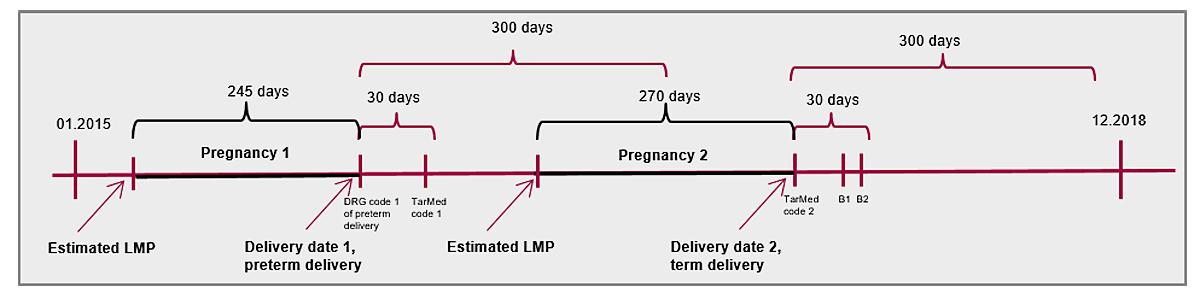
Figure 1 Definition of delivery and last menstruation period (LMP) scheme.
DOI: https://doi.org/10.4414/smw.2021.20386
Valproate has been available in Switzerland since 1972 for the treatment of epilepsy, and since the mid-1990s also for bipolar disease [1]. Since the 1980s, a congenital valproate syndrome has been characterised, which occurs in up to 10% of children after in utero exposure to valproate during the first trimester [2]. The syndrome comprises neural tube defects, cardiovascular anomalies, limb defects, hypospadias, oral clefts, and dysmorphic facial features [3–6]. In the early and mid-2000s, case series described developmental delays in children with in utero valproate exposure [7–9]. In 2009, a prospective cohort study, including 309 children exposed in utero to antiseizure drugs, reported significantly lower mean cognitive test scores at 3 years of age in 96 children with valproate exposure when compared to children exposed to lamotrigine, carbamazepine, or phenytoin [10]. Since then, several observational studies substantiated these findings, consistently reporting an increased risk of autism, attention deficit hyperactivity disorders, decreased cognitive function, and learning difficulties in children after in utero valproate exposure [10–17]. Some studies suggest that the risk of developmental disorders may be as high as 30–40% for children with in utero exposure to high doses of valproate [18].
The US Food and Drug Administration (FDA) issued a warning in 2011 [19], followed by the European Medicines Agency (EMA) in 2014 [20] and Swissmedic in 2015 [21]. They advised that valproate should not be used during pregnancy or by women of childbearing age unless other substances are ineffective or not tolerated. Since 2015, an increasingly heated public debate has unfolded, first in France, and then in Switzerland and other European countries, about whether or not authorities issued sufficiently appropriate and timely warnings. Until today, the number of exposed pregnant women in Switzerland, and thus the estimated number of affected children, remains unknown. We conducted an observational study using the Helsana claims database to quantify the number of prescriptions of valproate and other antiseizure drugs filled by pregnant women and women of childbearing age between 2014 and 2018 in Switzerland.
We conducted a retrospective descriptive study using the healthcare claims database of the Swiss health insurance Helsana for the years 2014-18. The Helsana group is one of Switzerland’s leading health insurance companies, covering 1.1 million individuals with mandatory health insurance from all 26 cantons (approximately 15% of the Swiss population). The database includes information on inpatient and outpatient healthcare services, including drug dispensations, as well as demographics. For dispensation of prescription drugs, the corresponding codes of the anatomical therapeutic chemical (ATC) classification system are available [22]. All data are anonymous, and all analyses were conducted internally by the department of health sciences of the Helsana group.
We identified inpatient and outpatient deliveries (including live births and stillbirths) covered by mandatory health insurance in the Helsana claims database between January 2014 and December 2018. Inpatient deliveries were recorded by means of SwissDRG (Drug Related Diagnosis) codes, and outpatient deliveries by means of Tarmed codes or billed deliveries by midwifes (appendix 1, see separate PDF file for download). Delivery codes recorded within a period of 30 days were regarded as pertaining to the same pregnancy [23], and the date of delivery was defined as the first recorded code. Delivery codes separated by more than 300 days were considered as two separate pregnancies (fig. 1). When two subsequent codes were separated between 30 to 300 days, the date of delivery was set at the inpatient SwissDRG code, whereas women (n = 80 women) were excluded if no SwissDRG code was recorded. We excluded pregnancies during which women were not continuously covered by mandatory insurance at Helsana between their last menstrual period and delivery (n = 9166 pregnancies). Some women contributed more than one pregnancy to the cohort. Twins were treated as single births.

