The Swiss STAR trial – an evaluation of target groups for sexually transmitted infection screening in the sub-sample of women
DOI: https://doi.org/10.4414/smw.2020.20393
Pietro
Vernazzaa, Manuela
Rasia, Michael
Ritzlerb, Ferah
Dostc, Milena
Stoffelc, Karoline
Aebi-Poppde, Christoph V.
Hausere, Cate
Essonf, Katharina
Langed, Lorenz
Rischb, Axel J.
Schmidtag
a Division of Infectious Diseases and Infection Control, Cantonal Hospital St. Gallen, Switzerland
b labormedizinisches zentrum Dr Risch AG, Buchs, Switzerland
c Gynaecological consultation, Walk-in Clinic Kanonengasse, Städtische Gesundheitsdienste Zurich, Switzerland
d Ladycheck, Aids-Hilfe beider Basel, Switzerland
e Department of Infectious Diseases, Bern University Hospital, University of Bern, Switzerland
f PROFA – Consultations in Sexual Health, Renens, Switzerland
g Communicable Diseases Division, Swiss Federal Office of Public Health, Bern, Switzerland
Summary
OBJECTIVES
In Switzerland, universal health insurance does not cover any routine testing for sexually transmitted infections (STIs), not even in individuals at high risk, and extra-genital swabbing is not standard of care. We compared STI prevalence in a multicentre prospective observational cohort of multi-partner women with/without sex work and evaluated associated risk factors.
MATERIALS AND METHODS
Between January 2016 and June 2017, we offered free STI testing to women with multiple sexual partners (three or more in the previous 12 months), with follow-up examinations every 6 months. We used multiplex polymerase chain-reaction testing (for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium) for pooled swabs (pharynx, urethra/vagina, anus), and antibody tests for human immunodeficiency virus (HIV) and Treponema pallidum at every visit, and for hepatitis B and C at baseline.
RESULTS
We screened 490 female sex workers (FSWs), including 17 trans women, and 92 other multi-partner women. More than half reported a steady partner. Previously undiagnosed HIV was found in 0.2% vs 0.0%, respectively, and T. pallidum antibodies in 5.9% vs 0.0%. STIs requiring antibiotic treatment comprised: active syphilis 1.2% vs 0.0%; N. gonorrhoeae 4.9% vs 0.0%; C. trachomatis 6.3% vs 5.4%, T. vaginalis 10.4% vs 0.0%; M. genitalium 6.7% vs 6.5%. One in four FSWs vs one in nine other women had one or more of these STIs at baseline. 15.8% vs 3.8% had a history of hepatitis B, 45.5% vs 22.8% had no immunity (HBs-AB <10 IU/l). Two FSWs had hepatitis C virus antibodies (0.4%) without concurrent HIV infection. Non-condom-use (last three months) for anal/vaginal sex was not associated with STIs. Independent risk factors were group sex (adjusted odds ratio [aOR] 2.1, 95% confidence interval [CI] 1.1–4.0), age less than 25 (aOR 3.7, 95% CI 1.6–8.9), and being active in sex work for less than 1 year (aOR 2.7, 95% CI 1.3–5.3).
CONCLUSION
HIV and HCV do not appear to pose a major public health problem among FSWs in Switzerland, whereas vaccination against HBV should be promoted. FSWs showed high rates of STIs requiring treatment to reduce transmission to clients and/or steady partners. FSWs should be offered low-cost or free STI screening as a public health priority.
Editorial note
We decided to publish the main results of the Swiss STAR trial as two separate publications – one on the sub-sample of men, another on the sub-sample of women. Reasons for this include anatomical and epidemiological differences, and the medical disciplines in charge: urology and infectious diseases for men, and gynaecology for women. Furthermore, the two main target groups, men who have sex with men and female sex workers, differ with respect to the legal and societal context of sexual contacts, all of this probably resulting in distinct readerships. The detailed joint methods for both publications are available as online supplement.
Four key messages
- In Switzerland five FSWs need to be screened for non-viral STIs to find one with clinically relevant infection: syphilis, gonorrhoea, chlamydia, trichomoniasis (excluding
- In the interest of public health, regular screening for syphilis, gonorrhoea and chlamydia should be offered to FSWs in Switzerland free of charge.
- Hepatitis B vaccination is lacking in many FSWs in Switzerland and the promotion of free vaccination should be considered.
- HIV and HCV infections do not appear to be a public health priority for female sex workers in Switzerland
Introduction
In addition to behavioural changes and vaccination, early detection and effective treatment of sexually transmissible infections (STIs) are important for the prevention of STIs [1]. Any STI can occur to a variable extent in an asymptomatic stage. Mucosal shedding and transmission of infectious pathogens is known for all STIs in the absence of symptoms. However, the risk of transmission might vary for different pathogens and anatomical sites. The role of asymptomatic carriers depends on the number of partners and the type of sexual network [2]. Importantly, asymptomatic genital shedding of an STI in a sex worker might have an impact on the epidemiology of that STI, given the high number of partners of the asymptomatically infected individual.
Accordingly, the Swiss National Programme on human immunodeficiency virus (HIV) and other STIs 2011–2017 highlights the importance of early detection and correct treatment of STIs in the context of sex work [1]. However, due to the current design of the Swiss health insurance system, the cost for routine testing for STIs in otherwise healthy individuals has to be paid almost exclusively by the individual. This cost is a disincentive for an asymptomatic individual to undergo STI testing.
If Swiss published recommendations for STI testing are closely followed and swabs from different anatomical sites are therefore not pooled [3], testing costs for syphilis, gonorrhoea, and chlamydia easily add up to an equivalent of more than US$ 700 or, when adjusted for purchasing power parity, of almost US$ 600 PPP [4]. The STAR trial (STI-Testing of Asymptomatic individuals at Risk) was initiated to examine the likely cost of a new financing system for such procedures. The aim was to define ideal target groups for free STI screening based on behavioural risk factors.
