee (PO- 08-182).
A total of 405 patients and practitioners from Switzerland, France and Belgium contacted the centre during the study period. Most requests were from the French- and Italian-speaking parts of Switzerland (fig. 1). The activity of the centre increased notably in 2014 (fig. 2).

Figure 1 Numbers and origins of requests at the Swiss regional centre for trophoblastic disease.
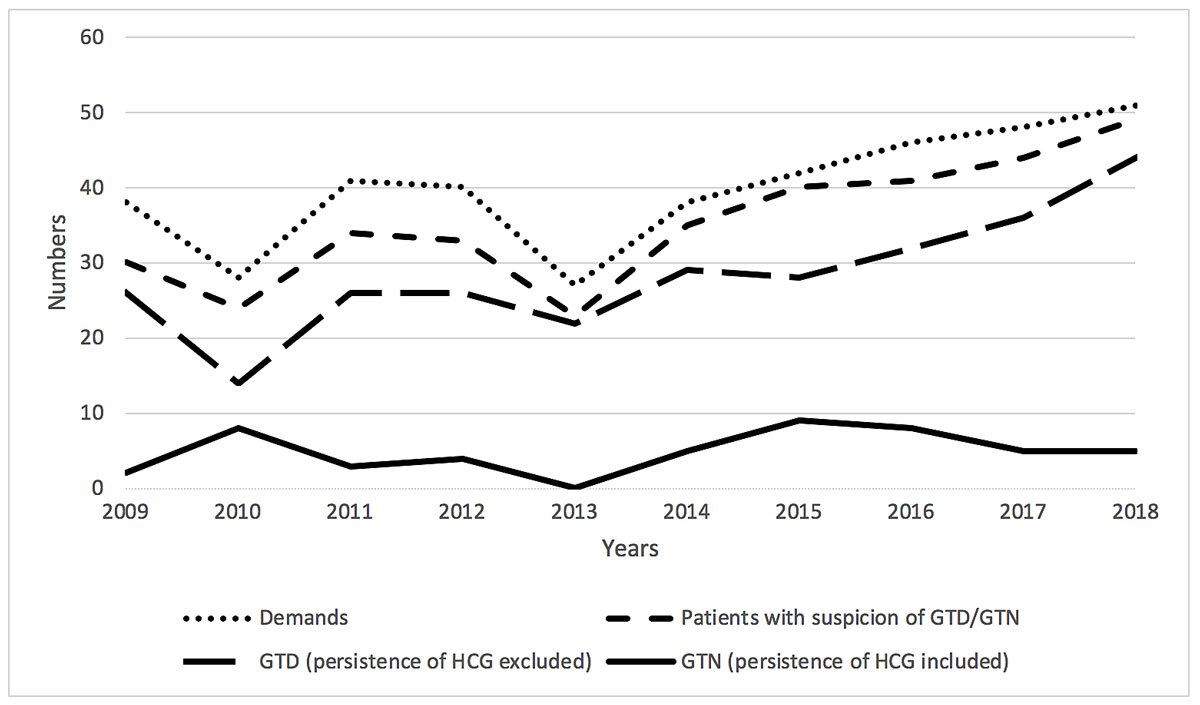
Figure 2 Evolution of recruitment. GTD = gestational trophoblastic disease; GTN = gestational trophoblastic neoplasia; HCG = human chorionic gonadotropin
Most of patients (354) consented to register at the centre. Of these, central pathology review established that 21 (6%) cases were not gestational trophoblastic disease, 156 (44%) patients had a partial hydatidiform mole, 163 (46%) a complete hydatidiform mole and 14 (4%) patients had a gestational trophoblastic neoplasm as evidenced by histology. During the follow-up of partial and complete hydatidiform moles, 35 (10%) patients developed a gestational trophoblastic neoplasm, defined by hCG persistence (fig. 3).