Figure 1 Definition of delivery and last menstruation period (LMP) scheme.
The date of the last menstrual period (i.e., start of pregnancy) was estimated, because gestational length or start of pregnancy are not recorded in healthcare claims data. According to an algorithm validated in US claims data [19], the date of the last menstrual period was assigned to be 245 days before the date of delivery for pregnancies that had a DRG code indicative of preterm delivery (<37 weeks, O01A, O01B, O01C, O01D, O60A), and 270 days before the date of delivery for all other pregnancies. A pregnancy trimester was defined to be 90 days, whereby the third trimester was shortened in case of a preterm delivery (fig. 1).
The cohort of women of childbearing age included all women aged 15-45 years between 2014 and 2018, irrespective of whether they were pregnant or not. Women were eligible to contribute data to any given year if they were continuously insured with Helsana’s mandatory health insurance for the entire year (January–December).
For all pregnancies, we captured maternal age at delivery, the year of delivery, and whether the recorded codes (SwissDRG and Tarmed) indicated a caesarean section. For women of childbearing age, we captured age in the year of interest and the year the drug was dispensed.
Antiseizure drugs were identified based on recorded ATC codes of recorded prescription fills of drugs in claims data, and included valproate (N03AG01), lamotrigine (N03AX09), carbamazepine (N03AF01), levetiracetam (N03AX14), topiramate (N03AX11), pregabalin (N03AX16), gabapentin (N03AX12), phenobarbital (N03AA02), and phenytoin (N03AB02). Due to the data structure of electronic claims data, we were not able to take into account the indication for the use of individual antiseizure drugs. We quantified the prevalence of prescription fills for specific antiseizure drugs in both cohorts, defined as women/pregnancies, with ≥1 recorded claim for an antiseizure drug divided by the total number of enrolled women/pregnancies during the respective time period. Prevalence of antiseizure drug exposure is presented as absolute numbers per 10,000 women/pregnancies by calendar year. In women of childbearing age, prevalence of antiseizure drug exposure is also presented by age groups.
For the pregnancy cohort, we further categorised whether the last valproate prescription was filled between the date of the last menstrual period and delivery, or within 90 days before the last menstrual period (discontinuers), excluding pregnancies with less than 90 days of enrolment before the last menstrual period.
We weighted results of both cohorts, accounting for the demographic distribution of the population insured with mandatory insurance with Helsana relative to the Swiss population. Weighting factors included calendar year, canton, age, and sex. Because sample size was small, absolute numbers of women/pregnancies exposed to specific antiseizure drugs are not shown to preserve patient confidentiality. In order to focus on the relevant antiseizure drugs with regard to prescription frequency, only antiseizure drugs with a minimum weighted prevalence of 50 exposed pregnancies are presented. All analyses were conducted using the statistical programming language R (R version 3.6.1).
This retrospective observational study using anonymous data did not require an ethics committee approval.
Between January 2014 and December 2018, we identified a weighted population of 387,418 pregnancies leading to a delivery. The mean age at delivery ranged between 31.8 (standard deviation 5.1) and 32.1 (standard deviation 5.0) years between 2014 and 2018 (table 1). Of those, between 24,832 (33.0%) and 27,392 (33.5%) deliveries were caesarean sections, which is in accordance with data of the Swiss federal statistical office [24].
