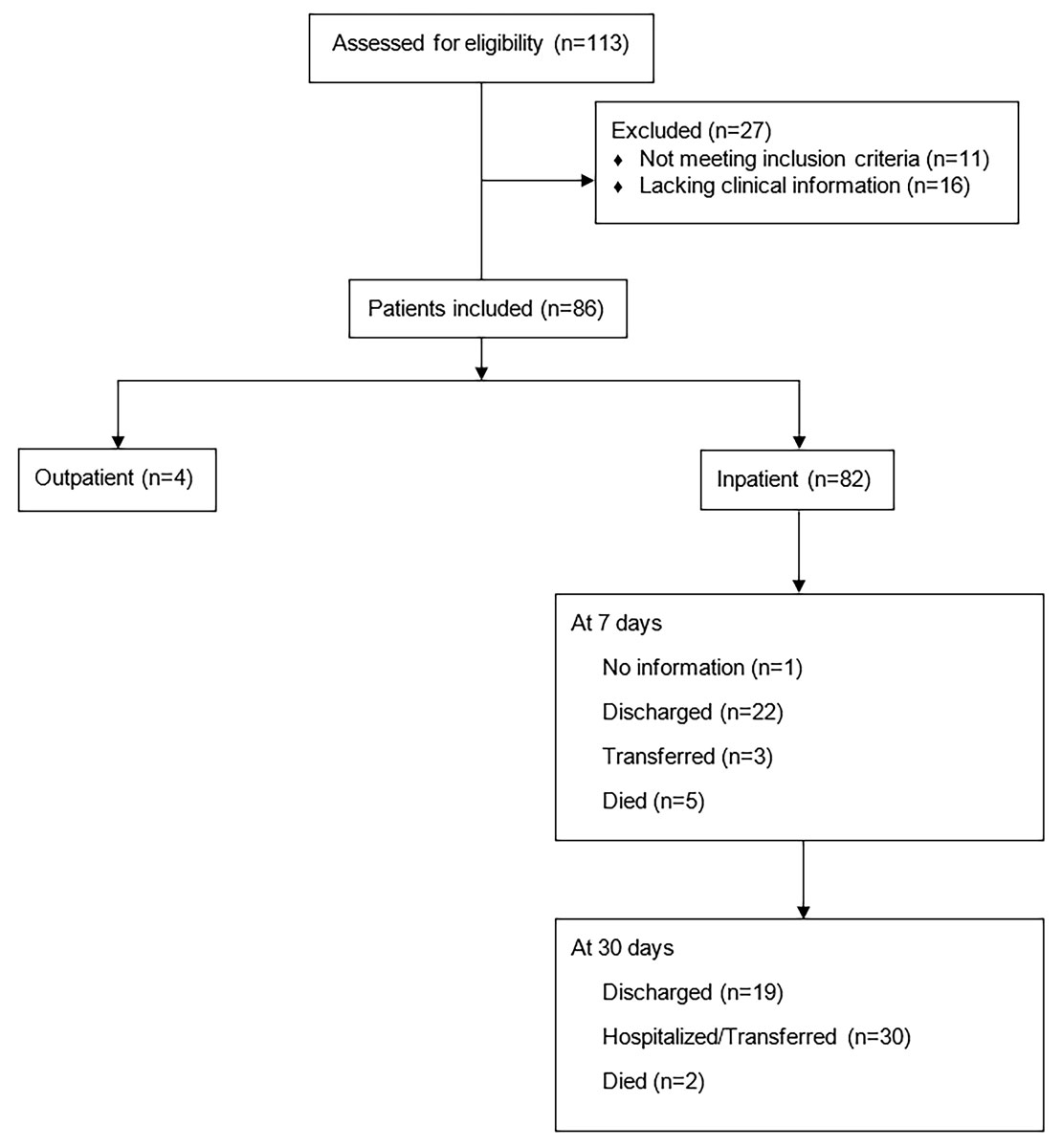
Figure 1 Consort diagram showing type of admission and outcome at 7 and 30 days post admission of 86 patients with invasive beta-haemolytic streptococcal infections.
