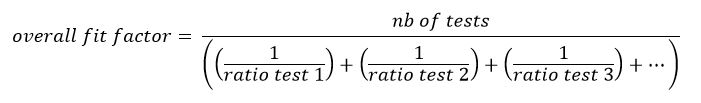
DOI: https://doi.org/10.4414/smw.2021.20459
SARS-CoV-2 is a respiratory virus that spreads mainly by contact or via droplets (aerodynamic diameter >5 μm; the diameter of an idealised spherical particle has the same aerodynamic behaviour as the physical airborne particle). However, laboratory tests suggest that SARS-CoV-2 may remain viable and infectious also in aerosols (aerodynamic diameter <5 μm) [1]. Droplets and aerosols are naturally produced by the human respiratory tract and both can contain the SARS-CoV-2 virus if produced by COVID-19 patients [2]. Furthermore, up to 40,000 droplets can be expelled during a single sneeze [3, 4].
Recent reports have suggested that healthcare workers are at increased risk of COVID-19 infection, particularly when access to personal protective equipment (PPE) is inadequate [5]. To protect healthcare workers from aerosols, particularly during aerosol-generating procedures, wearing a filtering facepiece (FFP) respirator is recommended [6]. In Europe, FFP respirators are divided according to their filtering efficiency into three different classes (FFP1 to FFP3) and are certified according to the European Standard EN 149. Healthcare settings use mainly FFP2 respirators. FFP2 respirators are equivalent to N95 and KN95 respirators, certified in the USA and China, respectively (for ease of reading, only “FFP respirator” is further used in the text and it should be understood as equivalent to other denominations such as protection mask type FFP2, N95, KN95, or equivalent). Since their introduction in hospitals in the 1990s, FFP respirators are routinely used by healthcare workers to protect themselves against bioaerosols such as those carrying tuberculosis, measles and selected respiratory viruses. Compared with surgical masks, FFP respirators fit tightly to the face with minimal leakage during inhalation, have a higher filtration efficiency and demonstrated a higher protection against SARS-CoV-2 transmission in recent studies [6–8].
The dramatic increase of COVID-19 patients hospitalised simultaneously in many countries during the first wave of the pandemic led to a very high demand for FFP respirators, which greatly outnumbered production capacity worldwide [9–11]. To cover current demand and in view of the plausible scenario of a future shortage, massive purchases of FFP respirators occurred and several producing countries decided to limit exports of PPE to protect their own market [11–14]. Therefore, the stocks of existing suppliers were rapidly exhausted, market prices for FFP respirators shot up to more than four times the original price [12] and new suppliers, often without prior experience in the manufacturing and/or distribution of medical and protection devices, established new channels of FFP respirator import, including into Switzerland.
Purchasing FFP respirators while assuring the minimal requirements according to the corresponding standards, either European or others, was difficult, and was made even more so by the proliferation of fraudulent websites, or of websites that provided unsupported claims or erroneous information [15]. Consequently, and similarly to other countries, such as the Netherlands that recalled 600,000 FFP respirators mid-March 2020, a number of FFP respirators imported into Switzerland during the first wave of the COVID-19 pandemic, mainly from China, were of low quality and potentially exposed healthcare workers to unnecessary risk [16–21].
Thus, Swiss federal and cantonal governments, some importers and buyers, as well as end-users wanted to test imported FFP respirators urgently. However, no Swiss testing laboratory was recognised as a notified body or accredited according to the European Standard EN 149. Furthermore, notified bodies across the European Union (EU) did not have enough resources to meet the increasing demand for testing. Therefore, three Swiss testing laboratories with sound experience in either testing PPE and/or in particle aerosol research were independently approached by stakeholders to assess the quality of FFP respirators.
The aims of this article are: (a) to raise awareness about the heterogeneous quality of FFP respirators imported into Switzerland during the first wave of the COVID-19 pandemic, (b) to increase the awareness of the Swiss medical community on the current directives regulating the market launch of FFP respirators in Switzerland, (c) to provide practical recommendations to identify suspicious products or documents, and finally (d) to offer strategies aimed at reducing the distribution of low-quality FFP respirators in the future.
Faced with the urgency of the situation and pressing demand for quality control of FFP respirators, three Swiss laboratories independently adapted their existing testing procedures, or set up new ones, to evaluate aerosol penetration and fit testing of FFP respirators imported into Switzerland: (a) Spiez Laboratory and (b) Unisanté in collaboration with TOXpro SA.
As a result of its growing experience with suspicious products and associated certificates, Spiez Laboratory also started to visually inspect the FFP respirators from mid-April 2020, to check product labelling and certification documents, and to crosscheck product information with published international databases.