Table 1 Demographics of the weighted pregnancy population (2014–18)
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| No. of pregnancies | 75,462 | 76,321 | 78,609 | 81,888 | 75,137 |
| Mean age at delivery in years (standard deviation) | 31.8 (5.1) | 31.9 (5.2) | 31.8 (5.2) | 32.0 (5.2) | 32.1 (5.0) |
| <20 years | 446 (0.6%) | 389 (0.5%) | 479 (0.6%) | 354 (0.4%) | 376 (0.5%) |
| 20–25 years | 8279 (11%) | 8599 (11.3%) | 8345 (10.6%) | 8324 (10.2%) | 6938 (9.2%) |
| 26–30 years | 21,082 (27.9%) | 21,286 (27.9%) | 22,487 (28.6%) | 23,113 (28.2%) | 21,232 (28.3%) |
| 31–35 years | 27,654 (36.6%) | 27,360 (35.8%) | 28,480 (36.2%) | 29,612 (36.2%) | 27,686 (36.8%) |
| 36–40 years | 14,836 (19.7%) | 15,215 (19.9%) | 15,374 (19.6%) | 16,596 (20.3%) | 15,725 (20.9%) |
| 41–45 years | 3000 (4.0%) | 3246 (4.3%) | 3166 (4.0%) | 3572 (4.4%) | 2931 (3.9%) |
| >45 years | 165 (0.2%) | 225 (0.3%) | 278 (0.4%) | 318 (0.4%) | 250 (0.3%) |
| Caesarean section | 25,983 (34.4%) | 26,177 (34.3%) | 26,200 (33.3%) | 27,392 (33.5%) | 24,832 (33.0%) |
After weighting, valproate was dispensed during 1.9/10,000 pregnancies between 2014 and 2018. Overall, valproate was less frequently dispensed during pregnancy than other antiseizure drugs. Lamotrigine was the most frequently dispensed antiseizure drug during pregnancy (20.0/10,000), followed by levetiracetam (11.0/10,000), and pregabalin (3.8/10,000, table 2, fig. 2). For 1.3/10,000 pregnancies, a valproate prescription was filled within 90 days prior to the last menstrual period but not during pregnancy (discontinuers). The prevalence of antiseizure drug exposure in the unweighted pregnancy population is not shown for confidentiality reasons.
Table 2 Weighted prevalence of exposure to antiseizure drugs (per 10,000) during pregnancy (2014–18)
| Valproate | Lamotrigine | Carbamazepine | Levetiracetam | Topiramate | Pregabalin | |
|---|---|---|---|---|---|---|
| Exposed pregnancies (per 10,000) | 1.9 | 20.0 | 3.0 | 11.0 | 2.4 | 3.8 |
| Discontinuers* | 1.3 | 0.7 | 0.4 | 0.8 | 3.3 | 9.3 |
| Exposed pregnancies by year | ||||||
| 2014 | NA† | 20.0 | 3.8 | 10.0 | 2.9 | 2.7 |
| 2015 | 3.8 | 18.0 | 3.4 | 12.0 | 5.2 | 5.6 |
| 2016 | 2.2 | 18.0 | 2.4 | 7.0 | 0.9 | 3.2 |
| 2017 | 2.6 | 22.0 | 0.4 | 13.0 | 1.3 | 2.3 |
| 2018 | 1.1 | 20.0 | 0.1 | 10.0 | 1.9 | 5.6 |
* ≥1 filled prescription between 90 days before the last menstrual period and the last menstrual period, but none during pregnancy. † No information available as the cell contained a 0 in the Helsana cohort.
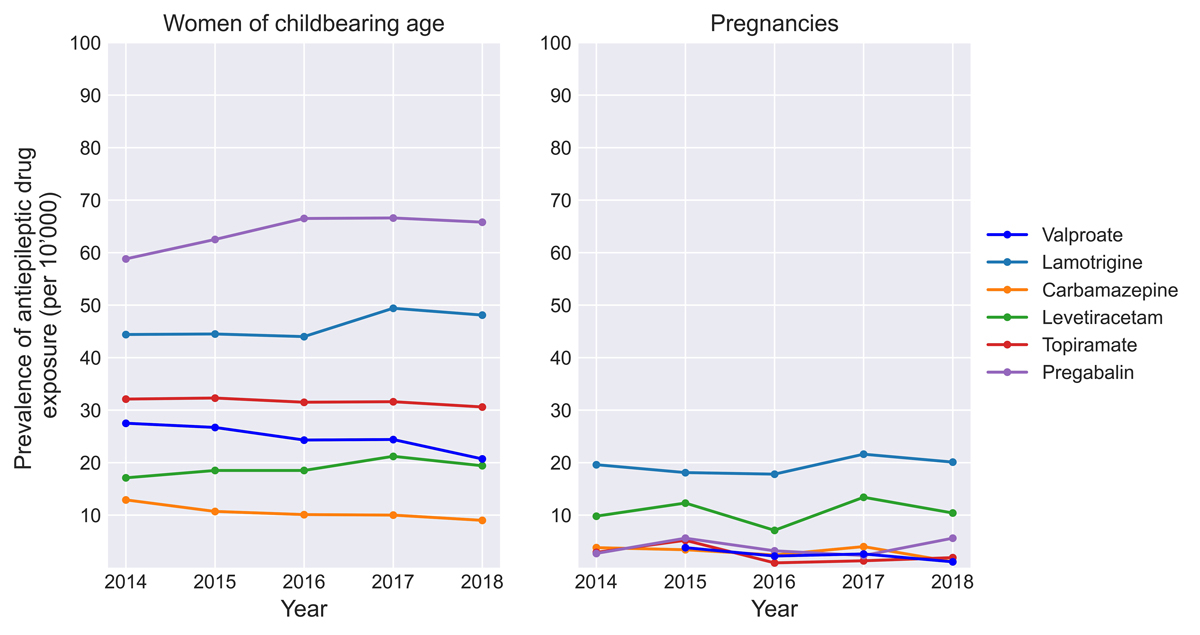
Figure 2 Prevalences of antiseizure drugs in pregnant women and women of childbearing age.