DOI: https://doi.org/10.4414/smw.2020.20378
The gram-positive beta-haemolytic bacterium Streptococcus pyogenes (group A Streptococcus, GAS) causes a wide spectrum of diseases, ranging from superficial diseases to severe invasive infections such as bacteraemia, necrotising fasciitis and streptococcal toxic shock syndrome (STSS) [1–5]. The estimated yearly global burden of invasive GAS infections is 663,000 new cases and 63,000 deaths [6, 7]. Although isolated less frequently than GAS, other beta-haemolytic streptococci, such as Streptococcus dysgalactiae ssp. equisimilis (groups C and G) and the Streptococcus anginosus group, may also cause severe invasive infections [8–13].
The clinical presentation of invasive streptococcal infections varies strongly depending on both the host and the microorganism [14], with necrotising fasciitis and STSS being the most severe presentations. Necrotising fasciitis is one of the most feared bacterial infections due to its rapid clinical course and high case fatality rate of almost 30% at 7 days. This rises to 50–60% if accompanied by STSS [7, 15–19]. Risk factors for invasive streptococcal infections are conditions affecting skin integrity such as skin lesions, penetrating trauma, skin breach, pregnancy, childbirth, gynaecological procedures, malignancy, obesity, alcohol use disorders, diabetes and immunosuppression [7, 9, 20–23].
The management of invasive beta-haemolytic streptococcal infections includes antibiotic treatment and, for necrotising fasciitis, immediate surgery [24]. Penicillin is the antibiotic of choice. Guidelines advise combining penicillin with clindamycin for GAS-associated necrotising fasciitis [24–32]. By reducing toxin production, clindamycin is expected to reduce tissue and host damage. In addition, polyspecific human intravenous immunoglobulins (IVIG) inhibit the activity of toxin and virulence factors in both STSS and necrotising fasciitis [33, 34]. Although a retrospective analysis failed to show a clinical benefit of IVIG [35], its use in treatment of necrotising fasciitis and STSS is recommended by several guidelines [34, 36, 37].
GAS expresses several virulence factors, including M protein, which is involved in the adhesion to host epithelial cells and escape from phagocytosis [38]. Classification of GAS strains is carried out by sequencing the emm gene’s variable region. Different emm serotypes are associated with strains with different degrees of virulence [39]. Necrotising infections caused by the highly virulent emm1 and emm3 GAS types are normally associated with STSS in about 50% of cases [40, 41]. T-antigen, a backbone subunit of the pilus protein, which is attached to the surface of S. pyogenes, can also be used for strain characterisation. In addition, GAS and S. dysgalactiae ssp. equisimilis produce various superantigens [42] that trigger a strong immune response, one of the causes of STSS [43–46].
Due to the severity of GAS infections, many countries have continuous surveillance monitoring programmes in place. This is not the case for Switzerland, where, except for sporadic case reports and one report of an outbreak, overarching epidemiological and microbiological data on GAS infections are lacking [47–49].
In this study, we aimed to characterise the clinical characteristics of patients diagnosed with invasive beta-haemolytic streptococcal infections and admitted to the University Hospital Zurich (UHZ), Switzerland between 2000 and 2014. We analysed epidemiological data and incidence rates, as well as the treatment regimens and clinical outcomes of the patients. We also reviewed the microbiological characteristics and antibiotic susceptibilities of the streptococcal bacterial isolates.
This retrospective analysis included patients presenting with invasive S. pyogenes, S. dysgalactiae ssp. equisimilis and S. anginosus infections who were treated at USZ between May 2000 and May 2014. Inclusion criteria were invasive infections originally verified by infectious disease specialists as being confirmed by isolation of the above-mentioned streptococci in sterile (blood, joint) or non-sterile sites (soft tissue, superficial swab, serous cavities). All samples were processed at the Institute of Medical Microbiology (IMM), Zurich. Exclusion criteria were incomplete clinical and microbiological data and non-invasive infections. Polymicrobial infections were defined as infections by beta-haemolytic streptococci and at least one additional bacterial or fungal species. The plausibility of the identified necrotising fasciitis incidence was assessed by an additional systematic review of medical records in the electronic patient information system (“KISIM Corp” Version 5.1.0.3, CISTEC AG, Zürich).
Microbiological identification of the clinical isolates was performed at the IMM using latex agglutination tests (Pastorex Strep, Sanofi, Pasteur, France) until 2010. From 2010 onwards, conventional culture-based and molecular biology techniques, including matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) with occasional verification by latex agglutination, were used [50, 51].
Emm-typing was performed either at the German National Reference Centre for Streptococci (GNRCS), University Hospital RWTH Aachen, Aachen, Germany, or in the Zinkernagel laboratory at the USZ, and was carried out according to the recommendations of the Centers for Disease Control and Prevention [52].
