The Swiss STAR trial – an evaluation of target groups for sexually transmitted infection screening in the sub-sample of men
DOI: https://doi.org/10.4414/smw.2020.20392
Axel J.
Schmidtab, Manuela
Rasia, Cate
Essonc, Vanessa
Christinetd, Michael
Ritzlere, Thomas
Lunge, Christoph V.
Hauserf, Marcel
Stoeckleg, Florent
Jouinotd, Andreas
Lehnerh, Katharina
Langei, Torsten
Konradf, Pietro
Vernazzaa
aDivision of Infectious Diseases and Infection Control,
bCommunicable Diseases Division,
cPROFA – Consultations in Sexual Health, Renens,
dCheckpoint Vaud,
elabormedizinisches zentrum Dr Risch AG, Buchs,
fDepartment of Infectious Diseases, Bern University Hospital,
gDivision of Infectious Diseases and Hospital Epidemiology,
h
iCheckpoint Basel,
The Swiss STAR trial – an evaluation of target groups for sexually transmitted infection screening in the sub-sample of men
Summary
OBJECTIVES
In Switzerland, universal health insurance does not cover any routine testing for sexually transmitted infections (STIs), not even in individuals at high risk, and extra-genital swabbing is not standard of care. We determined the prevalence and incidence of human immunodeficiency virus (HIV), viral hepatitis and non-viral STIs in a multicentre prospective observational cohort of multi-partner men who have sex with men (MSM) and other men.
MATERIALS AND METHODS
Between January 2016 and June 2017, we offered free STI testing to all men with multiple sexual partners (three or more in the previous 12 months), with follow-up examinations every 6 months. We used multiplex polymerase chain-reaction testing (for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium) on pooled swabs (pharynx, urethra/vagina, anus), and antibody tests for HIV and Treponema pallidum at every visit, and for hepatitis B/C at baseline.
RESULTS
We screened 779 multi-partner MSM and 92 other men. Previously undiagnosed HIV was found in 0.5% vs 0.0%, respectively and T. pallidum antibodies in 15.3% vs 1.1%. STIs requiring antibiotic treatment comprised: active syphilis 1.7% vs 0.0%; N. gonorrhoeae 10.3% vs 0.0%; C. trachomatis 8.7% vs 1.1%. One in four MSM versus 1 in 100 other multi-partner men had any of these three STIs at baseline. 10.4% vs 1.3% had a history of hepatitis B, 31.9% vs 47.3% had no immunity (HBs-AB <10 IU/l). Ten MSM had HCV antibodies (1.4%), with 8 out of the 10 being MSM with HIV; HCV seroprevalence was 0.3% among HIV-negative MSM. In MSM, incidence of the three bacterial STIs was 25.5 per year over 333 person years of follow-up, HIV incidence was 0.3%. Non-condom-use (in the last 3 months) for anal/vaginal sex was not associated with STIs. Independent risk factors were sex with men (adjusted odds ratio [aOR] 16.4) and the number of sexual partners (aOR 2.3 for >20).
CONCLUSION
Among MSM, but not among other multi-partner men, STIs, mostly asymptomatic, are common. Given the high risk of onward transmission, low-cost or free routine screening of multi-partner MSM is a public health priority.
Editorial note
We decided to publish the main results of the Swiss STAR trial as two separate publications – one on the sub-sample of men, another on the sub-sample of women. Reasons for this include anatomical and epidemiological differences, and the medical disciplines in charge: urology and infectious diseases for men, and gynaecology for women. Furthermore, the two main target groups, men who have sex with men and female sex workers, differ with respect to the legal and societal context of sexual contacts, all of this probably resulting in distinct readerships. The detailed joint methods for both publications are available as online supplement.
Four key messages
- In Switzerland five multi-partner MSM need screening for bacterial STIs to find one with a notifiable and clinically relevant infection: syphilis, gonorrhoea, or chlamydia.
- Biannual STI screening among multi-partner MSM, regardless of condom use, should be offered as part of a free/low-cost package that also includes an HIV test.
- In Switzerland, multi-partner men who only have sex with women will not benefit from STI screening in the absence of symptoms, or from regular routine HIV testing.
- In Switzerland, hepatitis C prevalence among non-HIV-diagnosed MSM is low with no evidence for an increasing trend.
Introduction
The Swiss National Programme on human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) 2011–2017 highlights the importance of early detection and correct treatment of STIs in addition to behavioural changes and vaccinations, where applicable [1]. Reducing numbers of sexual partners is a behavioural change that likely results in a decreased risk of STIs [2], although the role of condom use to prevent syphilis and gonorrhoea, and particularly chlamydia, has been debated [3–6].
The Swiss healthcare system, however, is a substantial barrier to the implementation of this recommendation as universal health insurance does not cover any routine testing for STIs, even in individuals at high risk. Although individuals with symptoms of an STI are likely to contact a physician to receive appropriate treatment, asymptomatic individuals may be reluctant to do so, since the costs for STI screening have to be paid out of their own pocket. If Swiss published recommendations are closely followed and swabs from different anatomical sites are not pooled [7], testing costs for syphilis, gonorrhoea, and chlamydia add up to more than US$ 700 or, when adjusted for purchasing power parity, of almost US$ 600 PPP [8]. Since this characteristic of the Swiss healthcare system hinders the implementation of a diagnostic procedure in the interest of public health, the Swiss government is currently investigating the implementation of a new financing system for such diagnostic procedures. However, to establish a system for the financing of these tests, the ideal target group/s for such a diagnostic procedure need to be defined.
The prevalence and incidence of STIs in clients of testing sites in Switzerland is largely unknown. The primary objective of the STAR trial was to describe the prevalence of HIV and common non-viral STIs across different behavioural/demographic risk categories. Although routine testing for hepatitis C virus (HCV) in men who have sex with men (MSM) without known HIV infection is not recommended in Switzerland, studies have recommended close monitoring of trends in the spread of HCV among MSM [9]. For this reason, we included testing for HCV antibodies as a secondary objective for MSM and female sex workers (FSWs). Other secondary objectives were to describe the incidence of infections with HIV and common non-viral STIs in MSM and FSWs, the prevalence of chronic hepatitis B, and to compare self-reported with actual hepatitis B vaccination status / immunity. We aimed to determine prevalence and incidence of HIV and STIs in two groups considered at high risk for STIs: FSWs [10] and MSM. This paper presents the results for MSM and a small comparison group of multi-partner men who exclusively had sex with women.
