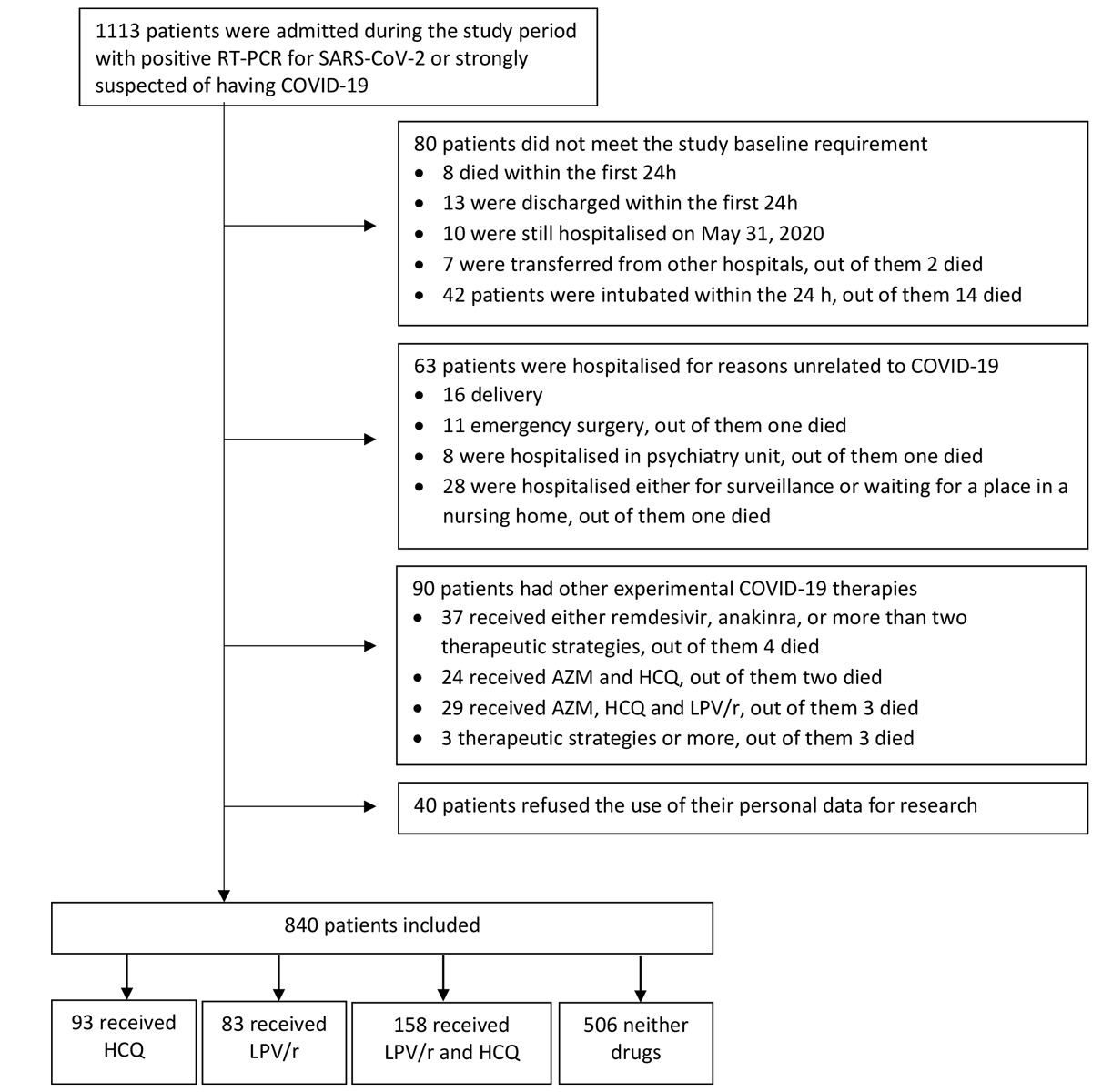
Figure 1 Flow chart of the study cohort. RT-PCR = reverse-transcriptase-polymerase-chain reaction; HCQ = hydroxychloroquine; LPV/r = lopinavir–ritonavir; AZM = azithromycin.
DOI: https://doi.org/10.4414/smw.2020.20446
During the first wave of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, the majority of countries experienced severe healthcare, social, and economic challenges. Huge efforts were directed towards finding an effective treatment to reduce morbidity and mortality. This study was conducted in Switzerland, a country severely affected by the pandemic, with one of the highest incidence rates of SARS-CoV-2 infections per capita [1], a total of 30,762 infected individuals and 1,656 deaths during the first wave (February to May 2020). We assessed two repositioned drugs that were commonly used worldwide in first wave of the pandemic: hydroxychloroquine, lopinavir/ritonavir, and their combination.
Overall, randomised controlled trials have thus far demonstrated no meaningful benefits of these two therapies in patients hospitalised for severe or critical COVID-19, as summarised in a recent living network meta-analysis [2–4]. The hydroxychloroquine and lopinavir/ritonavir arms of a major randomised controlled trial were stopped in June 2020 due to the absence of clinical benefit over the standard of care [3–7]. However, real-life data are crucially needed to gain a better understanding of the potential harmful impact of these drugs and their burden on the healthcare system. Our study thus aimed to compare hydroxychloroquine, lopinavir/ritonavir, and their combination when routinely used during the first wave of Covid-19 with standard of care without specific antiviral therapy in terms of (1) length of hospital stay (LOS), (2) in-hospital mortality, and (3) related additional healthcare costs.
