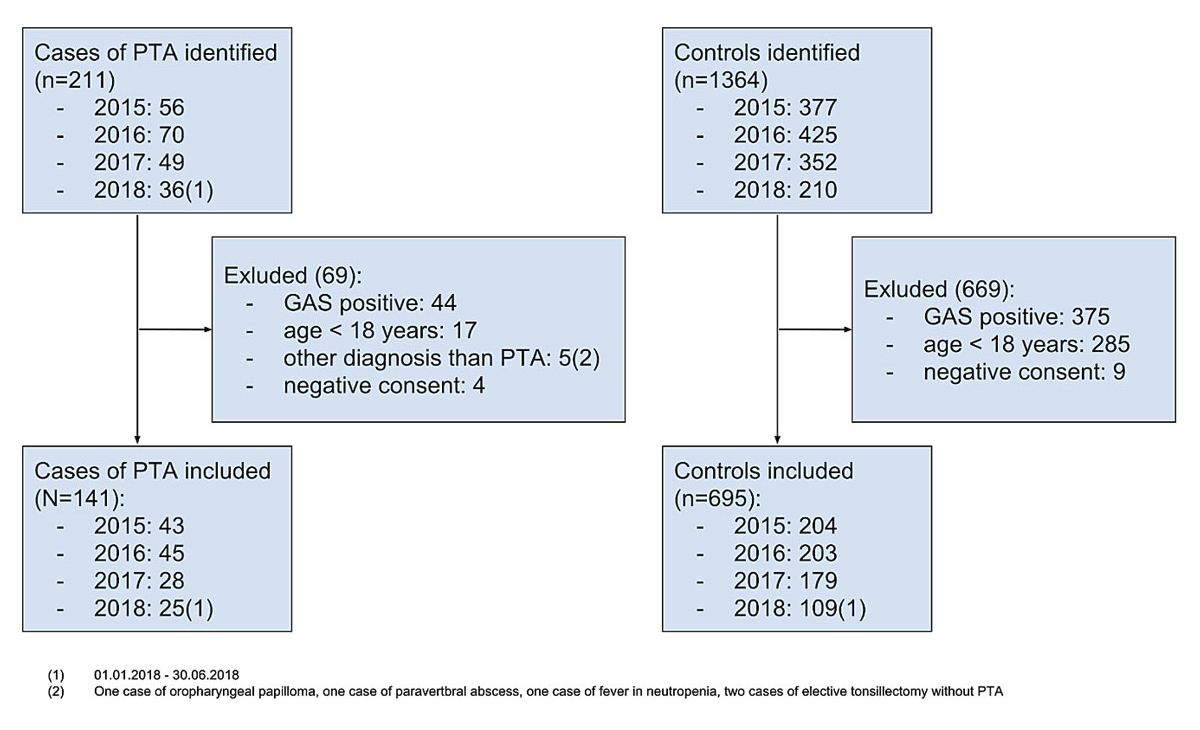
Figure 1 Cases and controls identified, excluded and included. GAS = group A β-haemolytic streptococcus; PTA = peritonsillar abscess
DOI: https://doi.org/10.4414/smw.2021.20404
Acute respiratory tract infections are a common problem in primary care. Clinical guidelines recommend against prescription of antibiotics for uncomplicated respiratory tract infections, including group A β-haemolytic streptococcus (GAS)-negative tonsillitis [1–3]. However, recent studies show that suppurative complications of tonsillitis such as peritonsillar abscess are more common than rheumatic fever [4–6], occurring in about 0.2–0.5% of patients with a sore throat [7]. Antibiotics have been shown to reduce the incidence of peritonsillar abscess compared with placebo within 2 months (risk ratio 0.15, 95% confidence interval 0.05–0.47) in a Cochrane systematic review [8].
The Centor criteria (pus, cervical lymphadenopathy, a history of fever and no history of cough) have been developed to identify patients at risk for GAS-positive tonsillitis [9]. The performance of these criteria in predicting GAS infection was validated in a large population of 206,807 patients [10]. Their use to identify patients with GAS-negative tonsillitis at risk for peritonsillar abscess has also been proposed [4, 11]; however, no validation study exists for this purpose. More recently, another clinical score (FeverPAIN; Fever, Pus, rapid Attendance (illness ≤3 days), severe Inflammation, and No cough or coryza) to predict infection with group A, C and G β-haemolytic streptococcus has been proposed [12]. Both scores have been shown to have poor predictive performance for suppurative complications of sore throat [7]. Similarly, laboratory tests such as C-reactive protein, procalcitonin, mean platelet volume and neutrophil-to-lymphocyte ratio have been proposed. Yet their performance in identifying patients likely to benefit from antibiotic treatment remains unclear [13–15].
In order to guide rational antibiotic prescription in clinical practice, new approaches to identifying patients at risk of complications are needed. The aims of this study were (1) to identify risk factors for peritonsillar abscess in acute GAS-negative tonsillitis, and (2) to estimate the diagnostic accuracy of clinical scores and laboratory tests to identify patients at risk for peritonsillar abscess in the setting of acute GAS-negative tonsillitis.
In a retrospective case-control study, we identified all cases with ICD-10 diagnosis code J36 “peritonsillar abscess” [16] using billing data at two regional hospitals in Northwestern Switzerland from 1 January 2015 to 30 June 2018. The two hospitals provide medical care for about a quarter of a million people in the rural area around Basel City. Two controls per case were selected from all patients who had a rapid antigen test for GAS in the emergency department in the same period. The emergency departments at these hospitals are divided into an emergency room, run by emergency physicians, and an emergency-care practice, where general practitioners treat ambulatory patients outside of office hours. This emergency-care practice accounted for 65% of the patients in the control pool.
At both hospitals, a written consent form allows patients to approve or refuse the use of their health records for research purposes, according to Swiss legislation. Patients with written refusal were excluded from the study. For patients who did not document refusal by signing this form, the need for consent was waived by the Ethikkommission Nordwest- und Zentralschweiz (EKNZ, Project-ID 2018-00760).