Materials and methods
Across Switzerland, between January 2016 and June 2017, we offered free STI testing to men and women with multiple sexual partners (three or more in the last 12 months) attending multiple STI testing sites. The study provided follow-up examinations every 6 months. We used multiplex polymerase chain-reaction testing (PCR) (for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium) of pooled swabs (pharynx, urethra/vagina, anus), and antibody tests for HIV and Treponema pallidum (IgG/M, plus rapid plasma reagin if positive) at every visit, and for hepatitis B and C at baseline. At every visit, participants self-completed an anonymous online questionnaire. The detailed methods are described in appendix 1.
Ethical approval was given on 21 July 2015 by the lead ethics committee Eastern Switzerland (EKOS) under BASEC PB_2016-00738, and subsequently approved by ethics committees in Bern (KEK BE), Basel (EKNZ), Vaud (CER-VD), and Zurich (KEK ZH).
Results
We enrolled 779 MSM (including 29 male sex workers) and 92 other multi-partner men who reported sex exclusively with women. Enrolment of male participants peaked in summer 2016. Overall, 535 men returned at least once for follow up, resulting in a follow-up rate of 61% and 338.0 person-years of follow-up. All participants received all HIV/STI tests; five men were not tested for HBV, and for 55 MSM HCV-RNA could not be determined because one centre used the wrong sampling tubes; they were excluded from the respective analyses.
Sociodemographic characteristics
Participants from the French-speaking part of Switzerland were over-represented. Most participants were recruited in dedicated MSM health centres (‘Checkpoints’). Among MSM, 0.3% were transgender men, 3.7% had sold sex since their last HIV test and 3.6% had HIV diagnosed prior to enrolment. Median age was 33 years for MSM vs 32 years among other multi-partner men. Nationality broadly reflected the composition of the Swiss general population. Almost all had health insurance in Switzerland. Almost half of MSM and about a third of other men were single. Table 1 shows the sociodemographics.
Table 1 Overview and sociodemographic parameters, risk and precautionary behaviours at baseline.
| |
MSM
n/N (%)
|
Other men
n/N (%)
|
|
Study recruitment overview
|
|
|
| Persons with baseline visit |
N = 779 |
N = 92 |
| Persons with follow-up visits |
n = 526 |
n = 9 |
| Follow up visits |
n = 623 |
n = 9 |
| Follow-up rate |
526/779 (67.5) |
9/92 (9.8) |
| Person years of follow up |
333.2 years |
4.8 years |
|
Location of VCT centre in Switzerland
|
French-speaking part |
373/779 (47.9) |
3/92 (3.3) |
| German-speaking part |
406/779 (52.1) |
89/92 (96.7) |
|
Service recruited at
|
Dedicated MSM health centre |
593/779 (76.1) |
4/92 (4.3) |
| General hospital |
156/779 (20.0) |
88/92 (95.7) |
| Other VCT centre |
30/779 (3.9) |
0/92 (0.0) |
|
Sociodemographic parameters
|
|
|
|
Especially vulnerable groups
|
Transgender (FtM) |
2/779 (0.3) |
0/92 (0.0) |
| Sold sex since last HIV test |
29/779 (3.7) |
0/92 (0.0) |
| Previously diagnosed HIV |
28/779 (3.6) |
0/92 (0.0) |
|
Age
|
<25 years |
148/779 (19.0) |
14/92 (15.2) |
| 25–39 years |
393/779 (50.4) |
55/92 (59.8) |
| 40+ years |
238/779 (30.6) |
23/92(25.0) |
| Median (IQR) |
33 (26; 42) |
32 (27; 40) |
|
Nationality
|
Swiss |
543/779 (69.7) |
85/92 (92.4) |
| Neighbouring countries: AT, DE, FR, IT |
116/779 (14.9) |
2/92 (2.2) |
| Latin American, ES, PT |
53/779 (6.8) |
0/92 (0.0) |
| Other Western European, US, CA |
21/779 (2.7) |
0/92 (0.0) |
| Eastern and South-eastern European |
21/779 (2.7) |
3/92 (3.3) |
| African |
10/779 (1.3) |
1/92 (1.1) |
| Asian |
15/779 (1.9) |
0/92 (0.0) |
| Unknown |
0/779 (0.0) |
1/92 (1.1) |
|
Legal status
|
Swiss |
553/779 (69.7) |
85/92 (92.4) |
| Settlement permit |
99/779 (12.7) |
3/92 (3.3) |
| Renewable/commuter permit |
114/779 (14.6) |
2/92 (2.2) |
| Short-term permit or tourist |
18/779 (2.3) |
2/92 (2.2) |
| No permit |
5/779 (0.6) |
0/92 (0.0) |
|
Health insurance in Switzerland
|
734/764 (94.2) |
89/91 (96.7) |
|
Single / no steady partnership
|
356/751 (47.4) |
33/92 (35.9) |
|
Non-heterosexual identity
*
|
757/779 (97.2) |
0/92 (0.0) |
|
|
Risk and precautionary behaviours
|
|
|
|
Reports hepatitis B vaccination†
|
462/592 (78.0) |
48/57 (84.2) |
|
Reports HPV vaccination†
|
25/609 (4.1) |
1/74 (1.4) |
|
Previous history of diagnosed STIs
‡
|
435/779 (44.2) |
76/92 (17.4) |
|
Number of sexual partners, previous 12 months
|
3–5 |
204/779 (26.2) |
57/92 (62.0) |
| 6–10 |
240/779 (30.8) |
25/92 (27.2) |
| 11–20 |
184/779 (23.6) |
8/92 (8.7) |
| 21–50 |
112/779 (14.4) |
2/92 (2.2) |
| 50+ |
39/779 (5.0) |
0/92 (0.0) |
|
Bought sex since last HIV test
|
51/779 (6.5) |
22/92 (23.9) |
|
Sex in a group, previous 12 months
|
No |
464/779 (59.6) |
83/92 (90.2) |
| Yes, longer than 6 weeks ago |
298/779 (38.3) |
9/92 (9.8) |
| Yes, in the last 6 weeks |
17/779 (2.2) |
0/92 (0.0) |
|
Online acquisition of sex partners
|
None, previous 12 months |
132/779 (16.9) |
51/92 (55.4) |
| Less than half, previous 12 months |
129/779 (16.6) |
26/92 (28.3) |
| Half or more, previous 12 months |
518/779 (66.5) |
15/92 (16.3) |
|
PrEP use§
|
8/779 (1.0) |
0/92 (0.0) |
|
CAVI, last 3 months
|
347/779 (44.5) |
49/92 (46.7) |
|
Negotiated safety
|
No steady partner |
356/749 (47.5) |
33/92 (35.9) |
| No agreements |
138/749 (18.4) |
37/92 (40.2) |
| Condom use outside partnership |
255/749 (34.0) |
22/92 (23.9) |
|
IDU, previous 12 months
|
4/773 (0.5) |
0/88 (0.0) |
|
Sexualised drug use
¶
|
235/743 (31.6) |
14/89 (15.7) |
|
Chemsex
¶, previous 12 months
|
7/63 (11.1) |
n.a. |
Risk/precautionary behaviour
Overall, 78% of MSM vs 84.2% of other multi-partner men reported full vaccination against hepatitis B. Human papilloma virus (HPV) vaccination was reported by 4.1% vs 1.4%, and in men under 27 by 8.3% vs 6.7%.