The prevalence and incidence of STIs in female sex workers (FSWs) in Switzerland is largely unknown. The primary objective of the STAR trial was to describe the prevalence of HIV and common non-viral STIs across different behavioural/demographic risk categories, with a focus on two populations known to have an increased risk for STIs: men who have sex with men (MSM) [5] and FSWs. Secondary objectives were to describe the incidence of infections with HIV and common non-viral STIs in these two groups, the prevalence of uncleared hepatitis B, and to compare self-reported with actual hepatitis B vaccination status / immunity.
This paper presents the results for the population of cisgender (assigned female at birth) and transgender (assigned male at birth) FSWs and a small comparison group of multi-partner women who had not been paid for sex.
Materials and methods
Across Switzerland, between January 2016 and June 2017, we offered free STI-testing to men and women with multiple (three or more in the last 12 months) sexual partners attending multiple STI testing sites. The study provided follow-up examinations every six months. We used multiplex polymerase chain-reaction testing (PCR) (for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium) of pooled swabs (pharynx, urethra/vagina, anus), and antibody tests for HIV and Treponema pallidum (IgG/M, plus rapid plasma reagin if positive) at every visit, and for hepatitis B and C at baseline. At every visit, participants self-completed an anonymous online questionnaire. The detailed methods are described in appendix 1.
Ethical approval was given on 21 July 2015 by the lead ethics committee Eastern Switzerland (EKOS) under BASEC PB_2016-00738, and subsequently approved by ethics committees in Bern (KEK BE), Basel (EKNZ), Vaud (CER-VD), and Zurich (KEK ZH).
Results
We enrolled 490 female sex workers (FSWs, including 17 trans women) and 92 other multi-partner women who were not paid for sex. Overall, 49 women returned at least once for follow-up, resulting in a rate of 8% and 29.5 person-years of follow-up. All participants received all HIV/STI tests; for two FSWs hepatitis C virus (HCV)-RNA could not be determined because one centre used the wrong sampling tubes; they were excluded from the respective analyses.
Sociodemographic characteristics at baseline
Participants resided almost entirely in the German-speaking part of Switzerland. Enrolment of FSW participants started slowly and peaked more than a year after study initiation, in March 2017. Most FSWs were recruited in dedicated FSW health centres or through outreach work organised by those centres; most other women were recruited in participating clinics. Among FSWs, 3.5% were transgender women and 0.2% had HIV diagnosed prior to enrolment (i.e., one cisgender FSW from a high-prevalence country). Median age was 31 years among FSWs vs 30 among other multi-partner women. Less than 3% of FSWs were Swiss, almost half of them were nationals of eastern or south-eastern Europe (predominantly Bulgaria and Romania), one third were nationals of Latin America, Portugal or Spain; among other multi-partner women Swiss nationality was over-represented compared with the general population. Of the FSWs, 13% had no permit and 70% had entered the country with a short-term permit or as a tourist. Overall, 78% said that their entire income was based on sex work and only 22% had health insurance in Switzerland. The median time spent in sex work was 3 years, with an interquartile range (IQR) of 1–7. Table 1 shows the sociodemographics of participants.
Table 1 Overview and sociodemographic parameters, risk and precautionary behaviours at baseline.
| |
FSWs
n/N (%)
|
Other women
n/N (%)
|
|
Study recruitment overview
|
|
|
| Persons with baseline visit |
N = 490 |
N = 92 |
| Persons with follow-up visits |
N = 42 |
N = 7 |
| Follow-up visits |
N = 46 |
N = 7 |
| Follow-up rate |
42/490 (8.6) |
7/92 (7.6) |
| Person years of follow-up |
25.4 years |
4.1 years |
|
Location of VCT centre in Switzerland
|
French-speaking part |
13/490 (2.7) |
4/92 (4.3) |
| German-speaking part |
477/490 (97.3) |
88/92 (95.7) |
|
Service recruited at
|
Dedicated FSW health centre |
340/490 (69.4) |
0/92 (0.0) |
| General hospital |
117/490 (23.9) |
88/92 (95.7) |
| Other VCT centre |
33/490 (6.7) |
4/92 (4.3) |
|
Sociodemographic parameters
|
|
|
|
Especially vulnerable groups
|
Transgender (MtF) |
17/490 (3.5) |
5/92 (5.4) |
| Previously diagnosed HIV |
1/490 (0.2) |
0/92 (0.0) |
|
Age
|
<25 years |
95/490 (19.4) |
14/92 (15.2) |
| 25–39 years |
276/490 (56.3) |
70/92 (76.1) |
| 40+ years |
119/490 (24.3) |
8/92 (8.7) |
| Median (IQR) |
31 (26; 39) |
30 (26; 34) |
|
Nationality
|
Swiss |
13/490 (2.7) |
81/92 (88.0) |
| Neighbouring countries: AT, DE, FR, IT |
29/490 (5.9) |
7/92 (7.6) |
| Latin American, ES, PT |
158/490 (32.2) |
0/92 (0.0) |
| Other Western European, US, CA |
1/490 (0.2) |
0/92 (0.0) |
| Eastern and south-eastern European |
237/490 (48.4) |
3/92 (3.3) |
| African |
35/490 (7.1) |
1/92 (1.1) |
| Asian |
11/490 (2.2) |
0/92 (0.0) |
| Unknown |
6/490 (1.2) |
0/92 (0.0) |
|
Legal status
|
Swiss |
13/490 (2.7) |
81/92 (88.0) |
| Settlement permit |
24/490 (4.9) |
