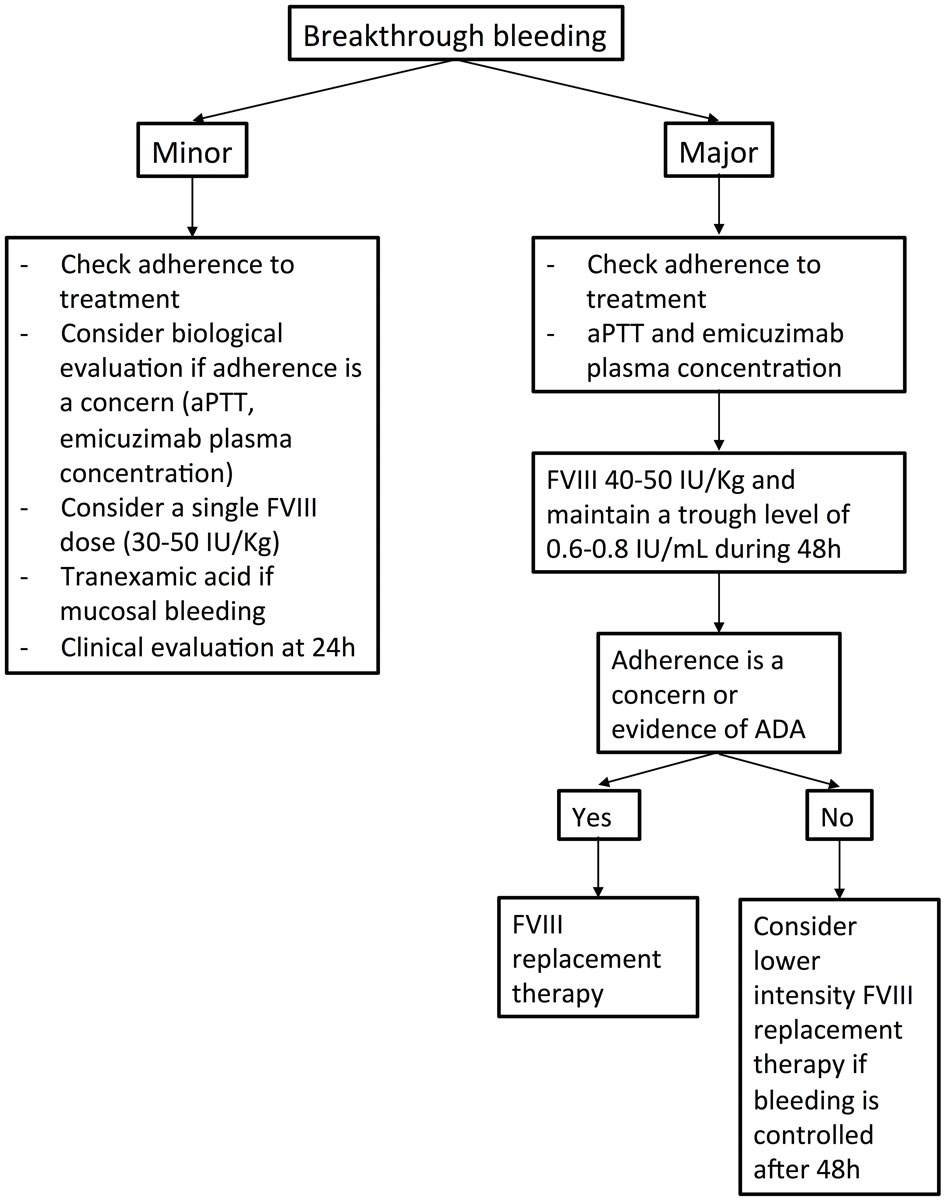
Figure 1 Summary of the proposed management of breakthrough bleeding events in persons with haemophilia A factor VIII without inhibitors and treated with emicizumab. ADA = anti-drug antibody.