T-typing and superantigen gene detection were performed on a subset of strains by the GNRCS, as described previously [53, 54].
Antimicrobial susceptibility testing was performed either at the IMM, the Zinkernagel laboratory USZ or the GNRCS. The IMM and the Zinkernagel laboratory USZ performed susceptibility testing using the Kirby–Bauer disk diffusion method [55]. The interpretation of the zone diameters was conducted according to the corresponding yearly guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Susceptibility testing at the GNRCS was performed using the micro-broth dilution method and susceptibility categorisation according to the Clinical and Laboratory Standards Institute and EUCAST breakpoints for trimethoprim/sulfamethoxazole [56].
All demographic, clinical and laboratory data were collected retrospectively from a clinical patient data management system. Diagnoses were categorised into soft tissue infections, STSS, bacteraemia, respiratory tract infections, urogenital/perineal infections and other infections. A soft tissue infection was defined as an infection spreading to skin, subcutaneous fat and skeletal muscle. Patients with soft tissue infections were divided into necrotising fasciitis and no necrotising fasciitis subgroups. We analysed the incidence per 100,000 population, epidemiology, known risk factors and complications (distributive shock, disseminated intravascular coagulation, acute renal failure, liver abnormality and acute respiratory distress syndrome [ARDS]) of invasive beta-haemolytic streptococcal infections (see table 1 below). Immunocompromised patients included individuals suffering from immunosuppressive diseases such as human immunodeficiency virus (HIV), undergoing immunosuppressive therapy, suffering from active cancerous disease, and not specified. Antibiotic treatment given within the first two weeks of hospitalisation was analysed. Antibiotics were categorised as β-lactam antibiotics, protein synthesis inhibitors (PSIs) or others. IVIG treatment was categorised as immediate (within 24 hours of admission) or delayed (>24 hours from admission). To assess whether the observed incidence was underestimated due to the study design, we additionally searched for patients with invasive streptococcal infections in the electronic patient medical records system. The medical data management system department retrieved cases admitted to the internal medicine, plastic surgery, dermatology, emergency and infectious diseases departments between May 2000 and May 2014 if the terms “fasciitis”, “STSS” or “Fournier gangrene” were found in the records. We manually reviewed these patients’ records to identify cases fulfilling the criteria for a beta-haemolytic necrotising fasciitis infection and used the number of cases identified to calculate the yearly incidence.
We analysed the dataset with the SPSS 22 software package (SPSS Inc., Chicago, IL, USA). For comparison of non-normally distributed variables we used the Mann–Whitney U test. Binomial data (dichotomous outcomes, categorical variables) were analysed with the chi-square test, or Fisher’s exact test if the numerator was <5. Results were considered statistically significant at p-values <0.05. Cut-off values for laboratory parameters provided by the diagnostics laboratory of the USZ were used: C-reactive protein (CRP) <5 mg/l, leucocytes 3.0‒9.6 g/l, neutrophils 1.40‒8.00 g/l.
The study was approved by the Cantonal Ethics Committee (Kantonale Ethikkommission Zurich, Switzerland, BASEC-Nr. 2016-00145).
Of the 113 cases of invasive beta-haemolytic streptococcal infection reported to the infectious disease specialists between May 2000 and May 2014 (fig. 1 and table S1 in appendix 1), 27 were excluded due to lack of data. In total, 86 patients were included in the analysis (fig.1), resulting in a yearly incidence of invasive infections of 0.47 per 100,000 population and a yearly incidence of necrotising fasciitis of 0.24 per 100,000 population for the canton of Zurich [57]. All included patients were over 18 years of age. Median age at presentation was 44 years and 13 patients were older than 65 years. Population characteristics, clinical presentation, outcome and risk factors are shown in table 1 (for the overall study population) and in table 2 (for the necrotising fasciitis and no necrotising fasciitis subgroups). The additional systematic search of the electronic medical records found a yearly necrotising fasciitis incidence of 0.33 cases per 100,000 population (fig. S1).

Figure 1 Consort diagram showing type of admission and outcome at 7 and 30 days post admission of 86 patients with invasive beta-haemolytic streptococcal infections.
