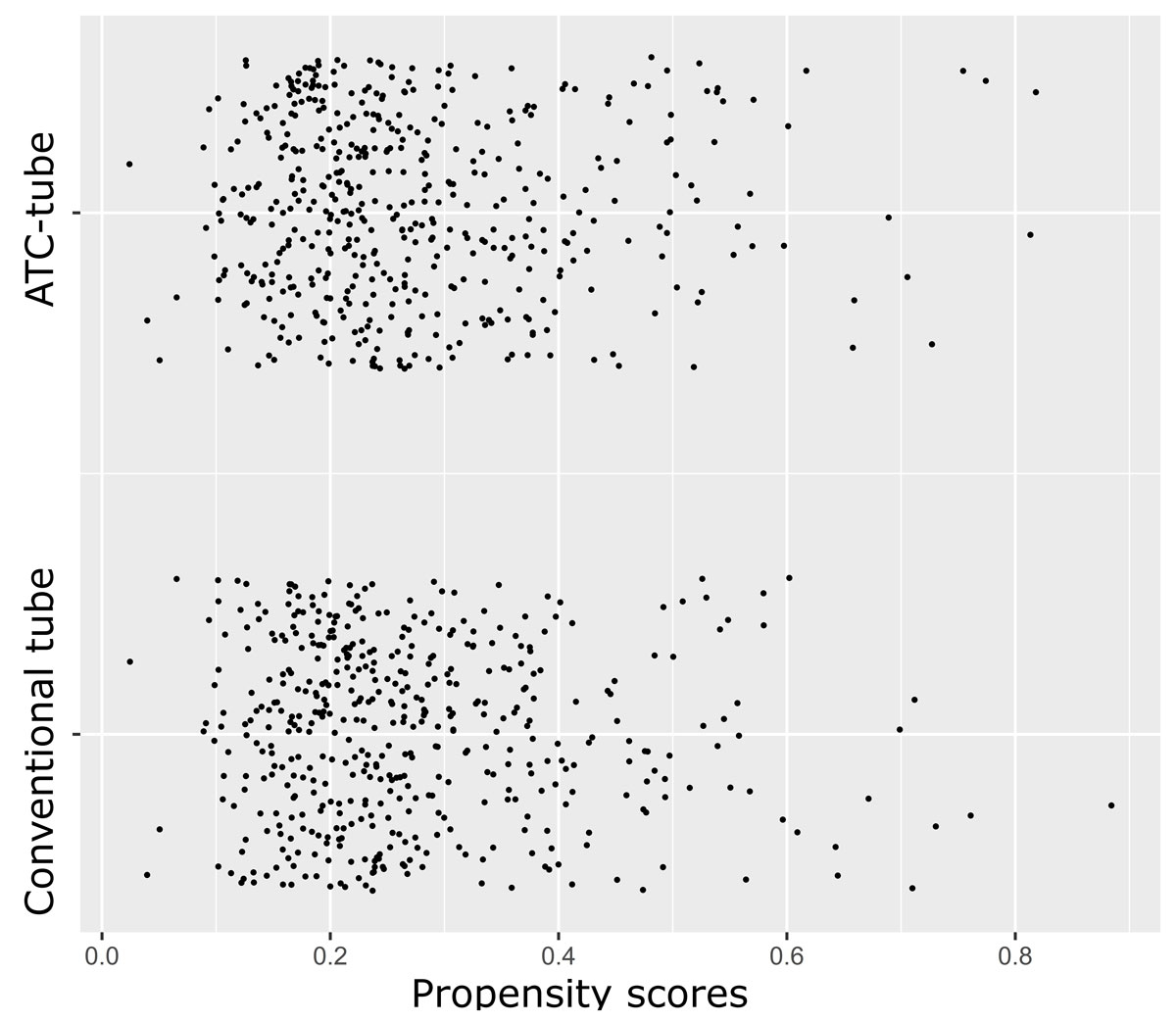
Figure 1 Jitter plot illustrating the distribution and overlap of propensity scores across the matched groups.
DOI: https://doi.org/10.4414/smw.2020.20394
Postoperative bleeding is a common complication of cardiac surgery and can lead to an increase in morbidity and mortality as well as healthcare costs [1, 2]. The evacuation of shed blood in the thoracic cavity following cardiac surgery is of great importance for awareness of sustained bleeding and to avoid retained blood syndrome [3, 4]. The retained blood syndrome arises from the collection of blood in the pericardial or pleural space, and the subsequent compression and impaired function of the heart and lungs. This syndrome can present clinically in a spectrum over time subdivided into acute, subacute and chronic retained blood syndrome. Typical manifestations are cardiac tamponade, haemothorax and pericardial or pleural effusions [5].
