Real-world effectiveness and safety of glecaprevir/pibrentasvir therapy in patients with chronic hepatitis C virus infection in Switzerland
DOI: https://doi.org/10.4414/smw.2021.20399
Beat
Müllhaupta, David
Semelab, Lisa
Ruckstuhlc, Lorenzo
Magentad, Olivier
Clerce, Ralph
Torglerc, Francesco
Negrof, Nasser
Semmog
a Division of Gastroenterology and Hepatology, University Hospital Zurich, Switzerland
b Division of Gastroenterology, Kantonsspital St. Gallen, Switzerland
c AbbVie Schweiz AG, Baar, Switzerland
d Fondazione Epatocentro Ticino, Lugano, Switzerland
e Infectious Diseases Department, Hospital Pourtalès, Neuchâtel, Switzerland
f Divisions of Gastroenterology and Hepatology and of Clinical Pathology, University Hospital, Geneva, Switzerland
g Hepatology, Department of BioMedical Research, University of Bern, Switzerland
Summary
AIM OF THE STUDY
In the era of pangenotypic treatment regimens against hepatitis C virus (HCV) infection, data from postmarketing observational studies are crucial to better understand the treatment patterns used in specific countries and treatment outcomes under real-life conditions. We report data from Switzerland from an ongoing, multinational postmarketing observational study on the pangenotypic treatment regimen of glecaprevir (GLE; NS3/4A protease inhibitor) and pibrentasvir (PIB; NS5A inhibitor), coformulated as GLE/PIB.
METHODS
Adults infected with chronic HCV genotypes 1–6 were eligible to participate in the postmarketing observational study if they started GLE/PIB at the treating physician’s discretion. The primary objective was to evaluate the effectiveness of GLE/PIB based on sustained virological response 12 weeks after completion of treatment (SVR12); secondary outcomes included patient-reported outcomes (Fatigue Severity Scale, Work Productivity and Activity Impairment Questionnaire, Pictorial Representation of Illness and Self Measure tool) and safety data.
RESULTS
In Switzerland, 109 patients were enrolled, and 107 patients received ≥1 dose GLE/PIB (94.4% non-cirrhotic; 43.9%/14.0%/29.0%/13.1% GT1/GT2/GT3/GT4; 89.7% treatment-naïve; 91.6% assigned to an 8-week GLE/PIB regimen). Overall, 95 of 98 patients with sufficient follow-up data (96.9%) achieved SVR12 (95% confidence interval [CI] 91.4% to 99.0%), and 91.6% in the safety population (including six non-virological failures). The three treatment failures were due to relapse. All three failures were GT3, without cirrhosis and treatment naïve. Patient-reported outcomes improved as well. GLE/PIB was well tolerated with no serious adverse events and no adverse events leading to discontinuation or interruption of GLE/PIB treatment.
CONCLUSION
These real-world effectiveness and safety data of GLE/PIB in patients from Switzerland were consistent with those seen in the multinational registration trials. (Trial registration number: Clinicaltrials.gov: NCT03303599.)
Introduction
Hepatitis C virus (HCV) infection is one of the main causes of chronic liver disease worldwide and 71 million people are expected to be chronically infected with HCV (1% of the global population) [1, 2]. Among the general population in Switzerland, the estimated anti-HCV prevalence is 0.7%. The most relevant high-risk groups for acquiring and spreading the disease are people who use drugs (PWUD), professional sex workers, prison inmates, migrant populations, children of infected mothers and men who have sex with men, and these risk groups have a much higher HCV prevalence with up to 45% for people who inject drugs [3].
Successful treatment cures the HCV infection, avoids further transmission and significantly reduces HCV-related complications, liver transplantations or death. HCV cure equates to sustained virological response (SVR), usually defined as “no HCV RNA detectable” at 12 weeks after treatment completion (SVR12) [4].
In clinical trials, direct-acting antiviral (DAA) treatments have achieved SVR12 rates of >90% across all HCV genotypes [4–8]. The development of the fixed dose combination of glecaprevir (GLE; NS3/4A protease inhibitor) and pibrentasvir (PIB; NS5A inhibitor) (GLE/PIB), allowed for the first time an abbreviated, 8-week treatment course for all treatment-naïve, non-cirrhotic patients, which marked a major step to simplifying HCV treatment algorithms. GLE/PIB demonstrated high virological efficacy in both treatment-naïve and previously treated patients across all genotypes [5–8]. Based on a pooled analysis of nine different phase II and phase III trials, 99.1% of non-cirrhotic patients across all HCV genotypes achieved SVR12 following 8 weeks of GLE/PIB treatment (12-week treatment: 99.6%), excluding patients with non-virological failure (core population with sufficient follow-up data [CPSFU] population) [7].
Now that effective and convenient pangenotypic treatment options are available, the World Health Organization aims to target the elimination of viral hepatitis (including HCV) as a public threat by 2030 (new infections reduced by 80%, mortality reduced by 65%) [1]. To achieve this goal, it is necessary to decrease viral transmission, reach the populations at the highest risk for transmission and infection, and ensure that patients are engaged in care and treatment. Right now, there is significant heterogeneity in DAA access and the types of patients treated across different geographical regions. Data from multinational postmarketing observational studies are crucial to a better understanding of the current, real-world HCV treatment patterns, healthcare resource utilisation, and treatment outcomes in different countries as well as in subpopulations underrepresented in clinical trials, such as elderly patients or people who use drugs.
In Switzerland, more people die every year from sequelae of HCV infection than from human immunodeficiency virus (HIV) infection [9]. Between 2012 and 2017, five times more deaths were caused by hepatitis C than by HIV [10]. Approximately 36,000 to 43,000 individuals are currently infected with chronic HCV [10], with an incidence of 50 new HCV cases per year [11]. Approximately 30% of all HCV-infected individuals in Switzerland are not aware of their HCV infection and therefore cannot benefit from the new, highly potent DAA regimens such as GLE/PIB [3]. To date, the available real-world data on DAA treatment in Switzerland are very limited.
We report the first real-world data for GLE/PIB from Switzerland, generated from a multinational postmarketing observational study that evaluated the effectiveness and safety of GLE/PIB in daily clinical routine. We report SVR12, adverse events, healthcare resource utilisation and patient-reported outcomes including burden of illness as assessed with the Pictorial Representation of Illness and Self Measure (PRISM) tool for 107 HCV patients treated with GLE/PIB (104 treated according to local labelling). The same parameters will be reported overall and for relevant subgroups (e.g., PWUD, treatment-naïve and treatment-experienced patients, cirrhotic and non-cirrhotic patients).
