Towards novel reimbursement models for expensive advanced therapy medicinal products (ATMPs)
DOI: https://doi.org/10.4414/smw.2020.20355
Dario
Picecchia, Katrin
Bertramb, Dominik
Brücherc, Michael
Bauerb
a Law School, University of Lucerne, Switzerland
b Institute of Molecular Cancer Research, University of Zurich, Switzerland
c Department of Biochemistry, University of Zurich, Switzerland
Summary
Currently, a major focus of biomedical research and clinical application are the so-called advanced therapy medicinal products (ATMPs), which are highly complex medicines that enable the targeted and personalised treatment of patients. The potential of ATMPs in future cancer treatment is invaluable. However, this novel class of treatments is often extremely expensive. Consequently, these therapies push established reimbursement models to their limits. Because of the high costs, as well as the lack of appropriate reimbursement models, access to these potentially lifesaving therapies is currently not guaranteed to all patients. This paper analyses the current legal framework in Switzerland and critically evaluates existing reimbursement models, particularly with respect to their adaptation for ATMPs. As a promising reimbursement arrangement, this paper proposes a model combining outcome-based instalment payments with aspects of the pay for performance and the annuity payment model. According to this performance-based shared risk model, instalment payments are due when defined treatment goals are achieved.
Introduction
Treatment of cancer patients is one of the biggest burdens for modern healthcare. In Europe alone, each year more than 3.9 million patients are diagnosed with cancer and, with 1.9 million deaths per year, cancer is also the second most common cause of death [1]. However, in recent years new possibilities have been explored for the treatment of cancer. The focus of research and clinical application are the so-called advanced therapy medicinal products (ATMPs), which are highly complex medicinal products that enable targeted and personalised treatment of patients. Although the potential of these novel therapies is invaluable in future cancer treatment, ATMPs often carry extraordinary costs. In the US, for instance, a single treatment with the ATMPs Kymriah® or Yescarta® – both CAR-T cell therapies – costs USD 475,000 or USD 373,000, respectively [2]. These extraordinary costs can be explained by the high costs of research, the complex manufacturing of such personalised therapeutics and great production costs for the individual therapy since mass production is currently not feasible. Furthermore, the pharmaceutical companies are also justifying the high costs with the high value of their products, especially in terms of health gains. In short, future strategies for cancer patient treatments are progressively heading towards a personalised approach, resulting in promising benefits but extensively higher costs per patient.
In consequence, these expensive therapies push our healthcare system and its established reimbursement models to their limits. Because of the high costs and the lack of appropriate reimbursement models, access to these potentially lifesaving therapies is currently not guaranteed to all patients. Therefore, it is necessary to devise novel reimbursement models in order to ensure greater and fair accessibility of therapies using ATMPs in the future.
The focus of this paper lies neither on the medical background of CAR-T cell therapies nor on the current reimbursement regulations for drugs. Rather, the aim is to identify possible reimbursement models for expensive ATMPs. However, to understand the problem as such, certain general remarks on the medical background and the current legal framework regarding drug reimbursement in Switzerland are indispensable. Following the general explanations, this paper will critically evaluate existing models for reimbursement and discuss their versatility regarding cancer therapy with ATMPs. Based on this analysis, a reimbursement approach is presented that could be used to pay for the costs of novel therapeutics, i.e., the performance-based shared risk model. Ultimately, the paper concludes with several final remarks.
Medical background
Chemotherapy and irradiation remain standard elements of many cancer treatment plans, with highly variable outcome which is frequently accompanied by partially severe side effects. In the last decade, a novel approach that aims to induce or boost patients’ immune response to eradicate tumour cells has attracted the attention of researchers and clinicians. In this context, immune checkpoint inhibitors that counteract the immune suppressive effect of the tumour by, for example, targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) or the programmed cell death protein 1 pathway (PD-1/PD-L1) have revolutionised cancer therapy [3]. Cancer therapy with immune checkpoint inhibitors has shown treatment success for a variety of malignancies such as melanoma, non-small cell lung cancer, head and neck squamous cell cancer, urothelial cancer, classical Hodgkin’s lymphoma and renal cell cancer [4, 5]. However, only a subset of patients initially responds to the treatment, and a substantial proportion shows innate resistance to the inhibitors. Furthermore, increasing evidence indicates that patients may acquire resistance and relapse within months or years later [6].
As a result, there is great need for new improved treatment strategies. One novel and highly promising approach is the application of ATMPs, which, according to the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) are classified into three categories: (i) somatic-cell therapy medicinal products (STMPs), (ii) tissue-engineered medicinal products (TEMPs) and (iii) gene therapy medicinal products (GTMPs) (see, for example, the definition laid out in art. 2 para. 1 lit. a EU-Regulation (EC) No. 1394/2007 of 13 November 2007). Somatic-cell therapies contain engineered autologous cells, allogeneic cells, or tissues changed in their biological characteristics or functions in the body. One of the most recent EMA-approved somatic-cell therapies is darvadstrocel (Alofisel®) produced by Takeda Pharma [7]. Alofisel® is comprised of a suspension of human allogeneic adipose-derived mesenchymal stem cells (eASCs) and approved for the treatment of adults with luminal Crohn’s disease [8, 9]. Tissue-engineered medicines comprise therapies containing modified cells or tissues to repair, regenerate or replace human tissue [10]. A recently approved example of a tissue-engineered medicinal product is Spherox® (manufactured by CO.DON AG) [11], which contains autologous spheroids of human matrix-associated chondrocytes to repair extensive defects of cartilage in the knee [12].