Chest tubes are inserted in the pericardial and pleural cavities postoperatively in almost all patients after cardiac surgery to facilitate the evacuation of shed blood. A common problem with the use of chest tubes is obstruction following clogging of blood inside the tube. A single centre prospective observational study has shown that the incidence of chest tube clogging after cardiac surgery is 36% [6]. In order to resolve this problem, larger-diameter tubes are preferred over smaller-diameter tubes, but these increase patient discomfort and suffer from the same problem as small diameter tubes [4]. Several controversial techniques such as milking, tapping, squeezing and stripping are commonly applied without being able to achieve an adequate degree of tube patency. Moreover, the use of these techniques raises concerns about the possibility of tissue damage though the negative pressure exerted on intrathoracic structures [7, 8].
The abovementioned problems of the conventional chest tubes and the complications associated with their occlusion postoperatively have led to the development of the active tube clearance (ATC) technology [9–11]. The PleuraFlow active clearance system consists of a moveable guide wire placed inside a specially manufactured chest tube able to restore patency in occluded tubes [12, 13]. The results of a single centre observational study showed a significant 43% reduction (p = 0.0087) in the rate of interventions for retained blood syndrome and a significant 33% reduction (p = 0.013) in the incidence of postoperative atrial fibrillation [14]. In 2019, guidelines for perioperative care in cardiac surgery from the ERAS (Enhanced Recovery After Surgery) Society were published, which recommend chest tube patency in all cases and recommend against chest tube stripping and milking [15]. Even though guidelines recommend the use of ATC tubes for the postoperative care of patients undergoing cardiac surgery, these recommendations are based on a small number of non-randomised studies. Therefore, the addition of more data about the performance of ATC tubes would further support evidence for their use. The aim of this study was the assessment of the efficacy of the PleuraFlow active chest tube clearance system in the reduction of retained blood syndrome after cardiac surgery and the validation of the aforementioned results in another hospital setting.
This propensity score matched study included 2461 adult patients undergoing major cardiac surgery (coronary artery bypass surgery, valve surgery, thoracic aortic surgery and their combinations) at the department of cardiac surgery of the Triemli City Hospital in Zurich, Switzerland, in the period 2013–2018. Underage patients or patients with documented refusal to participate in the research projected were excluded. The study was approved by the cantonal ethics board of Zurich (BASEC-Number 2016-02026). Data were collected prospectively in our database. Patients were divided into those consecutive adult cardiac surgery patients who received conventional chest tubes only (n = 1980), an ATC training phase (n = 50) and a cohort treated with ATC (n = 481). Assignment to treatment groups was purely time-dependent. All patients up to March 2017 were treated with conventional chest tubes only. An ATC tube in the retrosternal position was introduced in April 2017 as an initial ATC training phase without data collection. An ATC tube in the retrosternal position was used later on, with data collection about its performance starting in May 2017. Aspirin was not withheld prior to surgery. Clopidogrel (Plavix) and prasugrel (Effient) were generally stopped 7 days before the operation and ticagrelor (Brilique) 5 days preoperatively. In patients with preoperative ADP inhibitors needing urgent surgery, the surgery was postponed as long as possible up to these times. Preoperatively, kidney dysfunction was defined as (1) no dialysis but preoperative acute renal failure (anuria or oliguria <10 ml/hr); (2) dialysis for chronic renal failure with onset more than 6 weeks prior to cardiac surgery; and (3) dialysis for acute renal failure with onset within 6 weeks of cardiac surgery.
Generally, patients were implanted with four 24 Fr chest tubes: one in the retrosternal position over the closed pericardium, one inferiorly along the diaphragm inside the pericardial space and one in each pleural space if opened. Chest tube milking and stripping was used routinely on all conventional chest tubes. An ATC system (PleuraFlow Active Clearance Technology; ClearFlow, Inc., Anaheim, CA) was used for the retrosternal chest tube over the closed pericardium in the ATC group. ATC is a chest tube clearance device with a guide wire and a loop to clear the inner lumen of the chest tube from obstructing blood clot or fibrinous debris [13]. As described in prior studies, ATC was actuated every 15 minutes for the first 8 hours, then every 30 minutes for the next 16 hours, and then once an hour and as needed thereafter [12, 14]. Besides the single ATC in the retrosternal position, the other three chest tubes were conventional chest tubes and they were stripped and milked according to routine. No data were collected during the training phase in the intensive care unit (ICU). Other than the use of a single ATC system in the retrosternal position in the ATC group, there were no other differences in the size, placement location or management of chest tubes during this study. Chest tubes were removed when draining less than 100 ml over 8 hours postoperatively or when the drainage volume was less than 200 ml during the previous 24 hours. Most were removed in the ICU on the first postoperative day.