For each FFP respirator evaluated, the information published by the European Safety Federation and the related certification database of the notified body were consulted [18]. In the case of irregularities in relation to the certificates of the products to be tested, the laboratory contacted the client and requested further explanation, previous test reports or certificates. If the required information was not provided, the laboratory declined to test the respirators. Additionally, all FFP respirators already recalled by the European Rapid Exchange of Information System (RAPEX), a rapid alert system for unsafe consumer products, were not tested [22].
The thermal bond between the different parts of the FFP respirator, as well as the ear loops and the nose piece, were visually checked for defects after donning and doffing. Visual checks were performed on multiple products across each batch and compared for differences.
First, an aerosol penetration test was performed using an experimental protocol adapted from EN 13274-7 (Respiratory protective devices – Methods of test- Part 7: Determination of particle filter penetration). A sodium chloride aerosol (0.6%), continuously generated by nebulisation (flow rate 2.5 l/min; relative humidity 40–50% rH) was characterised in terms of particle number, N, per volume (size distribution <300 nm) once, passing through a sealed filter housing containing a sample of the filtering media of the FFP respirator to be analysed (about 10 cm2). Background measurements (concentration of the aerosol in the absence of sample measured as a control) were performed via a bypass, driving the aerosol generated directly to the particle measurement system. The size distribution (range 11–307 nm) and particle counting for particle characterisation was achieved using a Scanning Mobility Particle Sizer (model SMPS+C model 5400, Grimm Aerosol Technik Ainring GmbH und Co. KG, Germany). The SMPS measured data expressed as dN/dln(dp) (cm-3) was converted into particle number concentration (N/cm3) using the SMPS software.
The penetration rate (filtration efficiency) was calculated as follows:
penetration rate = 100 × [particle]FFP / [particle]background
At least three samples (circular punches 37 mm diameter) of each FFP respirator were tested. Three consecutive scans were systematically averaged to calculate the penetration rate of both background and sample. The penetration rate data (n ≥3) were finally classified into three qualitative categories for penetration tests, with the value of 6% as upper threshold for FFP respirator: recommended (mean + standard deviation [SD] <6%), sufficient (mean <6%; mean + SD >6%), not recommended (mean + SD >6%).
Second, and only if FFP respirators passed the aerosol penetration test, quantitative fit-testing, designed to measure the seal between the respirator and the face, was performed according to the ANSI/AIHA Z88.10 protocol [23]. The ratio of ambient aerosol concentration outside the FFP respirator versus inside was measured using a PortaCount® Pro+ Respirator Fit Tester 8038 (TSI Incorporated, Shoreview, USA) on volunteers performing the following tasks for 1 minute each: normal breathing, deep breathing, head turning side to side, head tilting up and down, talking, bending over and normal breathing again.
The PortaCount® was configured in N95 mode, according to TSI recommendations, to select appropriate aerosol size range (around 40 nm) and avoid inward leakage overestimation due to MPPS (most penetrating particle size particles, around 300 nm) passing through the filtering media.
One woman (170 cm height) and two men (175, 188 cm height) were the volunteers. Results were expressed as overall fit factor according to the equation below:
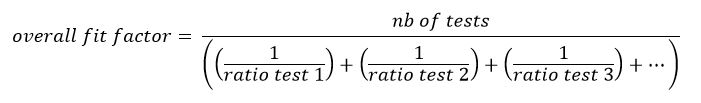
Average leakage ratio was then evaluated as the inverse of the overall fit factor. Overall leakage ratio was rated as recommended if the individual leakage ratios were ≤8% for all three volunteers and rated sufficient if two individual leakage ratios were ≤8%. If only two volunteers were available for testing, overall leakage ratio was rated as recommended if the individual leakage ratios were ≤8% for both volunteers and rated sufficient if one individual leakage ratio was ≤8%.
Fit testing (corresponding to the total inward leakage as defined in European Standard EN149) was quantified with three to ten test persons in an atmosphere charged with Paraffin aerosol. The aerosol concentration was measured with a PortaCount® Pro+ Respirator Fit Tester 8038 (TSI Incorporated, Shoreview, USA), configured in N95 mode, outside and inside the FFP respirator while the test persons were performing a series of tasks, each lasting 2 minutes, on a treadmill at 6 km/h, according to EN 149. The total inward leakage is the ratio of the inside to the outside concentrations, whereas the fit factor is the inverse thereof. The overall fit factors were calculated using the arithmetic mean of the fit factor measured during each task.
A similar experiment was performed with a Sheffield testing head form, equipped with an external artificial breathing circuit (Dräger Künstliche Lunge AS 50/2000, Drägerwerk AG), set at a breathing rate of 30 l/min. The edges of FFP respirators were sealed with duct tape on the testing head to insure that there was no leakage. These tests aimed at understanding whether the fit testing failed because of an inappropriate respirator shape (design) or because of an inefficient filtering material.