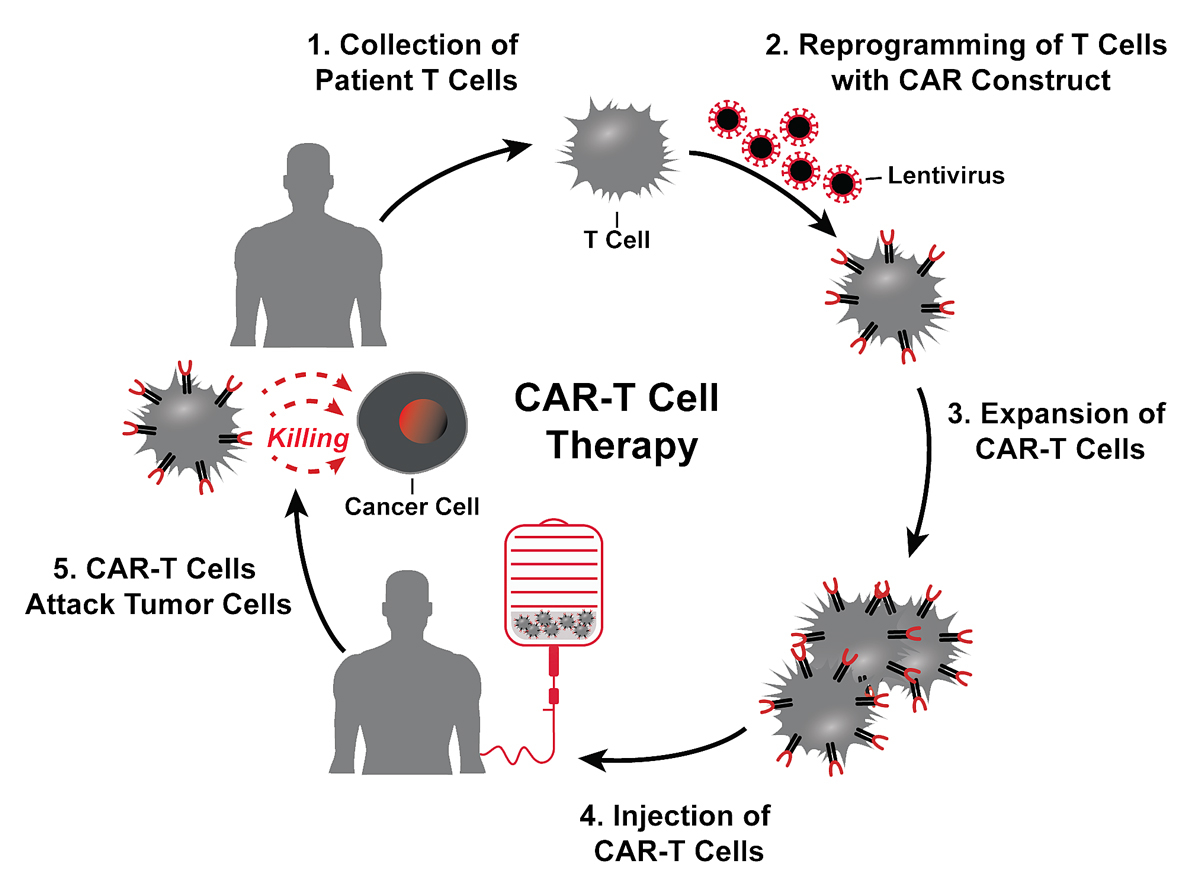
For the future of cancer treatments, gene therapy medicines introducing modified recombinant genes into patients or patient-derived cells represent the most encouraging type of ATMP. Unlike many standard treatment regimens, gene therapy medicinal products require only a single dose. The most prominent example for somatic gene therapy is the chimeric antigen receptor T cell (CAR-T cell) therapy [13]. This adoptive immunotherapy contains patient-autologous T lymphocytes that are engineered with synthetic chimeric antigen receptors (CARs) targeted at specific surface markers of tumours, redirecting their cytotoxic killing properties towards tumour cells (fig. 1) [13, 14]. For the production of CAR-T cells, patient-derived T cells are collected via leucapheresis and transduced with a lentivirus encoding for a CAR. Typically, CARs contain a cytoplasmic (including an endodomain), a transmembrane and an extracellular domain. The ectopic domain most commonly is an antibody fragment against a surface molecule of the target cell and is typically linked to the intracellular signalling domain of the T cell receptor (TCR) [15, 16]. In addition, often co-stimulatory receptors are introduced to the endodomain to provide an additional signal for T cell activation (cf. [17]).
The most intensively studied CAR targets the B cell surface protein CD19 (tisagenlecleucel) in the context of B cell malignancies. In several clinical trials, this novel therapy has shown remarkable results in patients suffering from B cell leukaemia and lymphomas. In a single-centre trial, tisagenlecleucel treatment resulted in a remission rate of about 90% in paediatric refractory or relapsed (r/r) acute lymphoblastic leukaemia patients [18]. Furthermore, in a global trial in paediatric r/r acute lymphoblastic leukaemia patients, the CD19-targeted CAR-T cell therapy led to a remission in 81% of participating patients [19]. Additionally, a multicentre trial with axicabtagene ciloleucel treatment, another CD19-directed CAR-T cell therapy, had a significant outcome in patients with refractory large B cell lymphoma, with a complete response in 54% of the patients [20]. Because of the extraordinary results in the first clinical trials using CAR-T cells, the FDA and EMA approved both, i.e., tisagenlecleucel (Kymriah®; Novartis Pharmaceuticals Corporation) [21, 22] as well as axicabtagene ciloleucel therapy (Yescarta®; Kite Pharma EU B.V.) [23, 24], for the treatment of diffuse large B cell lymphoma and acute lymphoblastic leukaemia. In Switzerland, the Swiss Agency for Therapeutic Products (Swissmedic) approved both therapies in 2018 and 2019 [25, 26].
Current legal framework for drug reimbursement in Switzerland
In Switzerland, the mandatory health insurance (Obligatorische Krankenpflegeversicherung, OKP) usually covers the costs of medical treatments. The insurance coverage is legally defined in the Federal Health Insurance Act (HIA; Krankenversicherungsgesetz, SR 832.10): The mandatory health insurance shall bear the costs for the services referred to in art. 25–31 HIA. These costs shall be reimbursed in accordance with the conditions laid down in art. 32–34 HIA (art. 24 para. 1 HIA). Thereby, the legislator establishes a catalogue of covered medical services [27, 28]. However, this catalogue in art. 25–31 HIA is very general and thus further specified at the ordinance level, i.e., the Health Insurance Ordinance (HIO; Krankenversicherungsverordnung, SR 832.102) and the Healthcare Services Ordinance (HCSO; Krankenpflege-Leistungsverordnung, SR 832.112.31).
In the following sections, this paper will first look at the usual reimbursement of drugs by the mandatory health insurance. Thereafter, the exception to the general drug reimbursement is examined in more detail, i.e., drug reimbursement in individual cases. Finally, this paper shows how the mandatory health insurance reimburses drugs as part of inpatient services. It should be noted that the reimbursement of ATMPs such as Kymriah® depends on their qualification as either drug or inpatient service, which has not been conclusively determined.1 Thus, in the Swiss healthcare system, the three following reimbursement mechanisms could apply.
Reimbursement according to the specialties list
The mandatory health insurance reimburses only those drugs that are positively listed on the so-called specialties list (Spezialitätenliste, SL) (art. 52 para. 1 lit. b HIA in conjunction with (i.c.w.) art. 34 HIO).2 According to art. 52 para. 2 lit. b HIA, the Federal Office of Public Health (FOPH; Bundesamt für Gesundheit) is responsible for the designation of certain drugs to be included in the SL. The listing of a drug on the SL always includes a price-setting decision and definition of possible limitations. The FOPH is advised by the Federal Drugs Commission (Eidgenössische Arzneimittelkommission) on the inclusion of drugs in the SL (art. 52 para. 1 Sentence 1 HIA i.c.w. art. 34 HIO and art. 31 HCSO).