The weighted study population of women aged 15–45 years consisted of 2,781,151 women, of whom 74,080 (2.66%) were exposed to at least one of the evaluated antiseizure drugs during the study period. Pregabalin was the most frequently dispensed antiseizure drug in women of childbearing age, with an exposure prevalence of 59-67/10,000 women, followed by lamotrigine (44–49/10,000), and topiramate (31–32/10,000). Valproate was the fourth most frequently dispensed antiseizure drug; 28/10,000 women filled ≥1 prescription in 2014, decreasing to 21/10,000 women in 2018 (absolute decrease −25%). The likelihood of filling at least one valproate prescription increased with increasing age, from 24/10,000 women aged 20–25 years to 44/10,000 women aged 41–45 years (table 3, fig. 2). The prevalence of antiseizure drug exposure in the unweighted population of women of childbearing age is not shown for confidentiality reasons.
Table 3 Weighted prevalence of exposure to antiseizure drugs (per 10,000) in women of childbearing age (2014–18).
| Valproate | Lamotrigine | Carbamazepine | Levetiracetam | Topiramate | Pregabalin | |
|---|---|---|---|---|---|---|
| Exposure prevalence (/10,000 women) by year | ||||||
| 2014 (N women total = 1,591,025) | 28.0 | 44.0 | 13.0 | 17.0 | 32.0 | 59.0 |
| 2015 (N women total = 1,598,009) | 27.0 | 45.0 | 11.0 | 19.0 | 32.0 | 63.0 |
| 2016 (N women total = 1,608,054) | 24.0 | 44.0 | 10.0 | 19.0 | 32.0 | 67.0 |
| 2017 (N women total = 1,610,897l) | 24.0 | 49.0 | 10.0 | 21.0 | 32.0 | 67.0 |
| 2018 (N women total = 1,601,858) | 21.0 | 48.0 | 9.0 | 19.0 | 31.0 | 66.0 |
| Exposure prevalence (/10,000 women) by age | ||||||
| ≤20 | 11.0 | 22.0 | 3.6 | 13.0 | 23.0 | 23.0 |
| 21–25 | 24.0 | 48.0 | 5.1 | 24.0 | 46.0 | 54.0 |
| 26–30 | 27.0 | 58.0 | 10.0 | 25.0 | 47.0 | 76.0 |
| 31–35 | 31.0 | 63.0 | 17.0 | 28.0 | 50.0 | 109.0 |
| 36–40 | 36.0 | 58.0 | 16.0 | 21.0 | 54.0 | 155.0 |
| 41–45 | 44.0 | 64.0 | 23.0 | 21.0 | 63.0 | 232.0 |
We estimated that approximately 1.9/10,000 pregnancies were exposed to valproate between 2014 and 2018 in Switzerland. Valproate exposure among women of childbearing age was more than 10-fold higher during the same time period, and decreased from 28/10,000 in 2014 to 21/10,000 women in 2018.
Our results are consistent with a reported valproate exposure of 2/10,000 pregnancies in Denmark in 2016 [25]. Contrary to the Danish study, however, our results are based on a relatively small sample of the Swiss population (15%), and thus our estimates may be subject to variability introducing some uncertainty. Reported prevalence of in utero valproate exposure vary by country and region; the Italian region of Tuscany reported that 15/10,000 pregnancies were exposed to valproate between 2007 and 2016 [26]. The French national agency of drug safety revealed a valproate exposure prevalence of 25/10,000 pregnancies in 2007, which declined to 14/10,000 pregnancies in 2014, and to 3/10,000 pregnancies in 2018 [27, 28], based on an analysis of national claims data. However, an EMA funded study suggested that valproate exposure prevalence in France was lower (11/10,000 pregnancies) as of 2011 [26]. Both the UK and the Emilia-Romagna region in Italy reported valproate exposure in 5/10,000 pregnancies in 2008 and 15/10,000 pregnancies in 2016 [26].
