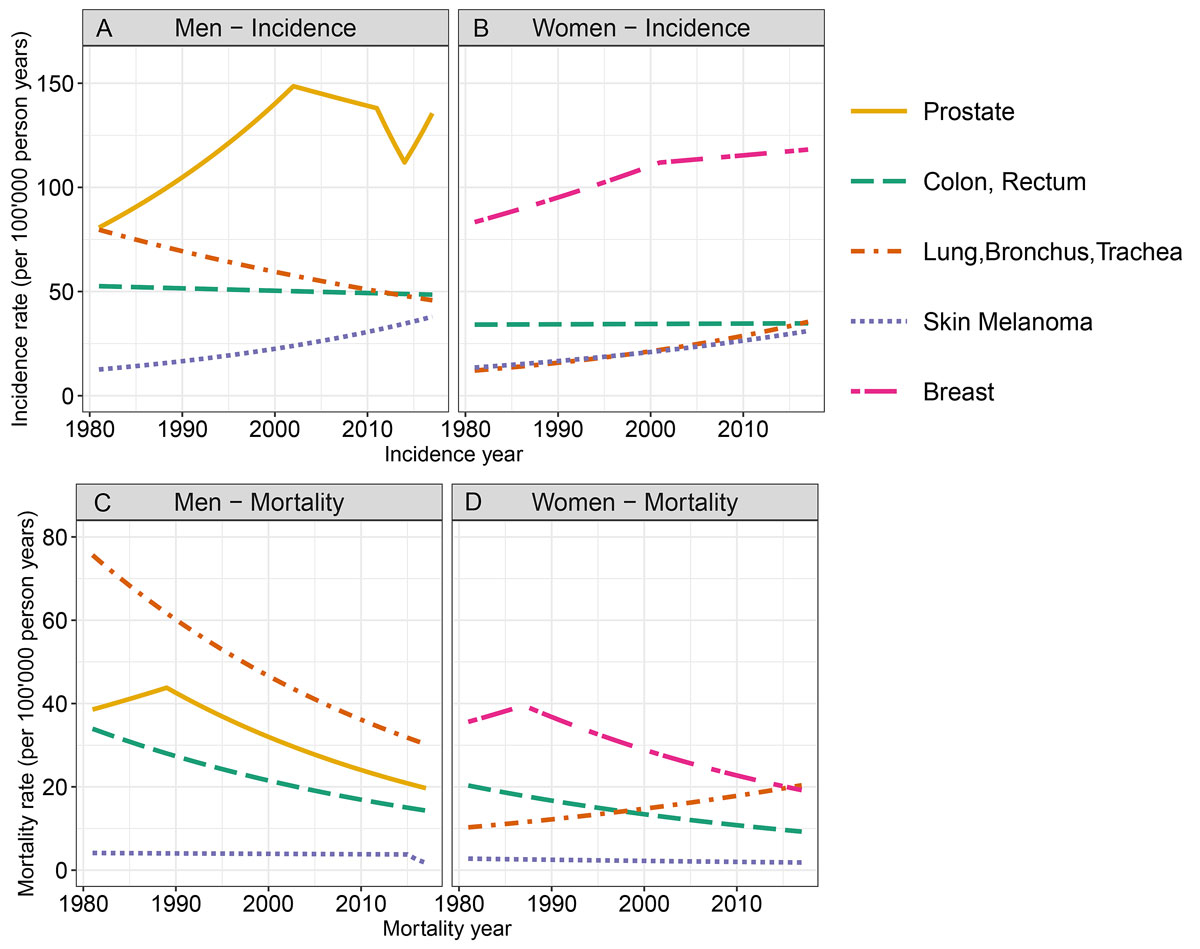
Figure 1 Age-standardised incidence and mortality rates for cancers of the prostate, breast, colon/rectum, and lung, as well as for melanoma from 1981 to 2017, by sex, in the canton of Zurich.
DOI: https://doi.org/10.4414/smw.2020.20388
As in many European countries, the most common types of cancer in Switzerland are breast (women) and prostate (men), lung and colon/rectum cancer [1]. In addition, melanoma ranks among the five most common types of cancer. These national trends are also observed in the canton of Zurich, which is the largest Swiss canton with a population of about 1.5 million in 2017 [2].
In 2017, more than 1,000 new cases of prostate and breast cancer were diagnosed in the canton of Zurich. Furthermore, we registered around 720 new colorectal cancer cases, 790 new lung cancer cases, and 620 new melanomas. Due to an increase in the population of the canton of Zurich of more than 30% between 1980 and 2017, the absolute numbers of new cancer cases increased. Therefore, age-standardised incidence and mortality rates per 100,000 are the most appropriate measures to study these changes, since they take into account the size of the population as well as its age structure. Nevertheless, changes in the absolute numbers are of interest, especially regarding health system costs.
Cancer registration in the canton of Zurich dates back to 1980. This provides the opportunity to look at time trends for more than 35 years regarding incidence and mortality of common types of cancer. Time trends are of interest for several reasons: they reflect changes in screening behaviour, effects of prevention efforts and health care planning, adaptions in treatment guidelines and treatment techniques, changes in the population distribution, etc. Furthermore, time trends may have an impact on future health care and prevention planning, and may affect the elaboration of guidelines regarding treatment and screening.
The availability of variables such as sex, age, and stage at tumour diagnosis further allows for stratifying analyses and investigating potentially diverging trends in subgroups. For example, screening efforts may lead to a specific increase in localised tumours [3]. Moreover, changes in stage distribution at diagnosis, as well as improvement in treatments, may affect mortality rates [3].
The aim of the present study was to present time trends in incidence and mortality rates for the most common types of cancer from 1981 to 2017 in the canton of Zurich, Switzerland. For more in-depth insights, trends were stratified by sex and age group and, for incidence rates, additionally by stage.
Cancer registration in Switzerland is mandatory from 2020 onwards, based on a national law. However, mainly based on cantonal legislation, several cantons have started to register cancer data much earlier, with the oldest registry dating back to the 1970s. The population-based cancer registry of the cantons of Zurich, Zug, Schaffhausen and Schwyz started registering data in 1980 for Zurich, in 2011 for Zug, while Schaffhausen and Schwyz joined the registry in 2020. The patient’s main place of residence at the time of diagnosis is the criterion for inclusion in the respective cantonal cancer registry. For the present analysis, we only included data from the canton of Zurich in order to investigate trends over more than 30 years. Compared to the whole country, the canton of Zurich is an urban canton with slightly more individuals between 20 and 39 years, and fewer above 60 years. Furthermore, the proportion of non-Swiss individuals is slightly higher in Zurich than in Switzerland.