For a drug to be included in the SL, the prerequisite is that Swissmedic has approved it (art. 65 para. 1 HIO). To do so, the drug must be of high quality, safe and effective in accordance with art. 10 para. 1 lit. a Therapeutic Products Act (Heilmittelgesetz, SR 812.21). Furthermore, to be included in the SL pursuant to art. 52 para. 1 HIA, a drug must meet the criteria of effectiveness (Wirksamkeit), appropriateness (Zweckmässigkeit) and cost-effectiveness (Wirtschaftlichkeit) (cf. art. 65 para. 3 HIO and art. 30 para. 1 lit. a HCSO).3 In short, effectiveness refers to the general suitability of a medical service, i.e., the service must be causal for the expected medical success [29]. Appropriateness describes the suitability of a medical measure in an individual case, which means that the measure must bear more benefits than risks for the specific patient [30]. Finally, cost-effectiveness requires that the medical measure has an appropriate cost-benefit ratio; whenever possible the most cost-effective measure must be chosen [31–33].
The drugs on the SL have a fixed maximum price determined by the FOPH (art. 65b para. 5 i.c.w. art. 67 para. 1 HIO).4 This means that the mandatory health insurance may reimburse listed drugs at no higher price than the one determined on the SL [34].
At the moment, neither Kymriah® nor Yescarta® are listed on the SL.5 As a consequence, the mandatory health insurance does not automatically reimburse these two drugs. Instead, an exceptional reimbursement is required, which must be approved individually by each health insurer before the start of every specific therapy.
Exceptional reimbursement in individual cases
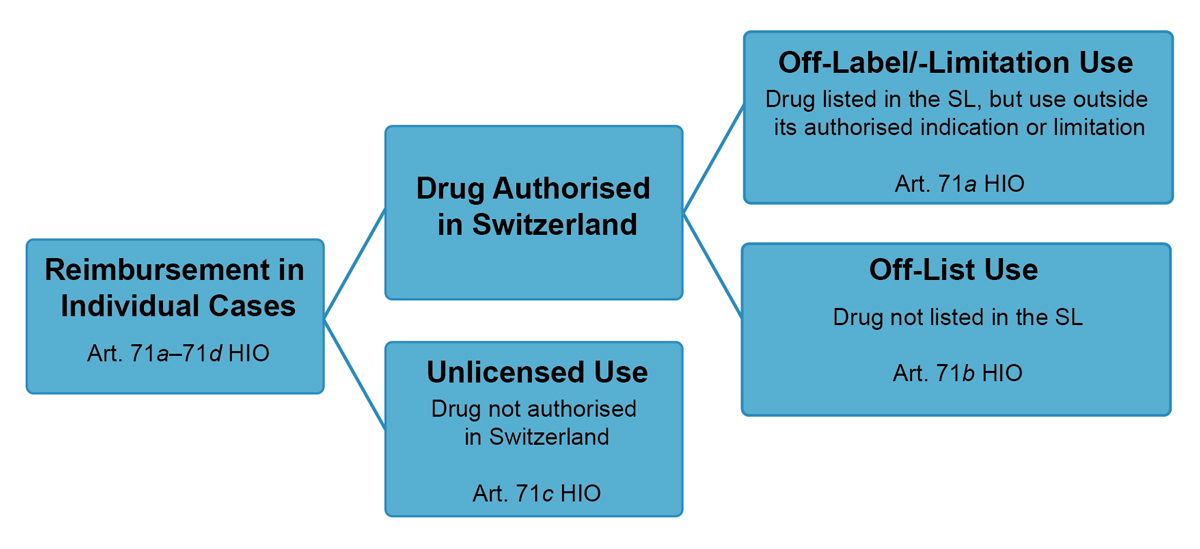
In addition to the reimbursement of drugs listed in the SL, reimbursement of non-listed drugs is possible in individual cases, i.e., off-list use according to art. 71b HIO (see fig. 2).6
The mandatory health insurance can reimburse non-listed drugs on a case-by-case basis if they are part of a treatment complex or in a case of hardship (cf. para. 1 of art. 71a–71c HIO):
- In the case of a treatment complex, the use of the drug is “an indispensable precondition for the performance of another service covered by the mandatory health insurance”, whereby this service must be clearly in the focus of the treatment (art. 71
- For the existence of a case of hardship, three conditions must be fulfilled (art. 71
In any case of exceptional reimbursement, the health insurer must issue prior approval of costs after consulting their medical examiner (Vertrauensärztin/Vertrauensarzt) (art. 71d para. 1 HIO). Finally, the costs assumed by the health insurer must be in proportion to the therapeutic benefit (art. 71d para. 2 HIO). In the case of off-list use pursuant to art. 71b HIO, the price of the drug is determined through negotiations between the health insurer and the manufacturer (para. 2). Unfortunately, the need for individual price negotiations can lead to different prices for the same drug, failed price negotiations or, in the worst case, a refusal to cover the treatment costs [35].
Overall, there has been an increase in exceptional reimbursement under art. 71a–71d HIO, particularly for the treatment of cancer [36]. However, such exceptional reimbursement causes various uncertainties, which also apply to the reimbursement of ATMPs such as Kymriah® or Yescarta®. First, reimbursement pursuant to art. 71a–71d HIO requires individual price negotiations for each individual reimbursement case between each health insurer and the pharmaceutical company. This leads to uncertainty about the price of a drug. Even worse, the current situation does not guarantee fair access to ATMPs [37–39]. The health insurers have considerable discretionary leeway as they assess the criteria according to art. 71a para. 1 lit. b HIO. For example, the concepts of great therapeutic benefit (grosser therapeutischer Nutzen) and seriousness of a chronic health impairment (schwere und chronische gesundheitliche Beeinträchtigungen) are subject to interpretation and can therefore be applied with varying degrees of rigour. Consequently, the lack of uniform assessment criteria and different assessment authorities (i.e., health insurance companies and their medical examiners) prevents equal access of patients to certain drugs. In fact, some health insurers used to cover the costs for Kymria® or Yescarta®, whereas other health insurers refused to reimburse the treatment costs [39]. However, the regulatory or legislative body should solve this fundamental problem independently of ATMPs.
Reimbursement as part of inpatient services
According to art. 49 para. 1 HIA, inpatient treatment – including hospitalisation and nursing services – is only reimbursed at a flat rate. For this purpose, the tariff partners, i.e., service providers and health insurers (fig. 3), determine flat rates per case that must be performance-related and based on uniform tariff structures throughout Switzerland (art. 46 para. 1 i.c.w. art. 49 para. 1 Sentence 2 and 3 HIA).7 Since 2012, Swiss Diagnosis Related Groups (SwissDRG) is the tariff system agreed upon and applied to the reimbursement of inpatient hospital services for which flat rates per case are paid throughout Switzerland.