A FFP respirator was considered as recommended if the fit testing results fulfilled the requirements given by EN 149 for FFP2 or equivalent respirators: ≥92% of individual tasks have a fit factor of at least 9 (i.e., a penetration below 11%) and ≥80% overall fit factors have a value of at least 13 (i.e., a penetration below 8%).
When only three or four samples were tested, the respirator was rated as recommended if the overall fit factors were ≥13 for all the volunteers and rated sufficient if all but one overall fit factors were ≥13.
The results of the tested FFP respirators received by Spiez Laboratory and Unisanté-TOXpro SA for evaluation between 31 March and 15 June 2020 are summarised in table 1. Thirty-five percent of the FFP respirators sent to Spiez Laboratory were declined for testing for at least one of the following reasons:
Table 1 Results of FFP respirators evaluated either by Spiez Laboratory or by Unisanté-TOXpro SA during the COVID-19 pandemic (n = 151).
| Spiez Laboratory | Unisanté-TOXpro SA | |
|---|---|---|
|
Total rejected before testing
(in % of test demands) |
35% | n.a. |
| Of which: | ||
| – Recall from EU | 7% | n.a. |
| – Rejected (visual inspection/certification) | 28% | n.a. |
|
Total rejected after testing
(in % of tested masks) |
52% | 60% |
| Details: | ||
| Aerosol penetration test (in % from performed aerosol tests) |
||
| – Recommended | 78% | 66% |
| – Sufficient | 10% | 4% |
| – Not recommended | 12% | 30% |
| Fit test (in % from performed fittest tests) |
||
| – Recommended | 48% | 30% |
| – Sufficient | 2% | 10% |
| – Not recommended | 50% | 60% |
Among the tested FFP respirators, aerosol penetration and fit testing failed in 24% and 55% of the tested products, respectively. In total, 52% and 60% of all products tested by Spiez Laboratory and Unisanté-TOXpro SA, respectively, did not meet the minimum performance required by the testing laboratories as defined in the “Material and methods” section.
The main outcome of this study is that more than half of the FFP respirators tested during the first wave of the COVID-19 pandemic did not meet the requirements defined by the three testing Swiss laboratories, which were based mainly on the European Standards EN 149 and EN 13274-7. Despite having set up their testing procedures and their requirements independently and having received FFP respirators from different sources, the total number of rejected FFP respirators after testing was consistent between the laboratories. Spiez Laboratory and Unisanté-TOXpro SA rejected 52% and 60% of FFP respirators, respectively.
These results are also in good agreement with those published by the US Centers for Disease Control and Prevention (CDC), who tested 345 FFP respirators [24]. Although their samples were tested using a modified version of the NIOSH (National Institute for Occupational Safety and Health) standard test procedure, only 46% of the FFP respirators met the N95 requirement for penetration (95% of filtering efficiency, equivalent to EN 149), and 19% showed a filtering efficiency lower than 50%. Furthermore, they reported substantial inhomogeneity in product quality within the same batch and, identified 46 instances of counterfeit and/or misused company names (updated on 29 August 2020).
During the peak of the COVID-19 pandemic in Switzerland, procurement of certified and tested FFP respirators became a challenge and the priority was to get as large a supply as possible to ensure sufficient FFP respirator availability in healthcare settings. As in other countries, low-quality FFP respirators entered the Swiss market, owing to either non-compliance or sheer ignorance of the current requirements for PPE by purchasers and clients. Even though the required procedure for the procurement of PPE was relaxed by federal decree during the COVID-19 crisis in Switzerland, with the aim of facilitating respirator availability, no concessions were made on safety. However, in order to ensure an adequate supply of PPE in Switzerland several derogations were issued (Ordinance on Measures to combat the coronavirus) [25]. In particular, since 13 March 2020, the procedure for the assessment of the conformity of the FFP respirators with Article 3 Paragraph 2 of the PPE Ordinance of 25 October 2017 (PPEO) was facilitated, although the FFP respirator still had to provide its user with a level of security comparable to the requirements of the PPEO. In short, according to directives published by the Swiss State Secretariat for Economic Affairs (SECO), FFP respirators could be distributed on the Swiss market if a valid European or American certification was issued or, at least, if they complied with the testing principle (Rev. 2 - 02.06.2020) for SARS-CoV-2 pandemic respiratory protective masks developed by the German Central Office of the Federal States for Safety Engineering (Zentralstelle der Länder für Sicherheitstechnik, ZLS) [26, 27]. This ordinance was revoked on 18 September 2020, but FFP respirators that have been authorised on the basis of this exception may continue to be launched on the market until 30 June 2021 [28].