Table 1 Population characteristics, clinical presentation, laboratory parameters, risk factors, complications and treatment (including antibiotic treatment, IVIG treatment, ICU admission and surgery performed due to infection) of 86 patients with invasive beta-haemolytic streptococcal infections.
|
Patients with invasive streptococcal infections (% of total)
(n = 86) |
|||
|---|---|---|---|
| Demographics | |||
| Age (years), median (IQR) | 44 (36‒55.0) | ||
| Gender, male | 41 (48.2) | ||
| Site of isolation | |||
| Soft tissue | 46 (53.5) | ||
| Superficial swab | 19 (22.1) | ||
| Blood culture | 15 (17.4) | ||
| Serous cavities | 3 (3.5) | ||
| Unknown site | 2 (2.3) | ||
| Joints | 1 (1.1) | ||
| Clinical presentation |
Sterile samples
n (%) |
Non-sterile samples
n (%) |
|
| Soft tissue infections | 66 (76.7) | ||
| Necrotising fasciitis | 42 (48.8) | 3 (7.1) | 39 (92.9) |
| – With STSS | 17 | ||
| – With bacteraemia | 11 | ||
| Non-necrotic soft tissue infections* | 24 (27.9) | 1 (4.2) | 23 (95.8) |
| – With STSS | 3 | ||
| – With bacteraemia | 2 | ||
| Respiratory tract infections | 6 (7.0) | 3 (50) | 3 (50) |
| Urogenital/perineal infections† | 9 (10.5) | 5 (55.6) | 4 (44.4) |
| Other clinical presentation‡ | 5 (5.8) | 4 (80) | 1 (20) |
| STSS (total) | 31 (36.0) | ||
| Bacteraemia (total) | 26 (30.2) | ||
| Laboratory parameters at admission | |||
| CRP (mg/l), median (IQR) | 183.0 (84.5–284.0) | ||
| Leucocytes (g/l), median (IQR) | 14.3 (9.77–19.34) | ||
| Neutrophils (g/l), median (IQR) | 12.4 (8.5–16.84) | ||
| Risk factors | |||
| Acute skin lesions | 24 (27.9) | ||
| Trauma | 10 | ||
| Surgery within 7 days | 7 | ||
| Ongoing intravenous drug use | 3 | ||
| Other§ | 4 | ||
| Immunosuppression¶ | 20 (23.3) | ||
| Chronic skin lesions | 6 (7.1) | ||
| Hospitalisation before occurrence of infection | 7 (8.2) | ||
| Complications | |||
| Distributive shock | 39 (45.3) | ||
| Acute renal failure | 30 (34.9) | ||
| DIC | 12 (13.9) | ||
| Liver abnormality | 12 (13.9) | ||
| ARDS | 9 (10.5) | ||
| Antibiotic treatment | |||
| β-lactam antibiotics | 82 (96.4) | ||
| – Penicillins | 64 (75.3) | ||
| – Amoxicillin/clavulanic acid | 43 (50.6) | ||
| – Piperacillin/tazobactam | 19 (22.4) | ||
| – Cephalosporins | 43 (50.6) | ||
| – Ceftriaxone | 41 (48.2) | ||
| – Others | 19 (22.3) | ||
| Protein synthesis inhibitors | 59 (69.4) | ||
| – Clindamycin | 54 (63.5) | ||
| – Gentamicin | 11 (12.9) | ||
| – Clarithromycin | 7 (8.2) | ||
| Others | 31 (36.5) | ||
| – Vancomycin | 14 (16.5) | ||
| – Metronidazole | 8 (9.4) | ||
| Combinations | |||
| – Clindamycin + β-lactam antibiotics | 52 (61.2) | ||
| IVIG | |||
| Within the first 24 h | 16 (18.6) | ||
| After 24 h | 8 (9.3) | ||
| Surgery | 70 (81.4) | ||
| ICU | 50 (58.1) | ||
ARDS = acute respiratory distress syndrome; CRP = C-reactive protein; DIC = disseminated intravascular coagulation; h = hours; HIV = human immunodeficiency virus; ICU = intensive care unit; IQR = interquartile range; IVIG = intravenous immunoglobulins; STSS = streptococcal toxic shock syndrome Information on laboratory findings at admission was available as follows: CRP levels for 81 patients, leucocyte levels for 86 patients, neutrophil levels for 78 patients. Information on risk factors and complications was available for 85 patients. Information about antibiotic treatment was available for 85 patients. Patients could be characterised by multiple sites of infection, risk factors and complications. * Site of infection: 10 (41.6%) extremities, 11 (45.8%) oropharynx, 2 (8.3%) urogenital/perineal area, 1 (4.1%) face. † One patient with necrotic myoma, 1 with acute cystitis with GAS, 1 with abscess in the small bowel, 1 with puerperal sepsis and endometritis after birth, 2 with STSS with vaginal focus, 1 with postpartum STSS, 1 with bartholinitis and 1 with pelvic inflammatory disease. ‡ Two patients with meningitis, 1 patient with myocarditis, 1 patient with bacteraemia of unknown focus, 1 patient with ruptured abdominal aortal aneurysm with STSS. § One patient with insect bite, 1 patient with pustule as entry point for bacteria, 1 patient with wound after swimming, 1 patient with impetiginized erosions. ¶ Eight patients had diabetes mellitus, 3 immunosuppressive drug treatment, 3 2 chronic immunosuppressive disease, 2 HIV infection, 2 active cancerous disease, 1 alcohol use disorder, 1 not specified.