DOI: https://doi.org/10.4414/smw.2020.20422
Emicizumab (Hemlibra®, Hoffmann-La Roche, Basel, Switzerland) is a recombinant, humanised bispecific monoclonal antibody that substitutes for the pro-coagulant role of factor VIII by bridging factor IXa to factor X. It is licensed for prophylactic treatment in patients with congenital (usually severe) haemophilia A, with or without factor VIII inhibitors. Emicizumab can be administered subcutaneously and has a half-life of approximately 30 days. This allows a dosing of 1.5 mg/kg bodyweight once a week, or 3 mg/kg every two weeks, or 6 mg/kg once a month after an initial weekly loading dose of 3 mg/kg for four consecutive weeks. The average effective plasma concentration reached is around 50 µg/ml [1, 2]. Although the number of spontaneous bleeds are dramatically decreased in patients on emicizumab, breakthrough bleeds may still occur, and invasive procedures including surgery may be necessary. Guidelines for the management of bleeds and invasive procedures in patients treated with emicizumab and factor VIII inhibitors are available, and rely mostly on recombinant factor VIIa to restore haemostasis [3–5]. However, specific recommendations for haemophilia A patients without inhibitors treated with emicizumab, who may need factor VIII concentrates to treat breakthrough bleeds or prior to invasive procedures, are scarce [6].
Our practical guidance is based on the available literature and experiences with patients treated in Switzerland. Two authors (PF and JAKH) reviewed the literature available in PubMed using “emicizumab” as a keyword, as well as relevant abstracts presented at major congresses (the American Society of Hematology, the European Association for Haemophilia and Allied Disorders, the International Society on Thrombosis and Haemostasis and Gesellschaft für Thrombose- und Hämostaseforschung) during the past three years, and abstracts on file from Roche (last updated 30 June 2020). A first draft of the proposals was then circulated and a consensus among all authors was reached.
Emicizumab does not fully restore haemostasis and patients may face bleeding events after (even minor) trauma or, rarely, spontaneously. Patients treated with emicizumab should have factor VIII concentrate available for emergency situations. However, factor VIII treatment is not always necessary, and depends on the severity of the bleed, on its localisation and on the possibility of specific local treatment, such as compression or endoscopic procedures. Notably, the amount of on-demand factor VIII used per bleeding episode was comparable in a subset of 48 patients during the non-interventional study period prior to study enrolment and on study in the HAVEN 3 trial [7]. This suggests that for most bleeds, factor VIII replacement therapy followed the same dosing as before switching to emicizumab. Breakthrough bleeding events and their treatment should be documented and recorded, for example in a national haemophilia registry by the treating haemophilia centre (NCT02512250). The treatment with emicizumab must not be interrupted and the posology should not be altered if a bleed occurs, provided that the dose and administration frequency are correct. Breakthrough bleeding events, particularly spontaneous bleeds, may raise the issue of poor efficacy of emicizumab prophylactic treatment. Poor adherence with many missed injections over weeks might be possible. Anti-drug antibodies [8] have been rarely described [9] and have not been associated with the occurrence of clinical events up to now. When decreased efficacy of the drug is a concern, the activated partial thromboplastin time (aPTT) can be measured, although, because of the oversensitivity of the aPTT for emicizumab, a prolonged aPTT will only occur at very low emicizumab plasma concentrations [10]. Studying thrombin generation may be an option, but no association with clinical efficacy of emicizumab has been reported [4]. Finally, the determination of emicizumab plasma concentration using dedicated calibrators may be helpful [11, 12], and concentrations below 40 µg/ml should be a concern, especially with once-a-week or every two weeks dosing [2]. Of note, the lower limit of a proposed “efficacious” range has been evaluated as 30 µg/ml [13]. Management of breakthrough bleeding events is summarised in figure 1.

Figure 1 Summary of the proposed management of breakthrough bleeding events in persons with haemophilia A factor VIII without inhibitors and treated with emicizumab. ADA = anti-drug antibody.
For mucosal bleeds, tranexamic acid alone should be prescribed (2–4.5 g/24h for adults in two or three intakes, and 20–25 mg/kg/dose every 6–8 hours for children) for 1–3 days. In the case of minor traumatic bleeds, administration of a single dose of a factor VIII concentrate (30–50 IU/kg) should be considered, followed by a clinical evaluation 24 hours later. For target joint bleeds, although a single dose of factor VIII concentrate may be sufficient in patients with minimal joint damage, more intensive replacement therapy may be required in patients with advanced joint disease.