The SwissDRG flat rates are determined by different factors (diagnosis, treatment, severity, etc.) [40]. As with the prices in the SL, the flat rates agreed upon by the tariff partners constitute a maximum compensation [41]. However, the tariff partners may agree that special diagnostic or therapeutic services are not included in the flat rate but will be charged separately (art. 49 para. 1 Sentence 4 HIA). In these cases, the tariff partners agree on additional compensation (Zusatzentgelt) that is added to the regular flat rates [42, 43].
Such additional compensation is the current solution being sought by various health insurers and Novartis in relation to the reimbursement of Kymriah®. Some health insurers (Helsana, Sanitas, KPT, Swica and CS) agreed with hospitals (hospital association H+) that Kymriah® will be paid for in addition to the usual flat rate for inpatient treatment (tariff agreement). The amount of the additional compensation for Kymriah® is based on the agreement between the health insurers and Novartis (subcontract of the tariff agreement). In December 2019, the Federal Council approved the tariff agreement between the health insurers and the hospital association [44]. However, the exact price of Kymriah® is kept secret and certain health insurers refuse to participate in this contract solution [37, 45]. Instead, these health insurers tried to negotiate their own tariff agreement. As a result, uniform reimbursement was at first not guaranteed, but health insurers who were party to the tariff agreement paid for the treatment with Kymriah® for their clients. The other health insurers continued to reimburse Kymriah® as an off-list use according to art. 71b HIO [46]. In August 2020, the Federal Council approved a second tariff agreement between santésuisse and the hospital association H+ [47]. This second agreement complements the first one and thus leads to a generally uniform reimbursement situation for Kymriah®.
Possible reimbursement models
The currently used fixed price model (see below), in which a single treatment is paid for up front, worked well in the past for bulk-produced medicines. However, therapeutics are becoming more and more specialised with decreasing patient cohorts and, ultimately, personalised drugs will be produced for a single patient.8 CAR-T cell therapy is one of the first such personalised approaches. Despite the obvious advantages of high success rate and fewer side effects, personalised medicine also leads to enormous costs. Drug prices in these fields have skyrocketed in recent years with Zolgensma® reaching a record price of USD 2.1 million for a single-shot treatment [48]. In the case of CAR-T cell and other gene therapy approaches, the costs concentrate on a single treatment. At the same time, there is no guarantee that the therapies, which have not been tried and tested for very long, will be effective in every patient and will enable a long-term cure. As a result, in certain cases very high treatment costs are incurred, although the benefits of the therapies have not been proven in the long term. Whether the extremely high prices are justifiable or whether such therapies should become cheaper over time will not be the focus of the following discussion (for a more general price discussion, see [49, 50]). Instead, this paper focuses on models for reimbursement of novel therapeutics.
For the evaluation of potential reimbursement solutions, a basic understanding of the relationships between the different parties involved in the reimbursement of therapeutic products is necessary. In Switzerland, up to five main parties are usually involved in this process:
According to the established reimbursement model, the very high costs for the many upcoming cell and gene therapies [51] must be paid up front with the risk that the treatment might not be successful. To develop a possible future reimbursement arrangement, this paper will present the advantages and pitfalls of four reimbursement models currently used in different countries: the flat rate, pay for performance, annuity payments and expanded risk pool model.
Fixed price
The fixed price model, also referred to as “one-shot” or “buy-and-bill” payment is a single, direct payment up front for an administered therapeutic. It is the simplest of the presented reimbursement models and is currently the most commonly used one (including in Switzerland).
This established model reduces bureaucratic hurdles and minimises administrative costs for therapeutics. On the one hand, the fixed price model does not require any further clinical monitoring. Instead, the initial indication is sufficient to receive a reimbursement as the drug is effective on average and treatment success can be predicted. On the other hand, a single up-front payment can lead to high costs for payers. The situation is particularly problematic because no refunds of the initially high costs are planned in the event of treatment failure. In addition, the possibility that the patient leaves the health insurer can make a single up-front payment without subsequent cost transfers an economically burdensome challenge.
To summarise, the low administrative cost, low clinical monitoring, and high feasibility of this model makes it advantageous for treatments. However, the high up-front compensation with no guaranteed customer financial tracking or reimbursement in the event of treatment failure, as well as the increasing number of high-cost treatments, makes flat price models challenging as a standard reimbursement approach for very expensive therapies without guarantee of (long-term) treatment success.
Pay for performance
The so-called pay for performance model is a reimbursement model that is based on patient outcome. Treatments have to meet defined and timed clinical goals to be eligible for reimbursement. The key rationale for this compensation strategy is to reduce the risk of covering inefficient treatments because safety and effectiveness of novel therapies must be comprehensively demonstrated before they are reimbursed by a health insurer. Ultimately, the aim is to justify the paid treatment compensation with the promised outcome. Therefore, pay for performance models are particularly interesting for therapy options of which the benefits have not yet been comprehensively proven or monitored in the long term (for a general discussion of pay for performance models see [52, 53]).
For instance, the gene therapy Luxturna® is approved by the EMA, FDA and Swissmedic for the curative treatment of patients with an inherited retinal dystrophy [54–56]. To reimburse the therapy costs, the manufacturer Spark Therapeutics introduced an outcome-based payment plan implying that Luxturna® must demonstrate short-term effectiveness (30 to 90 days) and long-term durability (30 months) as measured by an eyesight test [57]. Different insurers in the US and the UK have agreed to cover the treatment costs of Luxturna® according to this reimbursement model and final reimbursement negotiations are ongoing in other European countries [57–59].
Major challenges of pay for performance models include the determination of outcome measurements and the endpoint performance. The payers need to agree on what constitutes a successful treatment outcome within a predefined timespan. Importantly, as diagnostic methods and tools may improve through innovation and better scientific knowledge, the standards that define and measure treatment outcome might need to be adjusted over time. Thus, extensive reporting of patient outcome poses a significant challenge. In addition, there may be a risk that patients whose chances of success are uncertain or can be poorly assessed will no longer be treated.
Annuity payments
The annuity payment model describes a reimbursement scheme that considers spreading therapy costs on a series of payments over a predetermined period, which means that the total expenses for a treatment are split over a timeline of many months or years to reduce upfront costs. Furthermore, the annuity payments may be linked to performance milestones achieved in order to guarantee reimbursement.
The reimbursement of therapy costs according to the pay-over-time model was suggested for the world’s most expensive medicinal product Zolgensma® (price USD 2.1 million), which is a gene therapy for paediatric patients with spinal muscular atrophy. In this case, the manufacturer Novartis proposed the costs to be covered via five yearly instalment payments of USD 425,000, based on patient outcome [60].
Dilution of the financial impact as well as linking payments to treatment performance could make the annuity payment reimbursement model a popular scheme for covering the therapy costs of ATMPs. Nevertheless, there are some issues that are raising concerns for payers, e.g., that payments could continue over years even after a patient has transferred to another insurance plan. Furthermore, it is unclear if instalments have to be paid in cases where patients die of non-drug related events. Finally, when combining the annuity payment model with elements of the pay for performance model, the challenges of a performance-based approach must be taken into account here as well.