Considering the high proportion of not recommended FFP respirators reported in the present study, it is reasonable to assume that numerous products distributed on the Swiss market did not fulfil the SECO requirements. However, it is important to emphasise that even though the rapid test for FFP respirators developed by the ZLS takes several weeks, whereas a European certification usually takes months, the necessary time for either of these procedures was clearly lacking during the crisis. Indeed, in many cases, the available supply of FFP respirators was so critical that, according to our own observations, buyers did not have time to properly assess product quality, fearing that the products would quickly find another buyer if they were not immediately ordered. To address this problem and to protect the Swiss healthcare system, Spiez Laboratory on one side and Unisanté and TOXpro SA on the other urgently implemented simplified qualification testing with the aims to assess whether the available FFP respirators fulfilled minimal protection standards and to support healthcare facilities in the selection of the most appropriate products.
Examination of certification documents and crosschecking international databases, as performed by Spiez Laboratory by mid-April 2020, allowed the rejection of about 35% of FFP respirators without any testing. Some respirators were identified as non-conforming products by the RAPEX system, whereas others had suspicious documentation according to European guidelines. Such products were rejected from testing, unless sufficient complementary information was provided. This preliminary check may explain the slightly lower number of rejected FFP respirators after testing by Spiez Laboratory compared with Unisanté-TOXpro SA.
Importantly, a majority of those rejected FFP respirators were not listed in the RAPEX database at the time of this study. Most likely, although several FFP respirators were directly imported from the producing countries to Switzerland, they may not, yet have been distributed in EU countries. Furthermore, due to the urgent situation, some clients preferred to use low quality FFP respirators rather than no FFP respirators at all and, therefore, did not require them to be tested.
To support purchasers and clients in the identification of suspicious FFP respirators, we propose the following do-it-yourself eleven-criterion checklist:
Depending on the origin of a FFP respirator’s certification, information summarised in figure 1 should be present on its external side [16].

Figure 1 Standard markings printed on FFP respirator. Examples with certifications from Europe (left), the United States of America (centre) and China (right).
EU = European Union; FFP = filtering facepiece; NB = notified body; NR = non reusable; R = reusable; USA = United States of America.
The authenticity of this mark can be verified by checking its specific graphic design. The letters “C” and “E” must look like two circles, each cut in half. The middle line of the letter “E” must not extend to the centre of its circle (fig. 2a). Additionally, the inner edge of the circle of the letter “C” must reach the outer edge of the circle of the letter “E” [29]. Failure to comply with any of these rules is a telltale sign of a mark that, although looking similar, is not the official CE mark. One such example causing confusion is the “China Export” mark (see figs 2b and 2c) [30].
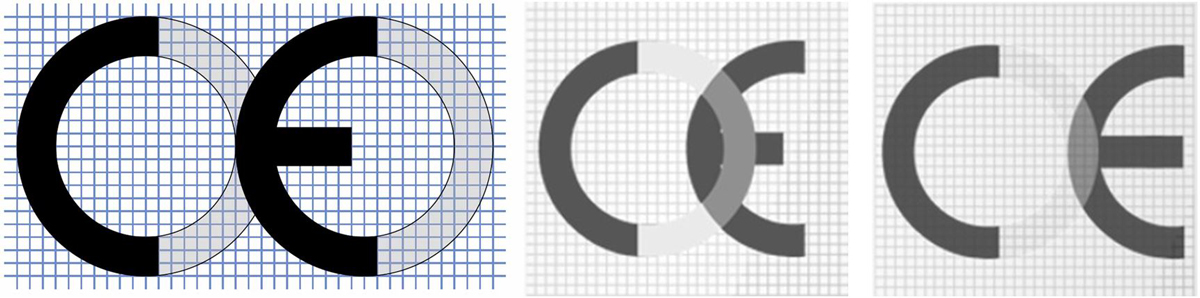
Figure 2 (a, above left) Original Communauté Européenne (CE) mark. Letters “C” and “E” are half circles and the middle line of the letter “E” does not extend to the center of its circle. The “China Export” marks can be identified by the overlapping circles of both letters (b, below), or by the middle line of the letter “E” extending to the centre of its circle (c, above right).
In the EU, a notified body is an organisation that assesses the conformity of certain products before their release onto the market. Official notified bodies accredited to test FFP respirators are listed in the NANDO (New Approach Notified and Designated Organisations) Information System and its identification number should be checked on the FFP respirator (fig. 3) [31].

Figure 3 Example of an identification number written on a FFP respirator designating a notified body not accredited to certify the product in question. The notified body 1282 certifies machinery and noise emission of equipment for outdoor use.
The number and the name of the notified body on the certificate should also be checked. If they do not correspond to each other, the authenticity of the certificate is not guaranteed (fig. 4). The European Safety Federation provides additional information about false certificates online [19].

Figure 4 Example of notified body identification number not corresponding to the laboratory identified by the logo (Universal certification has the number 2163 and not 2468)
When facing a Chinese certificate, the certificate number and the name of the laboratory should be recorded in the list of laboratories accredited for testing KN95 respirators edited by the China National Accreditation Service for Conformity Assessment (CNAS) [32].