Major bleeds, defined according to the ISTH criteria [14], such as large muscle haematomas, bleeds associated with a drop in haemoglobin ≥20 g/l and/or necessitating a transfusion with at least two units of packed red blood cell concentrate, life-threatening bleeds or a bleed in a critical organ must be immediately treated with an initial dose of 40–50 IU/kg of a factor VIII concentrate. Adherence to emicizumab treatment should be questioned in the event of a spontaneous major bleeding event and evaluation of the emicizumab plasma concentration is desirable. Timing and doses of the subsequent treatment should be adapted to the type of bleed, the factor used (standard or extended half-life) and the clinical course by maintaining a factor VIII trough level of 0.6–0.8 IU/ml for at least the first 48 hours. If the bleed is under control, a lower dose replacement therapy regimen may be prescribed (providing that adherence to emicizumab is not an issue) for up to 10 days after the major bleed. Tranexamic acid may be considered as an additional treatment. Of note, in cases of severe bleeds with massive transfusion, the plasma concentration of emicizumab may decrease because of loss of the compound due to bleeding and dilution with fluids and plasma replacement therapy, and assessing emicizumab concentration should be considered.
Of note, monitoring of actor VIII levels in patients treated with emicizumab requires a chromogenic factor VIII assay containing bovine factor X since emicizumab interferes with one stage aPTT-based coagulation assays and with chromogenic assays using human coagulation proteins [13].
Invasive procedures or surgery may be necessary, whether elective or emergency. In any case, the treatment with emicizumab must not be interrupted or the posology altered, provided that the dose and administration frequency are correct. We suggest that an elective procedure should be planned independently of the last emicizumab injection, except for patients with a once-a-month posology and elective surgery associated with a high bleeding risk. Once-a-month dosing is associated with emicizumab peaks and troughs levels ranging from approximately 30–60 µg/ml [2]. Therefore, the consensus