Expanded risk pool
In the expanded risk pool model, a third party – in most cases either public or private trusts or funds – will cover some of the payment for expensive treatments. Considering the simplest case, in which no performance-based evaluation is required by the third party, the medical costs are identical to the fixed price model but will be distributed between two or more different institutions.
The involvement of a third party might be beneficial with respect to the hesitation of current payers to reimburse novel high-cost single-shot therapeutics. In certain healthcare systems, public funds can guarantee access to treatments independently of the social or financial background of the patient, easing potential political tensions. For instance, Canada is taking a first step in this direction by announcing the “Express Scripts Canada Program”, which should help co-finance future therapeutics by third parties [61].
However, the involvement of the government or other third parties needs new regulations to determine in which cases the third party is required to pay and what will happen once the third party decides not to be involved. In cases where a third-party funding is denied, it must be clear whether the other payer (for example, the health insurer) is required to cover the payment or whether the reimbursement will be rejected with all the consequences thereof.
In conclusion, the expanded risk pool model may be a good option to create incentives for private or public actors to reduce the existing (social) economic hurdles and to loosen up the halt on expensive ATMPs. However, in the Swiss healthcare system, where cantons, health insurers as well as patients are all involved in the reimbursement of medical services, third-party funding does not seem to be a reasonable reimbursement solution for ATMPs. It is hardly conceivable that the inclusion of a third party would sustainably facilitate the reimbursement of ATMPs, as the payment would simply be split with another actor, but the specific challenges of the reimbursement of ATMPs (high costs, possible treatment failures, etc.) remain unsolved. Additionally, it would be unclear which third party – apart from the government or the health insurers – would have an interest in, or the resources, to reimburse a part of the therapy costs. Nevertheless, third-party funding could be a favourable model to bridge the reimbursement in exceptional cases or in cases of temporary lack of reimbursement arrangements.
An alternative approach: the performance-based shared risk model
A viable way to cover the costs of novel therapeutics could be a reimbursement model for ATMPs that goes beyond the standard fixed price model that focuses on a predefined value of a treatment. A promising reimbursement arrangement would rely on outcome-based instalment payments combining aspects and criteria of the pay for performance and the annuity payment model (fig. 4):
In concrete terms, this would mean that ATMPs will be paid for in instalments and not all at once. The instalments are due when certain previously agreed treatment goals are achieved. These predefined treatment goals should be based on medical performance, whereby economic considerations could also play a role. For example, performance can also be measured by whether or not a therapy can prevent subsequent treatments, which overall leads to lower healthcare costs [62]. Since data monitoring is part of a pay for performance approach, the proposed reimbursement model would increase the amount of available data on novel therapies. Hence, short- and long-term effects are closely assessed and can be used to further improve the ATMPs.
Furthermore, the performance-based shared risk model has the advantage that health insurers and cantons do not have to pay a large sum for a one-shot treatment up front, with the risk that the treatment will not achieve the desired success. Instead, a payment in instalments is linked to the success of the treatment. Connecting the reimbursement to the success of a therapy per defined time intervals significantly reduces the costs for an unsuccessful treatment as further payments would be discontinued in case of treatment failure. This also allows a distinct analysis of the overall cost-benefit ratio and, if required, reassessment of the original compensation or risk-sharing model. As a result, the proposed model reduces the financial risk in case a therapy is not as promising in reality or in the long run as initial data would indicate. In other words, the performance-based shared risk model guarantees that the high compensation is matched by a corresponding return on investment. Overall, the performance-based shared risk model reduces initial time and cost barriers allowing for an easier and faster access to newly approved ATMPs. Consequently, more patients would have access to promising lifesaving treatments.
If the reimbursement of a therapy is performance-based, it may be necessary to set up a fund for exceptional cases in which the chances of success of the therapy seem to be low. Otherwise, there is a risk that cases for which treatment success is uncertain – and therefore payment cannot be expected – will be rejected in order to avoid uncovered costs. Thus, as proposed here, it would be necessary to combine the performance-based reimbursement with a risk-pool model to guarantee access to ATMPs for cases that fall through strict performance considerations.
In conclusion, the performance-based shared risk model facilitates a smoother transition from generic therapies such as chemotherapy to highly personalised treatments tailored to the patient and the disease, which eventually promise a better outcome and decreased side effects. The proposed model ensures that the high compensation for ATMPs is matched with a corresponding return and any risks are shared between the pharmaceutical company and payers, which is not the case at the moment. The performance-based shared risk model is intended as an option to allow tailor-made reimbursement for tailor-made therapies.
However, every reimbursement model bears its limitations. The model proposed here is not directly aimed at reducing the initial price of a therapy but rather distributes the costs and financial risks to different parties and time points. It should also be considered that a performance-based shared risk model requires an advance contribution. In concrete terms, this means that either the payers must pay a certain amount in advance or the pharmaceutical company must initially provide a drug (in part) free of charge until it is clear whether the therapy is successful and reimbursement is due. Furthermore, defining the performance goals and success of a treatment will remain a difficult challenge that has to be agreed upon for every treatment as the individual characteristics of the specific therapy have to be taken into account. Such an agreement on concrete performance goals and their monitoring leads to a greater administrative burden. Finally, it must be examined in detail how a performance-based model can be implemented in the existing regulatory framework.9 The reimbursement approach proposed here should therefore also initiate further discussion in order to secure access to new therapies in the future.
Conclusion
This paper has shown that medical treatment, especially in the field of oncology, is in a state of flux. The future of medicine lies in personalised therapies tailor-made for individual patients. However, the development and production of personalised, highly advanced medicines is currently more expensive and complex than the same processes for the predominant bulk-produced medicines. At the same time, personalised drugs can provide an enormous additional health benefit for the individual patient. In particular, the recent advances in biomedical research have opened ground-breaking new avenues of personalised treatment opportunities for patients suffering from cancer, metabolic disorders or chronic diseases. Nevertheless, the enormously high prices demanded for ATMPs pose different challenges to the current Swiss healthcare system:
First, there are still some uncertainties regarding the benefits of new therapies. As it is intended to make therapies available to patients as soon as possible, long-term studies are often lacking and it is unclear whether the outstanding medical benefit of ATMPs will be proven in the longer term. However, if lifesaving therapies are available on the market, they will also be applied. This inevitably leads to a decision on their reimbursement by the mandatory health insurance. In reaching this decision, it is important to recall that the mandatory health insurance must, in principle, reimburse all effective, appropriate, and cost-effective treatments. This also applies to ATMPs. There, however, it is important to ensure that the mandatory health insurance does not suddenly bear high costs for therapies of which the long-term benefit has not yet been proven. Rather, it must be guaranteed that the mandatory health insurance covers costs that are in proportion to the ATMPs’ medical benefit. In this context, it also seems essential to discuss how the price of an ATMP is determined and what factors need to be taken into account (for example the level of innovation, durable clinical benefit, patient population size, impact on healthcare systems, etc.).10
Second, as soon as ATMPs are available, it must be clear whether they will be reimbursed by the mandatory health insurance. Unfortunately, this is not the case with the currently available ATMPs. Neither Kymriah® nor Yescarta® can be found on the SL and are therefore not reimbursed by default. Instead, health insurers decide on a case-by-case basis whether unlisted – but potentially lifesaving – drugs are covered by the mandatory health insurance. This can lead to unequal decisions and thus prevent fair access to therapies. In addition, the current reimbursement solution for Kymriah®, which introduces an additional compensation as part of an inpatient treatment, does not appear satisfactory: Some health insurers did not initially sign the corresponding tariff agreement, which meant that patients of certain health insurers did not have guaranteed access to the therapy whereas others did. Even after the other health insurers have negotiated a similar agreement, it would be much more satisfactory to establish a uniform reimbursement solution with respect to future ATMPs.