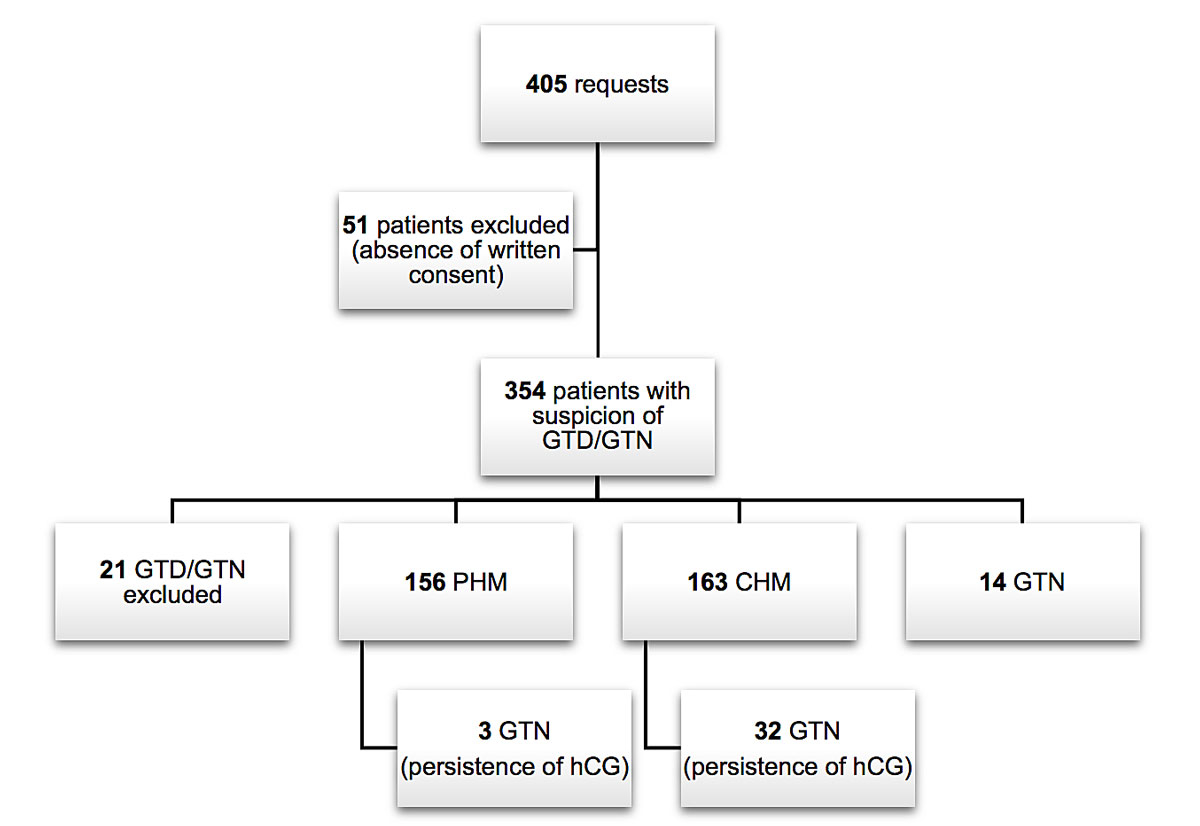
Figure 3 Flowchart of reference centre cases reviewed and final diagnosis. CHM = complete hydatidiform mole; GTD = gestational trophoblastic disease; GTN = gestational trophoblastic neoplasia; hCG = human chorionic gonadotropin; PHM = partial hydatidiform mole
Table 1 lists the results of the central pathology review by experienced pathologists, as well as the results of the genetic analysis. The overall rate of agreement between the referring centre and our centre was 82%. Histology was upgraded in almost 5% of reviewed cases; whereas, less than 1% of cases were downgraded. Central pathological review, which was performed 227 times, found the suspicion of gestational trophoblastic disease was not confirmed in 9% of the cases. Genetic testing was performed in 230 cases, either in the initial diagnostic analysis (for patients from our centre) or in a review, and included 203 QF-PCR tests, and 26 chromogenic in situ hybridisation and 20 fluorescence in situ hybridisation analyses. More than one test was performed for some patients. Approximately 15 samples resulted in inconclusive QF-PCR, as amplification profiles showed evidence of the presence of two genotypes, corresponding to massive maternal contamination. Inspection of complete hydatidiform moles identified 3 haploid cases, 2 cases of biparental diploidies (homozygous variants in NLRP7 gene (OMIM#609661) mutations), 1 digynic case and 82 androgenetic triploidies; the types of the remaining complete hydatidiform moles were not identified. From the 82 androgenetic complete hydatidiform moles, 12 were dispermic, 40 monospermic and 30 unspecified.