This study was a single-centre retrospective cohort study performed during the first wave of the pandemic (February to May 2020 [8]), between 26 February and 31 May 2020, with patients diagnosed with COVID-19 who were admitted to acute medical wards in internal medicine and geriatrics, including intermediate care and intensive care at Geneva University Hospitals (HUG), Geneva, Switzerland. All patients were hospitalised in COVID-19 wards. HUG is the leading Swiss university hospital and the only public hospital in the canton of Geneva, with 64,000 hospitalisations and 1.1 million outpatient visits per year. Ethics approval was granted by the Cantonal Ethics Research Committee of Geneva (REF 2020-01070).
We included all patients from 26 February to 30 April 2020, who were discharged from acute medical wards by 31 May 2020. Patients were included if they were hospitalised for a SARS-CoV-2 infection, with either a positive reverse-transcriptase polymerase chain reaction test (RT-PCR) for SARS-CoV-2 or a negative RT-PCR but a high suspicion of COVID-19 based on a plausible clinical presentation, chest imaging or the presence of SARS-CoV-2 IgG as a marker of past SARS-CoV-2 infection [9]. Hospitalisation criteria were standardised as much as possible in routine care and compatible with the World Health Organization (WHO) definition of either severe or critical COVID-19: pneumonia with CURB-65 score ≥2, sustained respiratory rate >20/min, need for oxygen (or increased requirement for oxygen) or respiratory distress, alternation of general status or rapid worsening, or other acute co-existing condition. These criteria remained the same from the beginning of the first wave and over the study period.
The following exclusion criteria were used: (1) death, intubation, transfer from other hospitals or discharge within the first 24 hours of hospitalisation [10]; (2) not discharged on 31 May 31 2020; (3) hospitalisation for reasons other than COVID-19, such as delivery, emergency surgery, presence on a waiting list for nursing home and psychiatric in-patient, hospitalisation for isolation; and (4) the use of other COVID-19 experimental therapies (e.g., remdesivir, anakinra, azithromycin).
The experimental therapies, given alone or in combination, were used at the following dosages, based on institutional recommendations [11, 12]: (1) 400 mg of lopinavir/ritonavir twice daily for 5 days under the age of 75 years and 400 mg of lopinavir/ritonavir in the morning and 200 mg of lopinavir/ritonavir in the evening for patients over 75; (2) a single dose of 800 mg of hydroxychloroquine; and (3) lopinavir/ritonavir and hydroxychloroquine dual therapy at the same dosages. Lopinavir/ritonavir was first prescribed on 5 March 2020, and hydroxychloroquine alone or combined with lopinavir/ritonavir on 20 March 2020. Institutional recommendations did not change over the study period.
Clinical outcomes: The LOS for patients who were readmitted for reasons related to COVID-19 within 18 days of the first discharge was aggregated [13]. We also collected in-hospital mortality.
Additional COVID-19-related costs: The additional costs of the experimental therapies, including direct drug costs and costs associated with an extended LOS. In the absence of specific COVID-19 hospitalisation costs, we chose the most recent year of reference (2018). Additional hospitalisation costs were measured according to the 2018 Swiss accounting REKOLE® system. The 2018 mean cost per patient per day hospitalised was US$ 1152 (EUR 983, CHF 1061) for geriatric wards and US$ 2568 (EUR 2190, CHF 2365) for internal medicine wards. The cost calculation considered the direct costs of the different lopinavir/ritonavir regimens adapted for patients over 75 years of age and the direct hospitalisation costs. Public prices for the drugs were extracted from the official Swiss prices. Costs were converted from Swiss francs to dollars and euros, at the exchange rate of 1 CHF = US$ 1.09 = EUR 0.93 (1 October 2020).
Administrative data included age, gender, dates of admission and discharge, acute care in internal medicine and geriatrics, and the month of hospitalisation (February and March aggregated or April).
COVID-19 severity: The modified National Early Warning Score (mNEWS) adapted for use in COVID-19 was used to assess COVID-19 severity [14]. Age (<65 or ≥65years), mean respiratory rate, heart rate, systolic blood pressure and oxygen saturation, administration of supplemental oxygen, level of consciousness, and the highest temperature during the first 24 hours were used to compute the mNEWS, which ranged from 0 to 23.
Comorbidities: We extracted the relevant medical history, including the presence of active asthma, chronic obstructive pulmonary disease or sleep apnoea, cancer (previous 5 years), renal impairment, diabetes, hypertension, heart disease, and cirrhosis. Comorbidities were summed and the total score ranged from 0 to 8 comorbidities. Obesity (body mass index [BMI] ≥30 kg/m2) was also recorded and analysed separately from other comorbidities.
Contraindications: Medications that should not be co-administered with hydroxychloroquine and/or lopinavir/ritonavir were extracted on the basis of the Liverpool recommendations. QT interval corrected with the Bazett formula before the administration of any therapy or at admission when a therapy had already been administered was also extracted. Two binary variables were created: (1) hydroxychloroquine contraindications (medications and/or corrected QT ≥500 ms), and (2) lopinavir/ritonavir contraindications (medications).