is to perform high-risk elective surgery within 10 days after an emicizumab injection. An alternative would be to switch from a once-a-month posology to a once-a-week or once every other week regimen 1 month before the procedure and for 2 weeks thereafter. Clinical experience up to now includes mostly low-risk procedures. However, a few cases of major surgeries have been described with favourable outcome with factor VIII replacement therapy. Management of invasive procedures is summarised in figure 2.
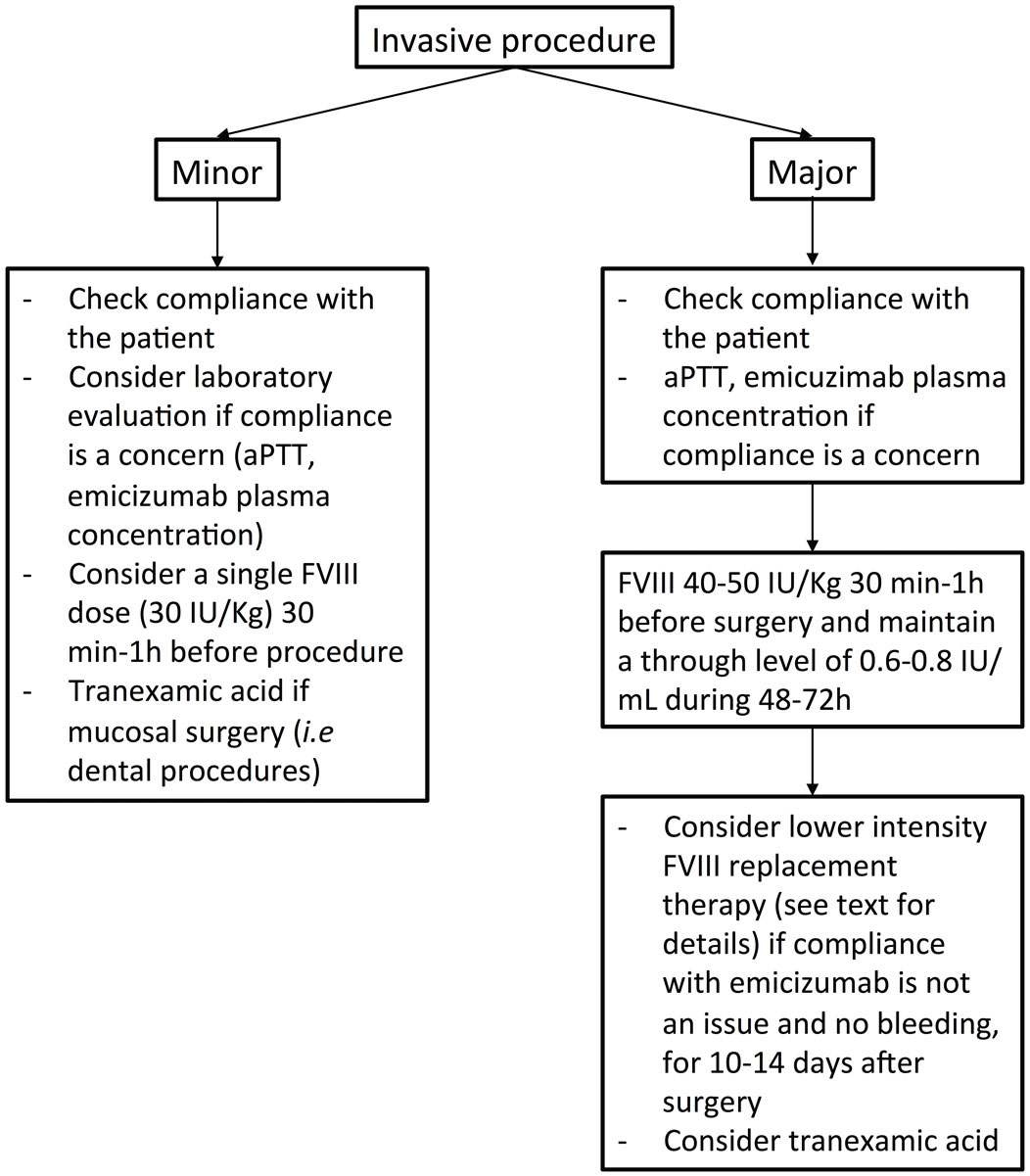
Figure 2 Summary of the proposed management of invasive procedures in persons with haemophilia A without factor VIII inhibitors and treated with emicizumab.
In the HAVEN 1–4 trials, 214 minor surgeries were performed in 113 study participants (108 minor surgeries performed in patients without inhibitors), including 63 dental and 34 central venous access procedures, without additional administration of coagulation factor concentrates in 41 and 25 procedures, respectively [15]. However, on 10 occasions, post-interventional treatment was necessary [15]. Real-world post-licensing experience is limited, and reports of 27 surgical procedures in patients without inhibitors have been published [16, 17]. The majority of procedures were minor surgeries – mostly removal of central venous access devices in children (16/27 procedures) – and were usually performed with a single dose of factor VIII concentrate prior to surgery, with two bleeding complications leading to one additional factor VIII infusion. No thrombotic events occurred. Altogether, emicizumab seems to confer good haemostatic coverage for minor surgeries, often even without the necessity for coagulation factor administration.
For minor invasive procedures, including dental care, dental avulsion, endoscopic procedures without biopsy and joint infiltration, we suggest abstaining from prior factor VIII product administration. Tranexamic acid should be prescribed, at least for all dental procedures. If the bleeding risk is a concern (e.g., in endoscopic procedures with biopsy, multiple dental avulsions), a factor VIII dose of 30 IU/kg 30 minutes to 1 hour before the procedure can be considered.