Inclusion criteria were age >18 years and a diagnosis peritonsillar abscess (ICD 10 diagnosis code J36) or having had a rapid antigen test for GAS in the emergency department for cases and controls respectively. Exclusion criteria were age <18 years, documented refusal and a positive test for GAS. The following five cases with diagnoses other than peritonsillar abscess were also excluded from the study (see fig. 1 below): one case of oropharyngeal papilloma, one case of paravertebral abscess, one case of fever in neutropenia, two cases of elective tonsillectomy without peritonsillar abscess.
We abstracted age, gender, patient history, physical examination and results of laboratory testing from health records into an electronic database, using a prespecified form. We adapted the variables used by Centor (fever, tonsillar swelling or exudate, cervical lymphadenopathy, and no cough) and Little (fever, pus [tonsillar exudate], rapid attendance [duration of symptoms ≤3days], inflammation [red throat], and no cough or coryza) in their original publications [9, 12] and added symptoms and signs typical of peritonsillar abscess (difficulty swallowing, trismus, dyspnoea), other symptoms of the common cold (conjunctivitis, myalgia and headache), as well as laboratory values of interest (white blood cell count, granulocyte, monocyte and lymphocyte counts, platelet count, mean platelet volume, C-reactive protein and procalcitonin). A list of variables and definitions as used in the data abstraction form is shown in supplementary table S1 in the appendix.
Selection, recall and observer bias are inherent to retrospective cohort studies. The use of a prespecified case report form, data abstraction by two independent researchers and the adjustment for matching criteria aimed to minimise these biases. To detect inter-observer reliability, 20% of cases and controls were abstracted by two independent raters.
Based on the validation study of Fine et al., we expected an exposure of at least three items of the Centor criteria in 25% of controls [10]. According to the formula of Kelsey [17], the calculated sample size was 135 cases and 268 controls to detect an odds ratio of at least 2.0 with a power of 90%, two-sided probability of type I error of 0.05, for an exposure of 25% in controls and a ratio of case to control of 1:2.
We used propensity score matching to select two controls, using the “optmatch” and “RItools” packages in R. Propensity scores were calculated using a binomial generalised linear model with age and gender as independent variables, and the command “pairmatch” was used to select two controls per case.
Interrater reliability was calculated using the “irr” package in R. We used the function “kappa2” to calculate Cohen’s kappa for binary variables and the function “icc” to calculate intraclass correlation coefficients for continuous variables, using a one-way random-effect model.
Owing to the retrospective design, missing values were inevitable. For the binary variables of signs and symptoms, we assumed that no mention in the health record signified the absence thereof. As the presence of a finding may not be reported in health records with the same probability as its absence, a reporting bias could not be excluded. Imputation would not have been valid in this case, as missing values are not random. For 5 cases and 30 controls, no duration of symptoms was reported in health records. We used multivariable imputation by chained equations (R package “mice”) to impute the variable rapid attendance (duration of symptoms ≤3 days), with age, gender and the binary variables of patient history and physical examination as predictors. For laboratory values, rates of missing values ranged from 20% to 98%. Because of the high rates of missing values, we did not attempt imputation and excluded laboratory values from the multivariable regression analysis.
We used descriptive statistics to explore data and Pearson's chi-square test to explore the relation between patient characteristics, patient history, physical examination and the occurrence of peritonsillar abscess. Laboratory values were compared between peritonsillar abscess cases and controls using the Welch Two Sample t-test. We used uni- and multivariable logistic regression analyses, adjusting for the matching variables (age and gender) to estimate the odd ratios (ORs) and 95% confidence intervals (CIs). Receiver operator characteristic (ROC) graphs were calculated for the Centor criteria, the FeverPAIN score, C-reactive protein and white blood cell count, using the R package “pROC” [18]. Optimal cut-offs were estimated by use of the Youden index. For all statistical analyses a significance level of 0.05 was used. Sensitivity analyses were performed (1) to compare conditional regression analysis and logistic regression adjusted for the matching variables, and (2) to assess the effect of multicollinearity in the full model, selectively omitting variables with variance inflation factors >5. All statistical analyses were performed in RStudio Version 1.1.463.
We examined 211 cases of peritonsillar abscess for eligibility and included 141 in the study. We excluded 44 GAS-positive patients, 17 with age <18 years, 4 with documented negative consent and 5 with a diagnosis other than peritonsillar abscess. We identified 1364 patients who were tested for GAS. We excluded 375 GAS-positive patients, 285 with age <18 years and 9 with documented negative consent. Out of the remaining 695 patients, we selected 282 controls using propensity score matching. All of the included patients were analysed. A study flow diagram is shown in figure 1.

Figure 1 Cases and controls identified, excluded and included. GAS = group A β-haemolytic streptococcus; PTA = peritonsillar abscess
In the subset of 20% of cases and controls that were abstracted by two independent raters, we assessed inter-rater reliability. Results are shown in table S2 in the appendix. Cohen’s kappa for binary variables ranged from 0.66 to 0.97, showing substantial agreement according to Landis et al. [19]. For laboratory values, intraclass correlation coefficients ranged between 0.73 and 0.98, showing good reliability according to Koo et al. [20].
Patient characteristics are shown in table 1. Out of the Centor criteria, tonsillar hyperplasia, cervical lymphadenopathy and the absence of cough were more common in peritonsillar abscess cases. Cases had higher scores than controls. The FeverPAIN score had no correlation with the outcome. Two of its components, pus (tonsillar exudate) and no cough or coryza were more common in peritonsillar abscess cases. Levels of C-reactive protein were higher in cases than in controls, as were all the elements of the white blood cell count, except lymphocytes. Platelet counts were also higher in cases.