We first described the cohort using descriptive statistics and tested which factors were associated with the outcomes using simple negative binomial (LOS) and logistic (in-hospital mortality) regressions. We then investigated the associations of experimental COVID-19 therapies with LOS and in-hospital mortality. We used propensity score matching analyses to minimise the effects of confounding factors. Propensity score matching was performed separately for each therapy (1) hydroxychloroquine, (2) lopinavir/ritonavir, and (3) hydroxychloroquine combined with lopinavir/ritonavir) compared with the standard of care. For each treatment group, the propensity score was estimated using a multiple probit regression that included the following factors: gender, location of hospitalisation, month of hospitalisation, mNEWS, number of comorbidities, BMI, and presence of contraindications for hydroxychloroquine (for analyses on hydroxychloroquine and hydroxychloroquine combined with lopinavir/ritonavir) or lopinavir/ritonavir (for analyses of lopinavir/ritonavir and hydroxychloroquine combined with lopinavir/ritonavir). We created separate matched control groups for each therapy using nearest-neighbour matching (full Mahalanobis). Multiple neighbours were allowed when controls had identical propensity scores. We calculated an average treatment effect on the treated for each outcome. Confidence intervals (CIs) were computed using bootstrapping. Crude associations (without propensity score matching) and associations for the matched groups (after propensity score matching) are reported for each therapy.
We performed deterministic sensitivity analyses to examine the robustness of our findings. We tested whether the results were similar using other propensity score methods: (1) covariate adjustment using the propensity score, (2) stratification based on the propensity score, (3) inverse probability weighting using the propensity score, and (4) nearest-neighbour matching using random draw. We also used the log of the LOS instead of the original count outcome. Finally, analyses were performed for different subsamples: (1) after the exclusion of cases not confirmed with RT-PCR, (2) after the exclusion of the most severe cases (patients admitted to the intensive care unit, patients who died), and (3) after exclusion of participants who had contraindications to the corresponding experimental therapy. Results were similar to those reported in the Results section.
Analyses were performed using Stata 15 (pscore for propensity score estimation, no imposition of common support and psmatch2 for propensity matching, option ties). Statistical significance was assessed at a two-sided 0.05 level for all analyses.
The study flowchart is depicted in figure 1. Among the 1113 patients who were admitted to HUG wards from 26 February to 30 April 2020, 1059 had a positive RT-PCR for SARS-CoV-2 and 54 patients tested negative but were strongly suspected of having the disease based on medical judgement. A total of 273 patients were excluded from the study because they were ineligible (see detail in fig. 1). Out of the 840 patients included in our analysis, 93 received hydroxychloroquine, 83 received lopinavir/ritonavir, 158 received hydroxychloroquine and lopinavir/ritonavir, and 506 received neither drug.

Figure 1 Flow chart of the study cohort. RT-PCR = reverse-transcriptase-polymerase-chain reaction; HCQ = hydroxychloroquine; LPV/r = lopinavir–ritonavir; AZM = azithromycin.
Descriptive statistics for the entire cohort are detailed in table 1. The mean age of included patients was 67.90 years (standard deviation [SD] 18.77), 50.5% were male (n = 424), and the mNEWS was 6.46 (SD 2.88) at admission. Most patients were hospitalised in internal medicine (69.9%, n = 587) and during the month of February/March 2020 (66.0%, n = 554). The average LOS was 10.38 days (SD 7.98), and a total of 15.6% (n = 131) patients died. Patients were more likely to receive lopinavir/ritonavir in February and March. The associations of variables included in the propensity scores with the outcomes are shown in supplementary table S1 (appendix 1). Before the propensity score matching, factors that were significantly associated with longer LOS included male gender, older age, hospitalisation in geriatrics, hospitalisation in April, a higher mNEWS, a high number of comorbidities, and having contraindications for hydroxychloroquine. Factors associated with mortality included male gender, older age, hospitalisation during the month of March, a higher mNEWS, and a high number of comorbidities.
Table 1 Baseline characteristics and outcomes of the full study cohort.
| Whole cohort | SOC | HCQ | LPV/r | HCQ + LPV/r | |