The FDA provides a comprehensive and regularly updated list of authorised imported non-NIOSH approved respirators manufactured in China. These have been issued an Emergency Use Authorization (EUA) by the FDA.
A “Certificate Of Compliance” is issued on a purely voluntary basis and is not a CE Certificate. Therefore, it absolutely cannot replace a correct EU declaration of conformity.
A “Certificate of FDA Registration” is a registration number for the US trade and does not imply that the producing company or its products are approved or certified according to the corresponding standards.
Visual inspection (fig. 5).
Figure 5 Example (a, above) nose piece wrongly mounted; example (b, below) the two halves of the FFP respirator are not properly sealed together.
Resistance evaluation (fig. 6).
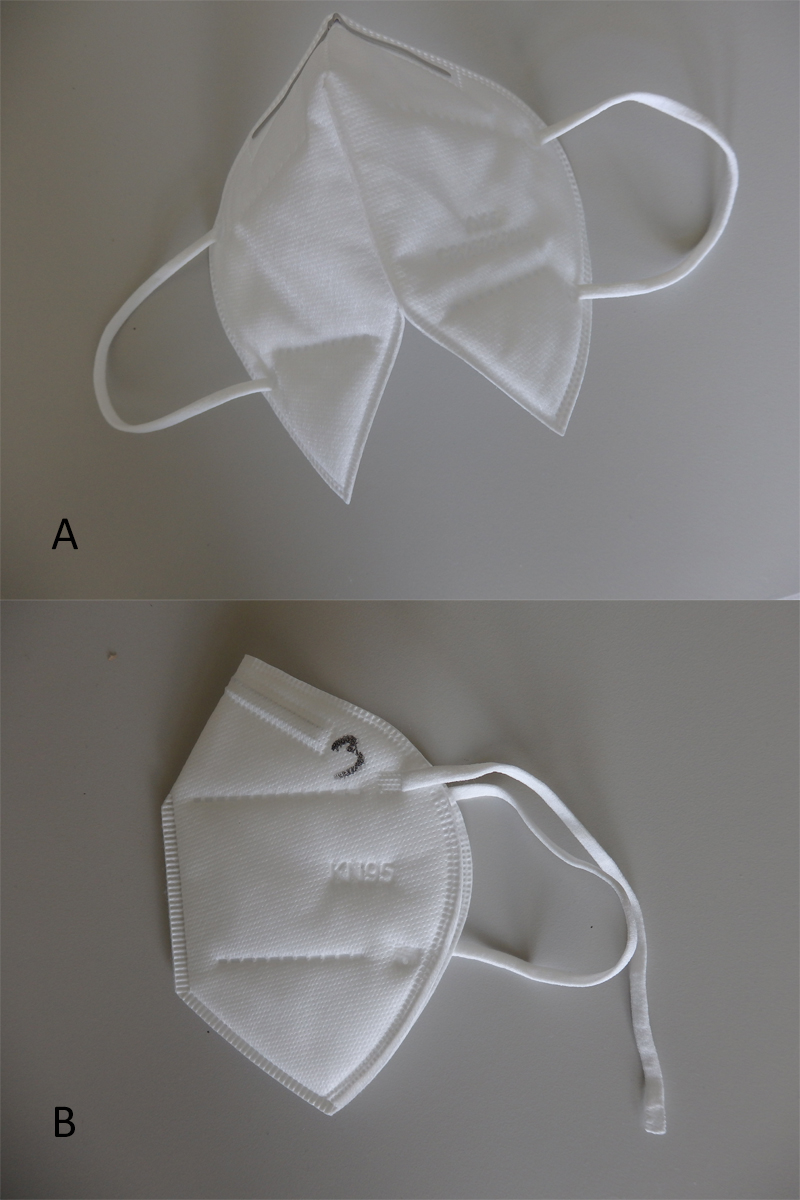
Figure 6 Example (a, above) thermal bond was weak and the FFP respirator split in half when it was unfolded. Example (b, below) ear loops were not properly attached to the FFP respirator.
Fit testing. Check the space around the chin and nose, also when moving the head in all directions. A FFP respirator that requires regular adjustments is either of poor quality or unsuitable for the wearer's morphology (fig. 7).