Table 2 Comparison of population characteristics, clinical presentation, laboratory parameters, risk factors, complications and treatment (including antibiotic treatment, IVIG treatment, ICU admission and surgery performed due to infection) of patients with invasive beta-haemolytic streptococcal infections categorised as necrotising fasciitis and or as without necrotising fasciitis.
|
Necrotising fasciitis
(n = 42) |
No necrotising fasciitis
(n = 44) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years), median (IQR) | 44 (37–62) | 42 (34–52) | 0.282 |
| Gender, male (%) | 23 (45.2) | 19 (43.2) | 0.146 |
| Site of infection (%)* | |||
| Extremities | 26 (61.9) | ||
| Oropharynx and mediastinum | 10 (23.8) | ||
| Bone and joint | 5 (11.9) | ||
| Urogenital/perineal area | 7 (16.7) | ||
| Other† | 4 (9.5) | ||
| STSS | 17 (40.5) | ||
| Bacteraemia | 11 (26.2) | ||
| Laboratory parameters at admission | |||
| CRP, mg/l, median (IQR) | 244 (163–322) | 134.5 (69–212.5) | 0.001‡ |
| Leucocytes, g/l, median (IQR) | 15.2 (10.4–22.7) | 13.5 (9.6–17.8) | 0.248‡ |
| Neutrophils, g/l, median (IQR) | 13.8 (9.6–19.6) | 11.4 (8.1–15.5) | 0.085‡ |
| Risk factors | |||
| No risk factors | 20 (47.6) | 23 (52.3) | – |
| Immunosuppression | 9 (21.4) | 10 (22.7) | 0.932§ |
| Diabetes | 4 (9.5) | 4 (9.1) | 1.000¶ |
| Acute skin lesions | 14 (33.3) | 10 (22.7) | 1.000§ |
| Trauma | 6 (14.3) | 3 (6.8) | 0.303¶ |
| Surgery within 7 days | 4 (9.5) | 3 (6.8) | 0.707¶ |
| Ongoing intravenous drug use | 2 (4.8) | 1 (2.3) | 0.607¶ |
| Other | 2 (4.8) | 3 (6.8) | – |
| Chronic skin lesions | 2 (4.8) | 4 (9.1) | 0.677¶ |
| Hospitalisation | 4 (9.5) | 3 (6.8) | 0.707¶ |
| Complications | 9 (21.4) | 10 (22.7) | 0.932§ |
| Distributive shock | 24 (57.1) | 16 (36.4) | 0.041 § |
| Acute renal failure | 22 (52.4) | 9 (20.5) | 0.001 § |
| DIC | 7 (16.7) | 5 (11.4) | 0.450§ |
| Liver abnormality | 7 (16.7) | 5 (11.4) | 0.450§ |
| ARDS | 5 (11.9) | 4 (9.14) | 0.733¶ |
| Antibiotics | |||
| β-lactam antibiotics | 42 (100) | 40 (91) | 0.241¶ |
| Protein synthesis inhibitors | 41 (97.6) | 19 (43.2) | <0.001 § |
| Clindamycin | 41 (97.6) | 13 (29.5) | <0.001 § |
| Clindamycin plus β-lactam | 41 (97.6) | 11 (25.0) | <0.001 § |
| Others‖ | 12 (28.5) | 18 (40.9) | 0.200§ |
| IVIG | |||
| Within 24 h | 14 (33.3) | 2 (4.5) | 0.001 § |
| After 24 h | 5 (11.9) | 3 (6.8) | – |
| ICU admissions | 33 (78.6) | 17 (38.6) | <0.001 § |
| Surgery | 42 (100) | 28 (63.6) | <0.001 § |
| Outcome | |||
| Death at 30 days | 5 (11.9) | 2 (4.5) | 0.255¶ |
ARDS = acute respiratory distress syndrome; CRP = C-reactive protein; DIC = disseminated intravascular coagulation; h = hours; ICU = intensive care unit; IQR = interquartile range; IVIG = intravenous immunoglobulin therapy; PSI = protein synthesis inhibitor; STSS = streptococcal toxic shock syndrome Information on laboratory parameters was available as follows. Necrotising fasciitis group: CRP for 41 patients; leucocytes for 40 patients; neutrophils for 39 patients. No necrotising fasciitis group: CRP for 40 patients; leucocytes for 40 patients; neutrophils for 39 patients. Information on IVIG and outcome was available for 41 patients in the necrotising fasciitis group. In the no necrotising fasciitis group, information on antibiotics was available for 43 out of 44 patients, and information on IVIG for 42 out of 44 patients. * Patients may have had many sites of infection † Four patients had necrotising fasciitis on the chest/flank ‡ p-value calculated using Mann-Whitney U-test ¶ p-value calculated using Fisher’s exact test § p-value calculated using the chi-square test ‖ Other antibiotics that were given additionally to β-lactam antibiotics and/or PSI