|---|---|---|---|---|---|
| n = 840 | n = 506 | n = 93 | n = 83 | n = 158 | |
| Gender † | |||||
| − Male | 50.5 (424) | 43.9 (222) | 59.1 (55) | 55.4 (46) | 36.1 (57) |
| − Female | 49.5 (416) | 56.1 (284) | 40.9 (38) | 44.6 (37) | 63.9 (101) |
| Age* | 67.90 (18.77, 0–101) | 70.75 (20.01, 0–101) | 66.14 (15.77, 16–96) | 63.40 (17.40, 28–90) | 62.15 (14.77, 21–95) |
| Location† | |||||
| − Main hospital | 69.9 (587) | 57.7 (292) | 83.9 (78) | 79.5 (66) | 95.6 (151) |
| − Hospital for elderly people | 30.1 (253) | 42.3 (214) | 16.1 (15) | 20.5 (17) | 4.4 (7) |
| Month of hospitalisation† | |||||
| − February/March | 66.0 (554) | 63.2 (320) | 54.8 (51) | 89.2 (74) | 69.0 (109) |
| − April | 34.0 (286) | 36.8 (186) | 45.2 (42) | 10.8 (9) | 31.0 (49) |
| mNEWS (0–23)* | 6.46 (2.88, 0–15) | 6.01 (2.96, 0–15) | 7.21 (2.86, 3–13) | 6.58 (2.59, 1–13) | 7.39 (2.49, 1–14) |
| Number of diseases (0–8)* | 1.59 (1.46, 0–7) | 1.75 (1.52, 0–5) | 1.72 (1.39, 0–5) | 1.31 (1.46, 0–5) | 1.13 (1.20, 0–5) |
| − Pulmonary diseases† | 13.2 (111) | 13.4 (68) | 16.1 (15) | 12.1 (10) | 11.4 (18) |
| − Asthma† | 3.6 (30) | 4.6 (23) | 2.2 (2) | 1.2 (1) | 2.5 (4) |
| − Cancer† | 9.6 (81) | 11.7 (59) | 8.6 (8) | 7.2 (6) | 5.1 (8) |
| − Renal impairment† | 26.7 (224) | 30.2 (153) | 25.8 (24) | 22.9 (19) | 17.7 (28) |
| − Diabetes† | 20.6 (173) | 20.8 (105) | 22.6 (21) | 20.5 (17) | 19.0 (30) |
| − Hypertension† | 48.5 (407) | 50.0 (253) | 55.9 (52) | 42.2 (35) | 42.4 (67) |
| − Heart diseases† | 31.1 (261) | 37.8 (191) | 34.4 (32) | 19.3 (16) | 13.9 (22) |
| − Liver diseases† | 5.2 (44) | 6.3 (32) | 6.5 (6) | 6.0 (5) | 0.6 (1) |
| BMI ≥30 kg/m2† | 17.7 (149) | 16.6 (84) | 22.6 (21) | 19.3 (16) | 17.7 (28) |
| Contraindications† | |||||
| − HCQ | 19.1 (153) | 24.1 (113) | 21.5 (20) | 11.1 (9) | 7.0 (11) |
| − LPV/r | 26.2 (220) | 33.0 (167) | 35.5 (33) | 9.6 (8) | 7.6 (12) |
| LOS* | 10.38 (7.98, 1.02–56.71) | 9.93 (7.78, 1.02–51.75) | 12.48 (9.56, 1.92–56.71) | 8.55 (6.98, 1.25–33.92) | 11.57 (8.07, 1.89–53.52) |
| Mortality† | 15.6 (131) | 18.6 (94) | 12.9 (12) | 10.8 (9) | 10.1 (16) |
BMI = body mass index; HCQ = hydroxychloroquine; LOS = length of stay); LPV/r = lopinivir/ritonavir; mNEWS = modified National Early Warning Score; SOC = standard of care * Means and (standard deviations with minimum and maximum) are reported. † Percentages and (n) are reported.
The top panel of table 2 reports the associations of factors before and after propensity score matching. In the entire cohort (hydroxychloroquine/standard of care, n = 599), the probability of receiving hydroxychloroquine was significantly higher in males (p = 0.007), patients hospitalised in internal medicine (p <0.001), and patients with a higher mNEWS (p <0.001, left panel of table 2). After propensity score matching, there were no significant differences between groups (right panel of table 2).
Table 2 Associations of factors and outcomes with HCQ, LPV/r, and their combination treatment before and after propensity score matching.
| Overall cohort | Propensity-based matched cohort | |||||
|---|---|---|---|---|---|---|
| HCQ | SOC | p-value | HCQ | SOC | p-value | |
| n = 93 | n = 506 | n = 93 | n = 105 | |||
| Variables of the propensity score | ||||||
| − Gender (ref. female)* | 0.59 | 0.44 | 0.007 | 00.59 | 00.60 | 0.882 |
| − Location (ref. internal medicine)* | 0.16 | 0.43 | <0.001 | 00.16 | 00.13 | 0.535 |
| − Month of hospitalisation (ref. Feb./March)* | 0.45 | 0.37 | 0.134 | 00.45 | 00.50 | 0.559 |
| − mNEWS† | 7.22 | 6.06 | 0.001 | 70.22 | 60.76 | 0.302 |
| − Number of diseases† | 1.72 | 1.80 | 0.627 | 10.72 | 10.62 | 0.661 |
| − BMI ≥30 kg/m2* | 0.23 | 0.17 | 0.169 | 00.23 | 00.23 | 10.00 |
| − Contraindications HCQ* | 0.22 | 0.24 | 0.592 | 00.22 | 00.16 | 0.351 |
| Outcomes | ||||||
| − LOS | 12.48 | 10.13 | 0.010 | 120.48 | 80.73 | 0.002 |
| − In-hospital mortality | 0.13 | 0.19 | 0.191 | 00.13 | 00.15 | 0.712 |
| LPV/r | SOC |
p-value
|
LPV/r | SOC |
p-value
|
|
| n = 83 | n = 506 | n = 83 | n = 98 | |||
| Variables of the propensity score | ||||||
| − Gender (ref. female)* | 0.55 | 0.44 | 0.050 | 00.55 | 00.49 | 0.440 |
| − Location (ref. internal medicine)* | 0.21 | 0.42 | <0.001 | 00.21 | 00.15 | 0.310 |
| − Month of hospitalisation (ref. Feb./March)* | 0.11 | 0.37 | <0.001 | 00.11 | 00.08 | 0.602 |
| − mNEWS† | 6.58 | 6.01 | 0.099 | 60.58 | 60.34 | 0.537 |
| − Number of diseases† | 1.31 | 1.75 | 0.016 | 10.31 | 10.28 | 0.873 |
| − BMI ≥30 kg/m2* | 0.19 | 0.17 | 0.548 | 00.19 | 00.12 | 0.202 |
| − Contraindications LPV/r* | 0.10 | 0.33 | <0.001 | 00.10 | 00.10 | 10.00 |
| Outcomes | ||||||
| − LOS | 8.55 | 9.93 | 0.124 | 80.55 | 70.32 | 0.319 |
| − In-hospital mortality | 0.11 | 0.19 | 0.086 | 00.11 | 00.08 | 0.639 |