Table 1 Descriptive analysis.
| Patient characteristics | N |
All
(n = 423) |
Cases
(n = 141) |
Controls
(n = 282) |
p-value* | |||
|---|---|---|---|---|---|---|---|---|
| Age (mean, standard deviation), years | 423 | 38.6 | 15.6 | 39.1 | 15.6 | 38.3 | 15.6 | 0.65 |
| Female (n, percent) | 423 | 191 | 45% | 66 | 47% | 125 | 44% | 0.70 |
| Duration of symptoms ≤3 days (n, percent)† | 423 | 225 | 53% | 65 | 46% | 160 | 57% | 0.050 |
| Sore throat (n, percent) | 423 | 387 | 92% | 136 | 97% | 251 | 89% | 0.016 |
| Difficulty swallowing (n, percent) | 423 | 145 | 34% | 107 | 76% | 38 | 14% | <0.001 |
| Trismus (n, percent) | 423 | 62 | 15% | 62 | 44% | 0 | 0% | <0.001 |
| Dyspnoea (n, percent) | 423 | 20 | 5% | 14 | 10% | 6 | 2% | <0.001 |
| Symptoms of the common cold‡ | 423 | 185 | 44% | 30 | 21% | 155 | 55% | <0.001 |
| Earache (n, percent) | 423 | 88 | 21% | 42 | 30% | 46 | 16% | 0.002 |
| Fever, reported or measured ≥38.5°C (n, percent) | 423 | 178 | 42% | 53 | 38% | 125 | 44% | 0.22 |
| Red throat (n, percent) | 423 | 297 | 70% | 99 | 70% | 198 | 70% | 1.0 |
| Tonsillar hyperplasia (n, percent) | 423 | 217 | 51% | 128 | 91% | 89 | 32% | <0.001 |
| Tonsillar exudate (n, percent) | 423 | 143 | 34% | 62 | 44% | 81 | 29% | 0.003 |
| Cervical lymphadenopathy (n, percent) | 423 | 215 | 51% | 107 | 76% | 108 | 38% | <0.001 |
| Unilateral signs and symptoms (n, percent) | 423 | 169 | 40% | 133 | 94% | 36 | 13% | <0.001 |
| Centor criteria (mean, standard deviation)§ | 423 | 2.30 | 1.15 | 3.00 | 0.78 | 1.95 | 1.15 | <0.001 |
| FeverPAIN (mean, standard deviation)¶ | 423 | 2.93 | 1.08 | 2.94 | 1.05 | 2.93 | 1.09 | 0.87 |
| C-reactive protein (mean, standard deviation), mg/l | 339 | 69.0 | 74.1 | 97.0 | 73.7 | 49.9 | 68.3 | <0.001 |
| White blood cell count (mean, standard deviation), 109/l | 339 | 12.3 | 6.05 | 14.8 | 7.03 | 10.6 | 4.55 | <0.001 |
| Granulocyte count (mean, standard deviation), 109/l | 270 | 9.4 | 6.94 | 12.4 | 9.24 | 7.6 | 4.09 | <0.001 |
| Monocyte count (mean, standard deviation), 109/l | 267 | 0.99 | 0.69 | 1.30 | 0.82 | 0.80 | 0.51 | <0.001 |
| Lymphocyte count (mean, standard deviation), 109/l | 270 | 2.33 | 3.82 | 2.71 | 5.91 | 2.09 | 1.43 | 0.30 |
| Granulocyte to lymphocyte ratio (mean, standard deviation) | 270 | 5.97 | 5.84 | 7.73 | 7.60 | 4.88 | 4.08 | <0.001 |
| Platelet count (mean, standard deviation), 109/l | 338 | 224 | 75.7 | 245 | 71.7 | 209 | 75.1 | <0.001 |
| Mean platelet volume (mean, standard deviation), fl | 85 | 9.8 | 1.22 | 9.7 | 1.42 | 10.0 | 0.93 | 0.25 |
| Procalcitonin (mean, standard deviation), μg/l | 7 | 1.42 | 0.93 | |||||
* p-value was calculated by Pearson’s Chi-square test with Yates’ continuity correction for binomial variables and by Welch Two Sample t-test for continuous variables † 35 missing values imputed using multivariable imputation by chained equations ‡ Cough, coryza, conjunctivitis, myalgia or headache § Fever, tonsillar swelling or exudate, cervical lymphadenopathy, no cough ¶ Fever, Pus (tonsillar exudate), rapid Attendance (duration of symptoms ≤3 days), Inflammation (red throat), No cough or coryza
Figure 2 shows the ROC curves for the Centor criteria and the FeverPAIN scores, C-reactive protein and the white blood cell count. The FeverPAIN score performanced was close to that of a random classifier (area under the curve [AUC] 0.51, sensitivity 0.66, specificity 0.39.). The Centor criteria, C-reactive protein and white blood cell count performed similarly (AUC 0.76, 0.73 and 0.74; sensitivity 0.77, 0.70 and 0.70; specificity 0.68, 0.67 and 0.67, respectively).