All patients were monitored continuously with telemetry in the ICU and vital signs were taken three times a day on the ward, at which point an ECG was ordered if an irregular heart beat was noted. Postoperative atrial fibrillation was defined as any monitored documentation of atrial fibrillation. Patients who had a documented history of atrial fibrillation/flutter prior to surgery were excluded from the postoperative atrial fibrillation sub-analysis. Postoperatively, kidney dysfunction was defined according to the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classification criteria [16].
The analysis summarised normal continuous variables as mean and standard deviation, non-normal continuous variables as median, first quartile and third quartile and binary variables as total count and frequency. Assessment of distribution normality was mainly graphical with the use of histograms, probability-probability plots (P-P plots) and quantile-quantile plots (Q-Q plots), and supplementary with the use of Shapiro-Wilk and Kolmogorov-Smirnov tests. It calculated standardised differences with non-normal continuous variables log-transformed before the calculation [17].
Statistical comparisons of the conventional chest tubes and the ATC tube with the unmatched data used independent samples t-tests for normal continuous variables, Wilcoxon’s rank sum test for non-normal continuous variables and Fisher’s exact test for binary variables. Statistical comparisons of the conventional chest tubes and the ATC tube with the matched data used paired t-tests for normal continuous variables, Wilcoxon’s signed rank test for non-normal continuous variables and McNemar’s test for binary variables. The analysis treated count variables as normal continuous variables when calculating standardised differences or performing statistical comparisons.
Propensity scores were calculated with a logistic regression model. Use of an ATC tube or conventional tubes only was the outcome variable and the following preoperative and intraoperative patient characteristics were the exploratory variables of the logistic regression model: age, gender, diabetes (yes/no), hypertension (yes/no), weight, body mass index (BMI), additive EuroScore, EuroScore II, dialysis (yes/no), pulmonary disease (yes/no), peripheral vascular disease (yes/no), preoperative heart arrhythmia (yes/no), ejection fraction less than or equal to 30% (yes/no), operative urgency (elective, urgent, emergency, or salvage), cardiopulmonary bypass (yes/no), operative category (coronary artery bypass graft [CABG], valve, CABG and valve, other), and operative access (sternotomy, partial upper sternotomy, other). Propensity score matching was performed with the nearest neighbour method, without replacement, in a one-to-one fashion (1:1), using the “pairmatch” function from the R package “optmatch”. The matching did not specify a calliper. Instead, the optimal assignment algorithm of the “pairmatch” function was used, which makes use of an efficient algorithm for minimising the total distance between matched pairs and maximising the number of matched pairs between the treated and control groups [18, 19]. Patients who were not matched were excluded from the paired comparisons. Balance of individual covariates across treatment and comparison groups within blocks of the propensity score was controlled through computation of the standardised differences of the individual covariates before and after propensity score matching. Patients receiving conventional chest tubes only were compared with patients receiving an ATC tube in the retrosternal position before and after propensity score matching.
The primary endpoint of the study was the rate of intervention for retained blood syndrome, a composite endpoint of re-exploration for bleeding or tamponade with washout of retained blood, intervention for pleural effusion or intervention for pneumothorax. Secondary outcomes of the study were the components of the composite primary endpoint, chest tube output on the first and second postoperative day, kidney replacement therapy, postoperative atrial fibrillation and sternal infection [3, 5, 14]. The p-values of endpoints were Bonferroni-corrected to account for multiple testing.
Unmatched and matched preoperative and intraoperative patient characteristics are presented in table 1. In the unadjusted data there were statistically significant differences for age (p <0.0001), pulmonary disease (p = 0.002), peripheral arterial disease (p = 0.002), preoperative dialysis (p <0.0001), emergency surgery (p = 0.002), use of cardiopulmonary bypass (p = 0.042), additive EuroScore (p = 0.002), Euroscore 2 (p = 0.03) and rate of sternotomy (p <0.0001) between patients treated with conventional chest tubes only and an ATC tube. After propensity score matching, 471 patients treated with conventional chest tubes only were matched for analysis to 471 patients treated with an ATC tube. There was an overlap in the range of propensity scores across matched patients of both groups (fig. 1). Matching balanced the two patient groups for preoperative and intraoperative characteristics. The standardised differences of all individual covariates after propensity score matching was <0.1, except for weight (0.43) and BMI (0.11), as seen in figure 2.