| HCQ + LPV/r | SOC | p-value | HCQ + LPV/r | SOC | p-value | |
| n = 158 | n = 506 | n = 158 | n = 117 | |||
| Variables of the propensity score | ||||||
| − Gender (ref. female)* | 0.64 | 0.44 | <0.001 | 00.64 | 00.58 | 0.251 |
| − Location (ref. internal medicine)* | 0.04 | 0.43 | <0.001 | 00.04 | 00.04 | 0.778 |
| − Month of hospitalisation (ref. Feb./March)* | 0.31 | 0.37 | 0.182 | 00.31 | 00.30 | 0.807 |
| − mNEWS† | 7.39 | 6.06 | <0.001 | 70.39 | 70.25 | 0.621 |
| − Number of diseases† | 1.13 | 1.80 | <0.001 | 10.13 | 10.04 | 0.539 |
| − BMI ≥ 30* | 0.18 | 0.17 | 0.752 | 00.18 | 00.16 | 0.653 |
| − Contraindications HCQ* | 0.07 | 0.24 | <0.001 | 00.07 | 00.08 | 0.829 |
| − Contraindications LPV/r* | 0.08 | 0.34 | <0.001 | 00.08 | 00.03 | 0.081 |
| Outcomes | ||||||
| − LOS | 11.57 | 10.13 | 0.044 | 110.57 | 70.38 | <0.001 |
| − In-hospital mortality | 0.10 | 0.19 | 0.013 | 00.10 | 00.12 | 0.697 |
BMI = body mass index; HCQ = hydroxychloroquine; LOS = length of stay); LPV/r = lopinivir/ritonavir; mNEWS = modified National Early Warning Score; SOC = standard of care * Proportions are reported. † Means are reported.
After propensity score matching, hydroxychloroquine treatment was significantly associated with a longer LOS, with on average 3.75 additional days compared with matched patients treated with the standard of care (95% CI 1.37–6.12, p = 0.002). There was no significant difference in mortality between the two groups, with a difference of –0.02% on average (95% CI –0.14 – 0.09, p = 0.712).
The second panel of table 2 reports the associations of factors before and after propensity matching. In the entire cohort (lopinavir/ritonavir/standard of care, n = 589), the probability of receiving lopinavir/ritonavir was significantly higher in males (p = 0.050), patients hospitalised in internal medicine (p <0.001), patients hospitalised in March (p <0.001), patients with more comorbidities (p = 0.016), and patients with no contraindications for lopinavir/ritonavir (p < 0.001, left panel of table 2). After propensity score matching, no significant differences between groups remained (right panel of table 2).
After propensity score matching, the LOS was not significantly different between patients who received lopinavir/ritonavir and patients who received standard of care (1.23 days, 95% CI −1.24 – 3.51, p = 0.319). Mortality was also not significantly different, with an average difference of −0.05% (95% CI −0.19 – 0.02, p = 0.639).
The third panel of table 2 reports the associations of factors before and after propensity matching. In the entire cohort (hydroxychloroquine combined with lopinavir/ritonavir/standard of care, n = 664), the probability of receiving hydroxychloroquine combined with lopinavir/ritonavir was significantly higher in males (p <0.001), patients in internal medicine (p <0.001), patients with higher mNEWS (p <0.001), patients with more comorbidities (p <0.001), and patients without contraindications for both hydroxychloroquine (p <0.001) and lopinavir/ritonavir (p <0.001, left panel of table 2). After propensity score matching, no significant differences between groups remained (right panel of table 2).
After propensity score matching, hydroxychloroquine combined with lopinavir/ritonavir was significantly associated with an average of 4.19 days longer LOS compared with patients treated with the standard of care (95% CI 1.52–5.31, p <0.001). There was no significant difference in mortality between the two groups, with a difference of −0.05% on average (95% CI −0.15–0.05, p =0.697).
Table 3 reports results of the cost analysis. The total additional days of hospitalisation were 348.75 (95% CI 127.41–569.16) for hydroxychloroquine and 662.02 additional days (95% CI 240.16–838.98) for hydroxychloroquine combined with lopinavir/ritonavir, accounting for a total of 1010.77 additional days (95% CI 367.57–1408.14) for hydroxychloroquine and hydroxychloroquine combined with lopinavir/ritonavir. Costs associated with lopinavir/ritonavir were not included, as there was no significant association between LOS and prescription of lopinavir/ritonavir. Using the different costs for one day of hospitalisation in internal medicine or geriatric wards, the total additional costs were US$ 816,073 (EUR 695,984, CHF 751,637) (95% CI US$ 298,211–1,331,760; EUR 254,328–1,135,780; CHF 274,665–1,226,610) for hydroxychloroquine and US$ 1,676,141 (EUR 1,429,490, CHF 1,543,790) (95% CI US$ 618,629–2,118,859; EUR 527,595–1,807,060; CHF 569,783–1,951,560) for hydroxychloroquine plus lopinavir/ritonavir, accounting for a total cost of US$ 2,492,214 (EUR 2,125,470, CHF 2,295,430) (95% CI US$ 916,839–3,450,619; EUR 781,921–2,942,840; CHF 844,446–3,178,160).