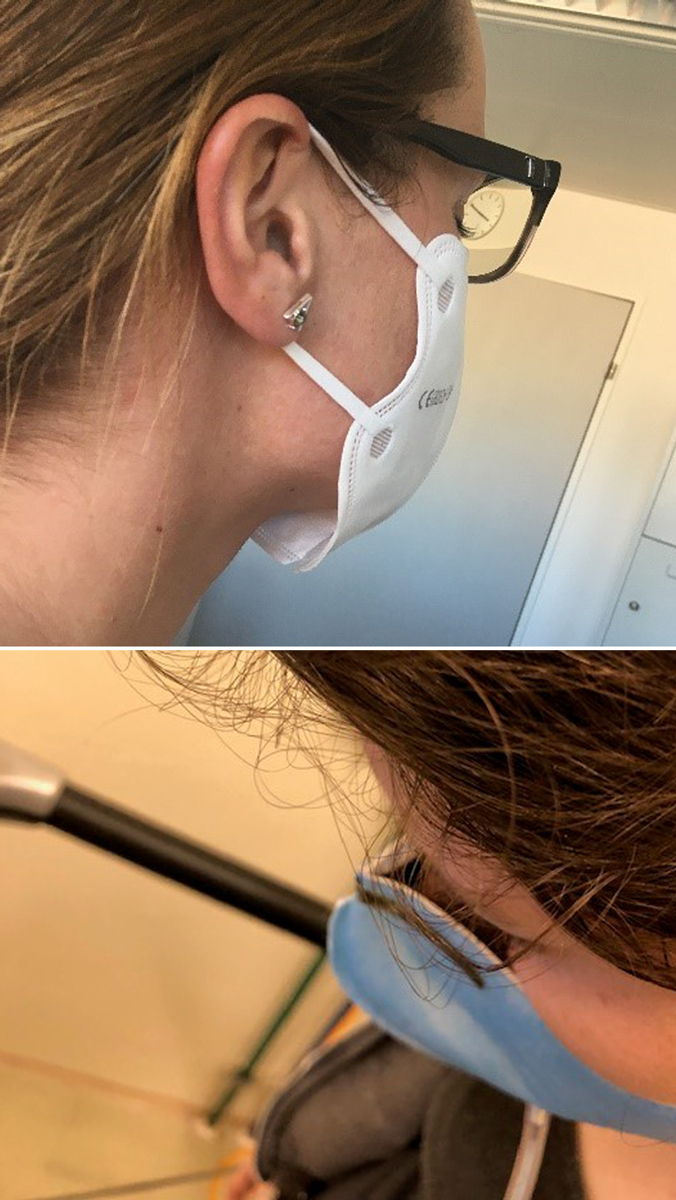
Figure 7 Example (a, above) the respirator does not properly seal onto the chin or (b, below) onto the nose.
If breathing is too hard, this may indicate that air permeability is too low. This may lead to air flowing through leaks around the FFP respirator seal to the face rather than through the filter material, reducing much of the FFP respirator’s filtering function.
The user exhales gently while blocking the paths for air to exit the FFP respirator by covering as much surface area as possible with her/his hands. A check is successful when the filtering facepiece is slightly pressurised before increased pressure causes outward leakage [34].
The present study has several limitations. First, the three laboratories are not accredited or certified according to EN 149 and the test methodology used to assess the quality of FFP respirators did not fully meet the requirements of the corresponding standard. The major differences were the aerosol detection principles and the number of test subjects for fit testing. Measuring principles of the PortaCount®, used in this study by both laboratories for fit testing, are different from the flame photometer, as required in EN 149. However, Sun et al. confirmed that a PortaCount® was an appropriate alternative to a flame photometer for aerosol detection [35].
The number of subjects (down to three) for fit testing was lower than the 10 persons required in EN 149 and the panel of subjects could not be selected to guarantee that the spectrum of facial characteristics of typical users would be covered. In the present study, the number and the selection of volunteers was limited, as the country was in partial lockdown and several employees were either sick, in quarantine, or working from home. Additionally, the laboratories received several times only a few samples of FFP respirators for testing. To account for this limited sample size, the laboratories introduced a sufficient rating, in addition to the “failed” (not recommended) and “passed” (recommended) ratings. According to our results, less than 10% of the tests were rated as sufficient, demonstrating that the procedure, even with a reduced panel of persons, was reliable.
Moreover, the number of subjects fulfilled the requirements of the revised test principle for SARS-CoV-2 pandemic respiratory protective masks (ZLS) where only three persons are required for the wear-test of FFP respirators [27].
Importantly, the results of this study provide an indication of the performance of FFP respirators available during the first wave of the COVID-19 pandemic. First, data generated in this work are neither a product certification nor are they to be used as a means of import approval. Second, the data presented here are not the results of a planned market study applying a sampling strategy, but a collection of data measured by independent laboratories. These tests were carried out to provide a quick response in an emergency situation in the context of an overstressed supply chain. Therefore, the data obtained are not exhaustive. Furthermore, the laboratories sometimes received identical samples from different clients, such that one FFP respirator model may be represented more than once in the database used for the overall analysis (table 1). Third, although the number of FFP respirators with insufficient results reported in this study clearly highlights the magnitude of the problem, they are not representative of the overall situation of FFP respirators used in Switzerland for the following reasons:
In the future, to avoid the problems reported in this article and to reduce the spread of unsuitable FFP respirators in Switzerland and elsewhere, we outline a few measures, in which we distinguish between normal and crisis situations.