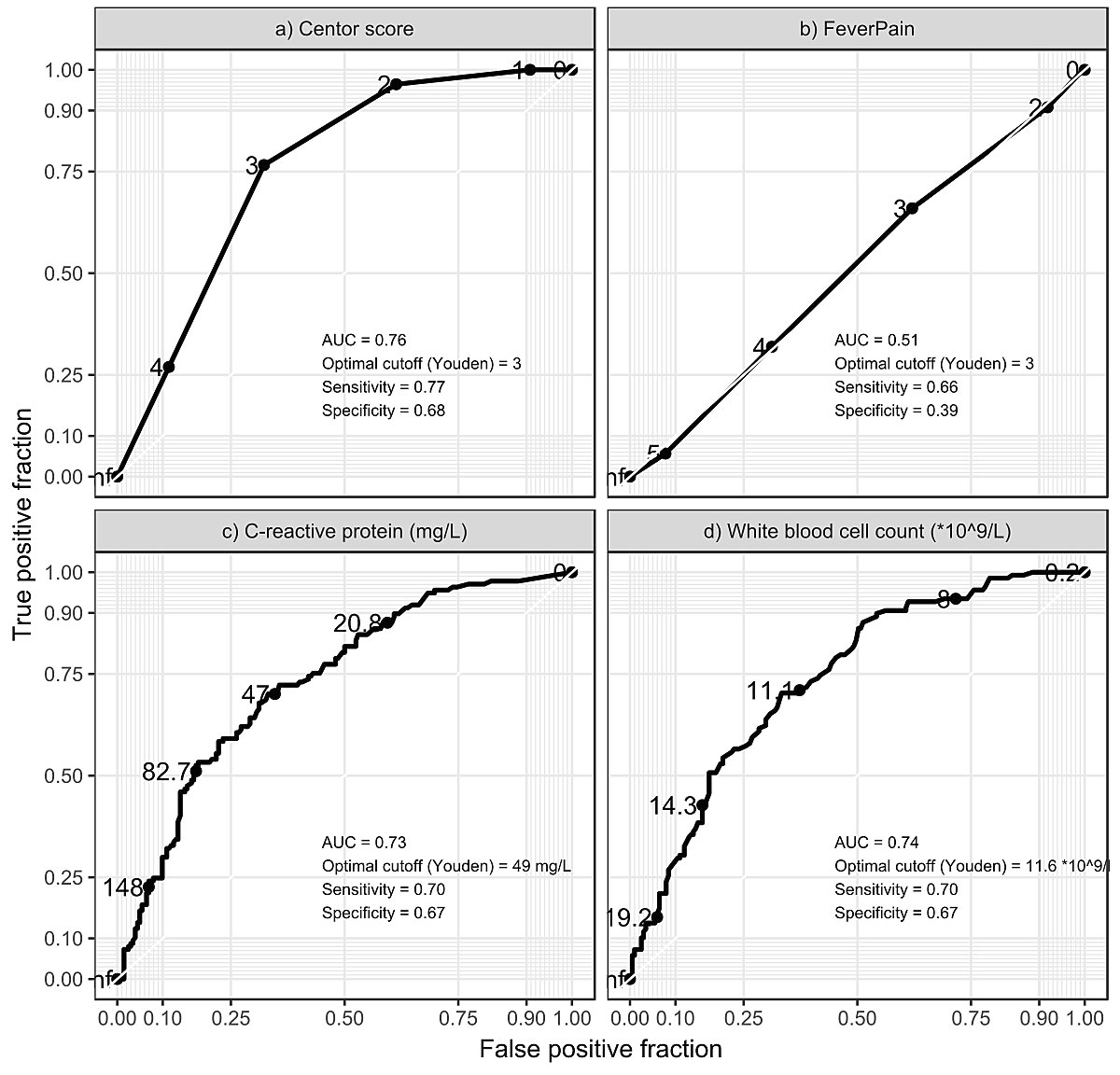
Figure 2 Receiver operator characteristic curves.
Results of the regression analyses are shown in table 2 and table 3. The primary models are presented on the left, models from the sensitivity analysis on the right. After adjustment for matching variables in the simple model, rapid attendance, sore throat, difficulty swallowing, dyspnoea, earache, tonsillar swelling, tonsillar exudate, cervical lymphadenopathy, unilateral signs and symptoms, and the absence of symptoms of the common cold were significantly associated with peritonsillar abscess. After multivariable adjustment, difficulty swallowing (OR 18.4, 95% CI 6.58–51.2), dyspnoea (OR 10.2, 95% CI 1.18–89.0), tonsillar swelling (OR 4.21, 95% CI 1.39–12.7) and unilateral signs and symptoms (OR 146, 95% CI 40.9–522) remained significant. The variable trismus did not occur in the control group and was not included in the regression analyses. Also, the variables mean platelet volume and procalcitonin were excluded from the regression analyses because of the high rates of missing values (80% and 98%, respectively). In the full model, we excluded the laboratory values because of the high number of missing values.
Table 2 Regression analyses: simple models.
| N = 423 | Adjusted for matching variables* | Conditional regression analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p-value | Odds ratio | 95% confidence interval | p-value | |||
| Age, per 1 year | 1.00 | 0.99 | 1.02 | 0.67 | 1.01 | 0.99 | 1.03 | 0.47 |
| Female vs male | 1.10 | 0.73 | 1.65 | 0.65 | 1.17 | 0.71 | 1.95 | 0.54 |
| Duration of symptoms ≤3 days vs > 3 days† | 0.65 | 0.43 | 0.98 | 0.041 | 0.62 | 0.40 | 0.95 | 0.030 |
| Sore throat, present vs not present | 3.46 | 1.42 | 10.3 | 0.012 | 3.18 | 1.23 | 8.26 | 0.017 |
| Difficulty swallowing, present vs. not present | 20.2 | 12.2 | 34.4 | <0.001 | 21.5 | 10.4 | 44.3 | <0.001 |
| Dyspnoea, present vs not present | 5.04 | 1.96 | 14.6 | 0.001 | 5.33 | 1.91 | 14.9 | 0.001 |
| Symptoms of the common cold‡, present vs not present | 0.22 | 0.13 | 0.34 | <0.001 | 0.22 | 0.13 | 0.36 | <0.001 |
| Earache, present vs not present | 2.16 | 1.33 | 3.51 | 0.002 | 2.19 | 1.35 | 3.59 | 0.002 |
| Fever, reported or measured ≥38.0°C, present vs not present | 0.77 | 0.50 | 1.16 | 0.21 | 0.76 | 0.50 | 1.15 | 0.19 |
| Red throat, present vs not present | 1.01 | 0.65 | 1.58 | 0.96 | 1.00 | 0.64 | 1.57 | 1.0 |
| Tonsillar swelling, present vs not present | 28.4 | 15.0 | 58.4 | <0.001 | 18.7 | 9.07 | 38.6 | <0.001 |
| Tonsillar exudate, present vs not present | 2.18 | 1.40 | 3.41 | <0.001 | 1.87 | 1.24 | 2.81 | 0.003 |
| Cervical lymphadenopathy, present vs not present | 5.71 | 3.59 | 9.32 | <0.001 | 5.86 | 3.44 | 9.99 | <0.001 |
| Unilateral signs and symptoms (4), present vs not present | 116 | 55.0 | 276 | <0.001 | 72.6 | 23.1 | 228 | <0.001 |
| Centor criteria‡, per 1 | 2.98 | 2.33 | 3.89 | <0.001 | 3.11 | 2.31 | 4.18 | <0.001 |
| FeverPAIN score§, per 1 | 1.03 | 0.85 | 1.25 | 0.77 | 1.02 | 0.84 | 1.23 | 0.87 |
| C-reactive protein, per 10 mg/l (n = 339) | 1.10 | 1.07 | 1.15 | <0.001 | 1.11 | 1.06 | 1.15 | <0.001 |
| White blood cell count, per 109/l (n = 339) | 1.19 | 1.13 | 1.25 | <0.001 | 1.17 | 1.11 | 1.24 | <0.001 |
| Granulocyte count, per 109/l (n = 270) | 1.22 | 1.15 | 1.31 | <0.001 | 1.18 | 1.10 | 1.27 | <0.001 |
| Monocyte count, per 108/l (n = 267) | 1.18 | 1.12 | 1.26 | <0.001 | 1.13 | 1.06 | 1.21 | <0.001 |