Materials and methods
Study design
This prospective, multi-centre, multinational, non-interventional postmarketing observational study was designed to evaluate the effectiveness and safety of the GLE/PIB regimen in patients with chronic HCV infection under real-world conditions and in a broad patient population as observed in real-world practice (ClinTrial.gov: NCT03303599). The study was performed in compliance with Swiss laws and regulations. The study was reviewed and approved by the responsible Swiss Ethics Committees, and was conducted in compliance with Swiss legislation.
HCV treatment-naïve or -experienced adults with confirmed chronic HCV infection of any genotype, with or without compensated cirrhosis, were eligible for participation in this trial, if they planned to start treatment with the GLE/PIB regimen according to standard of care, in line with the current local labelling. Treatment decisions were entirely at the discretion of the treating physician, based on the clinical characteristics of individual patients. The decision to initiate the GLE/PIB regimen was made independently from this study and preceded the decision to offer participation in the study. Patients were eligible for enrolment up to 4 weeks after treatment initiation.
The primary objective of the study was to describe the effectiveness of GLE/PIB, as evidenced by SVR12, overall and in the following subpopulations of interest: HCV genotype 1 to 6, cirrhotic or non-cirrhotic (investigator assessment), HCV treatment-experienced or -naïve, elderly or non-elderly (≥65 or <65 years), and former or current illicit drug users (PWUD [people who use [illicit] drugs]) or non-PWUD.
Secondary outcomes addressed in this publication include: clinical practice use of GLE/PIB regimen, adherence to the prescribed GLE/PIB regimen, comorbidities, concomitant medications, treatment tolerability and several patient-reported outcomes. Patient-reported outcomes included changes in fatigue, work productivity and disease burden.
In this study, 100 patients were planned to be enrolled. Here, we present data for 109 patients enrolled between March and December 2018 at six different sites in Switzerland.
Treatment
All participants received GLE/PIB treatment at the physician’s discretion. The recommended dosage (per Swiss prescribing information) is 300 mg GLE and 120 mg PIB once daily for either 8 weeks (non-cirrhotic, treatment-naïve patients), 12 weeks (all cirrhotic patients), or 16 weeks (pretreated patients with HCV genotype GT3 infection) [12].
Assessments and outcomes
Demographic, clinical, virological, patient reported outcomes, healthcare resource utilisation and safety data, as well as the actual regimen and any concomitant medications were collected. Visits, procedures and diagnostic methods were in accordance with the physicians’ routine clinical practice. Based on the anticipated regular follow up for patients undergoing HCV treatment, three main visits were recommended for each patient: baseline (within 4 weeks after initiation of GLE/PIB), end of treatment (week 8, 12 or 16, depending on the regimen), and 12 weeks after treatment completion (SVR12 assessment). Additional visits during and after treatment occurred as scheduled by the investigator.
HCV RNA tests, using a sensitive polymerase chain reaction test with a lower limit of quantification <50 IU/ml, were expected to be performed at each visit. The primary outcome, SVR12, was defined as HCV RNA below the lower limit of quantification at 12 weeks (allowed interval: 70 to 126 days) after the last actual dose of GLE/PIB.
Adverse events were collected at each post-baseline visit; healthcare resource utilisation and concomitant medications were collected at each visit. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA; Version 21.1). Laboratory data were assessed as per routine practice and changes in key hepatic parameters (e.g., alanine aminotransferase [ALT], bilirubin, etc.) were assessed. Healthcare resource utilisation was assessed for each patient based on the total number of face-to-face visits and the total number of telephone calls with a healthcare provider or designee in relation to the HCV infection. The study protocol recommended five visits per patient regardless of treatment duration: (1) baseline, (2) during treatment, (3) end of treatment, (4) early post-treatment, and (5) the visit to assess sustained virological response at post-treatment week 12 (SVR12). The number of visits was at the provider’s discretion based on routine clinical practice.
Patient-reported outcomes were collected at each main visit and included the PRISM tool, the Fatigue Severity Scale (FSS), and the Work Productivity and Activity Impairment (WPAI) Questionnaire. The PRISM tool provides a brief method to quantify the perceived burden of suffering due to illness. The tool consists of an A4-size metal board, which stands for the patient’s life. A yellow disk, representing the patient, is fixed at the bottom right-hand corner. The patient is asked to reflect the importance of the illness in his/her life by placing a red disk, which represents the illness, on the board. Thereby, the PRISM tools provides a graphic representation of the patient’s illness in relation to the patient. The distance between the centre of the yellow “self” and the red “illness” disk (in cm) is called “self-illness separation”, and can range from 0 to 27 cm (greater distance indicates less burden of illness). The validity and reliability of the PRISM tool have been assessed in more than 700 patients with various physical illnesses [13].
The WPAI questionnaire is a six-item scale instrument to measure work absenteeism and presenteeism, and daily activity impairment. Respondents are asked about time missed from work and time at work with reduced productivity during the last 7 days. The WPAI can easily be adopted for specific health problems (WPAI-SHP), its use has been validated in numerous diseases, and WPAI-SHP has been used in HCV studies [14, 15]. For this study, the HCV-specific version of the WPAI-SHP, WPAI HepC Version 2.0, was [15]. Results are expressed as % absenteeism (percentage of work time missed due to HCV), % presenteeism (percentage of impairment while working due to HCV), % overall work impairment due to HCV and % general activity impairment due to HCV.
The FSS is a nine-item scale which measures the severity of fatigue and its effect on a person’s activities and lifestyle. Each item is rated from 1 (completely disagree) to 7 (completely agree), and the FSS total score ranges from 1 (no fatigue) to 7 (very severe fatigue). The scale has been validated for use in patients with chronic HCV, and an increase of ≥0.7 in mean FSS scores can be considered as a clinically important difference [16].