In the HAVEN 1–4 trials, 19 major surgeries (including 8 major orthopaedic surgeries) were performed in 19 participants (including 8 patients without inhibitors) [15]. Most of these interventions were performed under prophylactic coagulation factor replacement, with postoperative bleeding in only one patient, and there were no thrombotic complications. For arthroplasty (hip or knee) in patients without inhibitors (n = 3), prophylaxis with factor VIII concentrates was used for 7, 16 and 19 days [15]. In the HAVEN 3 study, four patients had major orthopaedic surgery and the mean cumulative factor VIII replacement therapy was 135 IU/kg in the first 2 days after surgery, and 148 IU/kg over the following 5 days, suggesting decreased trough levels from day 3 after surgery [1, 15]. More recently, a single-centre experience of surgery in 122 children and adult patients treated with emicizumab for more than 6 months showed that the majority of orthopaedic surgeries were performed with an initial factor VIII dose of 50 IU/kg prior to surgery, replacement therapy once or twice a day (usually 50 IU/Kg) for the following 2 to 5 days and lower-intensity replacement therapy thereafter [18]. For major surgeries, adherence to the prophylactic treatment is of particular importance and a laboratory evaluation of emicizumab concentration, should be considered if compliance is a concern. Although poor compliance may not influence the initial factor VIII replacement therapy, it may influence factor VIII dosing during the postoperative days. We suggest administration of 40–50 IU/kg of factor VIII 30 minutes to 1 hour prior surgery and to maintain a factor VIII level above 0.6–0.8 IU/ml for 48–72 hours. In some situations, continuous infusion of factor VIII may be considered [19, 20]. We suggest that the decision to use iterative boluses or continuous infusion should be taken independently of emicizumab treatment. In the absence of bleeding complications, a lower intensity replacement therapy regimen with factor VIII trough levels of 0.5 IU/ml for another 4–6 days, followed by a through level ranging between 0.3 and 0.5 IU/ml for an additional 5–7 days may be adopted (total treatment duration 10–14 days). These suggestions have to be adapted to the clinical situation and the type of surgery. Of note, in cases of severe haemorrhagic complications, the plasma concentration of emicizumab may decrease because of loss of compound due to bleeding and dilution with fluids and plasma replacement therapy, and assessing emicizumab concentration should be considered.
We suggest that thromboprophylaxis similar to that for persons with haemophilia A who are not on emicizumab should be prescribed, according to the practice of each centre.
The management of breakthrough bleeding events and invasive procedures in patients treated with emicizumab challenges haemophilia centres. Experience is still scarce, with very few cases reported. Therefore, a common consensus and practical guidance is important to have, but also has to be updated regularly with growing experience. The management of patients treated with emicizumab requires a multidisciplinary approach and close collaboration with the haemostasis laboratory is mandatory, as in most settings involving patients with haemophilia.
Low-dose protocols for invasive procedures are considered in several situations already, including non-factor replacement therapies [21]. However, and according to the data available up to now, a lower intensity dosing is described for procedures associated with a low bleeding risk, whereas in most of the major surgeries performed in patients treated with emicizumab, factor VIII products were given at usual doses, at least during the first 48 hours. Clinical evaluation is of particular importance after major surgery in order to adapt replacement therapy. Indeed, a lower replacement dose protocol may be associated with increased bleeding complications, whereas a conventional protocol may increase the risk of venous and arterial thrombotic events, especially in geriatric patients with several comorbidities. Of note, no thrombotic event was described after major surgery up to now. In vitro studies using a thrombin generation assay showed a non-additive effect of factor VIII combined with emicizumab [22]. Indeed, emicizumab has a lower binding affinity to factor IXa and factor X than factor VIIIa and does not interfere with exogenous factor VIII binding in the intrinsic tenase complex [23], which probably contributes to its safety profile regarding thrombotic events when both treatments are prescribed. Integrative coagulation assays, such as a thrombin generation assay or thrombelastography, may be useful for the adaptation of periprocedural factor VIII replacement therapy [24] and should be included as part of research projects for optimal management of patients on emicizumab.
The authors thank Thomas Lecompte, University Hospitals of Geneva, for his critical review of the manuscript.