Third, once it has been established that certain ATMPs should be covered by the mandatory health insurance, the final issue to be discussed is what the reimbursement should or could look like. As outlined before, the tremendous costs of the ATMPs and lack of suitable reimbursement arrangements are major challenges for securing patients’ access to the treatments. Currently, compensation plans for covering the therapy costs of Kymriah®, which is the first cell therapy approved in Switzerland, need to be negotiated by the manufacturer Novartis and health insurers. A successful reimbursement process for currently used ATMPs has a pioneer role, as systematic uptake of other novel medicinal products in the Swiss healthcare system will be pursued in the future. Overall, the development of reimbursement models for new ATMPs needs equally innovative strategies to ensure that patients can benefit from these novel therapies.
To conclude, this paper has shown that highly personalised medicinal products are the future of medicine and offer huge opportunities. At the same time, they pose major challenges to our healthcare system in terms of their costs and reimbursement. Therefore, it is necessary to discuss these challenges broadly and to look for solutions that guarantee fair and equal access to medical services. The performance-based shared risk model presented here could ensure access to novel ATMPs for which the long-term therapeutic success has not yet been sufficiently established. Although this model may not solve all the problems around extraordinarily high drug prices, it would allow us to reimburse novel therapies more quickly and, at the same time, minimise the potential risks and impacts of the high costs on our healthcare system.
Footnotes
- According to the opinion taken here, genetically modified T cells are a product derived from blood (cf. art. 4 para. 1 lit. c of the Therapeutic Products Act) and intended for medical application in the human body. The genetically modified T cells are therefore to be qualified as a drug under art. 4 para. 1 lit. a of the Therapeutic Products Act. In the context of CAR-T cell therapies, one could also question whether the genetically modified T cells do fall within the scope of the Transplantation Act (Transplantationsgesetz, SR 810.21), as they are similar to a stem cell transplant. According to art. 2 para. 2 lit. b and c of the Transplantation Act, however, the law does not apply to the handling of blood or blood products, except blood stem cells. Since the modified T cells are not blood stem cells, but a blood product, they do qualify as a drug and not a transplant product, according to the wording of the law. However, according to Swissmedic, the definition of a transplant product is broader (cf. [63]).
- In this context, one also speaks of the list principle (Listenprinzip), which distinguishes medical services subject to reimbursement from services not subject to reimbursement. Contrary hereto, a presumption of mandatory insurance coverage applies to the medical and chiropractic services in the event of illness (art. 25 para. 2 lit. a no. 1 and 2 HIA). The presumption of mandatory insurance coverage means nothing other than that these services are legally presumed to meet the statutory conditions for reimbursement and are, therefore, covered by the mandatory health insurance (inter alia [64]).
- These three criteria are the so-called EAC-criteria (WZW-Kriterien), which, according to art. 32 HIA, all medical services must fulfil to be reimbursed by the mandatory health insurance.
- In reality, the FOPH negotiates the price of a drug with its manufacturer. There is some leeway in setting the price (for example, because of innovation surcharges under art. 65
- Since it is not clear whether Kymriah
- In individual cases (see fig. 2), reimbursement is also possible for drugs outside their approved indication (off-label use according to art. 71
- Pursuant to art. 46 para. 1 HIA, the tariff partners are at least one service provider and at least one health insurer or corresponding association.
- The recently produced drug Milasen
- In principle, health insurance law does not prevent performance-based reimbursement. In cases in which it is uncertain whether a therapy is effective, appropriate and cost-effective, the insured person is even better off with performance-based reimbursement, since it can be ensured that the legal criteria for reimbursement are met. However, it is important that all insured persons continue to have access to therapies and are not left untreated because of an uncertain prognosis. Thus, it must be examined in detail how exactly performance-based reimbursement can fit into the regulatory framework.
- Especially with the ever increasing prices for advanced therapies, a fundamental price discussion should take place, whereby the question must also be asked whether prices of up to more than USD 2 million for a single treatment can be justified. In the long term, the production of the expensive ATMPs should be simplified and the treatment should thus become significantly cheaper. However, it should be emphasised here that even the most sophisticated reimbursement model does not provide an answer to open questions in medicinal product or drug pricing. For first efforts of Swiss hospitals to reduce the prices for ATMPs, see [67].
References
1
Ferlay
J
,
Colombet
M
,
Soerjomataram
I
,
Dyba
T
,
Randi
G
,
Bettio
M
, et al.
Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. doi:.https://doi.org/10.1016/j.ejca.2018.07.005
2Henn V. Kymriah: CAR-T-Zellen bekämpfen Leukämien und Lymphome. In: Wissensschau.de [cited 2020 August 4]. Available from: https://www.wissensschau.de/krebs_tumor/car-t-zellen_kymriah_leukaemie.php.
3
Darvin
P
,
Toor
SM
,
Sasidharan Nair
V
,
Elkord
E
. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. doi:.https://doi.org/10.1038/s12276-018-0191-1
4
Buchbinder
EI
,
Desai
A
. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:.https://doi.org/10.1097/COC.0000000000000239
5
Prasad
V
,
Kaestner
V
. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin Oncol. 2017;44(2):132–5. doi:.https://doi.org/10.1053/j.seminoncol.2017.06.007
6
Syn
NL
,
Teng
MWL
,
Mok
TSK
,
Soo
RA
. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–41. doi:.https://doi.org/10.1016/S1470-2045(17)30607-1
7European Medicines Agency. Darvadstrocel-Alofisel [cited 2020 August 4]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel.