Table 1 Baseline characteristics.
| Characteristic | Unadjusted data | Propensity score matched data | ||||
|---|---|---|---|---|---|---|
|
Standard
(n = 1980) |
ATC
(n = 481) |
p-value |
Standard
(n = 471) |
ATC
(n = 471) |
p-value | |
| Age, years | 68.6 ± 11.6 | 65.7 ± 10.5 | <0.0001 | 65.9 ± 12.5 | 65.6 ± 10.5 | 0.72 |
| Male gender | 1541 (78) | 364 (76) | 0.33 | 347 (74) | 356 (76) | 0.54 |
| Weight, kg | 80 (70–89) | 80 (71–90) | 0.16 | 80 (70–89) | 80 (71–90) | 0.12 |
| BMI, kg/m2 | 26.6 (24.2–29.4) | 26.6 (24.3–30.3) | 0.19 | 26.5 (24.3–29.4) | 26.6 (24.3–30.2) | 0.27 |
| Arterial hypertension | 1433 (72) | 366 (76) | 0.11 | 359 (76) | 358 (76) | 1 |
| Diabetes mellitus | 423 (21) | 93 (19) | 0.35 | 77 (16) | 92 (20) | 0.21 |
| Smoking | 622 (31) | 137 (28) | 0.23 | 142 (30) | 134 (28) | 0.61 |
| Pulmonary Disease | 90 (4.5) | 40 (8.3) | 0.002 | 34 (7.2) | 39 (8.3) | 0.61 |
| Peripheral arterial disease | 200 (10) | 28 (5.8) | 0.002 | 27 (5.7) | 28 (5.9) | 1 |
| Preoperative Arrhythmia | 75 (3.8) | 20 (4.2) | 0.69 | 16 (3.4) | 20 (4.2) | 0.62 |
| Dialysis | 30 (1.5) | 22 (4.6) | <0.0001 | 19 (4) | 20 (4.2) | 1 |
| LVEF ≤30% | 99 (5) | 20 (4.2) | 0.55 | 19 (4) | 20 (4.2) | 1 |
| Operative urgency | ||||||
| – Elective | 1609 (81) | 367 (76) | 0.015 | 361 (77) | 362 (77) | 1 |
| – Urgent | 252 (13) | 68 (14) | 0.41 | 64 (14) | 67 (14) | 0.85 |
| – Emergency | 108 (5.5) | 45 (9.4) | 0.002 | 46 (9.8) | 41 (8.7) | 0.65 |
| – Salvage | 11 (0.56) | 1 (0.21) | 0.48 | 0 (0) | 1 (0.21) | 0.5 |
| Use of CPB | 984 (50) | 264 (55) | 0.042 | 259 (55) | 258 (55) | 1 |
| Additive EuroScore | 6 (4–8) | 6 (4–9) | 0.002 | 6 (4–9) | 6 (4–9) | 0.91 |
| EuroScore 2 | 1.78 (0.97–3.62) | 1.95 (1.09–4.50) | 0.03 | 1.87 (1.01–4.21) | 1.95 (1.08–4.50) | 0.35 |
| Operative category | ||||||
| – CABG | 883 (45) | 228 (47) | 0.28 | 225 (48) | 224 (48) | 1 |
| – Valve | 337 (17) | 69 (14) | 0.17 | 63 (13) | 68 (14) | 0.7 |
| – CABG and valve | 235 (12) | 51 (11) | 0.48 | 42 (8.9) | 51 (11) | 0.39 |
| – Other | 525 (27) | 133 (28) | 0.61 | 141 (30) | 128 (27) | 0.39 |
| Operative access | ||||||
| – Sternotomy | 1628 (82) | 454 (94) | <0.0001 | 452 (96) | 444 (94) | 0.29 |
| – Partial upper sternotomy | 114 (5.8) | 27 (5.6) | 1 | 19 (4) | 27 (5.7) | 0.29 |
| ‒ Other | 238 (12) | 0 (0) | <0.0001 | 0 (0) | 0 (0) | 1 |
ATC = active tube clearance; BMI = body mass index; CABG = coronary artery bypass grafting; CPB = cardiopulmonary bypass; LVEF = left ventricular ejection fraction Continuous variables are reported as mean ± standard deviation or median (interquartile range) and categorical variables as counts and percentages, n (%).