| Lymphocyte count, per 109/l (n = 270) | 1.06 | 0.98 | 1.24 | 0.33 | 1.04 | 0.94 | 1.16 | 0.43 |
| Granulocyte to lymphocyte ratio (n = 270), per 1 | 1.12 | 1.06 | 1.19 | <0.001 | 1.12 | 1.05 | 1.21 | <0.001 |
| Platelet count, per 1010/l (n = 338) | 1.07 | 1.04 | 1.10 | <0.001 | 1.08 | 1.04 | 1.12 | <0.001 |
* Age and gender † 35 missing values imputed using multivariable imputation by chained equations ‡ Cough, coryza, conjunctivitis, headache or myalgia § Unilateral sore throat, headache, earache or swelling
Table 3 Regression analyses: full models.
| N = 423 | VIF* | Multivariable adjustment† | Multivariable ajustment‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | p-value | Odds ratio | 95% confidence interval | p-value | ||||
| Age, per 1 year | 1.21 | 1.00 | 0.97 | 1.04 | 0.84 | 1.02 | 0.99 | 1.04 | 0.084 |
| Female vs male | 1.11 | 1.34 | 0.53 | 3.41 | 0.53 | 1.40 | 0.73 | 2.70 | 0.31 |
| Duration of symptoms ≤3 days vs >3 days§ | 1.07 | 0.73 | 0.28 | 1.90 | 0.52 | 0.58 | 0.29 | 1.14 | 0.12 |
| Sore throat, present vs not present | 1.30 | 0.27 | 0.04 | 1.61 | 0.15 | 1.05 | 0.28 | 3.94 | 0.94 |
| Difficulty swallowing, present vs not present | 1.33 | 18.4 | 6.58 | 51.2 | <0.001 | 12.8 | 6.57 | 24.8 | <0.001 |
| Dyspnoea, present vs not present | 1.06 | 10.2 | 1.18 | 89.0 | 0.035 | 5.43 | 1.39 | 21.3 | 0.015 |
| Symptoms of the common cold¶, present vs not present | 1.14 | 0.62 | 0.22 | 1.77 | 0.37 | 0.37 | 0.19 | 0.75 | 0.006 |
| Earache, present vs not present | 1.48 | 0.44 | 0.14 | 1.35 | 0.15 | 2.24 | 0.97 | 5.18 | 0.059 |
| Fever, reported or measured ≥38.0°C, present vs not present | 1.27 | 1.69 | 0.63 | 4.55 | 0.30 | 1.05 | 0.53 | 2.08 | 0.89 |
| Red throat, present vs not present | 1.23 | 0.74 | 0.27 | 2.08 | 0.57 | 0.48 | 0.22 | 1.01 | 0.051 |
| Tonsillar swelling, present vs not present | 1.39 | 4.21 | 1.39 | 12.7 | 0.01 | 16.7 | 7.19 | 39.0 | <0.001 |
| Tonsillar exudate, present vs not present | 1.41 | 1.18 | 0.42 | 3.30 | 0.76 | 0.81 | 0.40 | 1.66 | 0.56 |
| Cervical lymphadenopathy, present vs not present | 1.24 | 1.74 | 0.64 | 4.74 | 0.28 | 2.12 | 1.02 | 4.41 | 0.045 |
| Unilateral signs and symptoms‖, present vs not present | 1.78 | 146 | 40.9 | 522 | <0.001 | ||||
* Variable inflation factors † Full model adjusting for age, gender, duration of symptoms, sore throat, difficulty swallowing, dyspnoea, symptoms of the common cold, earache, fever, red throat, tonsillar swelling, tonsillar exudate, cervical lymphadenopathy and unilaterality ‡ Full model adjusting for age, gender, duration of symptoms, sore throat, difficulty swallowing, dyspnoea, symptoms of the common cold, earache, fever, red throat, tonsillar swelling, tonsillar exudate, cervical lymphadenopathy § 35 missing values imputed using multivariate imputation by chained equations ¶ Cough, coryza, conjunctivitis, headache or myalgia ‖ Unilateral sore throat, headache, earache or swelling
Conditional regression analysis showed results similar to those of simple logistic regression (see table 2). None of the variables reached the predefined variance inflation factor of >5 in the multivariable adjusted model. The variable with the highest variance inflation factor was unilaterality (1.78). Omitting this variable from the full model showed results comparable to those of the presented model, with the exception of symptoms of the common cold, which remained significant (see table 3).