Table 1 Central pathology review.
|
Total
(n = 354) |
PHM (%)
(n = 156) |
CHM (%)
(n = 163) |
p-value | |
|---|---|---|---|---|
| Review of histology | 227 | |||
| Unchanged | 186 | |||
| Upgrade | 12 | |||
| Downgrade | 2 | |||
| GTD excluded | 21 | |||
| Genetic tests | 230 | 121 (77.6) | 109 (66.9) | 0.033* |
| QF-PCR | 203 | 99 (63.5) | 104 (63.8) | 0.891 |
| Failed QF-PCR | 15 | 10 (6.4) | 5 (3.1) | 0.161 |
| CISH | 26 | 20 (12.8) | 6 (3.7) | 0.003* |
| FISH | 20 | 16 (10.3) | 4 (2.5) | 0.004* |
| Genetic characteristics | ||||
| Haploid | 3 | - | 3 (1.8) | |
| NLRP mutation | 2 | - | 2 (1.2) | |
| Biparental | 2 | - | 2 (1.2) | |
| Digynic | 1 | 0 | 1 (0.6) | |
| Diandric | 127 | 45 (28.8) | 82 (50.3) | <0.001* |
| – Dispermic | 57 | 45 (28.8) | 12 (7.4) | <0.001* |
| – Monospermic | 40 | 0 | 40 (24.5) | <0.001* |
CHM = complete hydatidiform moles; CISH = chromogenic in situ hybridisation ; FISH = fluorescence in situ hybridisation; GTD = gestational trophoblastic disease; PHM = partial hydatidiform moles; QF-PCR = quantitative fluorescence polymerase chain reaction * p <0.05
Table 2 compares results for partial and complete hydatidiform moles. The average age of patients at the time of diagnosis was higher in the partial than in the complete hydatidiform mole group (33.8 [32.4–35.1] years and 31.9 [31.0–32.8] years, respectively, p = 0.025). The average gestational age at the time of the intrauterine evacuation was lower for complete than for partial hydatidiform moles (8.4 weeks [8.1–8.8] and 9.3 weeks [8.8–9.7], respectively, p = 0.005). Gestational trophoblastic neoplasia, defined as the persistence of hCG after molar pregnancy evacuation, was more frequent for complete than for partial hydatidiform moles (19.6% and 1.9%, respectively). Complete follow-up after evacuation was more frequent for partial than for complete hydatidiform moles (71% and 63%, respectively). Pregnancies during follow-up were more frequent for patients with partial than complete hydatidiform moles (16% and 7.4%, respectively, p = 0.016). The ideal type of contraception was not specified by the centre and left to the discretion of each doctor; however, it was stated in all official communications that combined hormonal contraception was not contraindicated during follow-up.
Table 2 Gestational trophoblastic disease.