Statistical methods
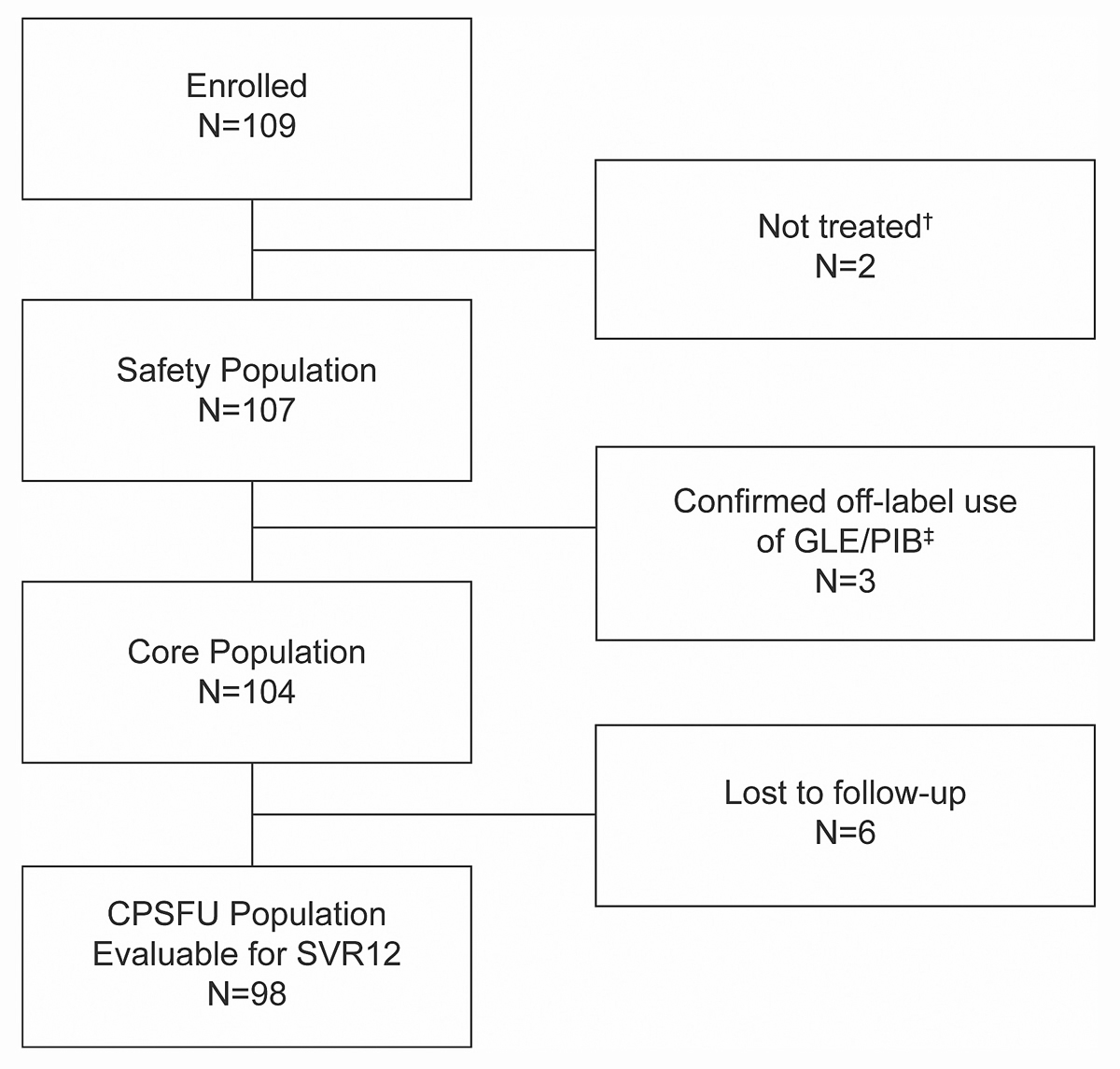
Analysis populations: Effectiveness, healthcare resource utilisation and patient-reported outcomes were evaluated for all patients who started the treatment regimen as recommended for their disease characteristics (core population) and who had sufficient follow-up. The primary analysis (SVR12) included all patients of the core population who had evaluable HCV RNA values ≥70 days after the last actual dose or whose ≥70 day HCV RNA values were missing for efficacy or safety reasons, i.e., patients with HCV RNA values ≥50 IU/ml at the last available measurement, and patients who discontinued the study due to adverse events (CPSFU population). Safety data were assessed for all patients who received at least one dose of GLE/PIB (safety population) (see fig. 1 below).
All analyses were mainly descriptive. For the primary SVR12 outcome and for secondary outcomes of treatment response (relapse rate), 95% confidence intervals (CIs; Wilson’s score method) were provided, for both the overall population and the main subgroups of interest. For patient-reported outcome scores, means and standard deviations (SDs) were provided for the changes from baseline to end of treatment and to post-treatment week 12 (70–126 days allowed).
Results
Study population
A total of 109 patients from Switzerland with chronic HCV infection were enrolled. Of these, 107 received at least one dose of GLE/PIB, 104 started the regimen as recommended for their disease characteristics (core population) and 98 were evaluable for SVR12 (fig. 1). Mean treatment adherence (patient-reported % of prescribed tablets taken) was ≥99% for all patients with data available (n = 107; range 88% to 100%).
Baseline characteristics for the safety population (n = 107) are summarised in table 1 (additional details can be found in supplementary tables S1 to S3
in appendix 1, available online). Most patients were non-cirrhotic (94.4%) and HCV treatment naïve (89.7%). The few patients with prior HCV treatment (n = 11) had mostly received interferon-based therapies (n = 10). GT1 was the most common HCV genotype (43.9%), followed by GT3 (29.0%), GT2 (14.0%) and GT4 (13.1%); there were no GT5 or GT6 patients enrolled. Prior or current use of illicit drugs was reported by 55.9% of all patients. Most patients had either normal renal function (47.3%) or minor renal impairment (49.5%). Only three patients with moderate, but none with severe, renal impairment were enrolled (table 1); no patient had received dialysis treatment. Overall, 17.8% of the patients had a history of depression or suicide attempts / self-injury (table S4). The most frequent medical histories included depression (10.3%), hypertension (9.3%), and substance dependence (4.7%) (table S5). Most frequently reported concomitant drugs included methadone (8.4%), acetylsalicylic acid (8.4%), mefenamic acid (7.5%) and amlodipine (5.6%) (table S6). Baseline characteristics for the different HCV genotype subgroups were generally similar (table S7).