Figure 1 Jitter plot illustrating the distribution and overlap of propensity scores across the matched groups.

Figure 2 Lineplot of the absolute standardised differences of all individual covariates before and after propensity score matching. The standardised differences of all individual covariates after propensity score matching was <0.1, except for weight (0.43) and body mass index (0.11).
Unmatched and matched postoperative outcomes are presented in table 2. Matched patients with an ATC-tube in retrosternal position had no statistically significant difference in the rate of intervention for retained blood syndrome (33% vs 31%, p = 1), re-exploration because of bleeding or tamponade (2.5% vs 4%, p = 1), intervention for pneumothorax (4.7% vs 4.9%, p = 1), intervention for pleural effusion (28% vs 28%, p = 1), postoperative kidney replacement therapy with dialysis (0.85% vs 0.85%, p = 1) and haemofiltration (4.5% vs 5.7%, p = 1), postoperative atrial fibrillation (19% vs 22%, p = 1), sternal infection (0.21% vs 0.64%, p = 1) and intensive care unit stay (median 4, interquartile range [IQR] 3–7 shifts vs median 3, IQR 3–6 shifts; p = 1) in comparison with patients with conventional chest tubes only. Matched patients treated with an ATC tube in the retrosternal position had statistically significantly less chest tube output on the first postoperative day (median 480, IQR 316–700 ml vs median 590, IQR 380–905 ml; p <0.0001) and second postoperative day (median 505, IQR 342–800 ml vs median 597, IQR 383–962 ml; p = 0.0012) in comparison with patients treated with conventional chest tubes only.
Table 2 Postoperative outcomes.
| Outcome | Unadjusted data | Propensity score matched data | ||||||
|---|---|---|---|---|---|---|---|---|
|
Standard
(n = 1980) |
ATC
(n = 481) |
Unadjusted p-value | Bonferroni-adjusted p-value |
Standard
(n = 471) |
ATC
(n = 471) |
Unadjusted p-value | Bonferroni-adjusted p-value | |
| Intervention for retained blood syndrome | 538 (27) | 158 (33) | 0.015 | 0.165 | 148 (31) | 155 (33) | 0.68 | 1 |
| Re-exploration because of bleeding or tamponade | 71 (3.6) | 12 (2.5) | 0.26 | 1 | 19 (4) | 12 (2.5) | 0.25 | 1 |
| Intervention for pneumothorax | 94 (4.7) | 23 (4.8) | 1 | 1 | 23 (4.9) | 22 (4.7) | 1 | 1 |
| Intervention for pleural effusion | 458 (23) | 136 (28) | 0.02 | 0.22 | 132 (28) | 133 (28) | 1 | 1 |
| Kidney replacement therapy | ||||||||
| – Dialysis | 19 (0.96) | 5 (1) | 0.8 | 1 | 4 (0.85) | 4 (0.85) | 1 | 1 |
| – Haemofiltration | 102 (5.2) | 24 (5) | 1 | 1 | 27 (5.7) | 21 (4.5) | 0.45 | 1 |
| Drainage volume first postoperative day, ml | 585 (370-870) | 480 (312-700) | <0.0001 | <0.0001 | 590 (380-905) | 480 (316-700) | <0.0001 | <0.0001 |
| Drainage volume second postoperative day, ml | 595 (370–900) | 505 (340–800) | <0.0001 | 0.008 | 597.5 (383.8–962.5) | 505 (342.5–800) | 0.0001 | 0.0012 |
| Postoperative atrial fibrillation | 424 (22) | 85 (18) | 0.077 | 0.847 | 98 (22) | 84 (19) | 0.33 | 1 |
| Sternal infection | 27 (1.4) | 1 (0.21) | 0.029 | 0.319 | 3 (0.64) | 1 (0.21) | 0.62 | 1 |
| Intensive care unit stay, shifts | 3 (3–6) | 4 (3–7) | <0.0001 | 0.0045 | 3 (3–6) | 4 (3–7) | 0.18 | 1 |
ATC: active tube clearance. Continuous variables are reported as median (interquartile range) and categorical variables as counts and percentages, n (%).