8
Scott
LJ
. Darvadstrocel: A Review in Treatment-Refractory Complex Perianal Fistulas in Crohn’s Disease. BioDrugs. 2018;32(6):627–34. doi:.https://doi.org/10.1007/s40259-018-0311-4
9
Verstockt
B
,
Ferrante
M
,
Vermeire
S
,
Van Assche
G
. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53(5):585–90. doi:.https://doi.org/10.1007/s00535-018-1449-z
10European Medicines Agency. Advanced Therapy Medicinal Products: Overview [cited 2020 August 4]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview.
11European Medicines Agency. Sperox-spheroids of human autologous matrix-associated chondrocytes [cited 2020 August 4]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/spherox.
12
Armoiry
X
,
Cummins
E
,
Connock
M
,
Metcalfe
A
,
Royle
P
,
Johnston
R
, et al.
Autologous Chondrocyte Implantation with Chondrosphere for Treating Articular Cartilage Defects in the Knee: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoeconomics. 2019;37(7):879–86. doi:.https://doi.org/10.1007/s40273-018-0737-z
13
Zhao
Z
,
Chen
Y
,
Francisco
NM
,
Zhang
Y
,
Wu
M
. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8(4):539–51. doi:.https://doi.org/10.1016/j.apsb.2018.03.001
14
Hucks
G
,
Rheingold
SR
. The journey to CAR T cell therapy: the pediatric and young adult experience with relapsed or refractory B-ALL. Blood Cancer J. 2019;9(2):10. doi:.https://doi.org/10.1038/s41408-018-0164-6
15
Dotti
G
,
Gottschalk
S
,
Savoldo
B
,
Brenner
MK
. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–26. doi:.https://doi.org/10.1111/imr.12131
16
Jackson
HJ
,
Rafiq
S
,
Brentjens
RJ
. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–83. doi:.https://doi.org/10.1038/nrclinonc.2016.36
17
Neelapu
SS
,
Tummala
S
,
Kebriaei
P
,
Wierda
W
,
Gutierrez
C
,
Locke
FL
, et al.
Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi:.https://doi.org/10.1038/nrclinonc.2017.148
18
Maude
SL
,
Frey
N
,
Shaw
PA
,
Aplenc
R
,
Barrett
DM
,
Bunin
NJ
, et al.
Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi:.https://doi.org/10.1056/NEJMoa1407222
19
Maude
SL
,
Laetsch
TW
,
Buechner
J
,
Rives
S
,
Boyer
M
,
Bittencourt
H
, et al.
Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. doi:.https://doi.org/10.1056/NEJMoa1709866
20
Neelapu
SS
,
Locke
FL
,
Bartlett
NL
,
Lekakis
LJ
,
Miklos
DB
,
Jacobson
CA
, et al.
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. doi:.https://doi.org/10.1056/NEJMoa1707447
21European Medicines Agency. Kymriah (tisagenlecleucel) [cited 2020 August 4]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah
22Food and Drug Administration. Kymriah (tisagenlecleucel) [cited 2020 August 4]. Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel.
23European Medicines Agency. Yescarta (axicabtagene ciloleucel) [cited 2020 August 4]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/yescarta
24Food and Drug Administration. Yescarta (axicabtagene ciloleucel) [cited 2020 August 4]. Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel.
25Swissmedic. Yescarta, 0,4–2 x 108 Zellen Infusionsdispersion (axicabtagene ciloleucel) [cited 2020 August 4]. Available from: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/yescarta_zellen_infusionsdispersion_axicabtagene_ciloleucel.html
26Swissmedic. Kymriah, Zellsuspension zur Infusion (Tisagenlecleucelum) [cited 2020 August 4]. Available from: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/kymriahtm_zellsuspensionzurinfusiontisagenlecleucelum.html.
27Supreme Court Decision 129 V 167 c. 3.1 p. 169 et seq.
28Federal Administrative Court Decisions C-6243/2014 of 2 May 2017 c. 4.1; C-6460/2011 of 24 June 2014 c. 4.2.1.
29Supreme Court Decisions 133 V 115 c. 3.1 p. 117; 128 V 159 c. 5c/aa p. 165.
30Supreme Court Decisions 130 V 299 c. 6.1 p. 304; 127 V 138 c. 5 p. 146; 9C_1011/2012 of 18 April 2013 c. 2.2.
31Federal Council, Statement on the revision of the health insurance of 6 November 1991, BBl 1992 I 93 et seq. p. 159.
32Supreme Court Decisions 142 V 26 c. 5.2.1 p. 35; 136 V 395 c. 7.4 p. 407 et seq.
33Federal Administrative Court Decision 2015/51 c. 8.2.2 p. 768.
34Supreme Court Decision 136 V 395 c. 5.1 p. 399.
35Rüefli C, Bolliger C. Off-Label-Use in der obligatorischen Krankenpflegeversicherung. Evaluation der Umsetzung von Artikel 71a und 71b KVV. Schlussbericht im Auftrag des Bundesamts für Gesundheit. Berne; 2014. p. 47 et seq.
36Cerny T, Lenz F. Innovative Medikamente: faire Preise und fairer Zugang für alle – ein Widerspruch? im dialog CSS 2019;3:10–11. p. 10; Wanner C. Die Krankenkasse zahlt nicht immer – der Bund will wissen wieso. Off-Label-Use von Medikamenten. In: SRF HeuteMorgen of 5 June 2019 [cited 2020 August 4]. Available from: https://www.srf.ch/news/schweiz/off-label-use-von-medikamenten-die-krankenkasse-zahlt-nicht-immer-der-bund-will-wissen-wieso.
37Bauer K. Umstrittener Geheimvertrag für teure Krebstherapie. Preispoker in Pharmabranche. In: SRF 10vor10 of 17 June 2019 [cited 2020 August 4]. Available from: https://www.srf.ch/news/schweiz/preispoker-in-pharmabranche-umstrittener-geheimvertrag-fuer-teure-krebstherapie
38Handelszeitung. Abrechnung neuer Gentherapien: Santésuisse skizziert Lösung. In: Handelszeitung of 30 March 2019 [cited 2020 August 4]. Available from: https://www.handelszeitung.ch/unternehmen/abrechnung-neuer-gentherapien-santesuisse-skizziert-losung
39Walter N. Die unglaubliche Heilung des Peter Rohr. In: Sonntagszeitung of 28 July 2019 [cited 2020 August 4]. Available from: https://www.tagesanzeiger.ch/sonntagszeitung/die-unglaubliche-heilung-des-peter-rohr/story/15720260.
40Swiss DRGAG. Basisinformationen für Gesundheitsfachleute. Berne; 2015. p. 2.
41Inter alia, Supreme Court Decision 141 V 206 c. 2.1.2 p. 210.
42Oggier W. Zusatz- und Innovationsentgelte unter SwissDRG. Warum und wie gehandelt werden sollte. Berne; 2012. p. 6 et seq.
43Kipfer B, Witzmann C. Die Vergütung von Arzneimitteln im Einzelfall nach Art. 71a–d KVV. LSR 2019;2:89–109. p. 91.