Table 3 Total additional cost of LOS for HCQ and HCQ combined with LPV/r in US$.
| HCQ | HCQ + LPV/r | |
|---|---|---|
| Extra LOS (95% CI) | 3.75 (1.37–6.12) | 4.19 (1.52–5.31) |
| Internal medicine | ||
| Number of patients | 78 | 151 |
| Additional LOS (95% CI) | 292.5 (106.86–477.36) | 632.69 (229.52–801.81) |
| Additional cost of LOS (95% CI) in $ | 751,152 (274,421–1,225,880) | 1,624,773 (589,417–2,059,080) |
| Cost of experimental therapies in $ | 96 | 16,954 |
| Geriatrics | ||
| Number of patients | 15 | 7 |
| Additional LOS (95% CI) | 56.25 (20.55–91.8) | 29 (10.64:3 7.17) |
| Additional cost of LOS (95% CI) in $ | 64,807 (23,676–105,766) | 33,792 (12,059–42,825) |
| Cost of experimental therapies in $ | 18 | 621 |
| Total additional LOS (95% CI) | 348.75 (127.41–569.16) | 662.02 (240.16–838,98) |
| Total additional cost in $ | 816,073 (298,211–1,331,760) | 1,676,141 (618,629–2,118,859) |
CI = confidence interval; HCQ = hydroxychloroquine; LOS = length of stay; LPV/r = lopinavir/ritonavir
Our study showed no significant beneficial association between LOS and the administration of either hydroxychloroquine, lopinavir/ritonavir or their use in combination among hospitalised patients with severe or critical COVID-19, in line with recently published findings of randomised controlled trials and observational studies [3, 4, 10, 15, 16]. In particular, none of the regimens was associated with shortened hospital stays or higher survival, compared with COVID-19 patients treated with the standard of care. On the contrary, hydroxychloroquine (alone or when combined with lopinavir/ritonavir) was significantly associated with an increased LOS. Treatment with lopinavir/ritonavir alone did not demonstrate any statistically significant association with LOS.
Regarding the findings on hydroxychloroquine (alone or in combination with lopinavir/ritonavir), a first hypothesis is that these regimens would worsen COVID-19 or resulted in complications. Hydroxychloroquine is an old and affordable medication that was widely promoted and endorsed worldwide, based on very low level of scientific evidence, at the time of the study: in-vitro studies and studies with important methodological limitations, such as lack of control group and small number of patients included or bias [17–20]. Even though our study used a hydroxychloroquine single dose of 800 mg, lower than in other studies (e.g., RECOVERY: 2.4 g during the first 24 hours and a cumulative dose of 9.2 g over 10 days; SOLIDARITY: cumulative dose of 4 g over 10 days [21]), we found a significant increase of LOS.
A second hypothesis is that patients would be more likely to be discharged later in order to monitor potential adverse events, in an unprecedented political and media context that placed physicians under extraordinary pressure to prescribe these therapies off-label [22]. Of note, the literature highlighted the hydroxychloroquine safety risks that could lead to QT interval prolongation or adverse events [17, 20, 23–25]. Despite local guidance requiring that hydroxychloroquine could only be prescribed in absence of contraindications, about 22% of patients who had contraindications nevertheless received it. This difference is important because the toxicity may outweigh potential benefits, especially among older patients with more prevalent cardiac diseases, who may be at higher risk of adverse effects [26].
A third hypothesis is that prognostic imbalances between groups have remained. As it was a naturalistic study, residual confounding that would not have been captured by available variables in the database might have remained. Even though we used propensity score analyses to address this methodological flaw, only known confounders could be included in the study [27]. Therefore, we could not exclude the possibility that the negative association of hydroxychloroquine and hydroxychloroquine combined with lopinavir/ritonavir and LOS was caused by an unknown confounding factor, including a time-dependent variable (such as change in recommendations during the study course).
Lopinavir/ritonavir did not show any significant association with LOS or in-hospital mortality. This is in line with recently published findings showing no benefit of lopinavir/ritonavir on clinical outcomes for patients hospitalised with COVID-19 [3].
In addition, these experimental therapies resulted in important additional healthcare costs. Our findings showed that the prolonged LOS resulted in 1010.77 additional days (95% CI 367.57–1408.14) over the study period, which resulted in a total additional cost of US$ 2,492,214 (95% CI US$ 916,839–3,450,619). Therefore, experimental COVID-19 therapy costs were not associated with clinical benefits, but might have resulted in a reduced availability of hospital beds and a substantial healthcare cost. During such a pandemic, it is crucial to keep healthcare systems afloat and every single additional day of hospitalisation may become detrimental [28]. Furthermore, we should keep in mind that the total additional 1010.77 days and costs of US$ 2,492,214 are underestimated. Indeed, we only considered hydroxychloroquine and hydroxychloroquine combined with lopinavir/ritonavir for which the LOS was significantly different from zero and thus excluded lopinavir/ritonavir alone. In addition, the costs were based on the most recent year of reference (2018) and obviously real COVID-19 costs were higher. We also excluded some patients under hydroxychloroquine and hydroxychloroquine combined with lopinavir/ritonavir from the analysis. Therefore, our estimate of COVID-19 therapy costs should be considered to be conservative.