Characteristics of Swiss billing codes did not allow inclusion of pregnancies that ended in termination or abortion into our study population. (This was the same for the studies from Denmark and Emilia-Romagna. In France, 38% of valproate exposed pregnancies accounted for terminations and abortions in 2013 [27], rising to 50% in 2018 [28], which is more than two-fold higher compared to the reported number of terminations and abortions reported for the overall French population [29]. It is unclear to what extent these numbers are applicable to the Swiss population, but it is likely that (elective) pregnancy terminations and abortions are also proportionally more frequent among valproate users than in the overall Swiss population [30].
In the study population of women of childbearing age, between 28/10,000 and 21/10,000 women in Switzerland filled at least one prescription for valproate per year between 2014 and 2018. Analyses of national claims data revealed a similar prevalence of valproate exposure among women of childbearing age for Denmark (21-19/10,000 women between 2001 and 2016) [25], Germany (30-25/10,000 women between 2004 and 2016) [31], Ireland (35-31/10,000 between 2008 and 2013) [32], and a higher prevalence for Finland (50-40/10’000 between 2012 and 2016) and France (75-25/10,000 between 2007-2018) [33,34].
The exact number of women of childbearing age who require valproate for seizure control in Switzerland is not known, but it has been shown that between 5-15% of women with idiopathic (genetic) generalised epilepsy do not achieve seizure control with antiseizure drugs other than valproate [35,36]. Other forms of epilepsy, bipolar disease, and other off-label indications such as migraine prophylaxis, should not be treated with valproate in this patient population. Based on data from other European countries, we estimate that between 0.3-1.5/10,000 pregnancies and between 0.3-1.7/10,000 women of childbearing age absolutely require valproate for seizure control (calculations in appendix 2, see separate PDF file for download). Small sample size of pregnancies exposed to valproate in our study population, and several assumptions underlying our estimation of women absolutely requiring valproate, prevents a conclusion on whether or not valproate was prescribed too often in Switzerland during pregnancy. However, the more than 10-fold higher prevalence of valproate exposure among women of childbearing age observed suggests that despite decreasing exposure prevalence, valproate is still prescribed to women for whom alternative drug treatment may exist. This is critical, since studies from US epilepsy registers have reported that up to 65% of pregnancies among women with epilepsy are unplanned [37]. We do not have information on birth control use in Swiss claims data, but given that for approximately 1.3/10,000 pregnancies the last valproate prescription was filled within 90 days before the last menstrual period, pregnancies seem to be unplanned in a large proportion of valproate users in Switzerland. Ideally, treatment switch to a new antiseizure drug should be completed a year before the last menstrual period to allow for a seizure-free year before conception [38].
In Switzerland, several media reports escalated public attention on children who had developed birth defects after in utero valproate exposure. This attention increased uncertainty among patients and healthcare providers and was driven by a lack of reliable data and subsequent speculations on the magnitude of the problem. Swissmedic concluded that valproate exposure in pregnant women has been low over the last two decades, based on the observation that only 29 cases of congenital malformations, and 10 cases of developmental disorders, had been reported to the Swiss spontaneous reporting system between 1994 and 2019 [21]. However, such systems do not capture drug exposure, and are highly susceptible to underreporting and bias. Rarely more than 10% of adverse drug events are reported, and the underreporting is especially pronounced if the events develop slowly (e.g., developmental disorders), or if the drug has been on the market for a long time [39]. In Switzerland, healthcare claims data are underused on an institutional level, and drug utilization on a national level is largely unknown. Other western countries, such as all Scandinavian countries, France, Germany, and the United States, have used healthcare claims data for surveillance of drug use and safety for decades, enhancing them with external data to study vulnerable populations such as pregnant women [40–43]. The Swiss federal council's health policy strategy 2020–2030 declared that the technological and digital transformation of the healthcare sector was one of its four main goals in order to improve public health over the next decade [44]. We hope that our study stimulates the discussion on how to make better use of existing data sources on an institutional level for the greater good of the Swiss population, and especially for vulnerable patient groups such as pregnant women.