|
PHM
(n = 156) |
CHM
(n = 163) |
p-value |
GTN
(n = 14) |
|
|---|---|---|---|---|
| Mean age (95% CI), years | 31.9 (31.0–32.8) | 33.8 (32.4–35.1) | 0.025* | 38.5 (33.5-43.5) |
| GTN by hCG persistence, n (%) | 3 (1.9) | 32 (19.6) | <0.001* | NA |
| Follow-up | ||||
| Complete hCG follow-up, n (%) | 110 (70.5) | 102 (62.6) | 0.133 | 9 (64.3) |
| Incomplete hCG follow-up, n (%) | 41 (26.3) | 40 (24.5) | 0.721 | 5 (35.7) |
| Actual hCG follow-up, n (%) | 5 (3.2) | 21 (12.9) | 0.002* | 0 |
| Pregnancy | ||||
| Pregnancy during/or after follow-up, n (%) | 35 (22.4) | 26 (16.0) | 0.141 | 0 |
| Pregnancy after hCG follow-up, n (%) | 10 (6.4) | 14 (8.6) | 0.461 | 0 |
| Pregnancy during hCG follow-up, n (%) | 25 (16.0) | 12 (7.4) | 0.016* | 0 |
| Dilatation and curettage | ||||
| Diagnosis by D and C, n (%) | 149 (95.5) | 158 (97.0) | 0.181 | 6 (42.9) |
| Diagnosis without D and C, n (%) | 7 (4.5) | 3 (1.8) | 0.181 | 8 (57.1) |
| Mean gestational age at the D and C (95% CI), weeks | 9.3 (8.8–9.7) | 8.4 (8.1–8.8) | 0.005* | NA |
| Second D and C, n (%) | 10 (6.4) | 17 (10.4) | 0.230 | 1 (7.1) |
| Second D and C for persistent hCG, n (%) | 2 (1.3) | 9 (5.5) | 0.062 | NA |
| Second D and C for other reasons, n (%) | 8 (5.1) | 8 (4.9) | 0.928 | NA |
CHM = complete hydatidiform moles; CI = confidence interval; D and C = dilation and curettage; GTN = gestational trophoblastic neoplasia; hCG = human chorionic gonadotropin; PHM = partial hydatidiform moles * p <0.05
Patients with gestational trophoblastic neoplasia are described in table 3. Forty-nine cases were registered in our centre, including 35 arising from persistent gestational trophoblastic disease and 14 histologically proven gestational trophoblastic neoplasms (three invasive hydatidiform moles, five choriocarcinomas, four intraplacental choriocarcinomas, one epithelioid trophoblastic tumour and one placental site trophoblastic tumour). Gestational trophoblastic neoplasia was confined to the uterus in 39 cases (FIGO stage I) and 6 patients were at FIGO stage III, mostly because of lung metastases. We found 34 patients with a FIGO prognostic score of 4 and less, 7 in the intermediate group (prognostic score of 5 or 6) and 5 in the high-risk group. Surgical treatment consisted of intrauterine evacuation and/or hysterectomy. Two patients had a second intrauterine evacuation, followed by a hysterectomy concomitant with chemotherapy (one persistent gestational trophoblastic disease FIGO III: 8 and one placental site trophoblastic tumour). Five patients treated by hysterectomy did not receive chemotherapy (two persistent gestational trophoblastic disease, two invasive hydatidiform moles and one epithelioid trophoblastic tumour), but all patients with a second intrauterine evacuation received chemotherapy.
Table 3 Gestational trophoblastic neoplasia.