Chest tubes are required for all cardiac surgery patients to evacuate shed mediastinal blood in the early hours after surgery. Chest tubes are prone to clog while the patient is still bleeding [4, 6]. This can result in increased complications and costs related to blood not fully evacuated by chest tubes from around the heart and lungs [3, 5, 20]. For many years, the standard in our hospital and others has been for nurses to make bedside efforts to keep chest tubes from clogging, such as milking and stripping chest tubes. In some extreme cases of clogging, the doctors are called to the bedside to open the chest tubes and blindly use a suction catheter to clear them [21, 22]. Chest tube stripping is very time consuming for nurses. This pulls them away from other critical tasks, especially when patients are most in need of intensive nursing efforts in early recovery [23]. Chest tube stripping has been studied in clinical trials summarised in a meta-analysis that failed to show any benefit, and could possibly harm [7, 8]. Furthermore, we have long discouraged open blind suction as potentially harmful, as it can potentially injure an internal organ, and may introduce bacteria into a sterile environment. Thus we recognised there was an unmet need in our ICU to explore new ways to routinely, safely and time efficiently maintain chest tube patency.
ATC was developed to address this unmet need for a simple, routine method to maintain chest tube patency in the ICU without breaking the sterile field [12]. In preclinical studies, ATC was more effective than stripping chest tubes in helping to evacuate blood from the chest, even with smaller diameters [10, 11]. In pilot clinical studies, it was shown that ATC systems could be introduced without over burdening the ICU staff and were effective in preventing chest tube occlusions [12]. We implemented this in our ICU, firstly training the nursing and ICU staff in order to become comfortable with its use before tracking data.
We carried out a pilot study with an ATC tube in the retrosternal position only, and the other three chest tubes were conventional tubes without active clearance. This tube was placed over the closed pericardium. One notable finding was a significant reduction in chest tube output on the first and second postoperative day. This was similar to the findings by Sirch and colleagues [14]. It is somewhat counterintuitive that one might see less overall drainage when chest tube patency is improved. However, it is well described that the tissue plasminogen activator content of shed mediastinal blood is high [24]. Tissue plasminogen activator results in fibrinolysis and microvascular bleeding. Pooling of unevacuated blood can perpetuate microvascular bleeding and thus more complete shed blood evacuation by patent chest tubes may allow bleeding to cease sooner. This is not inconsequential as the volume of chest tube output is closely correlated with mortality, complications and costs [2, 25, 26].
Several published studies have previously shown a significant reduction in retained blood syndrome, re-exploration for bleeding or tamponade and postoperative atrial fibrillation with the use of one ATC tube placed in a mediastinal position [14, 27, 28]. In our study, even though an ATC tube in the retrosternal position led to a significant reduction in chest tube output, no significant difference in the rate of intervention for retained blood syndrome, re-exploration for bleeding or tamponade and postoperative atrial fibrillation was observed. The rate of pleural intervention, kidney replacement therapy, sternal infection and ICU stay was not significantly different with the use of an ATC tube in our study, a finding similar to that of other studies [14, 27–29].
To our knowledge, our study is by far the largest sample-size study to date addressing the performance of an ATC tube in cardiac surgical patients, with 471 matched patient-pairs; the second largest sample size was that of Sirch et al., which included 256 matched patient pairs [14]. Additionally, our study was, as far as we know, the only one to apply the conservative Bonferroni method for p-value correction of outcomes in order to account for multiple comparisons. We were able to show a significant reduction in chest tube output with the use of an ATC tube in the retrosternal position, but no significant difference in the rate of intervention for retained blood syndrome in comparison with conventional chest tubes only. However, limitations of this study, inherently associated with the use of propensity score matching, have to be considered for the interpretation of the results. The aim of propensity score matching is reduction of confounding between treatment and control groups in order to assess the isolated effect of treatment on outcomes. As propensity score matching accounts only for confounders measured and added in the propensity score model, unmeasured confounders will still bias the treatment effect and will lead to unbalanced matched group populations. Additionally the forced balance of measured confounders may exacerbate the imbalance in the unmeasured confounders, thus leading to higher imbalance of the matched pairs driven by the higher imbalance of the unmeasured confounders.