Cases of peritonsillar abscess negative tonsillitis had greater odds of difficulty swallowing, dyspnoea, tonsillar swelling and unilateral signs and symptoms than controls with uncomplicated tonsillitis. This association remained significant after multivariable adjustment. Although the Centor criteria were significantly associated with peritonsillar abscess, the score had a low performance for identifying patients with peritonsillar abscess (sensitivity 0.77, specificity 0.68, ROC AUC 0.76). There was no association of the FeverPAIN score with peritonsillar abscess (sensitivity 0.66, specificity 0.39, ROC AUC 0.51). C-reactive protein and white blood cell count performed similarly to the Centor criteria.
We carried out a small study with minimal resources to assess risk factors of peritonsillar abscess. In addition to confirming the known weakness of existing clinical scores such as the Centor criteria and FeverPAIN [7], we included new clinical items in our analysis, as well as point-of-care laboratory values. This allowed the identification of new risk factors that might help developing new scores.
Our study has limitations. The use of billing data to identify cases did not provide a precise definition of the outcome. In fact, the ICD-10 diagnosis code J36 does not distinguish between the diagnoses of peritonsillar abscess and peritonsillar cellulitis or peritonsillitis. The latter might have been more accurate for some of the cases, because intervention or imaging did not show a collection of pus or because the patient improved under parenteral antibiotic therapy without surgical intervention. However, the diagnosis of peritonsillar abscess is clinical and, to our knowledge, there is no universally accepted definition. All of the cases were hospitalised, indicating a severe or complicated illness. Missing values are a problem inherent to the retrospective design of the study. We assumed that a characteristic was absent if not reported in the health record, introducing a possible observer bias. High rates of missing values for laboratory values, especially among controls, also limit the validity of the study. As underage patients were excluded, the results cannot be generalised to patients <18 years of age. The setting in two regional hospitals may lead to different results than, for example, in a generalist’s office.
We identified 211 cases of peritonsillar abscess and included 141 cases of GAS-negative tonsillitis. In a population of about 250,000 people over a time-period of 2.5 years, this translates into an overall annual incidence of 34/100,000. This aligns with the observations of Klug et al., who found incidence rates between 15 and 74/100,000 in the adult Danish population (20 years and older) [21].
The duration of symptoms ≤3 days was negatively associated with peritonsillar abscess in the simple model (p = 0.041). In the full model, no association was found (p = 0.515). This confirms previous studies on this subject. In a similar, retrospective cross-sectional study comparing cases of peritonsillar abscess to controls with peritonsillitis, Kilty et al. found that the duration of pain did not predict peritonsillar abscess [22]. Little et al. reported that previous symptom duration of ≤3 days could not predict suppurative complications such as peritonsillar abscess in a prospective cohort study of 14,610 patients with sore throat [7].
After multivariable adjustment, difficulty swallowing, dyspnoea, tonsillar swelling and unilateral signs and symptoms were associated with peritonsillar abscess. Having a sore throat, symptoms of the common cold, earache, sore throat and tonsillar exudate and cervical lymphadenopathy were not associated. Because none of the controls reported this symptom, trismus was not included in the regression analyses. In their similar case-control study, Kilty et al. also found no association of erythema/inflammation with the outcome. They found trismus to be associated with peritonsillar abscess, but did not include the variables dyspnoea, tonsillar swelling and unilaterality in their analysis.
In his cohort study, Little et al. reported severely inflamed tonsils, but not difficulty swallowing, to predict suppurative complications. This might be explained by differences in the definition of these items and of the outcome variable: Little et al. also included otitis media as suppurative complication. Also, Little et al. did not assess unilaterality of signs and symptoms, dyspnoea and tonsillar swelling, which were associated with peritonsillar abscess in our study [7, 22].
To our knowledge, two clinical scores have been proposed for identifying patients at risk for peritonsillar abscess: the Centor criteria (pus, cervical lymphadenopathy, a history of fever and no history of cough) and the FeverPAIN score (fever, pus, rapid attendance [illness ≤3 days], severe inflammation, and no cough or coryza). Little et al. developed the FeverPAIN score to predict Streptococcus C and G infection. Our results have shown no difference in this score between cases and controls. The predictive performance of the FeverPAIN score was close to that of a random classifier (ROC AUC 0.51). In our view, this score is not useful for predicting complications, as Little et al. did not report any suppurative complications in their trial [23].
Little et al. observed that most suppurative complications occur with low Centor scores [7]. In our study, we found a significant difference in the Centor criteria between cases and controls. Given the low prevalence of complications and the relatively poor test performance (sensitivity 0.77, specificity 0.68, ROC AUC 0.76), the clinical usefulness of the Centor criteria is nevertheless questionable.
Levels of C-reactive protein and white blood cell count were higher in cases than in controls. The predictive performance of C-reactive protein and white blood cell count were similar to the Centor criteria. This association has been demonstrated by Calviño et al., who found C-reactive protein to be associated with bacterial aetiology. Calviño et al. also concluded that C-reactive protein was not helpful in differentiating between GAS and other aetiologies [24]. Gahleitner et al. reported no difference in C-reactive protein or white blood cell count, comparing peritonsillar abscess with acute tonsillitis [15].
Meili et al. found that procalcitonin was more specific for bacterial aetiology than C-reactive protein in respiratory tract infections. However, both performed poorly concerning outcome prediction [13]. Because there is no point-of-care test available for procalcitonin, we found almost no values for this in our retrospective study.
Şentürk et al. analysed the neutrophil-to-lymphocyte ratio and mean platelet volume as inflammatory markers in peritonsillar abscess. In their study, mean platelet volume correlated with peritonsillar abscess [14]. We could not confirm this observation in our study. The lymphocyte count was the only part of the white blood cell count not associated with peritonsillar abscess, and the association of the neutrophil count alone was stronger than its ratio with the lymphocyte count. These findings have to be interpreted with caution because of the high number of missing values. Based on our results, point-of-care diagnostics such as white blood cell count and C-reactive protein cannot be recommended to guide antibiotic prescription in tonsillitis so far.