Our study showed that hydroxychloroquine and lopinavir/ritonavir were not associated with better clinical outcomes compared with standard of care. We rather found that patients who received hydroxychloroquine (alone or combined with lopinavir/ritonavir) had a significantly longer LOS. Prolonged LOS is associated with increased costs and therefore our study suggests negative issues among patients as well as for the whole healthcare system. In the context of COVID-19, these factors are key strategic elements because healthcare systems are facing tremendous pressure worldwide.
Findings from routine care studies, such as the present study, are crucial as a supplement to randomised controlled trials for the development of a better understanding of the potential benefits of therapies [29]. Indeed, our study population reflected the entire population hospitalised for COVID-19-related reasons, including elderly people, patients with comorbidities, and patients with contraindications to therapies. These subgroups are often excluded from randomised controlled trials and therefore their findings lack external validity [30]. Indeed, trials exclude some categories of patients, which limits the generalisability of their results [29]. Other issues related to external validity include potential better care in all arms and unrepresentativeness of healthcare professionals involved in the study [29]. Therefore, randomised controlled trials often provide information on achievement in the most favourable conditions, and not real-life conditions.
This study had important limitations that should be considered in the interpretation of these findings. First, we excluded patients with severe COVID-19 who were intubated at admission and most of the patients in the intensive care unit received other therapeutic strategies. Second, only a minority of patients included in the standard of care had identified contraindications, and therefore we may have missed the reasons for the non-prescription of experimental therapies. Subjective, prescriber-based opinions may be a factor explaining these differences. Third, we could not explain the reasons for increased LOS in patients treated with hydroxychloroquine or a combination of hydroxychloroquine and lopinavir/ritonavir (e.g., complications related to COVID-19 or treatment side effects). We formulated hypotheses, but they should be interpreted cautiously in light of residual confounding, and causal inference was not possible. Overall, there was an indication bias, with the reason of prescription being associated with the outcome of interest. Indeed, patients with more severe COVID-19 were more likely to receive experimental therapies. We captured severity of COVID-19 with the mNEWS, but it was used retrospectively and not to decide which experimental treatment should be used. Another limitation was that this study was a single-centre study and it might not be generalisable. Finally, the costs were underestimated, as they were based on the most recent year of reference (2018). COVID-19 costs were higher, due to the use of more expensive equipment. Our cost estimates should be updated when the cost for 2020 are available.
Our study showed that, in comparison with the standard of care, hydroxychloroquine or a combination of hydroxychloroquine and lopinavir/ritonavir was associated with an increased LOS and no significant association with in-hospital mortality. Prescribing experimental therapies for COVID-19 was also associated with additional costs (total additional costs US$ 2,492,214). Healthcare systems in a pandemic situation experience tremendous pressure and prolonged LOS and their related costs are key issues that all stakeholders should consider.
Table S1 Associations of factors included in the propensity score with outcomes.
| LOS* | Mortality† | |||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | OR (95% CI) | p-value | |
| Gender (ref. female) | 0.05 (−0.04 – 0.14) | 0.241 | 20.17 (10.32–30.56) | 0.002 |
| Age in years | 0.01 (0.003–0.01) | <0.001 | 10.10 (10.07–10.14) | <0.001 |
| Location (ref. internal medicine) | 0.16 (0.04–0.28) | 0.012 | 10.22 (00.67–20.25) | 0.516 |
| Month of hospitalisation (ref. Feb./March) | 0.12 (0.03–0.21) | 0.007 | 00.39 (00.22–00.67) | 0.001 |
| mNEWS | 0.03 (0.01–0.05) | <0.001 | 10.42 (10.28–10.57) | <0.001 |
| Number of diseases | 0.09 (0.06–0.13) | <0.001 | 10.43 (10.19–10.73) | <0.001 |
| BMI ≥30 kg/m2 | −0.02 (−0.14 – 0.10) | 0.752 | 10.08 (00.56–20.10) | 0.818 |
| Contraindications HCQ | 0.15 (0.03–0.26) | 0.012 | 10.01 (00.58–10.77) | 0.910 |
| Contraindications LPV/r | 0.07 (−0.04 – 0.17) | 0.211 | 10.03 (00.61–10.75) | 0.971 |
BMI = body mass index; CI = confidence interval; HCQ = hydroxychloroquine; LOS = length of stay); LPV/r = lopinivir/ritonavir; mNEWS = modified National Early Warning Score; OR = odds ratio * Simple negative binomial regressions (outcome: length of hospitalisation). † Simple logistic regressions (outcome: mortality).
We thank Dr Laurent Gétaz and Prof. Laurent Kaiser for their support and early contribution.
No financial support and no potential conflict of interest relevant to this trial were reported.
1 Salathé M , Althaus CL , Neher R , Stringhini S , Hodcroft E , Fellay J , et al. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150:w20225. doi:.https://doi.org/10.4414/smw.2020.20225
2 Siemieniuk RAC , Bartoszko JJ , Ge L , Zeraatkar D , Izcovich A , Kum E , et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi:.https://doi.org/10.1136/bmj.m2980
3 Horby PW , Mafham M , Bell JL , Linsell L , Staplin N , Emberson J , et al.; RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345–52 doi:.https://doi.org/10.1016/S0140-6736(20)32013-4
4 Horby P , Mafham M , Linsell L , Bell JL , Staplin N , Emberson JR , et al., RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383(21):2030–40. doi:.https://doi.org/10.1056/NEJMoa2022926
6 Griffin S . Covid-19: Lopinavir-ritonavir does not benefit hospitalised patients, UK trial finds. BMJ. 2020;370:m2650. doi:.https://doi.org/10.1136/bmj.m2650
7 https://www.recoverytrial.net/news/no-clinical-benefit-from-use-of-lopinavir-ritonavir-in-hospitalised-covid-19-patients-studied-in-recovery [accessed 2020 July 14].