|
hCG persistence
(n = 35) |
Invasive mole
(n = 3) |
Choriocarcinoma
(n = 5) |
Intraplacental choriocarcinoma
(n = 4) |
ETT
(n = 1) |
PSTT
(n = 1) |
Total
(n = 49) |
|
|---|---|---|---|---|---|---|---|
| Mean age (95% CI), years | 34.6 (31.7–37.6) | 51.3 (NA) | 38.6 (NA) | 31.3 (NA) | 37 | 30 | 35.8 (33.2–38.3) |
| FIGO stage | |||||||
| I | 29 | 2 | 2 | 4 | 1 | 1 | 39 |
| II | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| III | 4 | 0 | 2 | 0 | 0 | 0 | 6 |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| WHO prognostic scoring system (adapted by FIGO) | |||||||
| ≤4 | 26 | 2 | 2 | 4 | 0 | 0 | 34 |
| 5–6 | 4 | 0 | 1 | 0 | 1 | 1 | 7 |
| >6 | 3 | 0 | 2 | 0 | 0 | 0 | 5 |
| Unknown | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| Surgical treatment | 13 | 3 | 1 | 0 | 1 | 1 | 19 |
| Second D and C | 11 | – | – | 0 | – | 1 | 12 |
| Hysterectomy | 3 | 3 | 1 | 0 | 1 | 1 | 9 |
| Chemotherapy | 33 | 1 | 5 | 0 | 0 | 1 | 40 |
| Mtx | 24 | 1 | 1 | 0 | 0 | 1 | 27 |
| Mtx + ActD | 5 | 0 | 0 | 0 | 0 | 0 | 5 |
| Mtx + EMA-CO | 2 | 0 | 1 | 0 | 0 | 0 | 3 |
| EMA-CO | 2 | 0 | 3 | 0 | 0 | 0 | 5 |
| Incomplete chemotherapy | 4 | 0 | 0 | 0 | 0 | 1 | 5 |
| Consolidation | |||||||
| Complete | 12 | – | 3 | 0 | 0 | 0 | 15 |
| Partial | 6 | 1 | – | – | – | – | 7 |
| None | 6 | – | – | – | – | – | 6 |
| Outcome | |||||||
| Incomplete follow-up | 9 | 3 | 1 | 0 | 0 | 1 | 14 |
| Remission | 24 | - | 3 | 4 | 1 | - | 32 |
| Resistance | 8 | 1 | 1 | 0 | 0 | 1 | 11 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
ActD = actinomycin D; CI = confidence interval; D and C = dilation and curettage; EMA-CO = multi-agent chemotherapy (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine); ETT = epithelioid trophoblastic tumour; hCG = human chorionic gonadotropin; Mtx = methotrexate; PSTT = placental site trophoblastic tumour;
Methotrexate was our first line of single-agent chemotherapy in all low-risk and intermediate-risk cases, if indicated, and was used for 35 patients. The methotrexate protocol used was 1 mg/kg of intramuscular methotrexate on days 1, 3, 5 and 7 with folinic acid rescue (15 mg orally) on days 2, 4, 6 and 8. Of patients who followed this protocol, eight developed resistance (23%) as defined by the EOTTD [7], and chemotherapy was switched to either actinomycin D (0.5 mg intravenous bolus on days 1 to 5) (five patients) or EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine; three patients). Most of these patients were from the intermediate-risk group (four resistance, five intermediate-risk with indication of chemotherapy). Multi-agent chemotherapy (EMA-CO) was used as first-line treatment for five patients. Chemotherapy was stopped before reaching negative hCG levels (range 53–6566 U/l) in four patients, all during methotrexate treatment (range of cycles 1–17), because these patients refused to continue chemotherapy. Three of the patients spontaneously reached negative hCG levels, and to our knowledge, none have experienced reoccurrence so far. Of note, the fourth patient refused treatment after five cycles of methotrexate with hCG levels of over 6000 U/l, but is, 9 years later, alive and asymptomatic. Her hCG levels are not known. In the cases of intraplacental choriocarcinoma, the delivery of the placenta was considered as surgical treatment, and after careful hCG follow-up, none of the patients required further treatment.
Consolidation treatment was completed for 15 patients, incomplete for 7 patients, not given for 6 patients and unknown for the remaining 7 patients. Of the patients referred to our centre, 32 exhibited complete remission after finalisation of follow-up, whereas the follow up of 14 patients was considered as incomplete as the patients were either undergoing current follow-up (2 patients) or had discontinued follow-up (12 patients). To our knowledge, none of the patients followed up by our centre died of the described conditions. Two patients (not included in our table) were referred after starting a chemotherapy with methotrexate for a suspected gestational trophoblastic neoplasm that did not match criteria for a gestational trophoblastic neoplasia.
Gestational trophoblastic disease is rare: only 150 cases are expected to be diagnosed in Switzerland every year. On average Swiss pathologists will see one case a year, gynaecologists one case every 5 years, and medical oncologists only one case every 16 years. According to the literature, optimal hCG follow-up is lacking in one third of cases not followed in centres [12] and treatment is suboptimal in the same proportion [13]. The mortality of patients treated in reference centres is lower than for patients not treated in reference centres [14].