9/92 (9.8) |
| Renewable/commuter permit |
47/490 (9.6) |
1/92 (1.1) |
| Short-term permit or tourist |
342/490 (69.8) |
1/92 (1.1) |
| No permit |
64/490 (13.1) |
0/92 (0.0) |
|
Income through sex work
|
None / not applicable |
0/444 (0.0) |
92/92 (100.0) |
| Less than half |
64/444 (14.4) |
n.a. |
| More than half |
34/444 (7.7) |
n.a. |
| Entire income |
346/444 (77.9) |
n.a. |
|
Active in sex work for less than 1 year
|
88/490 (18.1) |
n.a. |
|
Health insurance in Switzerland
|
110/485 (22.4) |
88/90 (95.7) |
|
Single / No steady partnership
|
161/336 (47.9) |
43/91 (47.3) |
|
Non-heterosexual identity*
|
19/490 (3.9) |
18/92 (19.6) |
|
|
Risk and precautionary behaviours
|
|
|
|
Reports hepatitis B vaccination
†
|
64/130 (49.2) |
61/68 (89.7) |
|
Reports HPV vaccination
†
|
9/239 (3.8) |
13/77 (16.9) |
|
Previous history of diagnosed STIs
‡
|
105/490 (21.4) |
20/92 (21.7) |
|
Number of sexual partners, last 12 months
|
3–5 |
15/490 (3.1) |
66/92 (71.7) |
| 6–10 |
19/490 (3.9) |
17/92 (18.5) |
| 11–20 |
27/490 (5.5) |
5/92 (5.4) |
| 21–50 |
64/490 (13.1) |
2/92 (2.2) |
| 50+ |
365/490 (74.5) |
2/92 (2.2) |
|
Bought sex since last HIV test
|
13/490 (2.7) |
3/92 (3.3) |
|
Sex in a group, previous 12 months
|
No |
303/490 (61.8) |
84/92 (91.3) |
| Yes, longer than 6 weeks ago |
41/490 (8.4) |
8/92 (8.7) |
| Yes, in the last 6 weeks |
146/490 (29.8) |
0/92 (0.0) |
|
Online acquisition of sex partners
|
None, previous 12 months |
444/490 (90.6) |
63/92 (68.5) |
| Less than half, previous 12 months |
19/490 (3.9) |
17/92 (18.5) |
| Half or more, previous 12 months |
27/490 (5.5) |
12/92 (13.0) |
|
CAVI, previous 3 months
|
267/490 (54.5) |
47/92 (51.1) |
|
IDU, previous 12 months
|
2/481 (0.4) |
0/90 (0.0) |
|
Sexualised drug use
§
|
49/490 (14.4) |
21/91 (23.3) |
Risk and precautionary behaviour
Overall (excluding women with missing answers), 49% of FSWs vs 90% of other multi-partner women reported full vaccination against hepatitis B virus (HBV). Human papilloma virus (HPV vaccination was reported by 4% vs 17%.
A very similar proportion reported a history of previously diagnosed STIs, 21% of FSWs and 22% of other women. In contrast, the median number of sexual partners was 50+ among FSWs, but was a magnitude lower (3–5) in other women, and group sex was reported by 38% vs 8.7%. Among FSWs, 9% had met partners online in the previous 12 months, whereas online acquisition of partners was common among other women (32%).
Sexualised drug use was reported by 14% of FSWs vs 23% of other women. Injection drug use in the last 12 months was rare (0.4% of FSWs). About half of the participants (55% vs 51%) reported condomless anal or vaginal intercourse in the past three months. Table 1 shows the risk and precautionary behaviours of participants.
Clinical outcomes: mental health, HIV, STIs, hepatitis B and C
In both groups one third of women showed signs of major depression in the PHQ-2 screening tool; 17% of FSWs vs 8% of other multi-partner women were sexually unhappy (p <0.001), and in addition, 18% of FSWs reported having no sex life outside transactional sex. Previously undiagnosed HIV was found in 0.2% vs 0.0% (i.e., one transgender FSW from south-eastern Europe); TP-antibodies in 5.9% vs 0.0% (p = 0.006). For STIs requiring antibiotic treatment according to some guidelines at the time, we found active syphilis in 1.4% vs 0.0%; N. gonorrhoeae 4.9% vs 0.0% (p = 0.015); C. trachomatis 6.3% vs 5.4%; T. vaginalis 10% vs 0.0% (p <0.001); M. genitalium 6.7% vs 6.5%. The joint measure for any of those five STIs was 23% vs 11% (p = 0.004), corresponding to a number needed to screen of 4 or 9, respectively. When only infections with N. gonorrhoeae, C. trachomatis, and T. pallidum were considered to require treatment, the number needed to screen increased to 9 among FSWs vs 19 among other women (p = 0.068).
Concerning viral hepatitis, 16% of FSWs vs 3.8% of other multi-partner women had a previous hepatitis B infection (antibodies to hepatitis B core antigen [HBc-AB] positive, p = 0.002), 1.4% vs 0.0% had chronic hepatitis B (HBc-AB positive, antibodies to hepatitis B surface antigen [HBs-AB] negative); and 46% vs 23% had no immunity (HBs-AB <10 IU/l, p <0.001). Among FSWs, using this HBs-AB cut-off, the question relating to hepatitis B vaccination was 57% sensitive and 61% specific. Two FSWs (cisgender FSWs from Latvia and Hungary) had HCV antibodies (0.4%), one with HCV-RNA (0.2%).
Despite a rather small comparison group, the differences were statistically significant for a history of syphilis, current gonorrhoea, current T. vaginalis infection and for two combined outcomes, but not for C. trachomatis and M. genitalium. The differences were also statistically significant for a history of hepatitis B and lack of corresponding immunity.
Table 2 shows the clinical outcomes among all study participants. Among FSWs, with over 25 person-years of follow-up, the proportion with incident non-viral STIs over 1 year of follow-up was slightly higher than prevalent non-viral STIs at baseline (fig. 1). No incident HIV infection was found.
Table 2 Clinical outcomes. Mental health, HIV, STIs, hepatitis B and C, percentages with 95% confidence intervals.