In 2019, guidelines for perioperative care in cardiac surgery from the ERAS (Enhanced Recovery After Surgery) Society were published, which recommend chest tube patency in all cases and recommend against chest tube stripping and milking [15]. Based on our initial experience with ATC technology, these guidelines and the growing published peer reviewed literature on the subject of ATC, we intend to further evolve our use of ATC. In this pilot study we used only one ATC tube in the retrosternal space over a closed pericardium, whereas the tubes in the pleural and inferior pericardial space were conventional chest tubes. We plan to assess if adding additional ATC tubes might lead to a reduction in the rate of retained blood syndrome. Further, we did not use ATC in the inferior pericardial space under the closed pericardium. Given that we close our pericardium, the potential benefit of reducing blood pooling might not have extended to common sources of oozing postoperatively from inside the pericardium. A special point for consideration when using an ATC tube in the inferior pericardial space is the stiffness of the guide wire and the ATC system, as placement could be cumbersome. Thus further studies, preclinical for the assessment of the feasibility of ATC tube placement in sharply angled positions and randomised to add higher quality evidence about the outcomes after ATC tube use, are indicated to test the combination of ATC in the retrosternal and the inferior pericardial space.
No funding was received for this research project. The authors have no conflict of interest to disclose.
1 Kinnunen EM , Juvonen T , Airaksinen KE , Heikkinen J , Kettunen U , Mariscalco G , et al. Clinical significance and determinants of the universal definition of perioperative bleeding classification in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2014;148(4):1640–1646.e2. doi:.https://doi.org/10.1016/j.jtcvs.2014.07.040
2 Christensen MC , Krapf S , Kempel A , von Heymann C . Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg. 2009;138(3):687–93. doi:.https://doi.org/10.1016/j.jtcvs.2009.02.021
3 Balzer F , von Heymann C , Boyle EM , Wernecke KD , Grubitzsch H , Sander M . Impact of retained blood requiring reintervention on outcomes after cardiac surgery. J Thorac Cardiovasc Surg. 2016;152(2):595–601.e4. doi:.https://doi.org/10.1016/j.jtcvs.2016.03.086
4 Shalli S , Saeed D , Fukamachi K , Gillinov AM , Cohn WE , Perrault LP , et al. Chest tube selection in cardiac and thoracic surgery: a survey of chest tube-related complications and their management. J Card Surg. 2009;24(5):503–9. doi:.https://doi.org/10.1111/j.1540-8191.2009.00905.x
5 Boyle EM, Jr , Gillinov AM , Cohn WE , Ley SJ , Fischlein T , Perrault LP . Retained Blood Syndrome After Cardiac Surgery: A New Look at an Old Problem. Innovations (Phila). 2015;10(5):296–303. doi:.https://doi.org/10.1177/155698451501000502
6 Karimov JH , Gillinov AM , Schenck L , Cook M , Kosty Sweeney D , Boyle EM , et al. Incidence of chest tube clogging after cardiac surgery: a single-centre prospective observational study. Eur J Cardiothorac Surg. 2013;44(6):1029–36. doi:.https://doi.org/10.1093/ejcts/ezt140
7 Day TG , Perring RR , Gofton K . Is manipulation of mediastinal chest drains useful or harmful after cardiac surgery? Interact Cardiovasc Thorac Surg. 2008;7(5):878–90. doi:.https://doi.org/10.1510/icvts.2008.185413
8 Halm MA . To strip or not to strip? Physiological effects of chest tube manipulation. Am J Crit Care. 2007;16(6):609–12. doi:.https://doi.org/10.4037/ajcc2007.16.6.609
9 Vistarini N , Gabrysz-Forget F , Beaulieu Y , Perrault LP . Tamponade Relief by Active Clearance of Chest Tubes. Ann Thorac Surg. 2016;101(3):1159–63. doi:.https://doi.org/10.1016/j.athoracsur.2015.10.098
10 Arakawa Y , Shiose A , Takaseya T , Fumoto H , Kim HI , Boyle EM , et al. Superior chest drainage with an active tube clearance system: evaluation of a downsized chest tube. Ann Thorac Surg. 2011;91(2):580–3. doi:.https://doi.org/10.1016/j.athoracsur.2010.10.018
11 Shiose A , Takaseya T , Fumoto H , Arakawa Y , Horai T , Boyle EM , et al. Improved drainage with active chest tube clearance. Interact Cardiovasc Thorac Surg. 2010;10(5):685–8. doi:.https://doi.org/10.1510/icvts.2009.229393