PF has received support for the nurses’ programme of the Haemostasis Unit from Bayer, CSL Behring, NovoNordisk, Octapharma, Roche and Sobi. LA received grants/research support from: Bayer, CSL-Behring, Novartis, NovoNordisk, Roche, Shire-Takeda, and Sobi; support for the CHUV haemophilia nurses program from: CSL-Behring, Bayer, NovoNordisk, Octapharma, Roche, Shire, and Sobi; honoraria for participating in scientific advisory boards: Bayer, Boehringer Ingelheim, Daiichi Sankyo, NovoNordisk, OrPha Swiss, Pfizer, Roche, Takeda, Sobi; honoraria as consultant/speaker: Bayer, Sanofi-Genzyme, Siemens. MA received support for the haemophilia and physiotherapy nurse programme at the University Children’s Hospital, Zurich, from: Bayer, Biotest, CSL-Behring, NovoNordisk, Octapharma, Roche, and Sobi. She also participated in scientific advisory board activities for Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novonordisk, Roche and Sobi. AC has received grants from CSL Behring and NovoNordisk and non-financial support from Bayer and Shire. BG reports grants and personal fees from Pfizer; personal fees and funding for accredited continuing medical education from Sanofi and Alnylam, during the conduct of the study, funding for accredited continuing medical education program from Axonlab, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Janssen, Mitsubishi Tanabe Pharma, NovoNordisk, Octapharma, Takeda, Sanofi, Sobi; non-financial support from Axonlab and Thermo Fisher from outside the submitted work. LG received lecture and advisory honoraria from CSL Behring, Novo Nordisk, Octapharma, Roche and Sobi. IH received lecture and advisory honoraria from Bayer, CSL Behring, NovoNordisk, Octapharma, Pfizer, Roche, Shire-Takeda and Sobi. WK received grants/research support/travel support or honoraria from: Bayer, CSL-Behring, NovoNordisk, Shire-Takeda, and Sobi. JDS received advisory and lecture honoraria from Bayer, Shire/Takeda, BMS-Pfizer, Sanofi, Siemens Diagnostics. WW received grants/research support/travel support or honoraria from: Bayer, Bristol Myers Squibb, CSL-Behring, Daiichi-Sankyo, NovoNordisk, Pfizer, Sanofi and Shire-Takeda. JAKH has received a reserach grant from Baxter Inc, now part of Takeda group of companies, honoraria for participation in advisory boards or lectures in symposia of Bayer, CSL-Behring, NovoNordisk, Roche, Sanofi, Shire-Takeda, and Sobi are paid to her employer, Insel Gruppe AG; the support of the interprofessional haemophilia care at EHCCC Bern Inselspital by Bayer, CSL-Behring, Bayer, NovoNordisk, Octapharma, Roche, and Sobi is gratefully acknowledged. All other authors report no relevant conflict of interest.
1 Mahlangu J , Oldenburg J , Paz-Priel I , Negrier C , Niggli M , Mancuso ME , et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N Engl J Med. 2018;379(9):811–22. doi:.https://doi.org/10.1056/NEJMoa1803550
2 Pipe SW , Shima M , Lehle M , Shapiro A , Chebon S , Fukutake K , et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–305. doi:.https://doi.org/10.1016/S2352-3026(19)30054-7
3 Castaman G , Santoro C , Coppola A , Mancuso ME , Santoro RC , Bernardini S , et al.; ad hoc Working Group. Emergency management in patients with haemophilia A and inhibitors on prophylaxis with emicizumab: AICE practical guidance in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Blood Transfus. 2020;18(2):143–51 .
4 Susen S , Gruel Y , Godier A , Harroche A , Chambost H , Lasne D , et al. Management of bleeding and invasive procedures in haemophilia A patients with inhibitor treated with emicizumab (Hemlibra® ): Proposals from the French network on inherited bleeding disorders (MHEMO), the French Reference Centre on Haemophilia, in collaboration with the French Working Group on Perioperative Haemostasis (GIHP). Haemophilia. 2019;25(5):731–7. doi:.https://doi.org/10.1111/hae.13817
5 Collins PW , Liesner R , Makris M , Talks K , Chowdary P , Chalmers E , et al. Treatment of bleeding episodes in haemophilia A complicated by a factor VIII inhibitor in patients receiving Emicizumab. Interim guidance from UKHCDO Inhibitor Working Party and Executive Committee. Haemophilia. 2018;24(3):344–7. doi:.https://doi.org/10.1111/hae.13495
6 Holstein K , Albisetti M , Bidlingmaier C , Halimeh S , Heine S , Klamroth R , et al.; ‘Ständige Kommission Hämophilie’ (Haemophilia board) of the German, Swiss Austrian Society for Thrombosis Haemostasis Research (GTH). Practical Guidance of the GTH Haemophilia Board on the Use of Emicizumab in Patients with Haemophilia A. Hamostaseologie. 2020. doi:.https://doi.org/10.1055/a-1127-6476
7 Callaghan M , Trzaskoma B , Ko RH , Lee L , Patel AM , Tzeng E , et al. Factor VIII Use in the Treatment of Breakthrough Bleeds in Hemophilia A Patients without Inhibitors on Emicizumab Prophylaxis: The Phase 3 HAVEN 3 Study Experience. Blood. 2019;134(Supplement_1):2395. doi:.https://doi.org/10.1182/blood-2019-123654
8 Paz-Priel I , Chang T , Asikanius E , Chebon S , Emrich T , Fernandez E , et al. Immunogenicity of Emicizumab in People with Hemophilia A (PwHA): Results from the HAVEN 1-4 Studies. Blood. 2018;132(Supplement 1):633. doi:.https://doi.org/10.1182/blood-2018-99-118492
9Valsecchi C, Gobbi M, Schiavone L, Beeg M, Adams PE, Mancuso ME, et al. Characterization of Neutralizing Anti-emicizumab Antibody Developed in a Hemophilia A Patient. ISTH 2020 Virtual Congress. 2020. Abstract PB1159.