The feasibility of the measures proposed here must be verified by other regulatory bodies (such as market and law), which is outside the expertise of the authors.
In conclusion, the first wave of the COVID-19 pandemic highlighted how, in a largely globalised world, crises can rapidly lead to a breakdown of international supply chains and the appearance of manufacturers seeking to take advantage of the situation. As the demand for products such as FFP respirators far exceeded the supply capacity of the Swiss market, similarly to several other countries, new production and import channels emerged, leading to an increased number of poor-quality FFP respirators. Non-conforming FFP respirators remaining in stocks should be now checked for quality before being used, and eliminated or replaced if safety requirements are not met.
We thank Andreas Mortensen for helpful comments and assistance in editing the article. We also thank all the members of the reMask expert group for their support in this work.
The present study did not benefit from any financial support. The authors have no interests to declare.
1 van Doremalen N , Bushmaker T , Morris DH , Holbrook MG , Gamble A , Williamson BN , et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–7. doi:.https://doi.org/10.1056/NEJMc2004973
2 Leung NH , Chu DK , Shiu EY , Chan K-H , McDevitt JJ , Hau BJ , et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–80. doi:https://doi.org/10.1038/s41591-020-0843-2
3 Cole EC , Cook CE . Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26(4):453–64. doi:.https://doi.org/10.1016/S0196-6553(98)70046-X
4 Wei J , Li Y . Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9, Suppl):S102–8. doi:.https://doi.org/10.1016/j.ajic.2016.06.003
5 Nguyen LHDD , Drew DA , Joshi AD , Guo CG , Ma W , Mehta RS , et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. 2020;2020.04.29.20084111.
6Centers for Disease Control and Prevention [internet]. Understanding the Difference. Available from: https://www.cdc.gov/niosh/npptl/pdfs/UnderstandingDifference3-508.pdf.
7 Ueki H , Furusawa Y , Iwatsuki-Horimoto K , Imai M , Kabata H , Nishimura H , et al. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. MSphere. 2020;5(5):e00637-20. doi:.https://doi.org/10.1128/mSphere.00637-20
8 Chu DK , Akl EA , Duda S , Solo K , Yaacoub S , Schünemann HJ , et al.; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–87. doi:.https://doi.org/10.1016/S0140-6736(20)31142-9
9 Leung CC , Lam TH , Cheng KK . Mass masking in the COVID-19 epidemic: people need guidance. Lancet. 2020;395(10228):945. doi:.https://doi.org/10.1016/S0140-6736(20)30520-1
10 Strasser BJ , Schlich T . A history of the medical mask and the rise of throwaway culture. Lancet. 2020;396(10243):19–20. doi:.https://doi.org/10.1016/S0140-6736(20)31207-1
11The Organisation for Economic Co-Operation and Development [internet]. The face mask global value chain in the COVID-19 outbreak: Evidence and policy lessons [updated 2020 May 4]. Available from: http://www.oecd.org/coronavirus/policy-responses/the-face-mask-global-value-chain-in-the-COVID-19-outbreak-evidence-and-policy-lessons-a4df866d/
12Presse AF. Russia Bans Export of Masks, Hazmat Suits to Fight Coronavirus. The Moscow Times. 2020 Mar 4. Available from: https://www.themoscowtimes.com/2020/03/04/russia-bans-export-of-masks-hazmat-suits-to-fight-coronavirus-a69516.
13Gassmann M. Das deutsche Exportverbot ist die Antwort auf Frankreichs Masken-Embargo. Welt. 2020 Mar 4. Available from: https://www.welt.de/wirtschaft/article206324563/Coronavirus-Bundesregierung-verbietet-Export-von-Atemmasken.html.
14Bayer L, Vela JH, Tamma P. EU moves to limit exports of medical equipment outside the bloc [cited 2020 Mar 15]. Available from: https://www.politico.eu/article/coronavirus-eu-limit-exports-medical-equipment/.
15Europol [internet]. Corona crimes: Multi-million face mask scam foiled by police across Europe [cited 2020 Apr 14]. Available from: https://www.europol.europa.eu/newsroom/news/corona-crimes-multi-million-face-mask-scam-foiled-police-across-europe.
16British Occupational Hygiene Society [internet]. Spotting a Fake Respirator [cited 2020 Jul 14]. Available from: https://www.bohs.org/covid-19-hub/.
17Center for disease control and prevention [internet]. Factors to consider when purchasing respirators from another country [cited 2020 May 7]. Available from: https://www.youtube.com/watch?v=w7tVnjrmAmc.