Besides the strengths of this study, some limitations need to be considered. First, due to the claims data from 2019 being incomplete, the observational period ends in 2018; accordingly, we could not evaluate the effectiveness of the comprehensive ‘pregnancy prevention program’ for women treated with valproate, which has been implemented by EMA and Swissmedic in 2018 [45]. Second, healthcare claims data do not provide information on whether or not all tablets of a filled prescription were taken. We are therefore unable to evaluate how many of the women who filled a prescription for valproate within 90 days before the last menstrual period stopped the drug before conception, and how many still took it during the first most vulnerable weeks of pregnancy. Third, we were not able to evaluate the indication for the use of antiseizure drugs in this study. It is likely that the majority of women using pregabalin and topiramate did not have an underlying epilepsy diagnosis, but used these drugs for neuropathic pain and migraine respectively. Fourth, results have to be interpreted carefully, as they were estimated based on 15% of the Swiss population from all parts of the country with mandatory health insurance insured with Helsana, and weighted based on specific demographic factors. Thus, reported numbers do not necessarily represent the exact true exposure prevalence in the overall Swiss population of pregnant women or women of childbearing age. Fifth, given the descriptive nature of this study, we did not perform an a priori sample size calculation. However, results have to be interpreted carefully given the small sample size of women exposed to certain antiseizure drugs, and further studies should aim to replicate our results using different data sources. Sixth, as characteristics of Swiss billing codes did not allow inclusion of pregnancies which ended in termination or abortion into our study population, our prevalence of use is underestimated. The underestimation range is however difficult to predict, as the prevalence of terminations and spontaneous abortions in Switzerland is unknown.
In conclusion, the prevalence of valproate exposure in pregnancy was comparable to Denmark, and lower than in other European countries. As in most other European countries, the use of valproate in women of childbearing age between 2014 and 2018 was higher than what we estimated to be indicated. This study demonstrates the value of electronic claims databases in the evaluation of drug exposure during pregnancy. Future projects are needed to systematically monitor utilisation of drugs using healthcare claims data, especially in vulnerable patient populations such as pregnant women. Results of such projects may build the basis for interventions to increase drug safety in the pregnant women in Switzerland.
This study has been supported by the Swiss RBP IV fund for health quality and patient safety.
1Heilmittelinstitut SS. Product Information Depakine (R) [Internet]. 2020 [cited 2020 Jan 13]. Available from: https://www.dopps.org/dpm/DPMSlideBrowser.aspx?type=ComGrp&id=1
2 Robert E , Guibaud P . Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2(8304):937. doi:.https://doi.org/10.1016/S0140-6736(82)90908-4
3 Holmes LB , Harvey EA , Coull BA , Huntington KB , Khoshbin S , Hayes AM , et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344(15):1132–8. doi:.https://doi.org/10.1056/NEJM200104123441504
4 Jentink J , Loane MA , Dolk H , Barisic I , Garne E , Morris JK , et al.; EUROCAT Antiepileptic Study Working Group. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362(23):2185–93. doi:.https://doi.org/10.1056/NEJMoa0907328
5 Hernández-Díaz S , Smith CR , Shen A , Mittendorf R , Hauser WA , Yerby M , et al.; North American AED Pregnancy Registry; North American AED Pregnancy Registry. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692–9. doi:.https://doi.org/10.1212/WNL.0b013e3182574f39
6 Diav-Citrin O , Shechtman S , Bar-Oz B , Cantrell D , Arnon J , Ornoy A . Pregnancy outcome after in utero exposure to valproate : evidence of dose relationship in teratogenic effect. CNS Drugs. 2008;22(4):325–34. doi:.https://doi.org/10.2165/00023210-200822040-00004
7 Adab N , Kini U , Vinten J , Ayres J , Baker G , Clayton-Smith J , et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75(11):1575–83. doi:.https://doi.org/10.1136/jnnp.2003.029132
8 Adab N , Jacoby A , Smith D , Chadwick D . Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(1):15–21. doi:.https://doi.org/10.1136/jnnp.70.1.15
9 Gaily E , Kantola-Sorsa E , Hiilesmaa V , Isoaho M , Matila R , Kotila M , et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62(1):28–32. doi:.https://doi.org/10.1212/WNL.62.1.28
10 Meador K , Baker G , Browning N , Clayton-Smith J , Combs-Cantrell D , Cohen M , et al. Cognitive Function at 3 Years of Age after Fetal Exposure to Antiepileptic Drugs. N Engl J Med. 2008;359(16):1543–54.
11 Bromley R , Weston J , Adab N , Greenhalgh J , Sanniti A , McKay AJ , et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev. 2014;2014(10):CD010236. doi:.https://doi.org/10.1002/14651858.CD010236.pub2
12Gerard E, Meador K. An Update on Maternal Use of Antiepileptic Medications in Pregnancy and Neurodevelopment Outcomes. J Pediatr Genet. 2015;04(02):094–110.