A history of previously diagnosed STIs was reported by 44.2% of MSM vs 17.4% of other multi-partner men. Furthermore, 43.0% vs 10.9% had more than 10 partners in the past 12 months; sex in a group was reported by 40.5% vs 9.8%. Among MSM, two thirds had met at least half of their partners online and 6.5% had paid for sex since their last HIV test. Among other multi-partner men, 16.3% had met at least half of their partners online and 23.9% had paid for sex since their last HIV test.
About half of male participants (44.5% vs 46.7%) reported condomless anal or vaginal intercourse in the past 3 months. Among respondents with a steady partner, MSM were more likely to report explicit agreements on condom use with non-steady partners (“negotiated safety”: 64.9% vs 37.3%). Use of oral HIV chemoprophylaxis (PrEP) was not included in the clients’ questionnaire but was documented for eight MSM (1.0%) at baseline.
Sexualised drug use was reported by 31.6% vs 15.7%. Injection drug use in the last 12 months was rare (0.5% of MSM), but 11.1% reported Chemsex “often or always when having sex”. Although the baseline measure for Chemsex was based on only 63 participants, the follow-up measure was similar (9.8% of N = 582). Table 1 shows risk and precautionary behaviours.
Clinical outcomes: mental health, HIV/STIs, hepatitis B and C
In both groups one third of men showed signs of major depression in the PHQ-2 screening tool, and 11.9% of MSM vs 4.3% of other multi-partner men were sexually unhappy (p = 0.015).
Previously undiagnosed HIV was found in 0.5% vs 0.0% and T. pallidum antibodies in 15.3% vs 1.1% (p <0.001). For STIs requiring antibiotic treatment according to some guidelines at the time, we found active syphilis in 1.4% vs 0.0%, N. gonorrhoeae in 10.3% vs 0.0% (p <0.001), C. trachomatis in 8.7% vs 1.1% (p = 0.003), T. vaginalis in 3.2% vs 2.2% and M. genitalium in 5.3% vs 1.1% (p = 0.051). The collective measure for any of those five STIs was 25.6% vs 4.4%, corresponding to a number needed to screen (NNS) of 4 vs 23 (p <0.001).
When only bacterial infections with N. gonorrhoeae, C. trachomatis, and T. pallidum were considered to require treatment, the number needed to screen among MSM was still low (one in five), vs one in a hundred among other multi-partner men (p <0.001).
For viral hepatitis, 10.4% of MSM vs 1.3% of other multi-partner men had a history of hepatitis B (antibodies to hepatitis B core antigen [HBc-AB] positive, p = 0.003), 1.4% vs 1.3% had chronic hepatitis B (HBc-AB positive, antibodies to hepatitis B surface antigen [HBs-AB] negative) and 31.9% vs 47.3% had no immunity (HBs-AB <10 IU/l, p = 0.003). Among MSM, with use of this HBs-AB cut-off, the question relating to hepatitis B vaccination was 89.7% sensitive and 44.7% specific. Ten MSM had HCV antibodies (1.4%), with 8 out of the 10 being MSM with HIV; HCV seroprevalence was 0.6% among MSM without HIV infection known at baseline, and 0.3% among HIV-negative MSM. Three MSM (0.4%) had detectable HCV-RNA – all of them also had HIV. Table 2 shows the clinical outcomes.
Table 2 Clinical outcomes. Mental health, HIV, STIs, Hepatitis B and C, percentages with 95% confidence intervals.
|
Prevalence at baseline
|
MSM
% (95% CI)
|
Other men
% (95% CI)
|
| Persons with baseline visit |
n = 779 |
n = 92 |
|
Mental Health
|
Sexually unhappy |
11.9 (9.8–14.5) |
4.3 (1.4–11.4) |
| Signs of major depression (PHQ-2 variant) |
31.8 (28.6–35.3) |
31.5 (22.5–42.2) |
|
HIV
|
Newly diagnosed HIV |
0.5 (0.2–1.4) |
0.0 (0.0–3.3) |
|
STIs
|
Active syphilis (treatment) |
1.7 (0.9–2.9) |
0.0 (0.0–3.3) |
|
T. pallidum IgG/M positive |
15.3 (12.9–18.0) |
1.1 (0.1–6.8) |
| High RPR/VDRL (reactive at 1: ≥8) |
1.3 (0.7–1.4) |
0.0 (0.0–3.3) |
|
N. gonorrhoeae
|
10.3 (8.3–12.7) |
0.0 (0.0–3.3) |
|
C. trachomatis
|
8.7 (6.9–11.0) |
1.1 (0.1–6.8) |
|
T. vaginalis
|
3.2 (2.1–4.8) |
2.2 (0.4–8.4) |
|
M. genitalium
|
5.3 (3.8–7.1) |
1.1 (0.1–6.8) |
| Active syphilis, NG, or CT |
19.0 (16.3–22.0) |
1.1 (0.1–6.8) |
| Active syphilis, NG, CT, or TV |
21.4 (18.6–24.5) |
3.3 (0.8–9.9) |
| Active syphilis, NG, CT, TV, or MG |
25.5 (22.5–28.8) |
4.3 (1.4–11.4) |
| Reporting STI symptoms*
|
15.0 (12.6–17.7) |
12.0 (6.4–20.8) |
|
Hepatitis B and C
|
No. persons with HBs-AB (HBc-AB, HCV-AB) |
n = 775 (511, 724) |
n = 91 (77, n.a.) |
| HBs-AB <10 IU/l |
31.9 (28.6–35.3) |
47.3 (36.8–57.9) |
| HBc-AB positive |
10.4 (8.0–13.5) |
1.3 (1.2–6.7) |
| HBc-AB positive, HBs-AB negative |
1.4 (0.6–3.0) |
1.3 (0.1–8.1) |
| HCV-AB |
1.4 (0.8–2.7) |
n.a. |
| HCV-RNA |
0.4 (0.1–1.3) |
n.a. |
| HCV-AB among HIV-negative |
0.3 (0.05–1.2) |
n.a. |
| HCV-RNA among HIV-negative |
0.0 (0.0–0.4) |
n.a. |
|
Yearly incidence during follow-up
|
MSM
% (95% CI) |
|
| Persons with follow-up visits |
n = 526 |
|
| Follow up visits |
n = 623 |
|
| Person-years of follow up |
333.2 years |
|
|
HIV
|
Newly diagnosed HIV |
0.3 (0.04–2.2) |
|
|
STIs
|
Active syphilis (treatment) |
4.2 (2.5–7.0) |
|
| High RPR/VDRL (reactive at 1: ≥8) |
2.1 (1.0–4.4 |
|
|
N. gonorrhoeae
|
14.7 (11.4–19.0) |
|
|
C. trachomatis
|
9.0 (6.4–12.7) |
|
|
T. vaginalis
|
5.4 (3.4–8.5) |
|
|
M. genitalium
|
6.3 (4.2–9.5) |
|
| Active syphilis, NG, or CT |
25.5 (21.2–30.6) |
|
| Active syphilis, NG, CT, or TV |
30.6 (26.0–36.0) |
|
| Active syphilis, NG, CT, TV, or MG |
36.9 (32.1–42.5) |
|
Despite a rather small control group, the differences were statistically significant for a history of syphilis, current gonorrhoea, currant C. trachomatis infection, and for all combined outcomes. The differences were also statistically significant for a history of hepatitis B and lack of corresponding immunity.