18European Safety Federation [internet]. COVID 19 suspicious certificates for PPE [cited 2020 may 26]. Available from: https://www.eu-esf.org/covid-19/4513-covid-19-suspicious-certificates-for-ppe.
19Su A. Faulty masks. Flawed tests. China’s quality control problem in leading global COVID-19 fight. Los Angeles Times. [cited 2020 Apr 10] Available from: https://www.latimes.com/world-nation/story/2020-04-10/china-beijing-supply-world-coronavirus-fight-quality-control
20Dethurens C, Zumbach C. L’efficacité des masques chinois remise en question. 24 heures. [cited 2020 May 8] Available from: https://www.24heures.ch/suisse/efficacite-masques-chinois-remise-question/story/29236463
21Bradsher K. China Delays Mask and Ventilator Exports After Quality Complaints. The New York Times [cited 2020 Apr 11] Available from: https://www.nytimes.com/2020/04/11/business/china-mask-exports-coronavirus.html.
22European Safety federation [internet]. RAPEX 2020 Particle filter mask KN 95. Available from: https://eu-esf.org/personal-protective-equipment/146-non-compliant/rapex-respiratory-protection.
23American Industrial Hygiene Association [internet]. American National Standard-Respirator Fit Testing Methods. 2010. Available from: https://hse.nioc.ir/portal/file/?312746/ansi-aiha-z88.10-2010-respirator-fit-testing-methods.pdf
24The National Personal Protective Technology Laboratory [internet] NPPTL Respirator Assessments to Support the COVID-19 Response [cited 2020 Jun 1]. Available from: https://www.cdc.gov/niosh/npptl/respirators/testing/NonNIOSHresults.html.
25The Swiss Federal Council [internet]. Ordinance on Measures to Combat the Coronavirus (COVID-19) [updated 2020 Mar 13]. Available from: https://www.admin.ch/opc/en/classified-compilation/20200744/index.html.
26State Secretariat for Economic Affairs [internet]. FAQ Atemschutzmaske und andere PSA [updated 2020 Jun 16]. Available from: https://www.seco.admin.ch/seco/de/home/Arbeit/Arbeitsbedingungen/Produktsicherheit/produktesicherheit_faq_covid19.html.
27Deutsche Zentralstelle der Länder für Sicherheitstechnik [internet]. Prüfgrundsatz für Corona SARS-Cov-2 Pandemie Atemschutzmasken Rev.2 - 02.06.2020. 2020 June 2, 2020. [cited 2020 Jun 02] Available from: https://www.zls-muenchen.de/Corona/Atemschutzmasken/200602_Pruefgrundsatz%20Corona%20SARS-Cov-2%20Pandemie%20Atemschutzmasken%20Rev.%202.pdf.
28State Secretariat for Economic Affairs [internet]. FAQ Atemschutzmaske und andere PSA [updated 2020 Sep 24]. Available from: https://www.seco.admin.ch/seco/de/home/Arbeit/Arbeitsbedingungen/Produktsicherheit/produktesicherheit_faq_covid19.html
29European Commission [internet]. CE marking. Available from: https://ec.europa.eu/growth/single-market/ce-marking_en.
30European Parliament [internet]. China Export and the CE marking [cited 2017 Mar 16]. Available from: https://www.europarl.europa.eu/doceo/document/E-8-2017-001822_EN.html.
31European Commission [internet]. Notified Body NANDO. Available from: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=155501.
32China National Accreditation Service for Conformtiy Assessment [internet]. Laboratories Accredited by CNAS for Testing of Masks,Gloves,Medical Protective Clothing and Other Personal Protective Equipment [cited 2020 Apr 27]. Available from: https://www.cnas.org.cn/english/photonews/06/903064.shtml.
33US Food and Drug Administration. [internet]. Appendix A: Authorized Respirators [cited 2020 May 28]. Available from: https://www.fda.gov/media/136663/download.
34Center for Disease Control and Prevention [internet]. Filtering out Confusion: Frequently Asked Questions about Respiratory Protection, User Seal Check [cited 2018 Apr]. Available from: https://www.cdc.gov/niosh/docs/2018-130/pdfs/2018-130.pdf?id=10.26616/NIOSHPUB2018130.
35 Sun C , Thelen C , Sancho Sanz I , Wittmann A . Evaluation of a New Workplace Protection Factor-Measuring Method for Filtering Facepiece Respirator. Saf Health Work. 2020;11(1):61–70. doi:.https://doi.org/10.1016/j.shaw.2019.11.001
36The National Personal Protective Technology Laboratory [internet]. NIOSH-Approved Particulate Filtering Facepiece Respirators [cited 2020 Apr 9]. Available from: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/default.html.
37FLAWA [internet]. CPA respiratory protection mask. Available from: https://flawaconsumer-shop.ch/fr/masque-respiratoire/.
The present study did not benefit from any financial support. The authors have no interests to declare.