10 Adamkewicz JI , Chen DC , Paz-Priel I . Effects and Interferences of Emicizumab, a Humanised Bispecific Antibody Mimicking Activated Factor VIII Cofactor Function, on Coagulation Assays. Thromb Haemost. 2019;119(7):1084–93. doi:.https://doi.org/10.1055/s-0039-1688687
11 Tripodi A , Santoro RC , Testa S , Molinari AC , Bernardini S , Golato M , et al. Position paper on laboratory testing for patients with haemophilia. A consensus document from SISET, AICE, SIBioC and SIPMeL. Blood Transfus. 2019;17(3):229–36.
12 Tripodi A , Chantarangkul V , Novembrino C , Scalambrino E , Boscolo-Anzoletti M , Clerici M , et al. Emicizumab, the factor VIII mimetic bi-specific monoclonal antibody and its measurement in plasma. Clin Chem Lab Med. 2020;/j/cclm.ahead-of-print/cclm-2020-0696/cclm-2020-0696.xml .
13 Müller J , Pekrul I , Pötzsch B , Berning B , Oldenburg J , Spannagl M . Laboratory Monitoring in Emicizumab-Treated Persons with Hemophilia A. Thromb Haemost. 2019;119(9):1384–93. doi:.https://doi.org/10.1055/s-0039-1692427
14 Schulman S , Kearon C ; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. doi:.https://doi.org/10.1111/j.1538-7836.2005.01204.x
15 Santagostino E , Oldenburg J , Chang T , Xu J , Chebon S , Doral M , et al. Surgical Experience from Four Phase III Studies (HAVEN 1- 4) of Emicizumab in Persons with Haemophilia A (PwHA) with or without FVIII Inhibitors. Res Pract Thromb Haemost. 2019;3:115.
16 Ebbert PT , Xavier F , Seaman CD , Ragni MV . Emicizumab prophylaxis in patients with haemophilia A with and without inhibitors. Haemophilia. 2020;26(1):41–6. doi:.https://doi.org/10.1111/hae.13877
17 McCary I , Guelcher C , Kuhn J , Butler R , Massey G , Guerrera MF , et al. Real-world use of emicizumab in patients with haemophilia A: Bleeding outcomes and surgical procedures. Haemophilia. 2020;26(4):631–6. doi:.https://doi.org/10.1111/hae.14005
18Lewandowska M, Randall N, Maahs J, Bakeer J, Sagar J, Greist A, et al. Real-World Experience with Emicizumab in Persons with Hemophilia A (HA) with or without Inhibitors. ISTH 2020 Virtual Congress. 2020. Abstract PB0928.