|
FSWs
% (95% CI)
|
Other women
% (95% CI)
|
|
Prevalence at baseline
|
|
|
| Persons with baseline visit |
N = 490
|
N = 92
|
|
Mental health
|
Sexually unhappy / No private sex life*
|
35.8 (31.4–40.4) |
7.8 (3.5–15.9) |
| Signs of major depression (PHQ-2 variant) |
29.3 (25.2–33.7) |
36.7 (28.9–33.8) |
|
HIV
|
Newly diagnosed HIV |
0.2 (0.0–1.3) |
0.0 (0.0–3.3) |
| All prevalent HIV |
0.4 (0.1–1.6) |
0.0 (0.0–3.3) |
|
STIs
|
Active syphilis (treatment) |
1.2 (0.5–2.8) |
0.0 (0.0–3.3) |
|
T. pallidum IgG/M positive |
5.9 (4.1–8.5) |
0.0 (0.0–3.3) |
| High RPR/VDRL (reactive at 1: ≥8) |
0.2 (0.0–1.3) |
0.0 (0.0–3.3) |
|
N. gonorrhoeae
|
4.9 (3.2–7.3) |
0.0 (0.0–3.3) |
|
C. trachomatis
|
6.3 (4.4–9.0) |
5.4 (2.0–12.8) |
|
T. vaginalis
|
10.4 (7.9–13.5) |
0.0 (0.0–3.3) |
|
M. genitalium
|
6.7 (4.7–9.4) |
6.5 (2.7–14.2) |
| Active syphilis, NG, or CT |
11.0 (8.5–14.2) |
5.4 (2.0–12.8) |
| Active syphilis, NG, CT, or TV |
19.0 (15.7–22.8) |
5.4 (2.0–12.8) |
| Active syphilis, NG, CT, TV, or MG |
23.1 (19.5–27.1) |
10.9 (5.6–19.5) |
| Reporting STI symptoms†
|
21.0 (17.5–24.9) |
21.7 (14.1–31.8) |
|
Hepatitis B and C
|
No. persons with HBs-AB (HBc-AB, HCV-AB) |
N = 490 (431, 488)
|
N = 92 (78, n.a.)
|
| HBs-AB <10 IU/l |
45.5 (41.1–50.0) |
22.8 (15.0–33.0) |
| HBc-AB positive |
15.8 (12.6–19.7) |
3.8 (2.8–7.7) |
| HBc-AB positive, HBs-AB negative |
1.4 (0.8–1.8) |
0.0 (0.0–3.8) |
| HCV-AB positive |
0.4 (0.1–1.6) |
n.a. |
| HCV-RNA |
0.2 (0.0–1.3) |
n.a. |
| HCV-AB among HIV-negative |
0.4 (0.1–1.6) |
n.a. |
| HCV-RNA among HIV-negative |
0.2 (0.0–1.3) |
n.a. |
|
Yearly incidence during follow-up
|
FSWs
% (95% CI)
|
|
| Persons with follow-up visits |
n = 42
|
|
| Follow-up visits |
n = 46
|
|
| Person-years of follow-up |
25.4 years
|
|
|
HIV
|
Newly diagnosed HIV |
0.0 (0.0–11.8) |
|
|
STIs
|
Active syphilis (treatment) |
3.9 (0.6–26.9) |
|
| High RPR/VDRL (reactive at 1: ≥8) |
0.0 (0.0–11.8) |
|
|
N. gonorrhoeae
|
11.8 (4.1–34.2) |
|
|
C. trachomatis
|
7.9 (2.1–29.8) |
|
|
T. vaginalis
|
19.7 (9.0–43.2) |
|
|
M. genitalium
|
0.0 (0.0–11.8) |
|
| Active syphilis, NG, or CT |
19.7 (9.0–43.2) |
|
| Active syphilis, NG, CT, or TV |
35.4 (21.0–59.9) |
|
| Active syphilis, NG, CT, TV, or MG |
35.4 (21.0–59.9) |
|
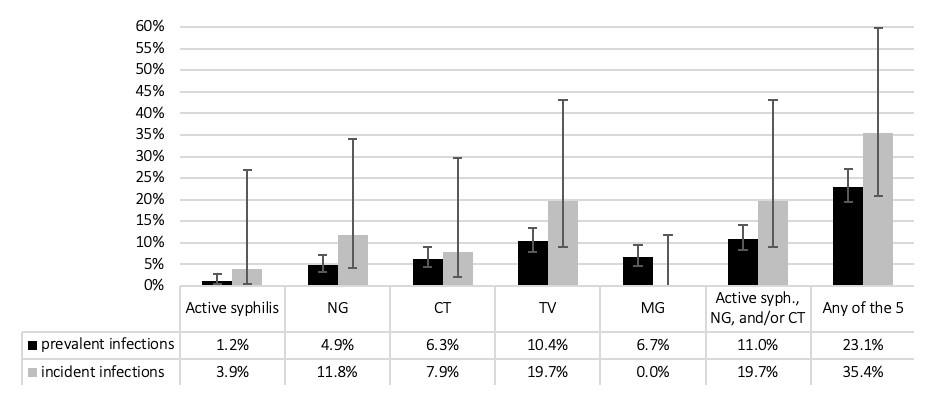
Figure 1 Sexually transmitted infections among female sex workers at baseline (n = 490) and during follow-up (n = 42 over 46 follow-up visits; 25.4 years of follow-up); percent with 95% confidence interval. CT = C. trachomatis; MG = M. genitalium; NG = N. gonorrhoeae; TV = T. vaginalis.
Multivariable models
In multivariable regression analysis, controlling for gender identity and the numbers of sexual partners, inconsistent condom-use (last 3 months) for anal/vaginal sex was not associated with STIs. Independent risk factors were group sex (adjusted odds ratio [aOR] 2.1, 95% confidence interval [CI] 1.1–4.0), age less than 25 (aOR 3.7, 95% CI 1.6–8.9) and being active in sex work less than 1 year (aOR 2.7, 95% CI 1.3–5.3).
When also controlled for reporting symptoms, our findings were almost identical, and the variance explained by our model was only marginally increased. Using the alternative outcome by adding M. genitalium and T. vaginalis did not substantially change these findings; however all effect size measures decreased, as did the explained variance (table 3).