12 Perrault LP , Pellerin M , Carrier M , Cartier R , Bouchard D , Demers P , et al. The PleuraFlow Active Chest Tube Clearance System: initial clinical experience in adult cardiac surgery. Innovations (Phila). 2012;7(5):354–8. doi: .https://doi.org/10.1177/155698451200700508
13 Shalli S , Boyle EM , Saeed D , Fukamachi K , Cohn WE , Gillinov AM . The active tube clearance system: a novel bedside chest-tube clearance device. Innovations (Phila). 2010;5(1):42–7. doi:.https://doi.org/10.1177/155698451000500109
14 Sirch J , Ledwon M , Püski T , Boyle EM , Pfeiffer S , Fischlein T . Active clearance of chest drainage catheters reduces retained blood. J Thorac Cardiovasc Surg. 2016;151(3):832–838.e2. doi:.https://doi.org/10.1016/j.jtcvs.2015.10.015
15 Engelman DT , Ben Ali W , Williams JB , Perrault LP , Reddy VS , Arora RC , et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019;154(8):755–66. doi:.https://doi.org/10.1001/jamasurg.2019.1153
16 Bellomo R , Kellum JA , Ronco C . Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30(1):33–7. doi:.https://doi.org/10.1007/s00134-003-2078-3
17 Austin PC . Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. doi:.https://doi.org/10.1002/sim.3697
18 Daniel SR , Armstrong K , Silber JH , Rosenbaum PR . An Algorithm for Optimal Tapered Matching, With Application to Disparities in Survival. J Comput Graph Stat. 2008;17(4):914–24. doi:.https://doi.org/10.1198/106186008X385806
19 Hansen BB , Olsen Klopfer S . Optimal Full Matching and Related Designs via Network Flows. J Comput Graph Stat. 2006;15(3):609–27. doi:.https://doi.org/10.1198/106186006X137047
20 Tauriainen T , Kinnunen EM , Koski-Vähälä J , Mosorin MA , Airaksinen J , Biancari F . Outcome after procedures for retained blood syndrome in coronary surgery. Eur J Cardiothorac Surg. 2017;51(6):1078–85. doi:.https://doi.org/10.1093/ejcts/ezx015
21 Boyacıoğlu K , Kalender M , Özkaynak B , Mert B , Kayalar N , Erentuğ V . A new use of Fogarty catheter: chest tube clearance. Heart Lung Circ. 2014;23(10):e229–30. doi:.https://doi.org/10.1016/j.hlc.2014.04.255
22 Halejian BA , Badach MJ , Trilles F . Maintaining chest tube patency. Surg Gynecol Obstet. 1988;167(6):521.
23 Cook M , Idzior L , Bena JF , Albert NM . Nurse and patient factors that influence nursing time in chest tube management early after open heart surgery: A descriptive, correlational study. Intensive Crit Care Nurs. 2017;42:116–21. doi:.https://doi.org/10.1016/j.iccn.2017.03.008
24 Fabre O , Vincentelli A , Corseaux D , Juthier F , Susen S , Bauters A , et al. Comparison of blood activation in the wound, active vent, and cardiopulmonary bypass circuit. Ann Thorac Surg. 2008;86(2):537–41. doi:.https://doi.org/10.1016/j.athoracsur.2008.02.076
25 Dixon B , Santamaria JD , Reid D , Collins M , Rechnitzer T , Newcomb AE , et al. The association of blood transfusion with mortality after cardiac surgery: cause or confounding? (CME). Transfusion. 2013;53(1):19–27. doi:.https://doi.org/10.1111/j.1537-2995.2012.03697.x
26 Christensen MC , Dziewior F , Kempel A , von Heymann C . Increased chest tube drainage is independently associated with adverse outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 2012;26(1):46–51. doi:.https://doi.org/10.1053/j.jvca.2011.09.021
27 Grieshaber P , Heim N , Herzberg M , Niemann B , Roth P , Boening A . Active Chest Tube Clearance After Cardiac Surgery Is Associated With Reduced Reexploration Rates. Ann Thorac Surg. 2018;105(6):1771–7. doi:.https://doi.org/10.1016/j.athoracsur.2018.01.002
28 Maltais S , Davis ME , Haglund NA , Perrault L , Kushwaha SS , Stulak JM , et al. Active Clearance of Chest Tubes Reduces Re-Exploration for Bleeding After Ventricular Assist Device Implantation. ASAIO J. 2016;62(6):704–9. doi:.https://doi.org/10.1097/MAT.0000000000000437
29 St-Onge S , Ben Ali W , Bouhout I , Bouchard D , Lamarche Y , Perrault LP , et al. Examining the impact of active clearance of chest drainage catheters on postoperative atrial fibrillation. J Thorac Cardiovasc Surg. 2017;154(2):501–8. doi:.https://doi.org/10.1016/j.jtcvs.2017.03.046
No funding was received for this research project. The authors have no conflict of interest to disclose.