We included malignant tumours of the breast (C50 according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD10)), prostate (C61), colon/rectum (C18–C21), and lung (C33–C34), as well as melanoma (C43), diagnosed in the canton of Zurich between 1981 and 2017. The reason for inclusion from 1981 instead of 1980 is that population files are only available from 1981 onwards. For stage-stratified analyses, we used only the period 2003–2017 because the recording of cancer stage was not systematic prior to 2003.
The incidence dataset includes data on the date of diagnosis, the ICD10 code for tumour localisation, age at diagnosis, as well as clinical and pathological TNM (tumour – node – metastasis) to define stage.
In general, the quality of the Zurich cancer registry data is good [4]. The percentage of death certificate only cases (DCO) was 2.6% for the period 1997–2014, and the percentage of morphologically verified cases (MV) was 93.3%. The percentage of DCO was highest for lung cancer (3.4%) and lowest for melanoma (0.2%). The percentage of MV was lowest for lung cancer (90.4%) and highest for melanoma (99.6%).
The mortality data were provided by the Swiss Federal Statistical Office and are based on the national statistics for cause of death. We used data from 1981 to 2017. The main cause of death was coded according to the 8th revision of the ICD (ICD8) up to 1994, and according to ICD10 from 1995 onwards. In our mortality analyses, we used the same cancer codes as for incidence.
Age-standardised incidence and mortality rates per 100,000 person-years were computed using the 1976 European Standard Population [5] (which has often been used in other studies and thus allows for comparison with other studies) and mid-year population estimates.
For the age-stratified analyses, the following age groups were used for prostate, lung, and colon/rectum cancer: <60 years, 60–69 years, 70–79 years, ≥80 years. For breast cancer and melanoma, an additional (younger) age group was included (<50 years, 50–59 years, 60-69 years, 70–79 years, ≥80 years), because these cancers frequently occur at younger ages. For the stage-stratified analyses, we used the stages I, II, III, IV and a ‘missing stage’. Based on pathological and/or clinical TNM, stage was defined separately for each localisation according to the TNM classification of malignant tumours version 6 (for incidence year up to 2009) and version 7 (from incidence year 2010 onwards) [6, 7]. Pathological TNM (pTNM) was used when available; otherwise, clinical TNM (cTNM) was used. Missing cM was assumed to be zero, while missing N and T were set to ‘missing’ if both clinical and pathological N/T were missing. For breast cancer and melanoma, missing pN/cN were set to zero if cT/pT=1. For example, melanomas with a thickness of 1 mm or thinner (T1) are typically localised and considered non-metastatic, hence why patients with these lesions are often not offered sentinel node operation and further examination [8].
Stata/SE Version 15 was used to prepare the data for further processing in the JoinPoint Trend Analysis Software. Incidence and mortality time trends were assessed using joinpoint regression analysis (JoinPoint Trend Analysis Software Version 4.6.0.0, April 2018; US National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program). This method is used to determine the number of joinpoints that are adequate for assessing significant changes in trends over time. The analysis starts with 0 joinpoints (corresponding to a straight regression line), and tests whether one or more joinpoints are significant, using the permutation test. The Grid Search Method and the Monte Carlo permutation method, with 4499 replicates and a significance level of 0.05, were used [9]. We defined the maximum number of joinpoints as five for general and age-stratified analyses (1981–2017), and as two for stage-stratified analyses (2003-2017), based on the recommendations in the Joinpoint Manual. Furthermore, we defined the minimum number of observations from a joinpoint to either end of the data as two, and the minimum number of observations between two joinpoints as two (including any joinpoint that falls on an observation). The dependent variables were the age-standardised incidence and mortality rates; the independent variable was calendar year. Age group/stage were defined as by-variables. The relevant results from these analyses are the annual percentage changes (APC) for each identified trend between two joinpoints, as well as the average annual percentage change (AAPC), a summary measure over the whole period of observations (1981–2017 for overall and age-stratified incidence and mortality; 2003-2017 for stage-stratified incidence). Log-transformation of the dependent variable (age-standardised incidence and mortality) was used because of non-normality of the data. The AAPC are presented with 95% confidence intervals (95% CI). R Version 3.6.1 was used to create the graphs.
Table 1 displays the number of cancer cases by localisation, sex, and age group for incidence and mortality.
Table 1 Number of cancer cases by tumour localisation, sex and age group, 1981–2017, canton of Zurich, Switzerland.
| Localisation | ICD10 | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | <50 | 50-59/<60 | 60-69 | 70-79 | ≥80 | Total | <50 | 50-59/<60 | 60-69 | 70-79 | ≥80 | ||
| Incidence | |||||||||||||
| Breast | C50 | 31,349 | 6744 | 6,598 | 7348 | 6259 | 4400 | ||||||
| Prostate | C61 | 31,673 | 3403 | 10,333 | 11,243 | 6694 | |||||||
| Colon/rectum | C18-C21 | 13,080 | 2758 | 3451 | 4115 | 2756 | 11,963 | 2,391 | 2544 | 3563 | 3465 | ||
| Lung | C33-34 | 15,361 | 3414 | 4840 | 4820 | 2287 | 7020 | 1,787 | 2005 | 2019 | 1209 | ||
| Melanoma | C43 | 6187 | 1403 | 1026 | 1418 | 1470 | 870 | 6247 | 2156 | 1,079 | 1102 | 1048 | 862 |
| Mortality | |||||||||||||
| Breast | C50 | 9671 | 879 | 1467 | 1998 | 2374 | 2953 | ||||||
| Prostate | C61 | 8356 | 177 | 905 | 2679 | 4595 | |||||||
| Colon/rectum | C18-C21 | 5656 | 701 | 1112 | 1876 | 1967 | 5404 | 569 | 771 | 1523 | 2541 | ||
| Lung | C33-34 | 12,048 | 2119 | 3455 | 4081 | 2393 | 4911 | 971 | 1311 | 1527 | 1102 | ||
| Melanoma | C43 | 970 | 116 | 142 | 222 | 255 | 235 | 738 | 118 | 92 | 134 | 173 | 221 |
Figures 1A and 1B present the sex-stratified incidence trends for cancers of the prostate, breast, colon/rectum, and lung, as well as for melanoma, from 1981 to 2017 in the canton of Zurich, Switzerland. The AAPC are displayed in supplementary table S1 (appendix 1). In men, the incidence trend linearly decreased for colon/rectum and lung cancer with statistically significant AAPC of −0.2 (95% CI −0.4 to −0.0) and −1.5 (95% CI −1.7 to −1.4), respectively. For prostate cancer, a joinpoint was detected in 2002 marking a change in the incidence trend from increasing to decreasing (APC 1981–2002: 2.9 (95% CI 2.6 to 3.3) vs APC 2002–2011: –0.8 (95% CI –2.0 to 0.4)). A joinpoint in 2011 indicated an even steeper decrease until 2014 (APC 2011–2014: –6.7 (95% CI –16.3 to 4.0), and a joinpoint in 2014 indicated a change towards an increasing trend (APC 2014–2017: 6.6 (95% CI 1.1 to 12.3)). In men, no joinpoints were detected for the other localisations.