The Centor criteria, as well as point-of-care tests such as C-reactive protein and white blood cell count, have a low discriminatory performance, and the FeverPAIN score is not useful to identify patients at risk for peritonsillar abscess in the setting of acute GAS negative tonsillitis. In order to guide a more rational antibiotic prescription, new clinical decision tools need to be developed. These might include items such as difficulty swallowing, dyspnoea, tonsillar swelling and unilaterality of signs and symptoms.
Table S1 List of variables and definitions.
| Name | Label | Variable type | Description |
|---|---|---|---|
| ParticipantId | Participant ID | Integer | |
| caseDate | Date of presentation | DateTime | Date of presentation. |
| age | Age (years) | Integer | Age at presentation in years. |
| female | Female | True/False (Boolean) | Is the participant female (true) or male (false)? |
| bruderholz | Bruderholz | True/False (Boolean) | Presentation at Bruderholz (true) or Liestal (false)? |
| case | Case | True/False (Boolean) | Is a case (true) or a control (false)? |
| duration | Duration of symptoms (days) | Integer | Duration of symptoms (days) reported by the patient? |
| soreThroat | Sore throat | True/False (Boolean) | Sore throat reported by the patient? |
| unilateralSoreThroat | Unilateral sore throat | True/False (Boolean) | Presence of unilateral sore throat? |
| difficultySwallowing | Difficulty swallowing | True/False (Boolean) | Presence of difficulty swallowing? |
| trismus | Trismus | True/False (Boolean) | Presence of difficulty or pain opening the mouth? |
| dyspnea | Dyspnea | True/False (Boolean) | Presence of difficulty breathing? |
| cough | Cough | True/False (Boolean) | Presence of cough? |
| headache | Headache | True/False (Boolean) | Presence of headache? |
| unilateralHeadache | Unilateral headache | True/False (Boolean) | Presence of unilateral headache? |
| coryza | Coryza | True/False (Boolean) | Presence of runny nose? |
| conjunctivitis | Conjunctivitis | True/False (Boolean) | Presence of red or itchy eyes? |
| earache | Earache | True/False (Boolean) | Presence of pain in or radiating to the ears? |
| unilateralEarache | Unilateral earache | True/False (Boolean) | Presence of unilateral pain in or radiating to an ear? |
| myalgia | Myalgia | True/False (Boolean) | Presence of muscle pain? |
| fever | Fever | True/False (Boolean) | Fever as a symptom or sign? |
| redThroat | Red throat | True/False (Boolean) | Presence of redness in oral inspection? |
| cervical | Cervical lymphadenopathy | True/False (Boolean) | Presence of cervical lymphadenopathy or tenderness? |
| tonsillarSwelling | Tonsillar swelling | True/False (Boolean) | Presence of tonsillar swelling or hyperplasia? |
| unilateralSwelling | Unilateral swelling | True/False (Boolean) | Presence of unilateral swelling in oral inspection? |
| tonsillarExsudate | Tonsillar exsudate | True/False (Boolean) | Presence of tonsillar exudate? |
| abnormalEar | Abnormal otoscopy | True/False (Boolean) | Abnormal tympanic membrane in otoscopy? |
| abnormalLung | Abnormal lung auscultation | True/False (Boolean) | Abnormal lung auscultation? |
| temperature | Body temperature (°C) | Number (Double) | |
| CRP | CRP (mg/L) | Number (Double) | |
| Hb | Haemoglobin (g/L) | Number (Double) | |
| Lc | Leucocyte count (10^9/L) | Number (Double) | |
| Gc | Granulocyte count (10^9/L) | Number (Double) | Granulocyte or neutrophil count. |
| Mono | Monocyte count (10^9/L) | Number (Double) | |
| Lym | Lymphocyte count (10^9/L) | Number (Double) | |
| Plt | Platelet count (10^9/L) | Number (Double) | |
| MPV | Meant platelet volume (fL) | Number (Double) | |
| PCT | Procalcitonin (μg/L) | Number (Double) |
Table S2 Inter-rater reliability.
| n = 84 | Cohen's kappa | Intraclass correlation |
|---|---|---|
| Rapid attendance | 0.84 | |
| Sore throat | 0.82 | |
| Difficulty swallowing | 0.97 | |
| Trismus | 0.84 | |
| Dyspnoea | 0.79 | |
| Symptoms of the common cold* | 0.88 | |
| Earache | 0.85 | |
| Red throat | 0.78 | |
| Cervical lymphadenopathy | 0.91 | |
| Tonsillar swelling | 0.79 | |
| Tonsillar exudate | 0.80 | |
| Abnormal otoscopy | 0.79 | |
| Abnormal lung auscultation | 0.79 | |
| Unilaterality of signs and symptoms† | 0.66 | |
| C-reactive protein | 0.98 | |
| Haemoglobin | 0.96 | |
| Leucocyte count | 0.96 | |
| Granulocyte count | 0.95 | |
| Monocyte count | 0.73 | |
| Lymphocyte count | 0.88 | |
| Platelet count | 0.94 | |
| Mean platelet volume | 0.83 |
* Cough, coryza, conjunctivitis, myalgia or headache † Unilateral sore throat, earache, headache or swelling
We would like to thank Laura Diaz Hernandez from the Universitäres Zentrum für Hausarztmedizin beider Basel for her valuable help with the statistical analysis, Prof. Kurt Tschopp, director of the ENT clinic at Kantonsspital Baselland, and Prof. Nicolas Geigy, for the access granted to the health records, and Dr Regina Classen, Director of Medical Controlling at Kantonsspital Baselland, for the provision of billing data.
This project was funded out of the annual budget of the Universitäres Zentrum für Hausarztmedizin beider Basel. No third party funding was required.
All authors report no competing interest.