Table 3 Uni- and multivariable regression models.
|
Regression Model
|
Univariable
OR (95% CI)
|
Multivariable 1
AOR (95% CI)
|
Multivariable 2
AOR (95% CI)
|
|
Persons with baseline visit
|
N = 532
|
N = 532
|
N = 532
|
|
Nagelkerke’s R2
|
– |
14.8%
9.5%
|
15.0%
9.5%
|
|
Age
|
40+ years |
1 |
1 |
1 |
| 25–39 years |
1.16 (0.51–2.64)
1.43 (0.81–2.51)
|
1.11 (0.48–2.58)
1.50 (0.84–2.67)
|
1.14 (0.49–2.66)
1.49 (0.83–2.66)
|
| <25 years |
4.60 (2.01–10.80)
3.37
(1.79–6.36)
|
3.70 (1.55–8.83)
3.04
(1.58–5.84)
|
3.73 (1.56–8.91)
3.03
(1.58–5.84)
|
|
Active in sex work for less than 1 year
|
No |
1 |
1 |
1 |
| Yes |
2.33 (1.25–4.36)
1.72
(1.03–2.86)
|
2.66 (1.33–5.33)
1.71 (0.99–2.96)
|
2.57 (1.28–5.19)
1.72 (0.99–2.99)
|
|
Transgender (MtF)
|
No |
1 |
1 |
1 |
| Yes |
0.88 (0.20–3.87)
0.58 (0.17–1.99)
|
1.11 (0.24–5.11)
0.62 (0.18–2.16)
|
1.13 (0.24–5.27)
0.61 (0.17–2.14)
|
|
Number of sexual partners, previous 12 months
|
3–5 |
1 |
1 |
1 |
| 6–10 |
0.55 (0.06–5.10)
1.00 (0.28–3.49)
|
0.28 (0.03–3.69)
0.75 (0.21–2.75)
|
0.29 (0.03–2.23)
0.75 (0.20–2.74)
|
| 11–20 |
0.62 (0.07–5.78)
2.67 (0.93–7.69)
|
0.37 (0.04–3.69)
2.29 (0.76–6.94)
|
0.36 (0.04–3.64)
2.31 (0.76–6.98)
|
| 20+ |
2.69 (0.94–7.64)
2.67
(1.19–5.10)
|
1.56 (0.51–4.83)
1.84 (0.85–4.00)
|
1.58 (0.51–4.90)
1.84 (0.85–4.00)
|
|
Sex in a group, previous 12 months
|
No |
1 |
1 |
1 |
| Yes |
2.46 (1.43–4.24)
2.03
(1.35–3.04)
|
2.09 (1.12–3.90)
1.74
(1.11–2.72)
|
2.14 (1.14–3.99)
1.73
(1.10–2.71)
|
|
CAVI, previous 3 months
|
No |
1 |
1 |
1 |
| Yes |
1.28 (0.74–2.20)
1.21 (0.81–1.81)
|
1.19 (0.66–2.14)
1.09 (0.71–1.66)
|
1.19 (0.66–2.15)
1.08 (0.71–1.66)
|
|
Reporting STI symptoms
|
No |
1 |
– |
1 |
| Yes |
1.31 (0.70–2.44)
0.94 (0.57–1.54)
|
–
|
1.29 (0.66–2.15)
0.93 (0.55–1.56)
|
Discussion
Our primary objective was to determine the prevalence of non-viral STIs (active syphilis, gonorrhoea, C. trachomatis, T. vaginalis and M. genitalium) among female sex workers in Switzerland. We found a combined prevalence of all five STIs of 23%, which was higher than the anticipated prevalence used in the sample size calculation. Non-viral STIs were common in FSWs, but not among other multi-partner women. Five FSWs needed to be screened for non-viral STIs to find one with a clinically relevant infection: syphilis, gonorrhoea, chlamydia or T. vaginalis. Even restricting our analysis to only the three pathogens with major clinical sequelae (active syphilis, gonorrhoea, C. trachomatis) a prevalence of 11% indicates that these pathogens merit further attention in this population.
The STAR trial also tried to estimate the incidence of STIs among FSWs, but this was hampered by the low follow-up rate (46 visits contributing to a total of 25 observation-years). Therefore, the incidence estimates need to be interpreted cautiously.
Syphilis
Among the 6% of FSWs with evidence of prior syphilis infection (T. pallidum IgG/M positive), one in five had active syphilis, compared with one in ten among MSM in the “men” part of the STAR trial [5]. This rate of active syphilis indicates inadequate diagnosis and therapy and highlights the importance of testing FSWs for syphilis (with subsequent treatment of individuals with no prior treatment) on a regular basis. The prevalence of active syphilis in this study (1.2%) was very similar to the 1.1% prevalence in a German study [6].
Syphilis remains asymptomatic for most of the post-primary phases, with the exception of secondary syphilis where fever and rash are common, but underreported. The risk of transmission of syphilis, however, remains, particularly within the first 2 years of infection [7]. The rate of transmission of primary or secondary syphilis from an infected individual has been estimated at 30% [8]; however, the study included repeated sexual exposures with the source. More recent studies investigating the per contact risk of transmission reported a transmission risk in the range of 1–2% [9]. Gray et al. estimated the per contact risk of transmission during the early stage of latent syphilis to be about half the risk compared with the primary or secondary stage [10].
Based on a Swiss survey on FSWs, Biberstein and Kilias estimated the number of sexual acts with clients to be approximately 2–6 million per year [11]. Assuming that 1% of FSWs are infected and a 1% risk of transmission per event, this would result in approximately 200 to 600 syphilis transmission events from FSWs to clients, per year. This figure is close to the reported yearly numbers of 200 syphilis cases among non-MSM men in Switzerland [12], especially if underreporting of syphilis cases among clients and the possibility of repeated exposures to the same FSW are considered. Since syphilis has potentially severe long-term consequences, prevention efforts to reduce the risk of transmission from FSWs to their clients are needed.
The high estimate of the incidence for active syphilis (3.6%) must be interpreted with caution owing to the low follow-up rate. However, even the lower margin of the 95% confidence interval indicates a relevant risk of incident infections above the national yearly average of 2.4/100,000 among women [12].
Gonorrhoea
In men, urethral infection with gonorrhoea is symptomatic in approximately 90% of individuals [13]. In women, the proportion of asymptomatic carriers appears significantly higher [14]. Since we used pooled sampling (vaginal, pharyngeal, rectal) we are unable to identify the fraction of pharyngeal infection in this sample. However, given that FSWs performing condomless oral sex on their clients is common [15], it is likely that a substantial part of the 5% gonorrhoea cases were pharyngeal carriers.
The risk of female to male transmission in the case of an asymptomatic carrier of gonorrhoea is not well known and the role of saliva in transmission of gonorrhoea has recently been disputed [16]. However, given the high prevalence of condomless fellatio with FSWs in Switzerland and the potential of oral transmission of gonorrhoea, sex with FSWs might contribute substantially to the spread of gonorrhoea among heterosexuals.