Table 1 Baseline characteristics (safety population, N = 107).
|
Variable, number (N) with data
|
|
Age (years)
|
| Mean (SD) |
52.4 (12.8) |
| <65 years, n (%) |
91 (85.0) |
| ≥65 years, n (%) |
16 (15.0) |
|
Gender, n (%)
|
| Male |
61 (57.0) |
| Female |
46 (43.0) |
|
BMI (kg/m2), N = 45*
|
| Mean (SD) |
24.5 (5.0) |
|
HCV genotype
†, n (%)
|
| GT1 |
47 (43.9) |
| GT2 |
15 (14.0) |
| GT3 |
31 (29.0) |
| GT4 |
14 (13.1) |
|
Viral load (HCV RNA), log10 IU/ml
|
| <6 |
45 (42.1) |
| ≥6 to <6.3 |
21 (19.6) |
| >6.3 |
39 (36.4) |
| Unknown |
2 (1.9) |
|
Cirrhosis status, n (%)
|
| Non-cirrhotic |
101 (94.4) |
| Cirrhotic |
6 (5.6) |
|
Liver fibrosis stage
‡, n (%)
|
| F0 to F1 |
56 (52.3) |
| F2 |
7 (6.5) |
| F3 |
4 (3.7) |
| F4 |
2 (1.9) |
| Unknown |
38 (35.5) |
|
HCV pretreatment status, n (%)
|
| Naïve |
96 (89.7) |
| Experienced§
|
11 (10.3) |
|
Years since diagnosis of HCV infection, N = 105*
|
| Mean (SD) |
14.6 (10.5) |
|
Illicit drug use, n (%), N = 102*
|
| No |
45 (44.1) |
| Yes, at any time |
57 (55.9) |
| Current |
15 (14.7) |
| <6 months ago |
3 (2.9) |
| 6 to 12 months ago |
1 (1.0) |
| >12 months ago |
38 (37.3) |
|
Illicit drug type
|
| Heroin |
37 (36.3) |
| Cocaine |
12 (11.8) |
| Marijuana |
7 (6.9) |
| Hashish |
5 (4.9) |
| Amphetamine |
2 (2.0) |
| Opioids |
1 (1.0) |
| Other |
3 (2.9) |
|
Illicit drug route
|
| Intravenous |
40 (39.2) |
| Respiratory (inhalation) |
13 (12.7) |
| Nasal |
9 (8.8) |
| Oral |
1 (1.0) |
|
Patients on stable opiate substitution, n (%), N = 99*
|
12 (12.1) |
|
Renal function (GFR), n (%), N = 91*
|
| Normal (≥90 ml/min) |
43 (47.3) |
| Mild impairment (≥60 to <90 ml/min) |
45 (49.5) |
| Moderate impairment (≥30 to <60 ml/min) |
3 (3.3) |
| Severe impairment (<30 ml/min) |
0 |
|
Mode of HCV infection, n (%)
|
| Contaminated needle or IV drug use (current/past) |
49 (45.8) |
| Blood product transfusion |
16 (15.0) |
| Contact with infected individual (other than vertical transmission) |
5 (4.7) |
| Vertical transmission (mother to child) |
3 (2.8) |
| Occupational exposure |
4 (3.7) |
| Unknown |
30 (28.0) |
|
Perceived burden of illness (PRISM tool), N = 102*
|
| Mean SIS (cm) |
17.2 |
|
Work Productivity and Activity Impairment (WPAI)*
|
| % overall work impairment due to HCV, mean, N = 49 |
14.0 |
| % general activity impairment due to HCV, mean, N = 102 |
18.6 |
| % absenteeism, mean, N = 49 |
6.4 |
| % presenteeism, mean, N = 47 |
7.9 |
|
Fatigue (FSS), N = 34*
|
| Mean total score |
3.7 |
In line with the recommended treatment regimen for their disease characteristics, 91.6% of all patients were assigned to the 8-week GLE/PIB regimen.
Clinical effectiveness
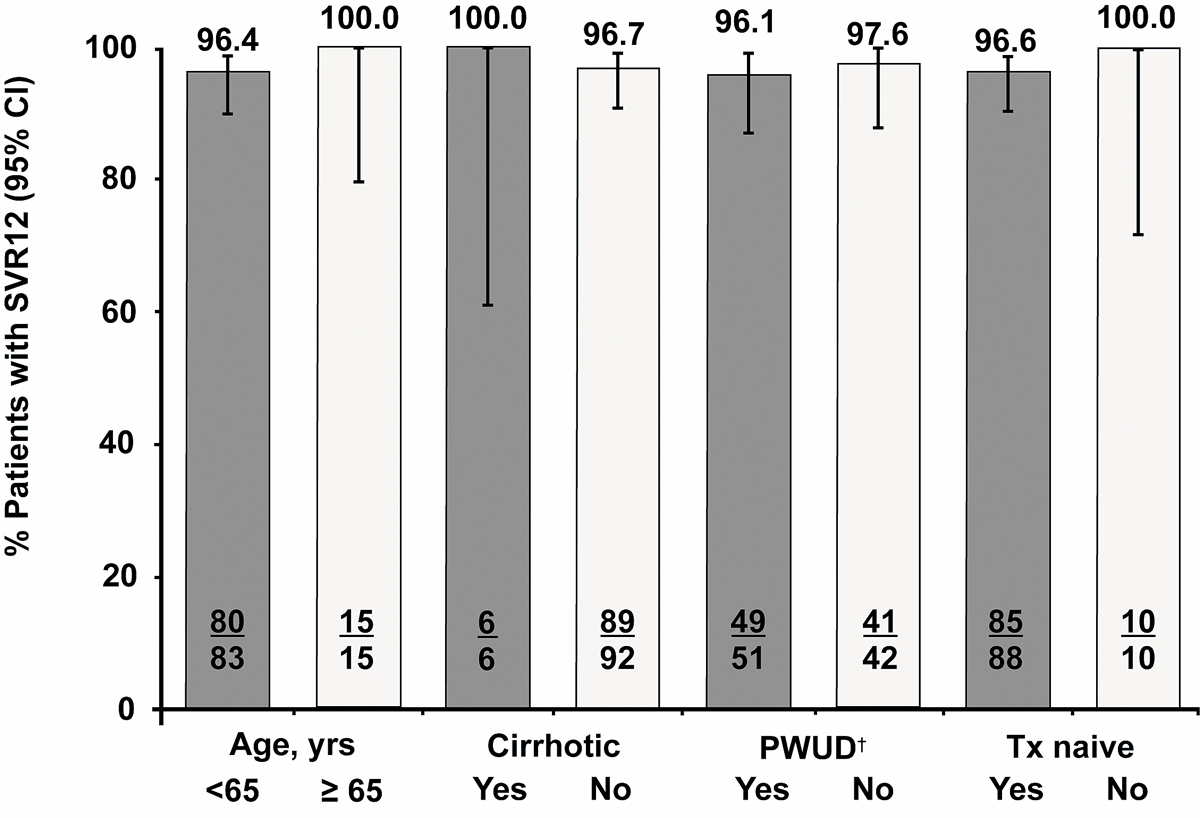
Of 98 patients evaluable for SVR12 (CPSFU population), 95 (96.9%) achieved SVR12 (95% CI 91.4% to 99.0%). The three failures were due to relapse and all had a GT3 infection. All three patients were treatment-naïve, two of them were current or former PWUDs (table 2, fig. 2). All three patients were reported to be treatment-adherent. Of the 104 patients that started the regimen as recommended for their disease characteristics (core population), 6 were lost to follow up.