Figure 1 Age-standardised incidence and mortality rates for cancers of the prostate, breast, colon/rectum, and lung, as well as for melanoma from 1981 to 2017, by sex, in the canton of Zurich.
In women, the incidence trends were significantly increasing for all localisations except for colon/rectum, with AAPC of 3.0 (95% CI 2.7 to 3.4) for lung cancer and 2.3 (95% CI 1.9 to 2.7) for melanoma. For breast cancer, we detected a joinpoint in 2001 marking a change in the incidence trend from ‘significantly increasing’ (APC 1981–2001: 1.5 (95% CI 1.1 to 1.9)) to ‘stable’ (APC 2001–2017: 0.3 (95% CI –0.2 to 0.8)). For colon/rectum cancer, the AAPC over the whole study period was 0.1 (95% CI –0.2 to 0.3).
Figures 1C and 1D present the sex-stratified mortality trends for the respective cancer localisations from 1981 to 2017 in the canton of Zurich, Switzerland. The AAPCs are displayed in table S1. In men, cancer mortality decreased significantly and linearly for colon/rectum and lung cancer, with AAPC of −2.4 (95% CI −2.6 to −2.1) and −2.5 (95% CI −2.7 to −2.3), respectively. A joinpoint was detected for prostate cancer in 1989 with a non-significantly increasing trend from 1981 to 1989 (APC: 1.6 (95% CI −0.7 to 4.0)) and a significantly decreasing trend from 1989 to 2017 (APC: −2.8 (95% CI −3.1 to −2.5)). A non-significantly decreasing trend was observed for melanoma throughout the study period (AAPC: −2.4 (95% CI −5.4 to 0.8)).
In women, cancer mortality decreased linearly between 1981 and 2017 for colon/rectum cancer and for melanoma, with statistically significant AAPC of −2.2 (95% CI −2.5 to −1.8) and −1.1 (95% CI −1.8 to −0.4), respectively. A joinpoint was detected for breast cancer in 1987, marking the change from a non-significantly increasing trend from 1981 to 1987 (APC: 1.8 (95% CI −1.3 to 4.9)) to a significantly decreasing trend from 1987 to 2017 (APC: -2.4 (95% CI −2.7 to −2.1)). For lung cancer, mortality increased significantly throughout the study period with AAPC of 1.9 (95% CI 1.5 to 2.3).
Figure 2 shows the sex-stratified incidence trends by age group. The AAPCs are displayed in table S2.
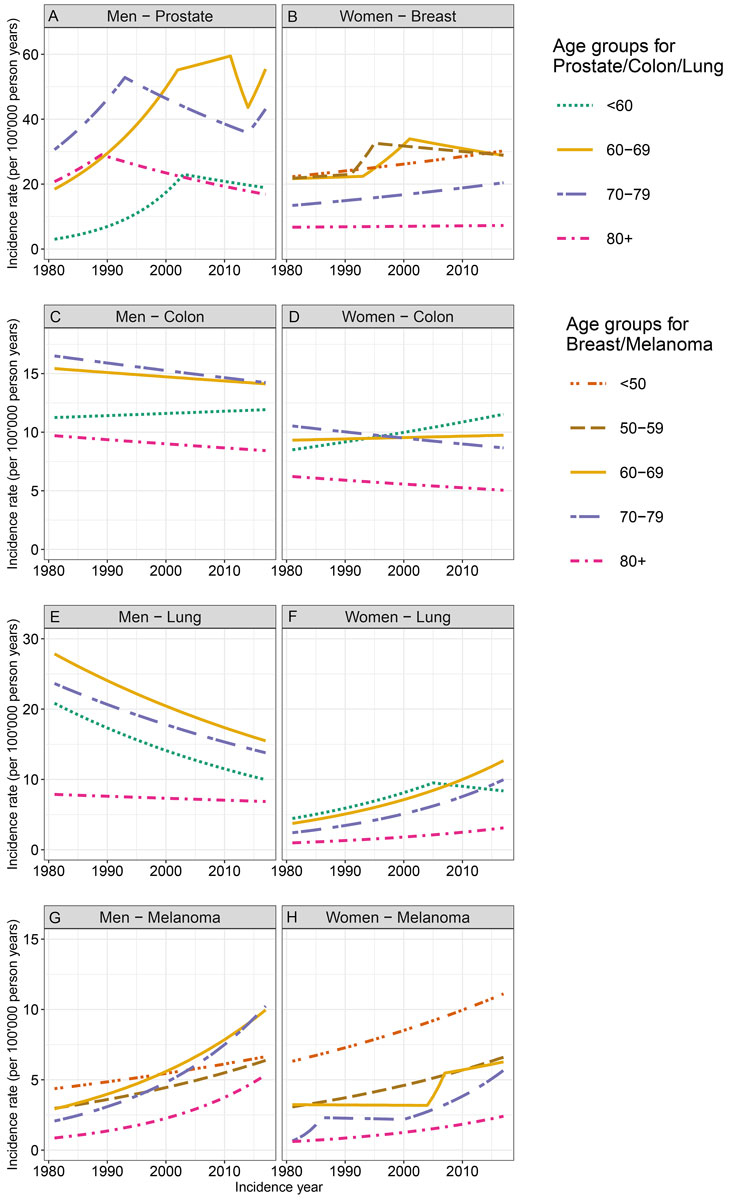
Figure 2 Age-standardised incidence rates by age group for cancers of the prostate, breast, colon/rectum, and lung, as well as for melanoma from 1981 to 2017, by sex, in the canton of Zurich.
In 1981, the age-standardised incidence of prostate cancer was highest in the 70-79 age group, while in 2017 it was highest in the 60-69 age group (fig. 2A). Prostate cancer incidence was overall significantly increasing for the younger age groups. In all age groups, we observed at least one joinpoint distinguishing between an increasing trend in earlier years and a decreasing trend in more recent years. However, in the older age groups, the joinpoint was observed earlier (around 1990), while for the younger age groups, the joinpoint was detected later (in 2002 and 2011, respectively), indicating that the decreasing incidence trend started earlier in older age groups. In the two middle age groups, the incidence trend started to increase again in 2014.