T. vaginalis
Among FSWs, T. vaginalis was the most frequently detected single pathogen (10%). Despite a large amount of epidemiological data on T. vaginalis in FSWs in Asia and Africa [17], information is limited for Europe. Ten years ago, a German survey on over 9000 FSWs found a 3% prevalence for T. vaginalis [6], whereas another German study in more marginalised FSWs found a higher prevalence (11%) [18].Here, we found a striking difference in prevalence rates among FSWs (10%) vs other women (0%). This clearly indicates sexual exposure as a primary risk factor for this infection. In men, symptomatic infections with T. vaginalis and complications are rare. Little is known about the rate of severe complications of T. vaginalis infection in women but an association with cervical neoplasia has been reported [19], possibly explained by an increased susceptibility to HPV.
For financial and logistic reasons, most screening programmes in Switzerland for FSWs do not include T. vaginalis. Nucleic acid amplification test kits are only available for multiple pathogens and therefore T. vaginalis is rarely tested in asymptomatic women. However, given the high prevalence of T. vaginalis particularly in FSWs, screening of asymptomatic FSWs might be a reasonable intervention, although alternative diagnostic procedures might need to be evaluated.
C. trachomatis
Since anal chlamydia infection in women is not associated with anal intercourse [20], we included anal swabbing in our pooling strategy regardless of sexual practices. C. trachomatis was found in 6.3% of FSWs – a prevalence not significantly higher than among other multi-partner women (5.4%). Whether C. trachomatis screening should be offered in women is highly debated, since such programmes do not appear to prevent a reasonable fraction of cases with pelvic inflammatory diseases or infertility in women [21], but result in an increased number of recurrent infections [22]. Unlike T. vaginalis, prevalence rates for C. trachomatis and M. genitalium were very similar in the two groups (table 2). The major epidemiological factors associated with risk of incident chlamydial infection were young age and less than 2 years of sex work [23, 24]. There is some evidence for the acquisition of an immune response against chlamydia if the infection is not treated [23], a potential argument against screening. On the other hand, early detection and treatment in FSWs might have a beneficial role on the spread of chlamydia due to the large number of exposed partners. As most detection assays for N. gonorrhoeae are combined with C. trachomatis, our options to select the pathogens for screening are limited.
M. genitalium
The prevalence of MG colonisation was exactly the same in FSWs as in other women. We are faced with a similar situation as for C. trachomatis. The majority of M. genitalium infections remain asymptomatic. The role of sexual exposure on the prevalence of M. genitalium remains unclear and we therefore suggest that M. genitalium screening should not be part of a screening programme in asymptomatic FSWs.
HIV
Only one FSW, a transgender woman, was found to be HIV positive in this study. Transgender FSWs have also been found to have a higher HIV prevalence in a study from Portugal [25].The low prevalence of HIV among cisgender FSWs might reflect a selection bias against FSWs who inject drugs. Low HIV prevalence was also found in other European studies of FSWs not injecting drugs [26–28]. This supports the finding from molecular epidemiological studies that ongoing HIV transmission among cisgender heterosexuals in Switzerland is extremely rare [29]. Nevertheless, given the high number of exposed partners and the potential risk of sexual transmission of untreated HIV, any strategy to offer STI testing to FSWs should include HIV testing.
Hepatitis B and C, self-reported HPV vaccination
This study confirms that (hetero)sexual contact is not a relevant mode of transmission of HCV [30]. Only one FSW was found with active HCV infection, which is in the range of prevalence in the general population [31].
Unlike HCV, HBV is often transmitted via sexual contacts. In Switzerland, hepatitis B vaccination is recommended and reimbursed for all adolescents, as well as for men and women with “frequently changing partners” [32]. All women in this study would thus be eligible for vaccination. Recruited FSWs had a high prevalence (16%) of past HBV infection, a relevant rate of active HBV infection (1.5%), and half lacked protection against HBV. This finding supports efforts to promote HBV vaccination among FSWs.
At the time of enrolment into this study, HPV vaccination was recommended and reimbursed for females younger than 27 [33]. FSWs in Switzerland lack protection and can therefore be regarded as relevant sources for HPV transmission to their (unvaccinated) clients.
Strengths and limitations
To our knowledge, this is the first and largest study ever performed on the prevalence of STIs in FSWs in Switzerland. The use of a multi-pathogen PCR method including trichomonas, mycoplasma and ureaplasma was useful to determine the relevance of some of these pathogens for public health.
Unfortunately, we did not reach the power needed for more precise estimates for incident STIs. There was a trend towards more diagnoses during follow-up as compared with baseline, possibly reflecting a self-selection bias, with women with previous STIs more likely to return for follow-up.
One methodological limitation was the pooling of vaginal, pharyngeal and anal samples for PCR analysis. As a consequence, we were unable to specify the location of pathogen shedding. Data collected from a 2016 randomised controlled trial and an in vitro study found that the mouthwash Listerine® significantly reduced the amount of N. gonorrhoeae on the pharyngeal surface [34]. In our questionnaire assessing specific risk behaviour we did not include the routine use of mouthwash as a precautionary behaviour for pharyngeal gonorrhoea.
A major limitation of this study was that FSWs were almost entirely (97%) recruited in the German-speaking part of Switzerland. Investigators from a large drop-in health centre for FSWs in Geneva (ASPASIE) conducted a similar study from March 2017 to March 2019 [35]. The 95%-confidence intervals of the prevalence rates of that study did overlap with the results presented here, hence there was no statistically significant difference in the prevalence of HIV, syphilis, N. gonorrhoea, C. trachomatis, and uncleared hepatitis B infection between FSWs in the German-speaking and the French-speaking part of Switzerland (table S1 in appendix 1).