This study examined the activity of the first regional trophoblastic disease centre created in 2009 in Switzerland, at the Geneva University Hospitals. In Switzerland, reporting of gestational trophoblastic disease is voluntary and, thus far, we estimate that the centre has been successful in recruiting about 80% of the cases from the French- and Italian-speaking parts of Switzerland. Unfortunately, we have not been as successful in recruiting the gestational trophoblastic disease cases from the German-speaking part of Switzerland. We do not think that the language barrier is an explanation for this disparity, as the website is in German as well as in French, and most of the doctors in the centre are fluent in German. Difficulties in recruiting patients from the German-speaking parts of Switzerland may be, in part, explained by their closer professional relationships with Germany. The University of Bern has become an active partner in the centre, and this may lead to recruitment of more gestational trophoblastic disease cases from the German-speaking areas. The visibility of our centre could also be increased by producing national guidelines for the management of gestational trophoblastic disease that are endorsed by national societies for medical oncology, pathology and gynaecology, as well as by facilitating direct access to the website of the reference centre.
Central histopathological review by a pathologist with expertise in trophoblastic disease is one of the cornerstones of any trophoblastic disease centre and should be standard care. In our series, we found a change in diagnosis in 15% of the reviewed cases. This is lower than the numbers reported by Golfier et al. [6]. We have no clear explanation for this, other than the fact that most pathologists from the French- and Italian-speaking parts of Switzerland did their residency in the hospitals that are part of the centre, and thus may have a higher awareness of gestational trophoblastic disease. This change in diagnosis allowed, on one hand, adequate monitoring of hCG in the 12 patients who were upgraded, and, on the other hand, interruption of follow-up of the 21 patients for whom gestational trophoblastic disease was excluded, with the consequent psychological and economic benefits. In 65% of the cases in which pathology was reviewed, genetic analysis, primarily by QF-PCR, was used to increase the accuracy in the diagnosis of hydatidiform mole. The proportion of dispermic complete hydatidiform moles in our study, 23%, is similar to other studies [4, 15].
The average gestational age at the time of the intrauterine evacuation was quite similar for complete and partial hydatidiform moles, with a small difference of 1 week. Data in the literature is varied on this subject, with certain studies showing that complete hydatidiform moles are diagnosed before partial hydatidiform moles, whereas other studies concluded that diagnosis was reached at similar gestational ages [16–19]. The similarity in our gestational age at diagnosis and treatment could be explained by the wide accessibility of the population to gynaecologists and ultrasound in Switzerland. In Switzerland the health system is private, so a patient can choose freely, and waiting times before appointments in the case of pregnancy are usually less than a week. All gynaecologists are trained in gynaecological ultrasound, which explains its accessibility.
Patients with a partial hydatidiform mole completed follow-up more frequently than patients with a complete hydatidiform mole (71% and 63%, respectively), probably because of a shorter follow-up time after partial hydatidiform mole. During the study period, follow-up after partial hydatidiform mole changed from 6 months to two negatives results after negativised hCG, which probably sped up the completion of follow-up. Follow-up of a complete hydatidiform mole is longer than for partial hydatidiform mole, with a minimum of 6 months, depending on the decrease of hCG. This decreased time of follow-up could also explain why pregnancy was more common after partial than complete hydatidiform mole.
A total of 49 cases of gestational trophoblastic neoplasia were followed up at our centre during the time period of the study. Most cases were persistent gestational trophoblastic disease arising from complete or partial molar pregnancy (19.8% and 1.8%, respectively), similar to other studies [19–21].
Chemotherapy was administered in all but nine cases of GTN: in five cases a hysterectomy was performed after persistent gestational trophoblastic disease and four cases had stage I intraplacental choriocarcinoma without persistent hCG [22]. After a follow-up period of 57 months (mean and median 53 months), none of these patients have presented a recurrence [22].