For colon/rectum cancer in men, a decreasing trend was observed in the two oldest age groups throughout the study period (fig. 2C). In the youngest age group, we observed a non-significantly increasing trend. The incidence rate was highest in the 70–79 age group in both 1981 and 2017. No joinpoints were detected, indicating linear trends. For lung cancer in men, the incidence trends were similar in all age groups, with highest incidence rates over the whole period for the 60–69 age group (fig. 2E). The incidence rates significantly decreased in all age groups over the study period except for those aged ≥80 years. An increasing trend for melanoma in men was observed in all age groups with AAPC ranging between 1.2 and 5.2; larger (positive) AAPC were observed for older age groups (fig. 2G). While in 1981 the incidence rate was highest in those under the age of 50, the highest rates in 2017 were observed for men aged 70–79 years.
Breast cancer incidence in women was highest in the three age groups up to 69 years both in 1981 and in 2017 (fig. 2B). The incidence trend was increasing in all age groups, although it was only significant in women in the <50 and 70–79 age groups. Joinpoints in the age groups between 50 and 69 years indicated the steepest increase in the 1990s. The incidence rate of colon/rectum cancer in women was highest for the 70–79 age group in 1981, but highest for the age group <60 years in 2017 (fig. 2D). An increasing trend was observed in the youngest age group, a stable trend in 60–69-year-old women, and decreasing trends in the older age groups. Lung cancer incidence increased significantly in women in all age groups with AAPC ranging between 1.8 and 4.0; it was highest in those younger than 60 years in 1981 and in those aged 60 to 69 years in 2017 (fig. 2F). An increasing trend for melanoma in women was observed in all age groups (although not significant for those aged 60-69 years) with AAPC ranging between 1.6 and 6.2; larger (positive) AAPC were observed for older age groups (fig. 2H). The incidence rate was highest in the age group <50 years throughout the study period.
Figure 3 shows the sex-stratified incidence trends by stage for the different localisations between 2003 and 2017. The AAPC are displayed in table S3. The incidence trend for ‘missing stage’ information decreased for all localisations and both sexes.
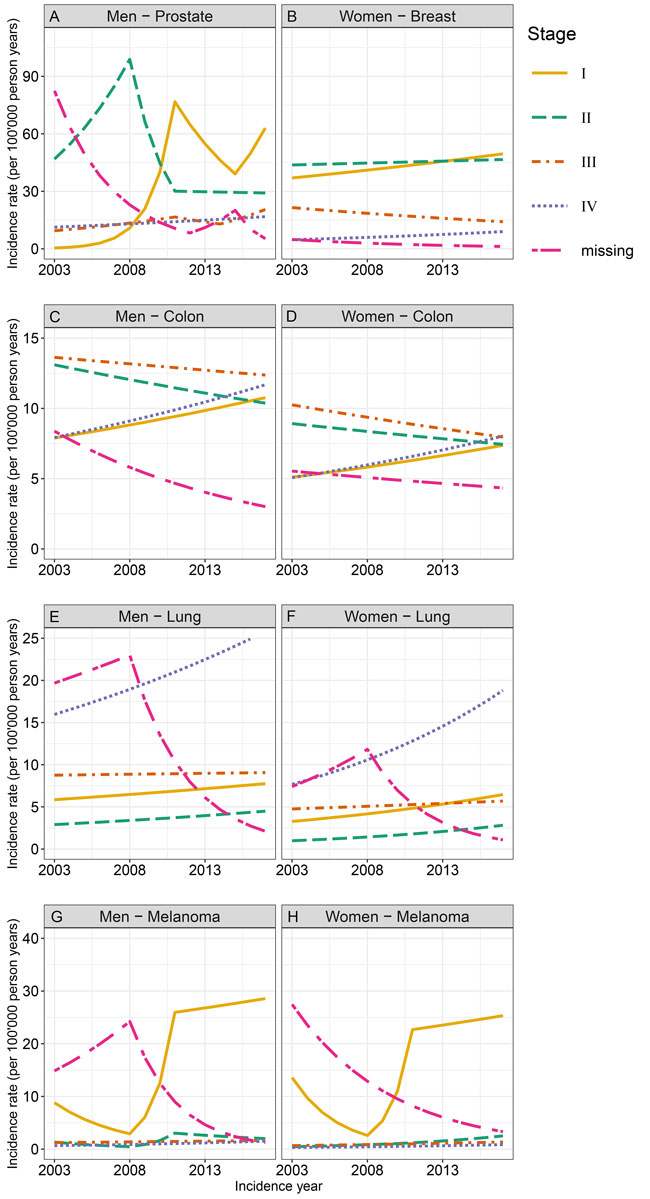
Figure 3 Age-standardised incidence rates by stage for cancers of the prostate, breast, colon/rectum, and lung, as well as for melanoma from 2003 to 2017, by sex, in the canton of Zurich.
For prostate cancer, the incidence was highest for stage II tumours up to about 2010, and for stage I tumours thereafter (fig. 3A). Overall, the trends increased for stage I, III and IV tumours and remained stable for stage II tumours of the prostate. Several joinpoints indicated changes in the trends by stage. Colon/rectum cancer in men significantly increased for stage I and IV tumours, and significantly decreased for stage II tumours; the trend for stage III tumours remained stable (fig. 3C). For lung cancer in men, an increasing trend was observed for stage I, II and IV tumours, although only significant for stage IV tumours (fig. 3E). A stable trend was observed for stage III tumours. The incidence rate was highest for stage IV tumours over the whole study period. No joinpoints were detected for any of the stages of lung cancer. The incidence of melanoma in men increased for all stages, although it was only significant for stage IV tumours (fig. 3G). The incidence rate was highest for stage I tumours over the whole study period.
In women, breast cancer incidence increased significantly for stage I and stage IV tumours between 2003 and 2017, while it decreased significantly for stage III tumours, and remained stable for stage II tumours (fig. 3B). Over the whole study period, the incidence of stage I and II tumours was highest. The incidence of colon/rectum cancer in women increased significantly for stage I and IV tumours, decreased significantly for stage III tumours, and remained stable for stage II tumours (fig. 3D). Overall, the incidence rate was highest for stage III tumours in 2003 and 2017, but similarly high for stage IV tumours in 2017. For lung cancer in women, statistically significantly increasing trends were observed for all stages, except for stage III tumours (fig. 3F). Like in men, the highest incidence was observed for stage IV tumours over the whole study period. For melanoma in women, an increasing trend with AAPC between 4.6 and 12.9 was observed for all stages, but it was only significant for stage II tumours (fig. 3H). Like in men, the incidence rate was highest for stage I tumours over the whole study period. For both men and women, the incidence trend in stage I tumours showed an interesting shape: a non-significant decrease between 2003 and 2008, extreme increases (AAPC >100) between 2008 and 2011, and a levelling-off afterwards.