13 Baker GA , Bromley RL , Briggs M , Cheyne CP , Cohen MJ , García-Fiñana M , et al.; Liverpool and Manchester Neurodevelopment Group. IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology. 2015;84(4):382–90. doi:.https://doi.org/10.1212/WNL.0000000000001182
14 Christensen J , Pedersen L , Sun Y , Dreier JW , Brikell I , Dalsgaard S . Association of Prenatal Exposure to Valproate and Other Antiepileptic Drugs With Risk for Attention-Deficit/Hyperactivity Disorder in Offspring. JAMA Netw Open. 2019;2(1):e186606. doi:.https://doi.org/10.1001/jamanetworkopen.2018.6606
15 Meador KJ , Baker GA , Browning N , Cohen MJ , Clayton-Smith J , Kalayjian LA , et al.; NEAD Study Group. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134(Pt 2):396–404. doi:.https://doi.org/10.1093/brain/awq352
16 Christensen J , Grønborg TK , Sørensen MJ , Schendel D , Parner ET , Pedersen LH , et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696–703. doi:.https://doi.org/10.1001/jama.2013.2270
17 Meador KJ , Baker GA , Browning N , Cohen MJ , Bromley RL , Clayton-Smith J , et al.; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–52. doi:.https://doi.org/10.1016/S1474-4422(12)70323-X
18 Cummings C , Stewart M , Stevenson M , Morrow J , Nelson J . Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child. 2011;96(7):643–7. doi:.https://doi.org/10.1136/adc.2009.176990
19FDA Drug Safety Communication. Children born to mothers who took Valproate products while pregnant may have impaired cognitive development [Internet]. 2011 [cited 2020 Jan 14]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-children-born-mothers-who-took-valproate-products-while-pregnant-may
20Agency EM. CMDh agrees to strengthen warnings on the use of valproate medicines in women and girls [Internet]. 2014 [cited 2020 Jan 14]. Available from: https://www.ema.europa.eu/en/news/cmdh-agrees-strengthen-warnings-use-valproate-medicines-women-girls
21Eidgenossenschaft S. Depakine-Skandal. Untersuchung der Situation in der Schweiz. Bericht des Bundesrates in Erfüllung des Postulates 18.3092, Sänderätin Maury Pasquier Liliane, 7- März 2018. Bern; 2019.
22World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. [Internet]. 2016 [cited 2018 Aug 14]. Available from: http://apps.who.int/classifications/icd10/browse/2016/en#/.
23 MacDonald SC , Cohen JM , Panchaud A , McElrath TF , Huybrechts KF , Hernández-Díaz S . Identifying pregnancies in insurance claims data: Methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf. 2019;28(9):1211–21. doi:.https://doi.org/10.1002/pds.4794
24Bundesamt für Statistik. Entbindungen und Gesundheit der Mütter im Jahr 2017 - Medizinische Statistik der Krankenhäuser | Publikation [Internet]. Medizinische Statistik der Krankenhäuser. 2019. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/reproduktive.html
25 Daugaard CA , Sun Y , Dreier JW , Christensen J . Use of antiepileptic drugs in women of fertile age. Dan Med J. 2019;66(8):1–5.
26 Hurault-Delarue C , Morris JK , Charlton R , Gini R , Loane M , Pierini A , et al.; EUROmediSAFE consortium. Prescription of antiepileptic medicines including valproate in pregnant women: A study in three European countries. Pharmacoepidemiol Drug Saf. 2019;28(11):1510–8. doi:.https://doi.org/10.1002/pds.4897
27Sante AN de securite du medicament et des produis de. Etude observationnelle ANSM-CNAMTS de l ’ exposition à l ’ acide valproïque et ses dérivés au cours de la grossesse en France. 2016.
28Agence national de sécurité du médicament et des produits de santé. Valproate et dérivés : l’exposition des femmes enceintes a fortement diminué mais persiste - Point d’information [Internet]. 2020 [cited 2020 Feb 5]. Available from: https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Valproate-et-derives-l-exposition-des-femmes-enceintes-a-fortement-diminue-mais-persiste-Point-d-information
29Organisation world health. European Health INformation Gateway, Abortions per 1000 live births [Internet]. [cited 2020 Feb 4]. Available from: https://gateway.euro.who.int/en/indicators/hfa_586-7010-abortions-per-1000-live-births/visualizations/#id=19681
30 Tomson T , Battino D , Bonizzoni E , Craig JJ , Lindhout D , Perucca E , et al.; EURAP Study Group. Antiepileptic drugs and intrauterine death: A prospective observational study from EURAP. Neurology. 2015;85(7):580–8. doi:.https://doi.org/10.1212/WNL.0000000000001840
31Wentzell N, Haug U, Schink T, Engel S, Liebentraut J, Linder R, et al. Prescribing valproate to girls and women of childbearing age in Germany: Analysis of trends based on claims data. Bundesgesundheitsblatt - Gesundheitsforsch - Gesundheitsschutz. 2018;61(8):1022–9.