8 Stringhini S , Wisniak A , Piumatti G , Azman AS , Lauer SA , Baysson H , et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–9. doi:.https://doi.org/10.1016/S0140-6736(20)31304-0
9 Prokop M , van Everdingen W , van Rees Vellinga T , Quarles van Ufford H , Stöger L , Beenen L , et al.; COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology. 2020;296(2):E97–104. doi:.https://doi.org/10.1148/radiol.2020201473
10 Geleris J , Sun Y , Platt J , Zucker J , Baldwin M , Hripcsak G , et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–8. doi:.https://doi.org/10.1056/NEJMoa2012410
11 Kalil AC . Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA. 2020;323(19):1897–8. doi:.https://doi.org/10.1001/jama.2020.4742
12 Windisch O , Zamberg I , Zanella M-C , Gayet-Ageron A , Blondon K , Schiffer E , et al. Using mHealth to increase the reach of local guidance to health professionals as part of an institutional response plan to the COVID-19 outbreak: Usage analysis study. JMIR Mhealth Uhealth. 2020;8(8):e20025. doi:.https://doi.org/10.2196/20025
13Swiss DRG. Règles et définitions pour la facturation des cas selon SwissDRG. Bern, Switzerland: SwissDRG; 2018.
14 Liao X , Wang B , Kang Y . Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med. 2020;46(2):357–60. doi:.https://doi.org/10.1007/s00134-020-05954-2
15 Joseph BA , Dibas M , Evanson KW , Paranjape G , Vegivinti CTR , Selvan PT , et al. Efficacy and safety of lopinavir/ritonavir in the treatment of COVID-19: A systematic review. Expert Rev Anti Infect Ther. 2020;14787210.2021.1848545. doi:.https://doi.org/10.1080/14787210.2021.1848545
16 Cortegiani A , Ippolito M , Ingoglia G , Iozzo P , Giarratano A , Einav S . Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care. 2020;59:176–90. doi:.https://doi.org/10.1016/j.jcrc.2020.06.019
17 Juurlink DN . Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450–3. doi:.https://doi.org/10.1503/cmaj.200528
18 Guastalegname M , Vallone A . Could Chloroquine /Hydroxychloroquine Be Harmful in Coronavirus Disease 2019 (COVID-19) Treatment? Clin Infect Dis. 2020;71(15):888–9. doi:.https://doi.org/10.1093/cid/ciaa321
19 Tanne JH . Covid-19: Trump is criticised for again promoting unorthodox medical information. BMJ. 2020;370:m3046. doi:.https://doi.org/10.1136/bmj.m3046
20 Yazdany J , Kim AHJ . Use of Hydroxychloroquine and Chloroquine During the COVID-19 Pandemic: What Every Clinician Should Know. Ann Intern Med. 2020;172(11):754–5. doi:.https://doi.org/10.7326/M20-1334
21Randomised evaluation of Covid-19 therapy (recovery) https://www.recoverytrial.net/files/protocol-archive/recovery-protocol-v6-0-2020-05-14.pdf/@@download [accessed 2020 July 14].
22 Kim AHJ , Sparks JA , Liew JW , Putman MS , Berenbaum F , Duarte-García A , et al. A Rush to Judgment? Rapid Reporting and Dissemination of Results and Its Consequences Regarding the Use of Hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172(12):819–21. doi:.https://doi.org/10.7326/M20-1223
23 Bonow RO , Hernandez AF , Turakhia M . Hydroxychloroquine, Coronavirus Disease 2019, and QT Prolongation. JAMA Cardiol. 2020;5(9):986–7. doi:.https://doi.org/10.1001/jamacardio.2020.1782
24 Mercuro NJ , Yen CF , Shim DJ , Maher TR , McCoy CM , Zimetbaum PJ , et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1036–41. doi:.https://doi.org/10.1001/jamacardio.2020.1834
25 Tang W , Cao Z , Han M , Wang Z , Chen J , Sun W , et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi:.https://doi.org/10.1136/bmj.m1849
26 Borba MGS , Val FFA , Sampaio VS , Alexandre MAA , Melo GC , Brito M , et al.; CloroCovid-19 Team. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4):e208857. doi:.https://doi.org/10.1001/jamanetworkopen.2020.8857
27 Straw S , Witte KK . Observational data during the COVID-19 pandemic: opportunity with uncertainty. Heart. 2020;106(19):1461–2. doi:.https://doi.org/10.1136/heartjnl-2020-317486
28 Lemos DRQ , D’Angelo SM , Farias LABG , Almeida MM , Gomes RG , Pinto GP , et al. Health system collapse 45 days after the detection of COVID-19 in Ceará, Northeast Brazil: a preliminary analysis. Rev Soc Bras Med Trop. 2020;53:e20200354. doi:.https://doi.org/10.1590/0037-8682-0354-2020
29 Black N . Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312(7040):1215–8. doi:.https://doi.org/10.1136/bmj.312.7040.1215
30 Rothwell PM . External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82–93. doi:.https://doi.org/10.1016/S0140-6736(04)17670-8
No financial support and no potential conflict of interest relevant to this trial were reported.