Among MSM, with over 333.2 person years of follow-up, the proportion with incident STIs over 1 year of follow-up was slightly higher than prevalent STIs at baseline (fig. 1), but only the aggregated measure of “any of the five STIs” showed a significantly higher incidence. We found one incident HIV infection, corresponding to an incidence of 0.3%.
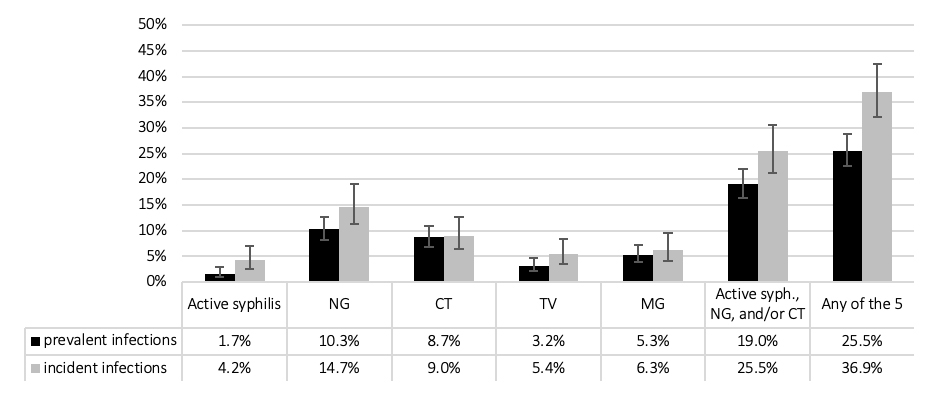
Figure 1 Sexually transmitted infections among men who have sex with men at baseline (n = 779) and during follow-up (n = 526 over 623 follow-up visits; 333.2 years of follow-up); percent with 95% confidence intervals. CT = C. trachomatis; MG = M. genitalium; NG = N. gonorrhoeae; TV = T. vaginalis.
Multivariable models
In multivariable regression analysis (table 3) – further controlling for age, previous HIV status, transactional sex and group sex – inconsistent condom use (in the last 3 months) for anal/vaginal sex was not associated with STIs (neither was “negotiated safety”, data not shown). Independent risk factors were sex with men (adjusted odds ratio [aOR] 16.4, 95% confidence interval [CI] 2.4–120.1) and the number of sexual partners (aOR 2.3 for >20, 95% CI 1.2–4.5).
Table 3 Uni- and multivariable regression models.
|
Regression model
|
Univariable
OR (95% CI)
|
Multivariable 1
AOR (95% CI)
|
Multivariable 2
AOR (95% CI
|
|
Persons with baseline visit
|
N = 871
|
N = 871
|
N = 871
|
|
Nagelkerke’s R2
|
– |
9.8%
7.8%
|
12.5%
9.0%
|
|
Age
|
40+ years |
1 |
1 |
1 |
| 25–39 years |
1.38 (0.90–2.11)
1.20 (0.83–1.74)
|
1.47 (0.94–2.28)
1.27 (0.86–1.86)
|
1.42 (0.91–2.22)
1.24 (0.84–1.82)
|
| <25 years |
1.54 (0.91–2.96)
1.34 (0.85–2.13)
|
1.62 (0.93–2.84)
1.43 (0.87–2.33)
|
1.51 (0.86–2.66)
1.37 (0.84–2.24)
|
|
Previous HIV diagnosis
|
No |
1 |
1 |
1 |
| Yes |
1.65 (0.69–3.94)
2.19
(1.01–4.76)
|
1.20 (0.48–2.96)
1.74 (0.78–3.88)
|
1.13 (0.45–2.86)
1.70 (0.76–3.83)
|
|
Sold sex since last HIV test
|
No |
1 |
1 |
1 |
| Yes |
2.66 (1.21–5.85)
2.07 (0.96–4.46)
|
1.81 (0.80–4.06)
1.47 (0.67–3.23)
|
1.63 (0.71–3.75)
1.37 (0.62–3.05)
|
|
Bought sex since last HIV test
|
No |
1 |
1 |
1 |
| Yes |
0.66 (0.32–1.36)
0.56 (0.29–1.09)
|
1.03 (0.48–2.21)
0.79 (0.39–1.59)
|
0.99 (0.46–2.15)
0.77 (0.38–1.56)
|
|
Number of sexual partners, previous 12 months
|
3–5 |
1 |
1 |
1 |
| 6–10 |
1.86 (1.09–3.17)
1.74
(1.12–2.71)
|
1.56 (0.88–2.73)
1.43 (0.89–2.32)
|
1.81 (1.01–3.23)
1.56 (0.96–2.53)
|
| 11–20 |
2.94 (1.71–5.03)
2.11
(1.33–3.37)
|
2.22 (1.20–4.10)
1.53 (0.89–2.63)
|
2.42 (1.29–4.53)
1.59 (0.92–2.75)
|
| 20+ |
3.38 (1.94–5.89)
2.76
(1.71–4.46)
|
2.31 (1.19–4.48)
1.84
(1.03–3.30)
|
2.45 (1.25–4.78)
1.89
(1.05–3.39)
|
|
Sex in a group, previous 12 months
|
No |
1 |
1 |
1 |
| Yes |
1.71 (1.20–2.44)
1.68
(1.22–2.30)
|
1.03 (0.66–1.59)
1.15 (0.77–1.72)
|
1.06 (0.68–1.65)
1.18 (0.79–1.76)
|
|
CAVI, previous 3 months
|
No |
1 |
1 |
1 |
| Yes |
1.49 (1.05–2.13)
1.30 (0.95–1.78)
|
1.33 (0.91–1.93)
1.15 (0.82–1.60)
|
1.25 (0.86–1.83)
1.10 (0.79–1.55)
|
|
Sex with men in the past 12 months
|
No |
1 |
1 |
1 |
| Yes |
21.34 (2.95–154.41)
7.55
(2.74–20.83)
|
16.40 (2.38–120.12)
5.77
(2.06–16.21)
|
16.06 (2.19–117.94)
5.65
(2.01–15.87)
|
|
Reporting STI symptoms
|
No |
1 |
– |
1 |
| Yes |
2.52 (1.65–3.87)
1.84
(1.23–2.77)
|
– |
2.53 (1.60–3.99)
1.84
(1.20–2.83)
|
When also controlled for reported symptoms, our findings were largely similar, but the variance explained by our model increased from 10% to 13%. Using alternative composite outcomes by adding M. genitalium and T. vaginalis did not substantially challenge these findings; however all effect size measures decreased and so did the explained variance.