32 Murphy S , Bennett K , Doherty CP . Prescribing trends for sodium valproate in Ireland. Seizure. 2016;36:44–8. doi:.https://doi.org/10.1016/j.seizure.2016.01.019
33 Virta LJ , Kälviäinen R , Villikka K , Keränen T . Declining trend in valproate use in Finland among females of childbearing age in 2012-2016 - a nationwide registry-based outpatient study. Eur J Neurol. 2018;25(6):869–74. doi:.https://doi.org/10.1111/ene.13610
34Démographiques institut national d’études. Population par sexe et âge au 1er janvier [Internet]. [cited 2020 Feb 5]. Available from: https://www.ined.fr/fr/tout-savoir-population/chiffres/france/structure-population/population-sexe-ages/
35 Marson AG , Al-Kharusi AM , Alwaidh M , Appleton R , Baker GA , Chadwick DW , et al.; SANAD Study group. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1000–15. doi:.https://doi.org/10.1016/S0140-6736(07)60460-7
36 Jallon P , Latour P . Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46(s9, Suppl 9):10–4. doi:.https://doi.org/10.1111/j.1528-1167.2005.00309.x
37 Herzog A . HB M, MacEachern D. Association of Unintended Pregnancy With Spontaneous Fetal Loss in Women With Epilepsy Findings of the Epilepsy Birth Control Registry. JAMA Neurol. 2019;76(1):50–5. doi:.https://doi.org/10.1001/jamaneurol.2018.3089
38 Tomson T , Battino D , Bromley R , Kochen S , Meador K , Pennell P , et al. Executive Summary: Management of epilepsy in pregnancy: A report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epilepsia. 2019;60(12):2343–5. doi:.https://doi.org/10.1111/epi.16395
39 Goldman SA . Limitations and strengths of spontaneous reports data. Clin Ther. 1998;20(Suppl C):C40–4. doi:.https://doi.org/10.1016/S0149-2918(98)80007-6
40 Frank AS , Lupattelli A , Nordeng H . Risk factors for discontinuation of thyroid hormone replacement therapy in early pregnancy: a study from the Norwegian Mother and Child Cohort Study and the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand. 2018;97(7):852–60. doi:.https://doi.org/10.1111/aogs.13339
41 Benevent J , Hurault-Delarue C , Araujo M , Montastruc JL , Lacroix I , Damase-Michel C . POMME: The New Cohort to Evaluate Long-Term Effects After Prenatal Medicine Exposure. Drug Saf. 2019;42(1):45–54. doi:.https://doi.org/10.1007/s40264-018-0712-9
42Innovationasausschuss GB. AMTS in utero - Untersuchung zur Arzneimitteltherapiesicherheit in der Schwangerschaft basierend auf Routinedaten in Deutschland [Internet]. 2020. Available from: https://innovationsfonds.g-ba.de/projekte/versorgungsforschung/amts-untersuchungen-zur-arzneimitteltherapiesicherheit-in-der-schwangerschaft-basierend-auf-routinedaten-in-deutschland.10
43 Platt R , Brown JS , Robb M , McClellan M , Ball R , Nguyen MD , et al. The FDA Sentinel Initiative - An Evolving National Resource. N Engl J Med. 2018;379(22):2091–3. doi:.https://doi.org/10.1056/NEJMp1809643
44Bundesrat D. Die gesundheitspolitische Strategie des Bundesrates 2020-2030 [Internet]. 2020. Available from: https://www.fda.gov/safety/fdas-sentinel-initiative/fdas-sentinel-initiative-background
45Swissmedic. Wichtige Sicherheitsinformationen - Valproat: Risiko kongenitaler Missbildungen und Entwicklungsstörungen bei der Exposition während der Schwangerschaft [Internet]. 2011. Available from: https://webcache.googleusercontent.com/search?q=cache:N377P9_J-5wJ:https://www.swissmedic.ch/dam/swissmedic/de/dokumente/marktueberwachung/dhpc_hpc/dhpc_-_valproat.pdf.download.pdf/dhpc_-_valproat.pdf+&cd=1&hl=de&ct=clnk&gl=ch
This study has been supported by the Swiss RBP IV fund for health quality and patient safety.