Table 2 Overview of treatment effectiveness of GLE/PIB in the Swiss cohort.
|
SVR12 rates (%), overall and by HCV genotype
|
| Overall (N = 98, CPSFU population) |
96.9
(95% CI 91.4% to 99.0%) |
| GT1 (N = 45) |
100.0 |
| GT2–4 (N = 53)†
|
94.3 |
|
Supporting information
|
| Patients with relapse, n |
3‡
|
| Patients with virological failure on-treatment, n |
0 |
| Patients with early discontinuation of GLE/PIB, n |
0 |
Healthcare resource utilisation
Healthcare resource utilisation data were available for 100 patients overall. These patients required a median of 4 face-to-face contacts (range 3–8) with healthcare professionals during the study, including any office visits, emergency room visits or hospitalisations. No additional telephone contacts were required. The small sample of cirrhotic patients (n = 6) tended to require more visits (median 6, range 4–7) than the non-cirrhotic group (n = 94, median 4, range 3–8).
Patient-reported outcomes
Burden of illness data, as assessed with the PRISM tool, were available for 102 patients at baseline and for 92 patients at the final visit 12 weeks after treatment completion. For these patients, the perceived burden of illness decreased during GLE/PIB treatment and was maintained at the final visit at 12 weeks after treatment completion, as shown by the mean increase of self-illness separation by 4.9 cm (SD 9.7 cm) from baseline to final visit (table 3).
Table 3 Summary of patient-reported outcomes (patients with data only).
| |
Baseline
|
End of Tx
|
SVR12 visit
|
|
Perceived burden of illness (PRISM tool)
|
| Number with data |
102 |
87 |
92 |
| Mean SIS (cm) |
17.2 |
20.3 |
21.7 |
| Mean (SD) change from baseline |
NA |
3.4 (7.1) |
4.9 (9.7) |
|
Work Productivity and Activity Impairment (WPAI)
|
|
% overall work impairment due to HCV
|
| Number with data |
49 |
36 |
37 |
| Mean percentage |
14.0 |
8.7 |
11.9 |
| Mean (SD) change from baseline |
NA |
−7.0 (21.9) |
−3.4 (19.8) |
|
% general activity impairment due to HCV
|
| Number with data |
102 |
88 |
92 |
| Mean percentage |
18.6 |
13.4 |
9.8 |
| Mean (SD) change from baseline |
NA |
−6.5 (20.8) |
−10.2 (24.2) |
|
% absenteeism
|
| Number with data |
49 |
36 |
37 |
| Mean percentage |
6.4 |
3.2 |
2.7 |
| Mean (SD) change from baseline |
NA |
−5.6 (21.3) |
−5.8 (20.8) |
|
% presenteeism
|
| Number with data |
47 |
34 |
35 |
| Mean percentage |
7.9 |
5.9 |
7.7 |
| Mean (SD) change from baseline |
NA |
−1.5 (6.6) |
0.6 (14.1) |
|
Fatigue Severity Scale (FSS) total score
|
| Number with data |
34 |
31 |
31 |
| Mean score |
3.7 |
3.1 |
2.9 |
| Mean (SD) change from baseline |
NA |
−0.6 (1.1) |
−0.8 (1.3) |
|
Improved ≥0.7 points from baseline, n (%)
|
NA |
14 (45.2) |
17 (54.8) |
Correspondingly, patients’ work productivity and general activity improved, as indicated by the WPAI data (table 3). The mean percentages for overall work impairment, general activity impairment, and absenteeism at work had all decreased by 5–7% at the end of treatment. The general activity impairment had continued to improve at 12 weeks after treatment completion, with an approximate 50% overall reduction in impairment (% impairment: 18.6% at baseline and 9.8% at 12 weeks after treatment completion).
Fatigue data, as assessed with the FSS, were available for 34 patients at baseline and for 31 patients at the final visit 12 weeks after treatment completion. Although this number is low, a mean decrease (improvement) of the FSS total score of 0.8 points at 12 weeks after treatment completion was observed. This was above the limit for clinical significance (decrease ≥0.7 points). Overall, 45.2% of patients (95% CI 27.6% to 62.7%) achieved a clinically meaningful improvement of ≥0.7 points at the end of treatment, and 54.8% of patients (95% CI 37.3% to 72.4%) still showed the clinically meaningful improvement at 12 weeks after treatment completion (table 3).
Safety data
Overall, GLE/PIB treatment was well tolerated in the real-life setting; there were no serious adverse events and no adverse events leading to discontinuation or interruption of GLE/PIB treatment (table 4). Fatigue, dyspepsia, headache, and nausea were the only adverse events occurring in more than one patient.
Table 4 Summary of adverse event data (safety population).
|
Adverse event category
|
Number of patients (%)
N = 107
|
|
Any adverse event
|
22 (20.6) |
| Possibly related (investigator assessment) |
12 (11.2) |
| Serious adverse event |
0 |
| Adverse event leading to treatment discontinuation |
0 |
| Fatal adverse event |
0 |
|
Adverse events reported in >1 patient (preferred term)
|
| Fatigue |
5 (4.7) |
| Dyspepsia |
3 (2.8) |
| Headache |
3 (2.8) |
| Nausea |
3 (2.8) |
There was no incidence of drug-induced liver injury: Overall, 3 of the 98 patients with liver function laboratory data available (3.1%) had postbaseline increases of total bilirubin to ≥2 times the upper limit of normal, no patient experienced ALT increases of >3 times the upper limit of normal. Gastrointestinal adverse events (dyspepsia, nausea, diarrhoea, constipation, abdominal pain) were reported in 7 patients (6.5%) overall.
Discussion
This postmarketing observational study on the use of GLE/PIB for the treatment of chronic HCV infections provides the first real-world data from Switzerland in a broad patient population with different concomitant conditions (supplementary tables S4 and S5
in appendix 1), as observed in routine practice. In this cohort from Switzerland, GLE/PIB treatment (an 8-week short regimen used in >90% of cases) yielded SVR12 rates of 96.9% in 98 patients evaluable for SVR12 (CPSFU population) and of 91.6% in the safety population, including 6 non-virological failures). This rate is similar to the 98% SVR12 rate identified in an integrated analysis of phase III studies for patients receiving the 8-week regimen [7]. The majority of patients had infections with HCV genotypes GT1 (43.9%) and GT3 (29.0%), closely reflecting the known worldwide distribution of HCV genotypes (44% and 25%, respectively) [17]. In this Swiss cohort, there were only three treatment failures due to relapse. All three patients had a GT3 infection, were non-cirrhotic and treatment naïve. Two of them were current or former PWUDs.