Discussion
Among multi-partner MSM, but not among other multi-partner men, STIs, mostly asymptomatic, are common. In voluntary counselling and testing centres in Switzerland five multi-partner MSM need to be screened for bacterial STIs to find one with a notifiable and clinically relevant infection: syphilis, gonorrhoea, or chlamydia. One in four MSM acquired at least one of these bacterial STIs per year. Given that less than 4% of MSM had previously diagnosed HIV at baseline – less than half of what would have been expected [11] – our estimates may be conservative.
HIV
We found an HIV incidence of 0.3% per year in MSM, matching previous findings based on MSM population estimates and notification data [11, 12]. The baseline prevalence of previously undiagnosed HIV in MSM was similar (0.5%), suggesting HIV testing is frequent in this population.
Hepatitis B and C
This study confirms previous findings that HCV infections are concentrated in HIV-diagnosed MSM, but among MSM without diagnosed HIV do not exceed the prevalence in the general population. When results of the two published studies on HCV in non-HIV-diagnosed MSM in Switzerland (total n = 1454) are combined, HCV seroprevalence was 0.3% (95% CI 0.1–0.7) and HCV-RNA was present in 0.1% (95% CI 0.01–0.4) [9].This is not statistically different from our findings, with 0.3% (0.05–1.2) and 0.0% (0.0–0.4), respectively. It also suggests that between 2009 and 2016, HCV in non-HIV-diagnosed MSM in Switzerland has not increased.
In Switzerland, hepatitis B vaccination is recommended and reimbursed for all adolescents as well as men and women with “frequently changing partners” [13]. All study participants were eligible for hepatitis B vaccination. Although previous hepatitis B infection correlated positively with age, MSM had an overall prevalence of 10% of HBc-AB, much higher than among other multi-partner men, which suggests the promotion of hepatitis B vaccination among MSM should be prioritised. A third of MSM showed evidence of no or insufficient vaccination, despite a conservative cut-off of <10 IU/l. Whereas the question on self-reported hepatitis B vaccination showed a high sensitivity for detecting individuals with previous vaccination, it was not very specific and thus overestimates true vaccination status. This has implications for monitoring vaccination coverage.
Non-viral STIs
Fifteen per cent of MSM showed evidence of prior syphilis infection, matching the 15.4% reported in one of the largest studies of MSM living in Switzerland (EMIS-2017) [14] After a historic nadir around the year 2000, syphilis incidence has steadily increased [15]. Epidemiological models have suggested that even sustainable interventions around partner reduction or increasing condom use would have limited effects [16], and the US Centers for Disease Control’s syphilis elimination plan officially ended in 2013 [17]. Among MSM in this study, the baseline prevalence of active syphilis was 1.7%, and the incidence was 4.2 per 100 person-years. This compares to 74 per 100 person-years among HIV-positive MSM in the Swiss HIV Cohort Study in 2014 [18], corroborating previous findings that the current syphilis epidemic is concentrated in sexual networks of MSM in general, and in sexual networks of HIV-positive MSM in particular. Given that syphilis incidence in this study was 10 times higher than HIV incidence, we agree with Australian guidelines that testing MSM for syphilis only once per year may not be sufficient to control the epidemic [19].
As urethral infections with N. gonorrhoeae are typically symptomatic and lead to medical consultation [20], most cases of gonorrhoea in this study are likely to have been present in the rectum, and/or in the oropharynx. The majority of N. gonorrhoeae / C. trachomatis infections in MSM are missed if asymptomatic testing is restricted to a urine specimen [21]. Pharyngeal screening is not implemented in many national guidelines, but the contribution of fellatio to transmission of not only N. gonorrhoeae but also C. trachomatis has been demonstrated [4]. This means all N. gonorrhoeae / C. trachomatis detected in this study would likely contribute to onward transmission if left untreated, as self-clearance of asymptomatic infections takes longer (>100 days) [22] than the time to the next sexual encounter – at least on average in MSM with three or more partners per year.
It has been proposed that C. trachomatis infection control programmes based on early detection and treatment might interfere with the effects of immunity on population susceptibility to infection. The same authors suggest two strategies to decrease C. trachomatis infections at the population level: developing a vaccine or strategies to alter sexual networks [23]. Whereas in settings with low C. trachomatis prevalence (e.g., <5% in young adults), opportunistic testing may not result in “sizeable reductions” in chlamydia prevalence [24]; C. trachomatis prevalence was higher in this and other studies [25] of MSM, and asymptomatic rectal chlamydial infections in MSM have been proposed as an important reservoir fuelling transmission [26]. As 90% (unpublished data FOPH) of N. gonorrhoeae / C. trachomatis diagnoses in Switzerland are based on nucleic acid amplification technique (NAAT), it would also be difficult to screen for gonorrhoea alone.
Infections with T. vaginalis among MSM were not uncommon, although less frequent than N. gonorrhoeae / C. trachomatis and not significantly higher than in other multi-partner men. Other research has suggested that T. vaginalis may circulate within MSM networks and not result from concurrent sexual contact with women [27]. Our multi-variable model suggests that transmission of T. vaginalis (and M. genitalium) is less specific to MSM, and less dependent on typical STI determinants such as the number of sexual partners. Given the substantial side-effects of metronidazole (standard treatment for T. vaginalis), the high rates of antimicrobial resistance of M. genitalium, and the unclear public health impact of infections with T. vaginalis / M. genitalium, we do not recommend routine testing of men for T. vaginalis or M. genitalium. M. genitalium will be analysed in a subsequent paper.