Methotrexate was our first line of single-agent chemotherapy for all low-risk patients. Resistance was encountered in 23% of the cases, corresponding to 21% of the patients in the low-risk group and 80% of the patients in the intermediate-risk group. This is in accordance with resistance as described in the literature [11, 23, 24]. Of the patients who were resistant to methotrexate, second-line treatment of actinomycin D was administered to five patients, three patients received EMA-CO, two patients had a hysterectomy with no further chemotherapy and one patient had no further treatment (she refused actinomycin D). All but one of the patients in the high-risk group were treated with EMA-CO as first-line treatment. No patients have presented recurrence thus far.
This paper supports the important role of a reference centre in the case of gestational trophoblastic disease for optimising quality and improving patient management and experience. A reference centre ensures appropriate diagnosis, treatment and follow-up of patients, and may potentially contribute to reducing the costs and psychological burden associated with this disease.
The data that support the findings of this study have restrictions and so are not publicly available. Data are, however, available from the authors upon reasonable request.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Horn L-C , Einenkel J , Hoehn AK . Classification and Morphology of Gestational Trophoblastic Disease. Curr Obstet Gynecol Rep. 2014;3(1):44–54. doi:.https://doi.org/10.1007/s13669-013-0075-2
2 Rougemont AL , Pelte MF , Béna FS , Paoloni-Giacobino A , Petignat P , Finci V . [Trophoblastic diseases: a multidisciplinary approach, a first Swiss center]. Rev Med Suisse. 2011;7(303):1496–501. Article in French.
3 Seckl MJ , Sebire NJ , Berkowitz RS . Gestational trophoblastic disease. Lancet. 2010;376(9742):717–29. doi:.https://doi.org/10.1016/S0140-6736(10)60280-2
4 Seckl MJ , Sebire NJ , Fisher RA , Golfier F , Massuger L , Sessa C ; ESMO Guidelines Working Group. Gestational trophoblastic disease: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi39–50. doi:.https://doi.org/10.1093/annonc/mdt345
5 Bagshawe KD , Dent J , Webb J . Hydatidiform mole in England and Wales 1973-83. Lancet. 1986;328(8508):673–7. doi:.https://doi.org/10.1016/S0140-6736(86)90179-0
6 Golfier F , Raudrant D , Frappart L , Mathian B , Guastalla JP , Trillet-Lenoir V , et al. First epidemiological data from the French Trophoblastic Disease Reference Center. Am J Obstet Gynecol. 2007;196(2):172.e1–5. doi:.https://doi.org/10.1016/j.ajog.2006.10.867
7 Lok C , van Trommel N , Massuger L , Golfier F , Seckl M , Abreu MH , et al.; Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228–40. doi:.https://doi.org/10.1016/j.ejca.2020.02.011
8 Bolze PA , Attia J , Massardier J , Seckl MJ , Massuger L , van Trommel N , et al.; EOTTD group. Formalised consensus of the European Organisation for Treatment of Trophoblastic Diseases on management of gestational trophoblastic diseases. Eur J Cancer. 2015;51(13):1725–31. doi:.https://doi.org/10.1016/j.ejca.2015.05.026
9 Ngan HY , Seckl MJ , Berkowitz RS , Xiang Y , Golfier F , Sekharan PK , et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2015;131(Suppl 2):S123–6. doi:.https://doi.org/10.1016/j.ijgo.2015.06.008
10 Ngan HYS , Seckl MJ , Berkowitz RS , Xiang Y , Golfier F , Sekharan PK , et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2018;143(Suppl 2):79–85. doi:.https://doi.org/10.1002/ijgo.12615
11 Sita-Lumsden A , Short D , Lindsay I , Sebire NJ , Adjogatse D , Seckl MJ , et al. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000-2009. Br J Cancer. 2012;107(11):1810–4. doi:.https://doi.org/10.1038/bjc.2012.462
12 Feltmate CM , Batorfi J , Fulop V , Goldstein DP , Doszpod J , Berkowitz RS . Human chorionic gonadotropin follow-up in patients with molar pregnancy: a time for reevaluation. Obstet Gynecol. 2003;101(4):732–6.