GT3 infections have emerged as the HCV genotype most difficult to treat in the era of DAAs [4]. The GLE/PIB combination is one of the DAA-based regimens recommended by the European Association and the Swiss Association for the Study of the Liver (EASL, SASL) for the treatment of any HCV genotype in non-cirrhotic patients [4, 18]. As for any other genotype, a treatment duration of 8 weeks for GLE/PIB is recommended for treatment-naïve GT3 non-cirrhotic patients [4, 18]. For the more problematic, treatment-experienced non-cirrhotic patients with GT3 infection, longer treatment durations of 12 weeks (EASL) [4] or 16 weeks (SASL expert statement [18], in alignment with the Swiss label [12]) are recommended. The fact, that GT3 remains the most difficult to treat HCV genotype is further reflected by the recommendation to either prolong treatment duration and/or add ribavirin when using other DAAs [19].
Real-world data for GLE/PIB are also available from other countries; results were consistent with the results from Switzerland. From the NAVIGATORE-Lombardia network in Italy, data are available for 723 patients (49% GT1, 10% GT3; 89% treated with the 8-week regimen). In the Italian cohort, GLE/PIB treatment resulted in an overall SVR12 rates of 99.3% in the per protocol analysis and 94% in the intention-to-treat analysis. A total of five treatment failures due to relapse were documented [20]. From the German Hepatitis C registry, prospective data are available for 552 patients (53% GT1, 33% GT3). Similar to the Swiss cohort, the majority of patients were treatment-naïve, had no cirrhosis and received the 8-week GLE/PIB regimen. The overall SVR12 rate in the German cohort was 96.7%, with one documented virological failure (relapse) and two documented HCV reinfections [21].
The high SVR12 rates observed in the Swiss cohort were accompanied by a decrease in burden of illness (PRISM tool), and improvements in work productivity and general activity (WPAI), as well as in fatigue (FSS). Improved FSS outcomes were also observed in the multi-country interim analysis of the same study, which included 720 patients (data pooled from six countries). Mean FSS scores decreased by 0.7 (Swiss cohort: decrease by 0.8; table 3), and 43.6% of patients showed a clinically meaningful improvement in fatigue from baseline to SVR12 visit [22].
GLE/PIB real-world treatment was well tolerated in the Swiss cohort. Overall, 20.6% of patients reported ≥1 adverse event, but there were no serious adverse events and no adverse events leading to discontinuation or interruption of GLE/PIB treatment. Safety data from the other real-world studies performed in Italy, Germany, and Japan had similar results. In the Italian study, mild adverse events were reported in 8.3% of the 723 patients treated; 0.7% of patients documented premature discontinuation of GLE/PIB [20]. In the study from Germany, 26% of patients reported ≥1 adverse event, 2% of patients had serious adverse events (nine AEs), and <1% of patients (two cases) had adverse events leading to discontinuation of GLE/PIB [21]. As known for real-world studies, the adverse event rates were generally lower when compared with clinical trials, where adverse event rates of 63% and 68% were reported for the 8- and 12-week regimens, respectively [7].
Fatigue was the most common adverse event (5 patients, 4.7%) in the Swiss cohort. Other adverse events reported more than once were dyspepsia, headache, and nausea. Headache and fatigue were also the most commonly adverse events reported in clinical trials [6, 7]. In the Swiss cohort, only three isolated cases of total bilirubin increases to ≥2 times the upper limit of normal, and importantly no ALT increases ≥3 times the upper limit of normal were observed. This is consistent with the integrated safety analysis of phase III data, which identified no incidence of drug-induced liver injury and a low rate (0.3%) of grade 3 total bilirubin increases (i.e., >3 times the upper limit of normal) [7]. Gastrointestinal adverse events were observed in 6.5% of patients, similar to the integrated analysis (6% of patients with diarrhoea) [7].
The main limitation of this study is the small sample size of the Swiss cohort (safety population: n = 107, evaluable for SVR12: n = 98), and the fact that many subgroups of interest were too small for valid subgroup analyses of effectiveness and safety, in particular cirrhotic patients (n = 6), patients with moderate or severe renal impairment (n = 3), or elderly patients (n = 16). However, such subgroup analyses may become available in the future, based on the results for the overall population of the ongoing multinational real-world study.
Overall, the first real-world evidence data of GLE/PIB were consistent with those from the large phase III trials, as well as with real-life data obtained in other countries (i.e., multi-country interim analysis and other postmarketing observational studies from Italy and Germany), indicating robust effectiveness and good tolerability of GLE/PIB across a wide range of different patient populations.
Appendix 1 Supplementary tables
Table S1 Additional baseline demographic characteristics (safety population, N = 107).
|
Variable
|
Number (%) of patients
|
| Ethnicity |
Not Hispanic or Latino |
102 (95.3) |
| Hispanic or Latino |
5 (4.7) |
| Race |
White |
102 (95.3) |
| Asian |
3 (2.8) |
| Black or African American |
2 (1.9) |
| Current occupational status |
Employed, full-time |
49 (45.8) |
| Retired |
17 (15.9) |
| Unemployed |
17 (15.9) |
| Employed, part-time |
14 (13.1) |
| Homemaker |
6 (5.6) |
| Student |
1 (0.9) |
| Unknown |
3 (2.8) |
| Highest level of education obtained |
Low level |
18 (16.8) |
| Medium level |
64 (59.8) |
| High level |
13 (12.1) |
| Unknown |
12 (11.2) |
| Smoking habits |
Current |
56 (52.3) |
| Former |
22 (20.6) |
| Never |
21 (19.6) |
| Unknown |
8 (7.5) |
| Drinking habits |
Current |
75 (70.1) |
| Former |
14 (13.1) |
| Never |
11 (10.3) |
| Unknown |
7 (6.5) |
Table S2 Additional disease characteristics (safety population, N = 107).
|
Variable
|
Number (%) of patients
|
| Cirrhotic (Child Pugh classification 5 to 6) |
6 (5.6) |
| APRI |
<0.5 |
58 (54.2) |
| ≥0.5 to ≤1 |
24 (22.4) |
| >1 |
16 (15.0) |
| Unknown |
9 (8.4) |
Table S3 Baseline laboratory data (safety population, N = 107).