Risk and precautionary behaviour
Slightly more than half of men reported consistent condom use for anal or vaginal sex, with no significant difference between the two groups. In Switzerland at the time of enrolment, PrEP was recommended for men and women at high risk for HIV [28], but not implemented and only available through online importation. Two years later, 4% of HIV-negative MSM reported current PrEP use [29], two thirds of them accessing PrEP informally online [14].
MSM reported much higher numbers of sexual partners than other multi-partner men. The distribution of partner numbers in the last 12 months almost perfectly reflected EMIS-2017 results, when the Swiss EMIS-2017 data was restricted to the sub-sample to men with 3+ partners (two thirds of the total): 3–10 partners, 62% (STAR) vs 56% (EMIS-2017); 11–20 partners, 19% vs 23%; 21–50 partners, 14% vs 14%, 50+ partners, 6% vs 7% [14]. Contrastingly, the majority of heterosexual men in STAR had only 3 to 5 sexual partners in the previous 12 months, but they were much more likely to report having paid for sex when compared with MSM.
Group sex and sexualised drug use were common among MSM. The proportion of MSM engaging in Chemsex was similar to other recently published studies from Switzerland – for example, the 11.8% of EMIS-2017 respondents in Switzerland reporting stimulant drugs in the last 12 months [14], or the 7.9% based on the same online tool but with a much larger sample size [30]. The difference from the latter publication might be attributable to our restriction to multi-partner men. What these studies have in common is the anonymous nature of reporting illicit/stigmatised behaviour. Surprisingly, Chemsex figures were not higher among HIV-positive MSM in the Swiss HIV Cohort Study [31], possibly indicating under-reporting of illicit/stigmatised behaviour in a clinical interview setting.
Since 2016, HPV vaccination has been recommended and reimbursed also for boys and men younger than 27 [32]. A result of 7% to 8% self-reported coverage among men in the eligible age group is an excellent outcome for the first year of implementation for men.
Strengths and limitations
The strengths of this study include the large sample size of MSM, the high rate of follow-up, and the rigorous methodology with respect to comprehensive STI testing. The study was sufficiently powered to detect even rare infections such as hepatitis C. The study participants represented a broad range of men with respect to age, nationality, legal status and place of residence. Although the country’s Italian-speaking part was clearly under-represented, we think that our MSM results are largely representative for sexually active gay and bisexual men in Switzerland.
This study has several limitations. It was not sufficiently powered to calculate the incidence of STIs among multi-partner men who exclusively have sex with women. The pooling of meatal/urethral, anal and pharyngeal swabs precludes the possibility of calculating site-specific prevalences, and also might lead to an underestimation of pharyngeal and thus overall N. gonorrhoeae infections [33]. Another major limitation is the absence of any test of cure. However, we think it is unlikely that syphilis, N. gonorrhoeae or C. trachomatis were not cured by standard treatment. Persisting infections would lead to an overestimation of incident cases, which is one of the reasons for not including MG or TV in our main outcome variable for incident STIs.
It needs to be highlighted that all behavioural data were self-reported. The finding that condom use was not protective against STIs (fig. S1 in appendix 1) has to be interpreted with caution. However, oral sex in MSM is typically condom-free – both fellatio [34] and oro-anal sex [35]. Although condoms effectively prevent contact with ejaculate, their effect of reducing mucosal contact over the whole course of sexual encounters is limited. In the common scenario of anal fingering prior to intercourse, transmission of STIs is possible via smear infection before or at the time of condom application.
Implications
The high incidence-to-prevalence ratio of the combined endpoint (active syphilis, gonorrhoea, or chlamydia) suggests that annual screening may be insufficient to control the epidemic of these three STIs in MSM. Biannual screening might be more adequate as a standard for multi-partner MSM [36], particularly for those with more than 10 partners per year. Other research has also suggested specifying situations in which culture-based testing is needed to provide information about anti-microbial resistance in N. gonorrhoeae [20]. Given the high prevalence and incidence of gonorrhoea among multi-partner MSM, this population is a good target for additional culture testing, with costs borne by the health care system rather than by the client. The best testing frequency MSM still needs to be determined by mathematical modelling, and in Switzerland the online counselling tool used in voluntary counselling and testing centres could include an algorithm to estimate the best individual interval for repeat testing based on personal data. Routine testing of heterosexual multi-partner men is not supported by our findings. Being part of a dense sexual network with a high turn-over of sexual partners may have a much larger impact on STI transmission than individual sexual behaviour [37, 38].
Conclusions
This study supports previous recommendations for MSM that syphilis testing and nucleic acid amplification technology-based screening for N. gonorrhoea (combined with C. trachomatis) at extra-genital sites [25] should be widely available, and providers should be educated about appropriate screening practice [19, 39, 40]. However, recommending regular testing in asymptomatic multi-partner individuals for the benefit of public health is pointless if it is not affordable to those at risk. MSM at highest risk of infection would have to spend more than US$ 2000 PPP [8] per year if screened for example every 3 months. The current price system in Switzerland may lead to substantial undertesting [20] and thus impede STI control among MSM. Given the high risk of onward transmission of bacterial STIs, low-cost or free routine screening of multi-partner MSM is a public health priority.
Preliminary data of this study were presented at the 31st annual IUSTI Europe conference in Helsinki, Finland (IUSTI17-47, IUSTI17-53) in August/September 2017, and at the Swiss HIV&STI Forum in March 2018.
Appendix 1: Methods in detail
The appendix is available as a separate file for download in the right side column.
Acknowledgements
We thank all men who participated in this arm of the study, all staff who reached out to gay saunas, provided counselling, performed testing procedures, entered laboratory results online, and/or contributed to the study in other ways.
We are particularly thankful to the following individuals: M. Kluschke (clinical study supervision at Checkpoint Zurich), K. Keckeis (help with cleaning of laboratory data); B. Aebersold, G. Aurora, C. Bischof, A. Christen, R.J. Fontana, D. Letsch, B. Leutwyler, W. Rim, S. Stölzl, M. Stratmann, (recruitment and counselling); J. Bläuer, C. Bischof, R.J. Fontana, R. Zbinden (outreach work); I. Goegele, F. Imeri, L. Risch, N. Wohlwend (laboratory). R. Staub, F. Schöni-Affolter, S. Derendinger (support with the online counselling tool); N. Low (support with study planning). We thank Peter Weatherburn for proof-reading the manuscript.