Figure 4 shows the sex-stratified mortality trends by age group and cancer localisation between 1981 and 2017. The AAPC are displayed in table S2.
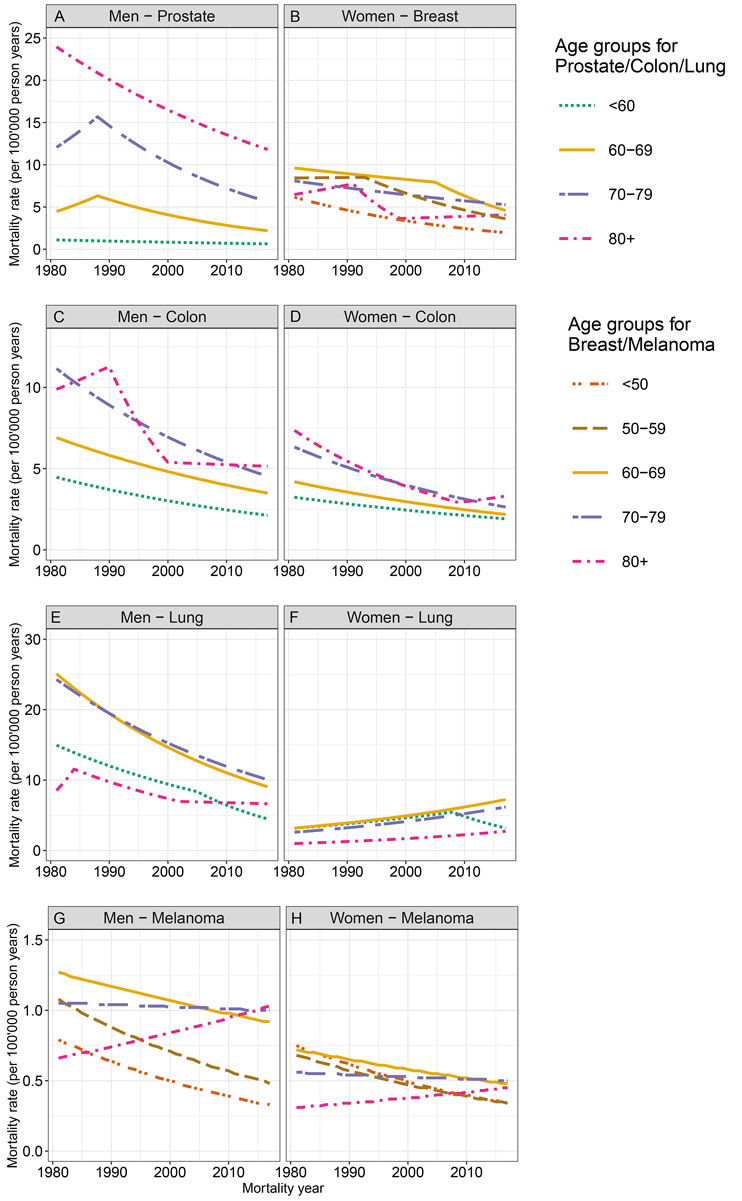
Figure 4 Age-standardised mortality rates by age group for cancers of the prostate, breast, colon/rectum, and lung, as well as for melanoma from 1981 to 2017, by sex, in the canton of Zurich.
Mortality associated with prostate, colon/rectum, and lung cancer decreased significantly for men in all age groups, except for lung cancer in men aged ≥80 years. For prostate cancer, the highest mortality rates were observed in the oldest age group (≥80 years), and the lowest rates in the youngest age group (<60 years) over the whole study period (fig. 4A). For prostate cancer, we observed joinpoints in 1988 for the two middle age groups (60–79 years), indicating non-significant increases in mortality before 1988 and significant decreases afterwards. For colon/rectum cancer mortality, the highest mortality rates were observed in the age groups above 70 years of age over the whole study period (fig. 4C). Here, joinpoints were only observed in the oldest age group (≥80 years), with a non-significantly increasing trend from 1981 to 1990, a significantly decreasing trend from 1990 to 2000, and a stable trend afterwards. Regarding lung cancer, the highest mortality rates were observed in the age groups 60 to 79 years throughout the study period, and joinpoints were detected in the youngest and oldest age group (fig. 4E). For melanoma mortality, the rates were significantly decreasing for men under the age of 60 years, while no significant trends were observed in older men (fig. 4G). The mortality rate was highest in those aged 60–69 years in 1981, and in those aged ≥80 years in 2017.
Except in the most recent years, breast cancer mortality was highest for women aged 60–69 (fig. 4B). In all age groups, the mortality rates decreased; however, the patterns were different. For example, in the age group 50-59, a joinpoint indicated a steeper and significant decrease after 1993, while in women aged 60–69 years, the decrease was steeper after 2005. For women aged ≥80 years, breast cancer mortality only decreased significantly between 1991 and 1999, with no significant trends before and after. Colon/rectum cancer mortality in women decreased significantly in all age groups (fig. 4D). Over the observation period, the mortality rates of colon/rectum cancer were higher in older age groups, with lowest rates observed for women under the age of 60. Only one joinpoint was observed for colon/rectum cancer in the oldest age group, indicating a decreasing trend only up to 2009. Lung cancer mortality in women was highest in women aged 60–69 years over the whole period (fig. 4F). It increased significantly in all age groups, except the youngest where it remained stable. In this age group, a joinpoint indicated a significantly increasing trend between 1981 and 2008, and a significantly decreasing trend thereafter. While the overall mortality rate for melanoma decreased in women between 1981 and 2017, the age-stratified decrease was only significant in the two youngest age groups (fig. 4H). In women aged ≥80 years, the melanoma mortality rate increased, although not significantly, throughout the study period.
Over the whole study period (1981–2017), the age-standardised incidence rates increased for prostate cancer, breast cancer, and melanoma in both sexes, while lung cancer incidence rates increased only in women. A decreasing rate was observed for lung cancer in men – potentially reflecting the decreasing smoking prevalence in men in Switzerland [10] – as well as for colon/rectum cancer. The diverging trends in lung cancer incidence for men and women are at least partly explained by the later onset of smoking in women compared to men [11]. Regarding cancer mortality, the rates were significantly decreasing for all localisations and both sexes, except for melanoma in men (stable trend) and lung cancer in women (increasing trend).
Looking at age- and stage-stratified incidence trends for prostate cancer, we observed joinpoints at different times depending on the age group. While for men older than 70 years of age, the turning point from an increasing to a decreasing rate was around 1990, it was later for younger men (after 2000). A steep increase in stage I tumours up to 2011 may be associated with increased PSA screening behaviour in the early 2000s [12]. Furthermore, the coding based on TNM classification of malignant tumours changed in 2010 from version 6 [6] to version 7 [7], with the result that T2a tumours were newly classified as stage I instead of stage II tumours, which may explain the decrease in stage II tumours and the simultaneous increase in stage I tumours.