|
Variable
|
Number with data
|
Mean (SD)
|
| HCV RNA level (IU/ml) |
105 |
2,901,194.3 (4,314,763.7) |
| Log10 IU/ml |
105 |
6.0 (0.87) |
| ALT (U/l |
102 |
81.2 (63.15) |
| ALT (ratio) |
102 |
1.8 (1.54) |
| AST (U/l) |
101 |
61.2 (54.17) |
| AST (ratio) |
101 |
1.5 (1.42) |
| Fibrosis-4 |
98 |
1.8 (1.69) |
| GGT (U/l) |
98 |
84.8 (109.62) |
| Total bilirubin (µmol/l) |
97 |
11.5 (5.22) |
| Albumin (g/l) |
97 |
39.9 (5.05) |
| Creatinine (µmol/l) |
91 |
73.8 (16.31) |
| AFP (ng/ml) |
32 |
5.0 (5.82) |
| Haemoglobin (g/l) |
102 |
144.1 (16.89) |
| Platelets (cells × 109/l) |
101 |
227.2 (61.83) |
| ALT = alanine aminotransferase; AST = aspartate aminotransferase; GGT = gamma-glutamyltransferase; AFP = alpha-fetoprotein |
Table S4 Viral coinfections and solicited comorbidities (safety population, N = 107).
|
Viral coinfection or solicited comorbidity
|
Number (%) of patients
|
| Viral coinfection |
HBV |
0 |
| HIV |
0 |
| History of depression or suicide/self-injury |
19 (17.8) |
| History of cardiovascular disease |
16 (15.0) |
| History of depression or bipolar disorder |
11 (10.3) |
| History of bleeding disorder |
6 (5.6) |
| History of diabetes |
3 (2.8) |
| Transplant history |
Liver |
0 |
| Renal |
0 |
| Other |
0 |
| Dialysis treatment required |
0 |
| Hepatocellular carcinoma |
0 |
| HBV = hepatitis B virus: HIV = human immunodeficiency virus |
Table S5 Most frequently reported medical histories (≥2% of patients) (safety population, N = 107).
|
Medical history (MedDRA preferred term)
|
Number (%) of patients
|
| Depression |
11 (10.3) |
| Hypertension |
10 (9.3) |
| Substance dependence |
5 (4.7) |
| Cholelithiasis |
4 (3.7) |
| Dyslipidaemia |
4 (3.7) |
| Hypertonia |
4 (3.7) |
| Vitamin D deficiency |
4 (3.7) |
| Drug dependence |
3 (2.8) |
| Gall bladder polyp |
3 (2.8) |
| Breast cancer |
3 (2.8) |
| Hepatitis B |
3 (2.8) |
| Migraine |
3 (2.8) |
| Obesity |
3 (2.8) |
Table S6 Most frequently reported concomitant medications (≥2% of patients) (safety population, N = 107).
|
Concomitant medication
|
Number (%) of patients
|
| Acetylsalicylic acid |
9 (8.4) |
| Methadone |
9 (8.4) |
| Mefenamic acid |
8 (7.5) |
| Amlodipine |
6 (5.6) |
| Metoprolol |
5 (4.7) |
| Mirtazapine |
5 (4.7) |
| Morphine |
5 (4.7) |
| Paracetamol |
5 (4.7) |
| Cholecalciferol |
4 (3.7) |
| Esomeprazole |
4 (3.7) |
| Fluoxetine |
4 (3.7) |
| Ibuprofen |
4 (3.7) |
| Budesonide/formoterol |
3 (2.8) |
| Chondroitin |
3 (2.8) |
| Escitalopram |
3 (2.8) |
| Lekovit CA |
3 (2.8) |
| Levothyroxine |
3 (2.8) |
| Lorazepam |
3 (2.8) |
| Pancreatin |
3 (2.8) |
| Pantoprazole |
3 (2.8) |
| Torasemide |
3 (2.8) |
| Trazodone |
3 (2.8) |
Table S7 Baseline characteristics by HCV genotype (core population, N = 104).
|
Variable
|
GT1
(n = 46)
|
GT2
(n = 15)
|
GT3
(n = 29)
|
GT4†
(n = 14)
|
Overall
(n = 104)
|
|
Age (years)
|
| Mean (SD) |
52.7 (12.93) |
63.5 (11.89) |
48.3 (9.86) |
48.7 (12.27) |
52.5 (12.74) |
| <65 years, n (%) |
40 (87.0) |
8 (53.3) |
28 (96.6) |
12 (85.7) |
88 (84.6) |
| ≥65 years, n (%) |
6 (13.0) |
7 (46.7) |
1 (3.4) |
2 (14.3) |
16 (15.4) |
|
Gender, n (%)
|
| Male |
29 (63.0) |
5 (33.3) |
22 (75.9) |
4 (28.6) |
60 (57.7) |
| Female |
17 (37.0) |
10 (66.7) |
7 (24.1) |
10 (71.4) |
44 (42.3) |
|
BMI (kg/m2)
|
| Mean (SD) |
25.2 (6.16) |
23.9 (2.92) |
23.6 (4.92) |
25.9 (3.73) |
24.6 (5.04) |
|
Cirrhosis status, n (%)
|
| Non-cirrhotic |
45 (97.8) |
13 (86.7) |
26 (89.7) |
14 (100) |
98 (94.2) |
| Cirrhotic |
1 (2.2) |
2 (13.3) |
3 (10.3) |
0 |
6 (5.8) |
|
HCV pretreatment status, n (%)
|
| Naïve |
40 (87.0) |
14 (93.3) |
28 (96.6) |
12 (85.7) |
94 (90.4) |
| Experienced‡
|
6 (13.0) |
1 (6.7) |
1 (3.4) |
2 (14.3) |
10 (9.6) |
|
Illicit drug use, n (%)
|
| Yes |
23 (52.3) |
3 (20.0) |
19 (70.4) |
9 (69.2) |
54 (54.5) |
| Current |
6 (13.6) |
1 (6.7) |
3 (11.1) |
4 (30.8) |
14 (14.1) |
| <6 months ago |
1 (2.3) |
0 |
2 (7.4) |
0 |
3 (3.0) |
| 6 to 12 months ago |
1 (2.3) |
0 |
0 |
0 |
1 (1.0) |
| >12 months ago |
15 (34.1) |
2 (13.3) |
14 (51.9) |
5 (38.5) |
36 (36.4) |
|
Mode of HCV infection, n (%)
|
| Contaminated needle or IV drug use (current/past) |
21 (45.7) |
3 (20.0) |
17 (58.6) |
6 (42.9) |
47 (45.2) |
| Blood product transfusion |
10 (21.7) |
5 (33.3) |
1 (3.4) |
0 |
16 (15.4) |
| Contact with infected individual (other than vertical transmission) |
1 (2.2) |
0 |
2 (6.9) |
2 (14.3) |
5 (4.8) |
| Vertical transmission (mother to child) |
2 (4.3) |
0 |
0 |
1 (7.1) |
3 (2.9) |
| Occupational exposure |
1 (2.2) |
0 |
2 (6.9) |
0 |
3 (2.9) |
| Unknown |
11 (23.9) |
7 (46.7) |
7 (24.1) |
5 (35.7) |
30 (28.8) |
|
Years since diagnosis of HCV infection (N = 102)
|
| Mean (SD) |
17.2 (9.56) |
13.0 (10.83) |
12.8 (12.03) |
12.4 (8.49) |
14.7 (10.47) |
Acknowledgements
We thank all patients and physicians who participated in the MYTHEN study. The authors additionally thank Zhenzhen Zhang, PhD, of AbbVie, for conducting the statistical analysis. Karin Helsberg, Trilogy Writing and Consulting GmbH, Frankfurt, Germany provided medical writing and editing services in the development of this manuscript. Glecaprevir was identified by AbbVie and Enanta.