References
1Federal Office of Public Health FOPH. National Programme on HIV and other STIs (NPHS) 2011–2017. Bern: Swiss Federal Office of Public Health; 2010. Available from: https://www.bag.admin.ch/bag/en/home/strategie-und-politik/nationale-gesundheitsstrategien/nationales-programm-hiv-und-andere-sexuell-uebertragbare-infektionen/strategie.html [accessed 2020 April].
2
Wasserheit
JN
,
Aral
SO
. The dynamic topology of sexually transmitted disease epidemics: implications for prevention strategies. J Infect Dis. 1996;174(Suppl 2):S201–13. doi:.https://doi.org/10.1093/infdis/174.Supplement_2.S201
3
Warner
L
,
Stone
KM
,
Macaluso
M
,
Buehler
JW
,
Austin
HD
. Condom use and risk of gonorrhea and Chlamydia: a systematic review of design and measurement factors assessed in epidemiologic studies. Sex Transm Dis. 2006;33(1):36–51. doi:.https://doi.org/10.1097/01.olq.0000187908.42622.fd
4
Marcus
JL
,
Kohn
RP
,
Barry
PM
,
Philip
SS
,
Bernstein
KT
. Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the female oropharynx to the male urethra. Sex Transm Dis. 2011;38(5):372–3. doi:.https://doi.org/10.1097/OLQ.0b013e3182029008
5
Rank
RG
,
Yeruva
L
. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun. 2014;82(4):1362–71. doi:.https://doi.org/10.1128/IAI.01244-13
6
Heijne
JCM
,
van Liere
GAFS
,
Hoebe
CJPA
,
Bogaards
JA
,
van Benthem
BHB
,
Dukers-Muijrers
NHTM
. What explains anorectal chlamydia infection in women? Implications of a mathematical model for test and treatment strategies. Sex Transm Infect. 2017;93(4):270–5. doi:.https://doi.org/10.1136/sextrans-2016-052786
7
Notter
J
,
Frey Tirri
B
,
Bally
F
,
Aebi Popp
K
,
Yaron
M
,
Nadal
D
, et al.
[Sexually transmitted infection with Chlamydia trachomatis]. Swiss Med Forum. 2017;17(34):705–11. Article in German and French. doi:.https://doi.org/10.4414/smf.2017.03020
8Organisation for Economic Co-operation and Development. Purchasing power parities (PPP). Paris: OECD; 2018. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [accessed April 2020].
9
Schmidt
AJ
,
Falcato
L
,
Zahno
B
,
Burri
A
,
Regenass
S
,
Müllhaupt
B
, et al.
Prevalence of hepatitis C in a Swiss sample of men who have sex with men: whom to screen for HCV infection?
BMC Public Health. 2014;14(1):3. doi:.https://doi.org/10.1186/1471-2458-14-3
10
Vernazza
P
,
Rasi
M
,
Ritzler
M
,
Dost
F
,
Stoffel
M
,
Aebi-Popp
K
, et al.
The Swiss STAR trial—An Evaluation of Target Groups for STI Screening in the Sub-sample of Women. Swiss Med Wkly. 2020;150:w20393.
11
Schmidt
AJ
,
Altpeter
E
. The Denominator problem: estimating the size of local populations of men-who-have-sex-with-men and rates of HIV and other STIs in Switzerland. Sex Transm Infect. 2019;95(4):285–91. doi:.https://doi.org/10.1136/sextrans-2017-053363
12
Clerc
O
,
Darling
K
,
Calmy
A
,
Dubois-Arber
F
,
Cavassini
M
. Hepatitis C Virus Awareness Among Men Who Have Sex With Men in Southwest Switzerland. Sex Transm Dis. 2016;43(1):44–8. doi:.https://doi.org/10.1097/OLQ.0000000000000378
13Federal Office of Public Health FOPH, Swiss Federal Vaccination Commission. [Swiss vaccination plan 2014. Guidelines and recommendations]. Bern: Swiss Federal Office of Public Health, 2014. Article in German. Available from: https://kssg.guidelines.ch/api/filestore/2nBFP8jzYazi0cK6CAyJvw1tf1OpuUonjYKt8A/data/impfplan_2014_de_final-2.pdf [accessed 2020 April].
14Weber P, Gredig D, Lehner A, Nideröst S. European MSM Internet Survey (EMIS-2017). [National Report for Switzerland]. Olten: School of Social Work, University of Applied Sciences and Arts Northwestern Switzerland; 2019. Report in French and German. Available from: http://sigmaresearch.org.uk/local/item/emis-2017-national-reports.
15
Spielmann
N
,
Münstermann
D
,
Hagedorn
HJ
,
an der Heiden
M
,
Houareau
C
,
Gunsenheimer-Bartmeyer
B
, et al., German HIV-1 Seroconverter Study Group. Time trends of syphilis and HSV-2 co-infection among men who have sex with men in the German HIV-1 seroconverter cohort from 1996-2007. Sex Transm Infect. 2010;86(5):331–6. doi:.https://doi.org/10.1136/sti.2009.040857
16
Gray
RT
,
Hoare
A
,
McCann
PD
,
Bradley
J
,
Down
I
,
Donovan
B
, et al.
Will changes in gay men’s sexual behavior reduce syphilis rates?
Sex Transm Dis. 2011;38(12):1151–8. doi:.https://doi.org/10.1097/OLQ.0b013e318238b85d
17
Clement
ME
,
Hicks
CB
. Syphilis on the Rise: What Went Wrong?
JAMA. 2016;315(21):2281–3. doi:.https://doi.org/10.1001/jama.2016.7073
18
Shilaih
M
,
Marzel
A
,
Braun
DL
,
Scherrer
AU
,
Kovari
H
,
Young
J
, et al.; and the Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96(2):e5849. doi:.https://doi.org/10.1097/MD.0000000000005849
19
Templeton
DJ
,
Read
P
,
Varma
R
,
Bourne
C
. Australian sexually transmissible infection and HIV testing guidelines for asymptomatic men who have sex with men 2014: a review of the evidence. Sex Health. 2014;11(3):217–29. doi:.https://doi.org/10.1071/SH14003
20
Low
N
,
Unemo
M
,
Skov Jensen
J
,
Breuer
J
,
Stephenson
JM
. Molecular diagnostics for gonorrhoea: implications for antimicrobial resistance and the threat of untreatable gonorrhoea. PLoS Med. 2014;11(2):e1001598. doi:.https://doi.org/10.1371/journal.pmed.1001598
21
Braun
DL
,
Marzel
A
,
Steffens
D
,
Schreiber
PW
,
Grube
C
,
Scherrer
AU
, et al.; Swiss HIV Cohort Study. High Rates of Subsequent Asymptomatic Sexually Transmitted Infections and Risky Sexual Behavior in Patients Initially Presenting With Primary Human Immunodeficiency Virus-1 Infection. Clin Infect Dis. 2018;66(5):735–42. doi:.https://doi.org/10.1093/cid/cix873
22
Chow
EP
,
Camilleri
S
,
Ward
C
,
Huffam
S
,
Chen
MY
,
Bradshaw
CS
, et al.
Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex Health. 2016;13(3):199–204. doi:.https://doi.org/10.1071/SH15175
23
Brunham
RC
,
Pourbohloul
B
,
Mak
S
,
White
R
,
Rekart
ML
. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192(10):1836–44. doi:.https://doi.org/10.1086/497341
24
Hocking
JS
,
Temple-Smith
M
,
Guy
R
,
Donovan
B
,
Braat
S
,
Law
M
, et al.; ACCEPt Consortium. Population effectiveness of opportunistic chlamydia testing in primary care in Australia: a cluster-randomised controlled trial. Lancet. 2018;392(10156):1413–22. doi:.https://doi.org/10.1016/S0140-6736(18)31816-6
25
Cornelisse
VJ
,
Chow
EP
,
Huffam
S
,
Fairley
CK
,
Bissessor
M
,
De Petra
V
, et al.
Increased Detection of Pharyngeal and Rectal Gonorrhea in Men Who Have Sex With Men After Transition From Culture To Nucleic Acid Amplification Testing. Sex Transm Dis. 2017;44(2):114–7. doi:.https://doi.org/10.1097/OLQ.0000000000000553
26
Annan
NT
,
Sullivan
AK
,
Nori
A
,
Naydenova
P
,
Alexander
S
,
McKenna
A
, et al.
Rectal chlamydia--a reservoir of undiagnosed infection in men who have sex with men. Sex Transm Infect. 2009;85(3):176–9. doi:.https://doi.org/10.1136/sti.2008.031773
27
Hoffman
CM
,
Fritz
L
,
Radebe
O
,
Dubbink
JH
,
McIntyre
JA
,
Kock
MM
, et al.
Rectal Trichomonas vaginalis infection in South African men who have sex with men. Int J STD AIDS. 2018;29(14):1444–7. doi:.https://doi.org/10.1177/0956462418788418
28
Federal Office of Public Healh FOPH. Recommendations of the Swiss Federal Commission for Sexual Health (FCSH) on pre-exposure prophylaxis (PrEP) for HIV prevention. Bulletin.
2016;4. Available at: https://www.bag.admin.ch/dam/bag/en/dokumente/mt/p-und-p/richtlinien-empfehlungen/prep-empfehlungen-der-eksg-januar-2016.pdf
. [accessed 2020 April 2020].
29The EMIS Network. EMIS-2017. The European Men-Who-Have-Sex-With-Men Internet Survey. Key findings from 50 countries. Stockholm: European Centre for Disease Prevention and Control; 2019. Available from: http://sigmaresearch.org.uk/reports/item/report2019a [accessed 2020 April].
30
Giraudon
I
,
Schmidt
AJ
,
Mohammed
H
. Surveillance of sexualised drug use - the challenges and the opportunities. Int J Drug Policy. 2018;55:149–54. doi:.https://doi.org/10.1016/j.drugpo.2018.03.017
31
Hampel
B
,
Kusejko
K
,
Kouyos
RD
,
Böni
J
,
Flepp
M
,
Stöckle
M
, et al.
Chemsex drugs on the rise: a longitudinal analysis of the Swiss HIV Cohort Study from 2007 to 2017. HIV Med. 2020;21(4):228–39. doi:.https://doi.org/10.1111/hiv.12821
32Federal Office of Public Health FOPH. [HPV: complementary vaccination recommendation for boys and men aged 11 to 26 years]. Bulletin. 2015;10:141–9. Article in French and German. available from: www.bag.admin.ch/dam/bag/fr/dokumente/cc/Kampagnen/Bulletin/2015/BU_10_15_f.pdf [accessed 2020 April].
33
Sultan
B
,
White
JA
,
Fish
R
,
Carrick
G
,
Brima
N
,
Copas
A
, et al.
The “3 in 1” Study: Pooling Self-Taken Pharyngeal, Urethral, and Rectal Samples into a Single Sample for Analysis for Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in Men Who Have Sex with Men. J Clin Microbiol. 2016;54(3):650–6. doi:.https://doi.org/10.1128/JCM.02460-15
34
Dudareva-Vizule
S
,
Haar
K
,
Sailer
A
,
Wisplinghoff
H
,
Wisplinghoff
F
,
Marcus
U
; PARIS study group. Prevalence of pharyngeal and rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among men who have sex with men in Germany. Sex Transm Infect. 2014;90(1):46–51. doi:.https://doi.org/10.1136/sextrans-2012-050929
35
Fairley
CK
,
Hocking
JS
,
Zhang
L
,
Chow
EP
. Frequent Transmission of Gonorrhea in Men Who Have Sex with Men. Emerg Infect Dis. 2017;23(1):102–4. doi:.https://doi.org/10.3201/eid2301.161205
36
Jenness
SM
,
Weiss
KM
,
Goodreau
SM
,
Gift
T
,
Chesson
H
,
Hoover
KW
, et al.
Incidence of Gonorrhea and Chlamydia Following Human Immunodeficiency Virus Preexposure Prophylaxis Among Men Who Have Sex With Men: A Modeling Study. Clin Infect Dis. 2017;65(5):712–8. doi:.https://doi.org/10.1093/cid/cix439
37
Potterat
JJ
,
Muth
SQ
,
Rothenberg
RB
,
Zimmerman-Rogers
H
,
Green
DL
,
Taylor
JE
, et al.
Sexual network structure as an indicator of epidemic phase. Sex Transm Infect. 2002;78(Suppl 1):i152–8. doi:.https://doi.org/10.1136/sti.78.suppl_1.i152
38
Potterat
JJ
,
Rothenberg
RB
,
Woodhouse
DE
,
Muth
JB
,
Pratts
CI
,
Fogle
JS, 2nd
. Gonorrhea as a social disease. Sex Transm Dis. 1985;12(1):25–32. doi:.https://doi.org/10.1097/00007435-198501000-00006
39
Marcus
JL
,
Bernstein
KT
,
Kohn
RP
,
Liska
S
,
Philip
SS
. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis. 2011;38(10):922–4. doi:.https://doi.org/10.1097/OLQ.0b013e31822a2b2e
40
Chan
PA
,
Robinette
A
,
Montgomery
M
,
Almonte
A
,
Cu-Uvin
S
,
Lonks
JR
, et al.
Extragenital Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A Review of the Literature. Infect Dis Obstet Gynecol. 2016;2016:5758387. doi:.https://doi.org/10.1155/2016/5758387