1 Selby K , Gaspoz JM , Rodondi N , Neuner-Jehle S , Perrier A , Zeller A , et al. Creating a list of low-value health care activities in Swiss primary care. JAMA Intern Med. 2015;175(4):640–2. doi:.https://doi.org/10.1001/jamainternmed.2014.8111
2 Kenealy T , Arroll B . Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev. 2013;10–2(6):CD000247. doi:.https://doi.org/10.1002/14651858.CD000247.pub3
3 Cooper RJ , Hoffman JR , Bartlett JG , Besser RE , Gonzales R , Hickner JM , et al.; American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine; Centers for Disease Control. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Intern Med. 2001;134(6):509–17. doi:.https://doi.org/10.7326/0003-4819-134-6-200103200-00019
4 Centor RM , Atkinson TP , Ratliff AE , Xiao L , Crabb DM , Estrada CA , et al. The clinical presentation of Fusobacterium-positive and streptococcal-positive pharyngitis in a university health clinic: a cross-sectional study. Ann Intern Med. 2015;162(4):241–7. doi:.https://doi.org/10.7326/M14-1305
5 Batty A , Wren MWD , Gal M . Fusobacterium necrophorum as the cause of recurrent sore throat: comparison of isolates from persistent sore throat syndrome and Lemierre’s disease. J Infect. 2005;51(4):299–306. doi:.https://doi.org/10.1016/j.jinf.2004.09.013
6 Ehlers Klug T , Rusan M , Fuursted K , Ovesen T . Fusobacterium necrophorum: most prevalent pathogen in peritonsillar abscess in Denmark. Clin Infect Dis. 2009;49(10):1467–72. doi:.https://doi.org/10.1086/644616
7 Little P , Stuart B , Hobbs FD , Butler CC , Hay AD , Campbell J , et al.; DESCARTE investigators. Predictors of suppurative complications for acute sore throat in primary care: prospective clinical cohort study. BMJ. 2013;347:f6867. doi:.https://doi.org/10.1136/bmj.f6867
8 Spinks A , Glasziou PP , Del Mar CB . Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;2013(11):CD000023. doi:htps://doi.org/10.1002/14651858.CD000023.pub4.
9 Centor RM , Witherspoon JM , Dalton HP , Brody CE , Link K . The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–46. doi:.https://doi.org/10.1177/0272989X8100100304
10 Fine AM , Nizet V , Mandl KD . Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch Intern Med. 2012;172(11):847–52. doi:.https://doi.org/10.1001/archinternmed.2012.950
11 Klug TE , Rusan M , Fuursted K , Ovesen T , Jorgensen AW . A systematic review of Fusobacterium necrophorum-positive acute tonsillitis: prevalence, methods of detection, patient characteristics, and the usefulness of the Centor score. Eur J Clin Microbiol Infect Dis. 2016;35(12):1903–12. doi:.https://doi.org/10.1007/s10096-016-2757-y
12 Little P , Hobbs FDRFR , Mant D , McNulty CAMA , Mullee M ; PRISM investigators. Incidence and clinical variables associated with streptococcal throat infections: a prospective diagnostic cohort study. Br J Gen Pract. 2012;62(604):e787–94. doi:.https://doi.org/10.3399/bjgp12X658322
13 Meili M , Kutz A , Briel M , Christ-Crain M , Bucher HC , Mueller B , et al. Infection biomarkers in primary care patients with acute respiratory tract infections-comparison of Procalcitonin and C-reactive protein. BMC Pulm Med. 2016;16(1):43. doi:.https://doi.org/10.1186/s12890-016-0206-4
14 Şentürk M , Azgın İ , Övet G , Alataş N , Ağırgöl B , Yılmaz E . The role of the mean platelet volume and neutrophil-to-lymphocyte ratio in peritonsillar abscesses. Rev Bras Otorrinolaringol (Engl Ed). 2016;82(6):662–7. doi:.https://doi.org/10.1016/j.bjorl.2015.11.018
15 Gahleitner C , Hofauer B , Stark T , Knopf A . Predisposing factors and management of complications in acute tonsillitis. Acta Otolaryngol. 2016;136(9):964–8. doi:.https://doi.org/10.3109/00016489.2016.1170202
16World Health Organization. ICD-10 : international statistical classification of diseases and related health problems, 10th revision. Geneva: World health Organization; 2016.
17Kelsey JL, Whittemore AS, Evans AS, Thompson WD. Methods in Observational Epidemiology. Oxford: Oxford University Press; 1996.
18 Robin X , Turck N , Hainard A , Tiberti N , Lisacek F , Sanchez J-C , et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(1):77. doi:.https://doi.org/10.1186/1471-2105-12-77
19 Landis JR , Koch GG . The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. doi:.https://doi.org/10.2307/2529310
20 Koo TK , Li MY . A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. doi:.https://doi.org/10.1016/j.jcm.2016.02.012
21 Klug TE . Incidence and microbiology of peritonsillar abscess: the influence of season, age, and gender. Eur J Clin Microbiol Infect Dis. 2014;33(7):1163–7. doi:.https://doi.org/10.1007/s10096-014-2052-8
22 Kilty SJ , Gaboury I . Clinical predictors of peritonsillar abscess in adults. J Otolaryngol Head Neck Surg. 2008;37(2):165–8.
23 Little P , Hobbs FD , Moore M , Mant D , Williamson I , McNulty C , et al.; PRISM investigators. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ. 2013;347(oct10 3):f5806. doi:.https://doi.org/10.1136/bmj.f5806
24 Calviño O , Llor C , Gómez F , González E , Sarvisé C , Hernández S . Association between C-reactive protein rapid test and group A streptococcus infection in acute pharyngitis. J Am Board Fam Med. 2014;27(3):424–6. doi:.https://doi.org/10.3122/jabfm.2014.03.130315
This project was funded out of the annual budget of the Universitäres Zentrum für Hausarztmedizin beider Basel. No third party funding was required.
All authors report no competing interest.