19 Boehlen F , Burkhard PR , Momjian S , Fontana P . Subthalamic nucleus deep brain stimulation for Parkinson’s disease in a patient with severe haemophilia A. Haemophilia. 2017;23(3):e246–8. doi:.https://doi.org/10.1111/hae.13235
20 Meijer K , Rauchensteiner S , Santagostino E , Platokouki H , Schutgens RE , Brunn M , et al. Continuous infusion of recombinant factor VIII formulated with sucrose in surgery: non-interventional, observational study in patients with severe haemophilia A. Haemophilia. 2015;21(1):e19–25. doi:.https://doi.org/10.1111/hae.12530
21 Hermans C , Apte S , Santagostino E . Invasive procedures in patients with haemophilia: Review of low-dose protocols and experience with extended half-life FVIII and FIX concentrates and non-replacement therapies. Haemophilia. 2020;hae.13978. doi:.https://doi.org/10.1111/hae.13978
22 Bravo MI , Raventós A , Pérez A , Costa M , Willis T . Non-additive effect on thrombin generation when a plasma-derived factor VIII/von Willebrand factor (FVIII/VWF) is combined with emicizumab in vitro. J Thromb Haemost. 2020;18(8):1934–9. doi:.https://doi.org/10.1111/jth.14887
23 Kitazawa T , Esaki K , Tachibana T , Ishii S , Soeda T , Muto A , et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. 2017;117(7):1348–57. doi:.https://doi.org/10.1160/TH17-01-0030
24 Fernandez-Bello I , Alvarez-Roman M , Martin-Salces M , Rivas-Pollmat I , et al. Thromboelastometry may be useful to guide treatment for breakthrough and perioperative bleeds of patients on prophylaxis with emicizumab. Haemophilia. 2019;25:106.
PF has received support for the nurses’ programme of the Haemostasis Unit from Bayer, CSL Behring, NovoNordisk, Octapharma, Roche and Sobi. LA received grants/research support from: Bayer, CSL-Behring, Novartis, NovoNordisk, Roche, Shire-Takeda, and Sobi; support for the CHUV haemophilia nurses program from: CSL-Behring, Bayer, NovoNordisk, Octapharma, Roche, Shire, and Sobi; honoraria for participating in scientific advisory boards: Bayer, Boehringer Ingelheim, Daiichi Sankyo, NovoNordisk, OrPha Swiss, Pfizer, Roche, Takeda, Sobi; honoraria as consultant/speaker: Bayer, Sanofi-Genzyme, Siemens. MA received support for the haemophilia and physiotherapy nurse programme at the University Children’s Hospital, Zurich, from: Bayer, Biotest, CSL-Behring, NovoNordisk, Octapharma, Roche, and Sobi. She also participated in scientific advisory board activities for Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novonordisk, Roche and Sobi. AC has received grants from CSL Behring and NovoNordisk and non-financial support from Bayer and Shire. BG reports grants and personal fees from Pfizer; personal fees and funding for accredited continuing medical education from Sanofi and Alnylam, during the conduct of the study, funding for accredited continuing medical education program from Axonlab, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Janssen, Mitsubishi Tanabe Pharma, NovoNordisk, Octapharma, Takeda, Sanofi, Sobi; non-financial support from Axonlab and Thermo Fisher from outside the submitted work. LG received lecture and advisory honoraria from CSL Behring, Novo Nordisk, Octapharma, Roche and Sobi. IH received lecture and advisory honoraria from Bayer, CSL Behring, NovoNordisk, Octapharma, Pfizer, Roche, Shire-Takeda and Sobi. WK received grants/research support/travel support or honoraria from: Bayer, CSL-Behring, NovoNordisk, Shire-Takeda, and Sobi. JDS received advisory and lecture honoraria from Bayer, Shire/Takeda, BMS-Pfizer, Sanofi, Siemens Diagnostics. WW received grants/research support/travel support or honoraria from: Bayer, Bristol Myers Squibb, CSL-Behring, Daiichi-Sankyo, NovoNordisk, Pfizer, Sanofi and Shire-Takeda. JAKH has received a reserach grant from Baxter Inc, now part of Takeda group of companies, honoraria for participation in advisory boards or lectures in symposia of Bayer, CSL-Behring, NovoNordisk, Roche, Sanofi, Shire-Takeda, and Sobi are paid to her employer, Insel Gruppe AG; the support of the interprofessional haemophilia care at EHCCC Bern Inselspital by Bayer, CSL-Behring, Bayer, NovoNordisk, Octapharma, Roche, and Sobi is gratefully acknowledged. All other authors report no relevant conflict of interest.