Regarding breast cancer incidence, there was a steep increase for women aged 50-69 years in the 1990s. Even though there is no organised breast cancer screening program in the canton of Zurich, increasing incidence in these age groups may be an effect of the guidelines for mammography screening for women aged ≥50 years [13, 14]. This is also in line with the increase in stage I tumours between 2003 and 2017.
Colon/rectum cancer incidence decreased in men in general; however, when age-stratified, this decreasing trend was only significant in men aged ≥70 years over the whole study period. In women, the colon/rectum incidence trend was stable overall; however, it was increasing in women aged <60 years but decreasing in women aged ≥70 years. An increasing trend in younger adults has been reported in other high-income countries [15]. Possible explanations are the changing prevalence and distribution of risk factors for colorectal cancer in recent generations, such as excess body weight, consumption of red and processed meat, alcohol consumption, smoking, and decrease in physical activity, among others [15]. The decreasing incidence of colon cancer in older individuals could be due to interventions leading to the removal of adenomatous polyps [15]. In both men and women, incidence of stage I and IV tumours of the colon/rectum was significantly increasing. The increase of stage I tumors may be associated with an increased use of colonoscopy in Switzerland in individuals aged 55 years and older [16].
The increase in melanoma incidence observed in Zurich, as in many other European countries [17], is probably the result of an increased exposure to ultraviolet (UV) radiation, especially during childhood, which, due to the lag time, causes rising incidence rates in older adults [17]. The smaller increase observed in younger adults may be a result of the increasing awareness of the risk factors for skin cancer in the younger age group [17].
For prostate cancer, an overall increasing incidence and decreasing mortality rate was reported for several European countries [18, 19], including Nordic countries [20]. We observed an increase in incidence up to 2002, and a decrease thereafter (with some indication of a new increasing trend after 2014), while for mortality we observed a significant decrease since 1989. An increase in incidence is supported by data from the French-speaking part of Switzerland for the period from 1974 until at least 1994 (canton of Vaud) [21], and from 1973 until about 2000 (canton of Geneva) [22]. Increasing incidence was also observed in the Italian-speaking part of Switzerland from 1996 until 2007 [12], even though the rates in these regions were considerably lower. The latter study was a comparison of prostate cancer incidence between Zurich and Ticino for the period 1996 to 2013 [12]. A strong increase in incidence from the 1970s or 1980s, up to around 2000-2005 and levelling off afterwards, was reported in other European countries [23, 24]. Increasing trends in prostate cancer are likely associated with the introduction of measuring blood-circulating levels of prostate-specific antigen (PSA) as a screening instrument in the 1990s. Even though some national and international organisations such as the US Preventive Services Task Force and the Swiss Medical Board advise against PSA testing for prostate cancer, this screening method is quite frequent in Switzerland and increased between 1992 and 2012 [25].
Regarding breast cancer, both incidence and mortality trends observed in Zurich are similar to trends reported from northern European countries [19, 20]. Decreasing trends in breast cancer mortality from around 1990 have been reported in the EU in general, and specifically in Germany, Italy, France, Spain, the UK [26], and in northern European countries [19]. A study comparing breast cancer mortality in the French-speaking part of Switzerland (with organised breast-screening since 1993 in the canton of Vaud) with two German-speaking cantons without organised breast screening, reported a decrease of about 30% between 1990 and 2000 in the French-speaking part compared to no decrease in the German-speaking part [27]. Similar to our study, breast cancer incidence in younger women increased in other European countries such as Spain, France, Italy, and Portugal [28], but also in Switzerland [29] and in Europe overall [30], potentially reflecting an increase in mammography use. Data from the Swiss Health Survey indicate that 44% of women aged 40 to 49 years already had a mammogram [31].
The relatively stable incidence rates for colon cancer in Zurich are comparable to the results from several cantons in Switzerland [16], as well as in other European countries (e.g., Ireland and Denmark), but also Canada [32]. Decreasing mortality rates are also widely observed in other northern and western European countries [19, 32, 33], and in the USA [34]. An increase in stage I and stage IV tumours was also reported for other regions in Switzerland [16].
A very similar picture to our study – with decreasing trends in both incidence and mortality – was reported for lung cancer in men all over the world, including Europe [19, 35, 36]. In women, an increase in incidence was observed in several countries, especially in western, southern and eastern Europe, and some northern European countries, and also in earlier publications of Zurich [10] and several other Swiss cantons [37] looking at histological subtypes. Similar to our study, an increasing mortality rate was observed in western, southern, eastern, and some northern European countries [35, 36]. Because of different smoking behaviour in women compared to men (later onset of smoking epidemic in women, lower smoking prevalence, and lower numbers of cigarettes smoked per day), lung cancer in women occurred later [10, 38].
Similar to our results, the incidence of melanoma increased in most other European countries for both sexes [17, 19, 39, 40], with mostly higher AAPC in the older age groups [17]. Increasing trends have also been reported for the French-speaking part of Switzerland between 1978 and 2002 [41], and in an earlier publication using data from the canton of Zurich that looked at histological subtypes [42]. While mortality rates increased up to 2000 in several European countries [39], we did not observe such an increase.
While for prostate cancer, breast cancer, and melanoma, the age-standardised incidence rates over the whole period were highest for stage I and/or II tumours, the highest incidence rates for colon/rectum and lung cancer were observed for stage III (colon/rectum) and stage IV (lung) tumours, respectively. This is in line with other studies [43-47], and may be due to a lack of symptoms in early-stage cancer for colon/rectum and (especially) lung cancer, but also due to a higher prevalence of mammography and PSA screening compared to lung and colorectal screening. For all localisations, except for melanoma in women, the age-standardised incidence rates increased for stage IV tumours, indicating an increased frequency of higher-staged tumours detected over time. However, this may also be associated with the simultaneous decrease in tumours with ‘missing stage’ information, especially if the missing information was not random (i.e., missing stage information more likely in higher-staged tumours at earlier times).
The strengths of this study include the long observation period, the completeness of the data in the cancer registry [4], and the stratification by age and stage. However, this study had some limitations. The relatively large amount of missing information regarding stage, especially in earlier years, only allowed the stratification by stage from 2003 onward. Moreover, the increase observed in stages I to IV for several tumours is potentially driven by the decrease in missing stage information over time, which was significant for all tumours of interest in this study. In addition, the choice of the joinpoint input parameters (such as the maximum number of joinpoints) influence, to some extent, the model output. Furthermore, due to the heterogeneity regarding cancer registration in Switzerland, we only included data from the canton of Zurich. Finally, parameters that may have influenced the observed trends (such as changes in screening opportunities or new therapies) are not available on the individual level, and thus not included in the joinpoint models, which needs to be taken into consideration when interpreting the results.