13 Golfier F , Labrousse C , Frappart L , Mathian B , Guastalla JP , Trillet-Lenoir V , et al. Évaluation de la prise en charge des tumeurs trophoblastiques gestationnelles enregistrées au Centre de référence des maladies trophoblastiques de Lyon de 1999 à 2005 [Evaluation of treatment relating to gestational trophoblastic tumor registered to the French Trophoblastic Disease Reference Center (TDRC) in Lyon from 1999 to 2005]. Gynécol Obstét Fertil. 2007;35(3):205–15. Article in French. doi:.https://doi.org/10.1016/j.gyobfe.2006.12.023
14 Kohorn EI . Worldwide survey of the results of treating gestational trophoblastic disease. J Reprod Med. 2014;59(3-4):145–53.
15 Lipata F , Parkash V , Talmor M , Bell S , Chen S , Maric V , et al. Precise DNA genotyping diagnosis of hydatidiform mole. Obstet Gynecol. 2010;115(4):784–94. doi:.https://doi.org/10.1097/AOG.0b013e3181d489ec
16 Lelic M , Fatusic Z , Iljazovic E , Ramic S , Markovic S , Alicelebic S . Challenges in the Routine Praxis Diagnosis of Hydatidiform Mole: a Tertiary Health Center Experience. Med Arh. 2017;71(4):256–60. doi:.https://doi.org/10.5455/medarh.2017.71.256-260
17 Joneborg U , Marions L . Current clinical features of complete and partial hydatidiform mole in Sweden. J Reprod Med. 2014;59(1-2):51–5.
18 Khachani I , Alami MH , Bezad R . Implementation and Monitoring of a Gestational Trophoblastic Disease Management Program in a Tertiary Hospital in Morocco: Opportunities and Challenges. Obstet Gynecol Int. 2017;2017:5093472. doi:.https://doi.org/10.1155/2017/5093472
19 Sun SY , Melamed A , Joseph NT , Gockley AA , Goldstein DP , Bernstein MR , et al. Clinical Presentation of Complete Hydatidiform Mole and Partial Hydatidiform Mole at a Regional Trophoblastic Disease Center in the United States Over the Past 2 Decades. Int J Gynecol Cancer. 2016;26(2):367–70. doi:.https://doi.org/10.1097/IGC.0000000000000608
20 Berkowitz RS , Goldstein DP . Clinical practice. Molar pregnancy. N Engl J Med. 2009;360(16):1639–45. doi:.https://doi.org/10.1056/NEJMcp0900696
21 Lurain JR . Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203(6):531–9. doi:.https://doi.org/10.1016/j.ajog.2010.06.073
22 Jiao L , Ghorani E , Sebire NJ , Seckl MJ . Intraplacental choriocarcinoma: Systematic review and management guidance. Gynecol Oncol. 2016;141(3):624–31. doi:.https://doi.org/10.1016/j.ygyno.2016.03.026
23 Yarandi F , Mousavi A , Abbaslu F , Aminimoghaddam S , Nekuie S , Adabi K , et al. Five-Day Intravascular Methotrexate Versus Biweekly Actinomycin-D in the Treatment of Low-Risk Gestational Trophoblastic Neoplasia: A Clinical Randomized Trial. Int J Gynecol Cancer. 2016;26(5):971–6. doi:.https://doi.org/10.1097/IGC.0000000000000687
24 Goldstein DP , Berkowitz RS , Horowitz NS . Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anticancer Ther. 2015;15(11):1293–304. doi:.https://doi.org/10.1586/14737140.2015.1088786
AF and MU carried out study conception, systematic search, manual search, data retrieval, analysis, draft of manuscript and revision according to other authors’ suggestions, and submission. GR carried out data retrieval and analysis. KB and UM carried out data retrieval, analysis and revision. JT, FS, AG, AR, AB, PM and PP carried out critical revision of the manuscript. All authors read and approved the final manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.