44Haas V. Bundesrat genehmigt Tarifvertrag für innovative Gentherapien. HSK Einkaufsgemeinschaft Press Release of 6 December 2019 [cited 2020 August 4]. Available from: https://ecc-hsk.info/de/aktuelles/2019/medienmitteilung-bundesrat-genehmigt-tarifvertrag-fuer-innovative-zell-und-gentherapien.
45Burkhardt P. Novartis erhält für neues Krebsmittel bis zu 370’000 Franken. In: Tagesanzeiger of 16 June 2019 [cited 2020 August 4]. Available from: https://www.tagesanzeiger.ch/wirtschaft/unternehmen-und-konjunktur/novartis-erhaelt-fuer-neues-krebsmittel-unter-370-000-franken/story/23472566.
46Kipfer B, Witzmann C. Die Vergütung von Arzneimitteln im Einzelfall nach Art. 71a–d KVV. LSR 2019;2:89–109. p. 108.
47FOPH. Der Bundesrat verabschiedet Tarifvereinbarung zur Vergütung einer innovativen Krebstherapie [cited 2020 August 30]. Available from https://www.admin.ch/gov/de/start/dokumentation/medienmitteilungen.msg-id-80181.html#:~:text=August%202020%20eine%20Tarifvereinbarung%20zur,ist%20g%C3%BCltig%20bis%20zum%2031.
48Cohen J. At Over $2 Million Zolgensma Is The World’s Most Expensive Therapy, Yet Relatively Cost-Effective. In: Forbes of 5 June 2019 [cited 2020 August 4]. Available from: https://www.forbes.com/sites/joshuacohen/2019/06/05/at-over-2-million-zolgensma-is-the-worlds-most-expensive-therapy-yet-relatively-cost-effective/#ddbc2aa45f5a.
49
Danzon
PM
. Differential Pricing of Pharmaceuticals: Theory, Evidence and Emerging Issues. Pharmacoeconomics. 2018;36(12):1395–405. doi:.https://doi.org/10.1007/s40273-018-0696-4
50Harris E. Potential Solutions To Current Pricing Models For Cell And Gene Therapies. In: Life Science Leader of 1 October 2019 [cited 2020 August 4]. Available from: https://www.lifescienceleader.com/doc/potential-solutions-to-current-pricing-models-for-cell-and-gene-therapies-0001.
51Alliance for Regenerative Medicine. Annual Regenerative Medicine Data Report 2018 [cited 2020 August 4]. Available from: http://alliancerm.org/wp-content/uploads/2019/03/ARM_AR2018_Web_FINAL.pdf.
52
Towse
A
,
Garrison
LP, Jr
. Can’t get no satisfaction? Will pay for performance help?: toward an economic framework for understanding performance-based risk-sharing agreements for innovative medical products. Pharmacoeconomics. 2010;28(2):93–102. doi:.https://doi.org/10.2165/11314080-000000000-00000
53
Antoñanzas
F
,
Rodríguez-Ibeas
R
,
Juárez-Castelló
CA
. Personalized Medicine and Pay for Performance: Should Pharmaceutical Firms be Fully Penalized when Treatment Fails?
Pharmacoeconomics. 2018;36(7):733–43. doi:.https://doi.org/10.1007/s40273-018-0619-4
54European Medicines Agency. Luxturna (voretigene neparvovec) [cited 2020 August 4]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/luxturna
55Food and Drugs Administration. Luxturna. (voretigene neparvovec-rzyl) [cited 2020 August 4]. Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna
56Swissmedic. Luxturna (Wirkstoff: Voretigen Neparvovec). Public Summary SwissPAR vom 11.06.2020 [cited 2020August 4]. Available from: https://www.swissmedic.ch/swissmedic/de/home/ueber-uns/publikationen/public-summary-swiss-par/public-summary-swisspar-luxturna.html.
57 Spark Therapeutics. Spark Therapeutics Announces First-of-their-kind Programs to Improve Patient Access to LUXTURNA™ (voretigene neparvovec-rzyl), a One-time Gene Therapy Treatment. Press Release of 3 January 2018 [cited 2020 August 4]. Available from: https://sparktx.com/press_releases/spark-therapeutics-announces-first-of-their-kind-programs-to-improve-patient-access-to-luxturna-voretigene-neparvovec-rzyl-a-one-time-gene-therapy-treatment/.
58NICE. NICE recommends novel gene therapy treatment for rare inherited eye disorder. Press Release of 4 September 2019 [cited 2020 August 4]. Available from: https://www.nice.org.uk/news/article/nice-recommends-novel-gene-therapy-treatment-for-rare-inherited-eye-disorder
59Jack Z. Novartis’s gene therapy Luxturna receives ‘considerable added benefit’ in Germany. Global Pricing Innovations Press Release of 18 October 2019 [cited 2020 August 4]. Available from: https://globalpricing.com/news/novartiss-gene-therapy-luxturna-receives-considerable-added-benefit-in-germany/
60Novartis. AveXis Announces Innovative Zolgensma® Gene Therapy Access Programs for US Payers and Families. Press Release of 24 May 2019 [cited 2020 August 4]. Available from: https://www.novartis.com/news/media-releases/avexis-announces-innovative-zolgensma-gene-therapy-access-programs-us-payers-and-families.
61Express Scripts Canada. Express Scripts Canada Introduces New Business Model. Press Release of 15 November 2011 [cited 2020 August 4]. Available from: https://www.express-scripts.ca/news-room/express-scripts-canada-introduces-new-business-model.
62
Malik
NN
. Pay-for-performance pricing for a breakthrough heart drug: learnings for cell and gene therapies. Regen Med. 2016;11(3):225–7. doi:.https://doi.org/10.2217/rme-2016-0014
63Swissmedic. Requirements rel. to the authorisation documentation for TP/GT/GMO. AW-Information sheet, version 5.0. Berne; 2019. p. 4.
64Supreme Court Decision 136 V 84 c. 2.1 p. 86.
65Blaser N. Roche setzt Bundesamt unter Druck, Poker um Medikamentenpreise. In: SRF Rundschau of 31 January 2019 [cited 2020 August 4]. Available from: https://www.srf.ch/news/schweiz/poker-um-medikamentenpreis-roche-setzt-bundesamt-unter-druck.
66Fossgreen A. Ein Medikament nur für Mila. In: Tagesanzeiger of 4 November 2019 [cited 2020 August 4]. Available from: https://www.tagesanzeiger.ch/wissen/medizin-und-psychologie/ein-medikament-nur-fuer-mila/story/20525344.
67Viciano A, Catanzaro M. Krankenhäuser stellen eigene Krebsmedikamente her. In: Süddeutsche Zeitung of 27 June 2020 [cited 2020 August 4]. Available from: https://www.sueddeutsche.de/wissen/krebstherapie-selbstgemacht-1.4949290.