This study presents the incidence and mortality trends for the most common types of cancer from 1981 to 2017 in the canton of Zurich, Switzerland. The overall increasing incidence trends for prostate and breast cancer, as well as for melanoma, are in line with data from other Western countries. While lung cancer incidence was decreasing in men, it is still on the rise in women. Despite increasing incidence rates, the mortality rates were decreasing for all localisations except for lung cancer in women, which reflects the changing smoking patterns and increasing lung cancer incidence in women. The opposite direction of the incidence and mortality curves could be attributed to increased awareness, screening, and better/more effective treatment options. Decreasing mortality rates are good news for cancer patients. With more advanced treatments, this decline will hopefully continue. The increasing trends in lung cancer incidence and mortality in women are alarming and highlight the need for public health measures, in order to reduce smoking prevalence and prevent young women from starting smoking.
Table S1: Average Annual Percentage Changes (AAPC) for incidence and mortality trends by tumour localisation and sex, 1981–2017, canton of Zurich, Switzerland.
Table S2: Average Annual Percentage Changes (AAPC) for incidence and mortality trends by tumour localisation, age group, and sex, 1981–2017, canton of Zurich, Switzerland.
Table S3: Average Annual Percentage Changes (AAPC) for incidence trends by tumour localisation, stage, and sex, 2003-2017, canton of Zurich, Switzerland.
The appendix is available as a separate file at https://smw.ch/article/doi/smw.2020.20388.
The authors declare that they have no conflict of interest.
We thank Linda Vinci for her support with the joinpoint analyses.
1Federal Statistical Office (FSO): Swiss Cancer Report 2015 - Current situation and developments. 2015.
2Statistical Office of the Canton of Zurich. Statistisches Jahrbuch des Kantons Zürich 2019 (Statistical Annual Report of the Canton of Zurich 2019). In. Zurich: Statistical Office of the Canton of Zurich; 2019: p10.
3 Verdial FC , Etzioni R , Duggan C , Anderson BO . Demographic changes in breast cancer incidence, stage at diagnosis and age associated with population-based mammographic screening. J Surg Oncol. 2017;115(5):517–22. doi:.https://doi.org/10.1002/jso.24579
4 Wanner M , Matthes KL , Korol D , Dehler S , Rohrmann S . Indicators of Data Quality at the Cancer Registry Zurich and Zug in Switzerland. BioMed Res Int. 2018;2018:7656197. doi:.https://doi.org/10.1155/2018/7656197
5Waterhouse JAH, Muir CS, Correa P, Powell J. Cancer incidence in five continents, Vol. III In: IARC Scientific Publications No 15. Lyon: IARC; 1976: 456.
6Wittekind C, Meyer HJ, Bootz F. TNM Klassifikation maligner Tumoren, 6. Auflage edn. Berlin: Springer; 2002.
7Wittekind C, Meyer HJ. TNM Klassifikation maligner Tumoren, 7. Auflage edn: Wiley-Blackwell; 2010.
8 Frøslev T , Grann AF , Olsen M , Olesen AB , Schmidt H , Friis S , et al. Completeness of TNM cancer staging for melanoma in the Danish Cancer Registry, 2004-2009. Clin Epidemiol. 2012;4(Suppl 2):5–10. doi:.https://doi.org/10.2147/CLEP.S32064
9Joinpoint Help Manual 4.3.1.0 [https://surveillance.cancer.gov/joinpoint/]
10 Oberli LS , Valeri F , Korol D , Rohrmann S , Dehler S . 31 years of lung cancer in the canton of Zurich, Switzerland: incidence trends by sex, histology and laterality. Swiss Med Wkly. 2016;146:w14327. doi:.https://doi.org/10.4414/smw.2016.14327
11Lillard DR. The Evolution of Smoking in Switzerland. In: Social Dynamics in Swiss Society Empirical Studies Based on the Swiss Household Panel. Volume 9, edn. Edited by Robin Tillmann MV, Peter Farago. Lausanne: Springer Open; 2018: 3-16.
12 Wanner M , Richard A , Matthes K , Ortelli L , Lorez M , Korol D , et al. Trends in prostate cancer incidence between 1996 and 2013 in two Swiss regions by age, grade, and T-stage. Cancer Causes Control. 2018;29(2):269–77. doi:.https://doi.org/10.1007/s10552-017-0993-9
13 Cardoso F , Kyriakides S , Ohno S , Penault-Llorca F , Poortmans P , Rubio IT , et al.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674. doi:.https://doi.org/10.1093/annonc/mdz189
14 Albert US , Schreer I . Arbeitsgruppe der Stufe-3-Leitlinie M [S3 guideline breast cancer: update on early detection, and mammography screening]. Radiologe. 2019;59(1):13–8. doi:.https://doi.org/10.1007/s00117-018-0473-6
15 Araghi M , Soerjomataram I , Bardot A , Ferlay J , Cabasag CJ , Morrison DS , et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4(7):511–8. doi:.https://doi.org/10.1016/S2468-1253(19)30147-5
16 Lorez M , Marbet U , Arndt V . Subsite-specific colorectal cancer trends in Switzerland (1989-2012). Swiss Cancer Bulletin. 2016;36(1):67–74.