Conclusions
HIV and HCV do not appear to pose a major problem among FSWs in Switzerland, while vaccination against HBV should be promoted in this population. Non-viral STIs, particularly syphilis and gonorrhoea, appear to have a major impact in this population and screening of asymptomatic FSWs might help to reduce the spread of these pathogens. The importance of T. vaginalis infection seems underestimated in FSWs and merits further studies. The epidemiology of C. trachomatis and M. genitalium appears to be less driven by sexual exposure, which renders screening of FSWs for these pathogens less important. Given the high prevalence of non-viral STIs in this study and the substantial risk of onward transmission, low-cost or free routine screening of FSWs should be a public health priority.
Preliminary data of this study were presented at the 31st annual IUSTI Europe conference in Helsinki, Finland (IUSTI17-47, IUSTI17-53) in August/September 2017, and at the Swiss HIV&STI Forum in March 2018.
Appendix 1: Methods in detail
The appendix is available as a separate file in the download section in the right side column.
Acknowledgements
We thank all men and women who participated in the study, all staff who reached out to brothels and gay saunas, did counselling, performed testing procedures, entered laboratory results online, and/or contributed to the study in other ways.
We are particularly thankful to the following individuals: K. Keckeis (help with cleaning of laboratory data); B. Aebersold, G. Aurora, C. Bischof, A. Christen, T. Konrad, B. Leutwyler, W. Rim, S. Stölzl, B. Zahno (recruitment and counselling); C. Bischof, S. Gresser, (outreach work); I. Goegele, F. Imeri, L. Risch, N. Wohlwendt (laboratory). R. Staub, F. Schöni-Affolter, S. Derendinger (support with the online counselling tool); N. Low (support with study planning). Finally, we would like to thank M. Furegato for statistical advice, Marianne Jossen for valuable inputs for the manuscript, and Peter Weatherburn for proof-reading the manuscript.
References
1Federal Office of Public Health FOPH. National Programme on HIV and other STIs (NPHS) 2011–2017. Bern: Swiss Federal Office of Public Health; 2010. Available from: https://www.bag.admin.ch/bag/en/home/strategie-und-politik/nationale-gesundheitsstrategien/nationales-programm-hiv-und-andere-sexuell-uebertragbare-infektionen/strategie.html [accessed 2020 April].
2
Boily
MC
,
Mâsse
B
. Mathematical models of disease transmission: a precious tool for the study of sexually transmitted diseases. Can J Public Health. 1997;88(4):255–65. doi:.https://doi.org/10.1007/BF03404793
3
Notter
J
,
Frey Tirri
B
,
Bally
F
,
Aebi Popp
K
,
Yaron
M
,
Nadal
D
, et al.
[Sexually transmitted infection with Chlamydia trachomatis]. Swiss Med Forum. 2017;17(34):705–11. Article in German and French. doi:..https://doi.org/10.4414/smf.2017.03020
4Organisation for Economic Co-operation and Development. Purchasing power parities (PPP). Paris: OECD; 2018. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm [accessed 2020 April].
5
Schmidt
AJ
,
Rasi
M
,
Esson
C
,
Christinet
V
,
Ritzler
M
,
Lung
T
, et al.
The Swiss STAR trial – an evaluation of target groups for sexually transmitted infection screening in the sub-sample of men. Swiss Med Wkly. 2020;150:w20392.
6
Bremer
V
,
Haar
K
,
Gassowski
M
,
Hamouda
O
,
Nielsen
S
. STI tests and proportion of positive tests in female sex workers attending local public health departments in Germany in 2010/11. BMC Public Health. 2016;16(1):1175. doi:.https://doi.org/10.1186/s12889-016-3847-6
7Holmes KK, Sparling PF, Walter E. Stamm, Piot P, Wasserheit JN, Corey L, et al. Clinical Manifestations of Syphilis (Chapter 37). Sexually Transmitted Diseases. 4th edition ed. New York: The McGraw-Hill Companies; 2008. p. 661ff.
8
Schroeter
AL
,
Turner
RH
,
Lucas
JB
,
Brown
WJ
. Therapy for incubating syphilis. Effectiveness of gonorrhea treatment. JAMA. 1971;218(5):711–3. doi:.https://doi.org/10.1001/jama.1971.03190180033006
9
Stoltey
JE
,
Cohen
SE
. Syphilis transmission: a review of the current evidence. Sex Health. 2015;12(2):103–9. doi:.https://doi.org/10.1071/SH14174
10
Gray
RT
,
Hoare
A
,
Prestage
GP
,
Donovan
B
,
Kaldor
JM
,
Wilson
DP
. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis. 2010;37(5):298–305. doi:.https://doi.org/10.1097/OLQ.0b013e3181ca3c0a
11Biberstein L, Killias M. [Erotic operations as a gateway for human trafficking? A study on the extent and structure of the sex work market in Switzerland - investigation commissioned by the Federal Office of Police]. Lenzburg: Killias Research & Consulting AG; 2015. In German; available from: https://www.alexandria.unisg.ch/252621 [accessed 2020 April].
12Federal Office of Public Healh FOPH. [HIV, syphilis, gonorrhoea, and chlamydia in Switzerland: 2018 epidemiological overview]. Bulletin. 2019;41:10-20. Article in French and German; available from: https://www.bag.admin.ch/dam/bag/fr/dokumente/cc/Kampagnen/Bulletin/2019/BU_41_19.pdf [accessed 2020 April].
13
Ong
JJ
,
Fethers
K
,
Howden
BP
,
Fairley
CK
,
Chow
EPF
,
Williamson
DA
, et al.
Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service. Clin Microbiol Infect. 2017;23(8):555–9. doi:.https://doi.org/10.1016/j.cmi.2017.02.020
14
Walker
CK
,
Sweet
RL
. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health. 2011;3:197–206. doi:.https://doi.org/10.2147/IJWH.S13427
15Lociciro S, Ernst M-L, Simonson T, Bize R. Les comportements face au VIH et autres IST des travailleuses et travailleurs du sexe en Suisse. Enquête SWAN 2016. Lausanne: Institut universitaire de médecine sociale et preventive (IUMSP); 2017. P 1–114.