References
1World Health Organization. Global Hepatitis Report. 2017. Available from: www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed 2020 March 04.
2
Stanaway
JD
,
Flaxman
AD
,
Naghavi
M
,
Fitzmaurice
C
,
Vos
T
,
Abubakar
I
, et al.
The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–8. doi:.https://doi.org/10.1016/S0140-6736(16)30579-7
3Swiss Hepatitis. Strategy 2014 – 2030 Process Paper. Version 4, 2019 January. Available from: https://www.hepatitis-schweiz.ch/files/Dokumente/PDF/Process_Paper_14_02_2019.pdf. Accessed 2020 March 04.
4
Pawlotsky
J
,
Negro
F
,
Aghemo
A
,
Berenguer
M
,
Dalgard
O
,
Dusheiko
G
, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69(2):461–511. doi:.https://doi.org/10.1016/j.jhep.2018.03.026
5
Carrion
AF
,
Martin
P
. Glecaprevir + pibrentasvir for treatment of hepatitis C. Expert Opin Pharmacother. 2018;19(4):413–9. doi:.https://doi.org/10.1080/14656566.2018.1444030
6
Zeuzem
S
,
Foster
GR
,
Wang
S
,
Asatryan
A
,
Gane
E
,
Feld
JJ
, et al.
Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med. 2018;378(4):354–69. doi:.https://doi.org/10.1056/NEJMoa1702417
7
Puoti
M
,
Foster
GR
,
Wang
S
,
Mutimer
D
,
Gane
E
,
Moreno
C
, et al.
High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: An integrated analysis of HCV genotype 1-6 patients without cirrhosis. J Hepatol. 2018;69(2):293–300. doi:.https://doi.org/10.1016/j.jhep.2018.03.007
8
Kwo
PY
,
Poordad
F
,
Asatryan
A
,
Wang
S
,
Wyles
DL
,
Hassanein
T
, et al.
Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. 2017;67(2):263–71. doi:.https://doi.org/10.1016/j.jhep.2017.03.039
9
Bruggmann
P
. Hepatitis C epidemiology in Switzerland and the role of general practitioners
[Die Hepatitis-C-Epidemiologie in der Schweiz und die Rolle der Grundversorgung].
Praxis (Bern). 2016;105:885–9. doi:.https://doi.org/10.1024/1661-8157/a002424
10Zahnd C, Brezzi M, Bertisch B, et al. Situationsanalyse zu Hepatitis B und C in der Schweiz 2017. Schweizerische Eidgenossenschaft, Bundesamt für Gesundheit. Available from: https://www.bag.admin.ch/bag/de/home/das-bag/publikationen/forschungsberichte/forschungsberichte-uebertragbare-krankheiten/situationsanalyse-hepatitis.html. Accessed 2020 March 04.
11Swiss Federal Office of Public Health. Hepatitis C. Schweizerische Eidgenossenschaft, Bundesamt für Gesundheit. Available from: https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/hepatitis-c.html. Accessed 2020 March 04.
12SwissMedic [Internet]. Switzerland: Maviret Product Information 2018. Available from: http://www.swissmedicinfo.ch/. Accessed 2020 March 04.
13
Büchi
S
,
Buddeberg
C
,
Klaghofer
R
,
Russi
EW
,
Brändli
O
,
Schlösser
C
, et al.
Preliminary validation of PRISM (Pictorial Representation of Illness and Self Measure) - a brief method to assess suffering. Psychother Psychosom. 2002;71(6):333–41. doi:.https://doi.org/10.1159/000065994
14
Reilly
MC
,
Zbrozek
AS
,
Dukes
EM
. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65. doi:.https://doi.org/10.2165/00019053-199304050-00006
15Reilly Associates. Work Productivity and Activity Impairment Index (WPAI). 2002. Available from: http://www.reillyassociates.net/Index.html. Accessed 2020 March 04.
16
Rosa
K
,
Fu
M
,
Gilles
L
,
Cerri
K
,
Peeters
M
,
Bubb
J
, et al.
Validation of the Fatigue Severity Scale in chronic hepatitis C. Health Qual Life Outcomes. 2014;12(1):90. doi:.https://doi.org/10.1186/1477-7525-12-90
17
Blach
S
,
Zeuzem
S
,
Manns
M
,
Altraif
I
,
Duberg
A
,
Muljono
D
, et al., Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. doi:.https://doi.org/10.1016/S2468-1253(16)30181-9
18Moradpour D, Fehr J, Semela D, et al. Treatment of Chronic Hepatitis C –Update SASL-SSI Expert Opinion Statement 2018 August. Available from: https://sasl.unibas.ch/guidelines/SASL-SSI_EOS_Aug2018.pdf. Accessed 2020 March 04.
19SwissMedic [Internet]. Switzerland: Epclusa Product Information, 2019. Available from: http://www.swissmedicinfo.ch/. Accessed 2020 March 04.
20
D’Ambrosio
R
,
Pasulo
L
,
Puoti
M
,
Vinci
M
,
Schiavini
M
,
Lazzaroni
S
, et al.; NAVIGATORE-Lombardia Study Group. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol. 2019;70(3):379–87. doi:.https://doi.org/10.1016/j.jhep.2018.11.011
21
Berg
T
,
Naumann
U
,
Stoehr
A
,
Sick
C
,
John
C
,
Teuber
G
, et al.
Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: data from the German Hepatitis C-Registry. Aliment Pharmacol Ther. 2019;49(8):1052–9. doi:.https://doi.org/10.1111/apt.15222
22Aghemo A, Bourgois S, Gschwantler M, et al. Real-world health care resource utilization and quality of life (QoL) with G/P treatment: A pooled analysis from post-marketing observational studies. Abstract. J Hepatol 2019; 70(Suppl N1S):e228. Poster presented at: International Liver Congress 2019, Vienna. 10–14 April 2019. 1.