17 Arnold M , Holterhues C , Hollestein LM , Coebergh JW , Nijsten T , Pukkala E , et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J Eur Acad Dermatol Venereol. 2014;28(9):1170–8. doi:.https://doi.org/10.1111/jdv.12236
18 Wong MC , Goggins WB , Wang HH , Fung FD , Leung C , Wong SY , et al. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur Urol. 2016;70(5):862–74. doi:.https://doi.org/10.1016/j.eururo.2016.05.043
19 Karim-Kos HE , de Vries E , Soerjomataram I , Lemmens V , Siesling S , Coebergh JW . Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44(10):1345–89. doi:.https://doi.org/10.1016/j.ejca.2007.12.015
20 Kvåle R , Myklebust TA , Engholm G , Heinävaara S , Wist E , Møller B . Prostate and breast cancer in four Nordic countries: A comparison of incidence and mortality trends across countries and age groups 1975-2013. Int J Cancer. 2017;141(11):2228–42. doi:.https://doi.org/10.1002/ijc.30924
21 Levi F , La Vecchia C , Randimbison L , Erler G , Te VC , Franceschi S . Incidence, mortality and survival from prostate cancer in Vaud and Neuchâtel, Switzerland, 1974-1994. Ann Oncol. 1998;9(1):31–5. doi:.https://doi.org/10.1023/A:1008209005622
22 Chen C , Naidoo N , Yang Q , Hartman M , Verkooijen HM , Loy EY , et al. A comparative population-based study of prostate cancer incidence and mortality rates in Singapore, Sweden and Geneva, Switzerland from 1973 to 2006. BMC Cancer. 2012;12(1):222. doi:.https://doi.org/10.1186/1471-2407-12-222
23 Zhou CK , Check DP , Lortet-Tieulent J , Laversanne M , Jemal A , Ferlay J , et al. Prostate cancer incidence in 43 populations worldwide: An analysis of time trends overall and by age group. Int J Cancer. 2016;138(6):1388–400. doi:.https://doi.org/10.1002/ijc.29894
24 Bray F , Lortet-Tieulent J , Ferlay J , Forman D , Auvinen A . Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46(17):3040–52. doi:.https://doi.org/10.1016/j.ejca.2010.09.013
25 Guessous I , Cullati S , Fedewa SA , Burton-Jeangros C , Courvoisier DS , Manor O , et al. Prostate cancer screening in Switzerland: 20-year trends and socioeconomic disparities. Prev Med. 2016;82:83–91. doi:.https://doi.org/10.1016/j.ypmed.2015.11.009
26 Carioli G , Malvezzi M , Rodriguez T , Bertuccio P , Negri E , La Vecchia C . Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95. doi:.https://doi.org/10.1016/j.breast.2017.06.003
27 Bulliard JL , La Vecchia C , Levi F . Diverging trends in breast cancer mortality within Switzerland. Ann Oncol. 2006;17(1):57–9. doi:.https://doi.org/10.1093/annonc/mdj035
28 Leclère B , Molinié F , Trétarre B , Stracci F , Daubisse-Marliac L , Colonna M ; GRELL Working Group. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol. 2013;37(5):544–9. doi:.https://doi.org/10.1016/j.canep.2013.05.001
29 Bodmer A , Feller A , Bordoni A , Bouchardy C , Dehler S , Ess S , et al.; NICER Working Group. Breast cancer in younger women in Switzerland 1996-2009: a longitudinal population-based study. Breast. 2015;24(2):112–7. doi:.https://doi.org/10.1016/j.breast.2014.11.004
30 Merlo DF , Ceppi M , Filiberti R , Bocchini V , Znaor A , Gamulin M , et al.; AIRTUM WG. Breast cancer incidence trends in European women aged 20-39 years at diagnosis. Breast Cancer Res Treat. 2012;134(1):363–70. doi:.https://doi.org/10.1007/s10549-012-2031-7
31Federal Statistical Office. Swiss Health Survey 2012. Overview. In. Neuchâtel: Federal Statistical Office FSO; 2013.
32 Arnold M , Sierra MS , Laversanne M , Soerjomataram I , Jemal A , Bray F . Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. doi:.https://doi.org/10.1136/gutjnl-2015-310912
33 Ait Ouakrim D , Pizot C , Boniol M , Malvezzi M , Boniol M , Negri E , et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015;351:h4970. doi:.https://doi.org/10.1136/bmj.h4970
34 Ansa BE , Coughlin SS , Alema-Mensah E , Smith SA . Evaluation of Colorectal Cancer Incidence Trends in the United States (2000-2014). J Clin Med. 2018;7(2):E22. doi:.https://doi.org/10.3390/jcm7020022
35 Wong MCS , Lao XQ , Ho KF , Goggins WB , Tse SLA . Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7(1):14300. doi:.https://doi.org/10.1038/s41598-017-14513-7
36 Zhang Y , Ren JS , Huang HY , Shi JF , Li N , Zhang Y , et al. International trends in lung cancer incidence from 1973 to 2007. Cancer Med. 2018;7(4):1479–89. doi:.https://doi.org/10.1002/cam4.1359
37 Lorez M , Rohrmann S , Heusser R , Arndt V , Group NW . Lung Cancer Trends by Histologic Subtype in Switzerland. Swiss Cancer Bulletin. 2017;37(2):179–85.
38 Alberg AJ , Samet JM . Epidemiology of lung cancer. Chest. 2003;123(1, Suppl):21S–49S. doi:.https://doi.org/10.1378/chest.123.1_suppl.21S
39 de Vries E , Bray FI , Coebergh JW , Parkin DM . Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–26. doi:.https://doi.org/10.1002/ijc.11360
40 Erdmann F , Lortet-Tieulent J , Schüz J , Zeeb H , Greinert R , Breitbart EW , et al. International trends in the incidence of malignant melanoma 1953-2008--are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. doi:.https://doi.org/10.1002/ijc.27616
41 Levi F , Te VC , Randimbison L , La Vecchia C . Trends in incidence of various morphologies of malignant melanoma in Vaud and Neuchatel, Switzerland. Melanoma Res. 2005;15(1):73–5. doi:.https://doi.org/10.1097/00008390-200502000-00012
42 Minini R , Rohrmann S , Braun R , Korol D , Dehler S . Incidence trends and clinical-pathological characteristics of invasive cutaneous melanoma from 1980 to 2010 in the Canton of Zurich, Switzerland. Melanoma Res. 2017;27(2):145–51. doi:.https://doi.org/10.1097/CMR.0000000000000312
43 Bay C , Kejs AM , Storm HH , Engholm G . Incidence and survival in patients with cutaneous melanoma by morphology, anatomical site and TNM stage: a Danish Population-based Register Study 1989-2011. Cancer Epidemiol. 2015;39(1):1–7. doi:.https://doi.org/10.1016/j.canep.2014.10.010
44 Ellis L , Abrahão R , McKinley M , Yang J , Somsouk M , Marchand LL , et al. Colorectal Cancer Incidence Trends by Age, Stage, and Racial/Ethnic Group in California, 1990-2014. Cancer Epidemiol Biomarkers Prev. 2018;27(9):1011–8. doi:.https://doi.org/10.1158/1055-9965.EPI-18-0030
45 Hoffman RM , Meisner AL , Arap W , Barry M , Shah SK , Zeliadt SB , et al. Trends in United States Prostate Cancer Incidence Rates by Age and Stage, 1995-2012. Cancer Epidemiol Biomarkers Prev. 2016;25(2):259–63. doi:.https://doi.org/10.1158/1055-9965.EPI-15-0723
46 Moller MH , Kristiansen IS , Beisland C , Rorvik J , Stovring H . Trends in stage-specific incidence of prostate cancer in Norway, 1980-2010: A population-based study. BJU Int. 2015.
47 Kennedy MPT , Cheyne L , Darby M , Plant P , Milton R , Robson JM , et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax. 2018;73(12):1128–36. doi:.https://doi.org/10.1136/thoraxjnl-2018-211842
The authors declare that they have no conflict of interest.