16
Chow
EP
,
Fairley
CK
. The role of saliva in gonorrhoea and chlamydia transmission to extragenital sites among men who have sex with men: new insights into transmission. J Int AIDS Soc. 2019;22(S6, Suppl 6):e25354. doi:.https://doi.org/10.1002/jia2.25354
17
Chemaitelly
H
,
Weiss
HA
,
Smolak
A
,
Majed
E
,
Abu-Raddad
LJ
. Epidemiology of Treponema pallidum, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and herpes simplex virus type 2 among female sex workers in the Middle East and North Africa: systematic review and meta-analytics. J Glob Health. 2019;9(2):020408. doi:.https://doi.org/10.7189/jogh.09.020408
18Jansen K, Bremer V, Steffen G, et al. Abstracts of the German STI Congress and Leopoldina Symposium 2016, 06.-09.07.2016, Berlin. J Dtsch Dermatol Ges. 2016;14(Suppl 3):2–55. doi:https://doi.org/10.1111/ddg.13106
19
Viikki
M
,
Pukkala
E
,
Nieminen
P
,
Hakama
M
. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000;39(1):71–5. doi:.https://doi.org/10.1080/028418600431003
20
Chandra
NL
,
Broad
C
,
Folkard
K
,
Town
K
,
Harding-Esch
EM
,
Woodhall
SC
, et al.
Detection of Chlamydia trachomatis in rectal specimens in women and its association with anal intercourse: a systematic review and meta-analysis. Sex Transm Infect. 2018;94(5):320–6. doi:.https://doi.org/10.1136/sextrans-2017-053161
21
Hoenderboom
BM
,
van Benthem
BHB
,
van Bergen
JEAM
,
Dukers-Muijrers
NHTM
,
Götz
HM
,
Hoebe
CJPA
, et al.
Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for chlamydia in a chlamydia screening trial. Sex Transm Infect. 2019;95(4):300–6. doi:.https://doi.org/10.1136/sextrans-2018-053778
22
Brunham
RC
,
Pourbohloul
B
,
Mak
S
,
White
R
,
Rekart
ML
. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192(10):1836–44. doi:.https://doi.org/10.1086/497341
23
Batteiger
BE
,
Xu
F
,
Johnson
RE
,
Rekart
ML
. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. J Infect Dis. 2010;201(Suppl 2):S178–89. doi:.https://doi.org/10.1086/652400
24
Brunham
RC
,
Kimani
J
,
Bwayo
J
,
Maitha
G
,
Maclean
I
,
Yang
C
, et al.
The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 1996;173(4):950–6. doi:.https://doi.org/10.1093/infdis/173.4.950
25
Gama
A
,
Martins
MRO
,
Mendão
L
,
Barros
H
,
Dias
S
. HIV Infection, risk factors and health services use among male-to-female transgender sex workers: a cross-sectional study in Portugal. AIDS Care. 2018;30(1):1–8. doi:.https://doi.org/10.1080/09540121.2017.1332736
26
Verscheijden
MMA
,
Woestenberg
PJ
,
Götz
HM
,
van Veen
MG
,
Koedijk
FDH
,
van Benthem
BHB
. Sexually transmitted infections among female sex workers tested at STI clinics in the Netherlands, 2006-2013. Emerg Themes Epidemiol. 2015;12(1):12. doi:.https://doi.org/10.1186/s12982-015-0034-7
27
Reeves
A
,
Steele
S
,
Stuckler
D
,
McKee
M
,
Amato-Gauci
A
,
Semenza
JC
. National sex work policy and HIV prevalence among sex workers: an ecological regression analysis of 27 European countries. Lancet HIV. 2017;4(3):e134–40. doi:.https://doi.org/10.1016/S2352-3018(16)30217-X
28
Tokar
A
,
Sazonova
I
,
Mishra
S
,
Smyrnov
P
,
Saliuk
T
,
Lazarus
JV
, et al.
HIV testing behaviour and HIV prevalence among female sex workers in Ukraine: findings from an Integrated Bio-Behavioural Survey, 2013-2014. Sex Transm Infect. 2019;95(3):193–200. doi:.https://doi.org/10.1136/sextrans-2018-053684
29
von Wyl
V
,
Kouyos
RD
,
Yerly
S
,
Böni
J
,
Shah
C
,
Bürgisser
P
, et al.; Swiss HIV Cohort Study. The role of migration and domestic transmission in the spread of HIV-1 non-B subtypes in Switzerland. J Infect Dis. 2011;204(7):1095–103. doi:.https://doi.org/10.1093/infdis/jir491
30
Terrault
NA
,
Dodge
JL
,
Murphy
EL
,
Tavis
JE
,
Kiss
A
,
Levin
TR
, et al.
Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57(3):881–9. doi:.https://doi.org/10.1002/hep.26164
31
Richard
JL
,
Schaetti
C
,
Basler
S
,
Mäusezahl
M
. The epidemiology of hepatitis C in Switzerland: trends in notifications, 1988-2015. Swiss Med Wkly. 2018;148:w14619. doi:.https://doi.org/10.4414/smw.2018.14619
32Federal Office of Public Healh FOPH, Swiss Federal Vaccination Commission. [Swiss vaccination plan 2014. Guidelines and recommendations]. Bern: Swiss Federal Office of Public Healh, 2014. Article in German; available from: https://kssg.guidelines.ch/api/filestore/2nBFP8jzYazi0cK6CAyJvw1tf1OpuUonjYKt8A/data/impfplan_2014_de_final-2.pdf [accessed 2020 April].
33Federal Office of Public Healh FOPH. [HPV: complementary vaccination recommendation for boys and men aged 11 to 26 years]. Bulletin. 2015;10:141–9. Article in French and German; available from: www.bag.admin.ch/dam/bag/fr/dokumente/cc/Kampagnen/Bulletin/2015/BU_10_15_f.pdf; [accessed 2020 April].
34
Chow
EP
,
Howden
BP
,
Walker
S
,
Lee
D
,
Bradshaw
CS
,
Chen
MY
, et al.
Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect. 2017;93(2):88–93. doi:.https://doi.org/10.1136/sextrans-2016-052753
35Wetzel D, Delicado N, Wehrli M, et al. [Establishment of a VCT HIV / STI consultation for people practicing prostitution in Geneva. Activity report of the pilot phase March 2017–March 2019]. Geneva: Aspasie, GsG, PSM (HUG); 2019. Report in French.