The majority of patients suffered from soft tissue infections (76.7%) (table 1). Of these, 48.8% had necrotising fasciitis and 27.9% had non-necrotic soft tissue infections. In patients with non-necrotic soft tissue infections, the site of infection was located on the extremities (41.6%), the oropharynx (45.8%), the urogenital/perineal region (8.3%) or the face (4.1%). Thirty-six percent of the patients presented with STSS and 30.2% had bacteraemia. Invasive infections manifested as respiratory tract infections in 7.0% of patients. Urogenital/perineal infections not categorised as soft tissue infections occurred in 10.5% of patients. Five patients showed a clinical presentation categorised as other, with two of these having meningitis. The median CRP at admission was 183.0 mg/ (interquartile range (IQR) 84.5–284.0). The median leucocyte count was 14.3 g/l (IQR 9.77–19.34) and the median neutrophil count was 12.4 g/l (IQR 8.5–16.84).
Forty-nine percent of the patients had one or more risk factors for a streptococcal infection (42 patients, table 1). Acute skin lesions (e.g., cut, wounds, surgery) were the most frequent risk factor (27.9%). Seven percent of patients had chronic skin lesions (e.g., underlying skin diseases such as psoriasis) and 22.1% were immunosuppressed, of which eight had diabetes mellitus, three were on immunosuppressive drugs (adalimumab, azathioprine and immunosuppression due to kidney-transplantation), three had immunosuppressive diseases (rheumatoid arthritis, systemic lupus erythematosis), two had HIV and two suffered from cancerous diseases. 8.2% of patients were hospitalised for other reasons, such as surgery, prior to diagnosis of invasive streptococcal infection.
At least one acute complication occurred in 55% of the patients. Distributive shock was the most common complication, occurring in 45.3% of patients, followed by acute renal failure (34.9%) (table 1). The case fatality rate was 6% at 7 days and 8.1% at 30 days post admission.
Rates of antibiotic treatment, surgical therapy and ICU therapy are depicted for the overall study population in table 1 and for the necrotising fasciitis and no necrotising fasciitis subgroups in table 2. All patients underwent antibiotic therapy. Eighty-two patients (96.4%) were treated with at least one β-lactam antibiotic, with amoxicillin/clavulanic acid the most commonly used (50.6%) followed by the third-generation cephalosporin ceftriaxone (48.2%) and by piperacillin/tazobactam (22.4%). Fifty-nine patients received a PSI (69.4%), most commonly clindamycin (63.5%). Antimicrobials from two or more classes were given to 63 patients (73.2%). The most common combination was a β-lactam plus clindamycin (61.2%). IVIG was received within 24 hours of admission in 16 patients (18.6%) and after more than 24 hours (9.3%) in 8 patients. Surgery was performed in 70 cases (81.4%) and 50 patients (58.1%) were admitted to the ICU.
Forty-two out of the 86 patients had necrotising fasciitis (48.8%). In most necrotising fasciitis cases the site of infection was the extremities (61.9%), followed by the oropharynx/mediastinum (23.8%) and the urogenital/perineal region (16.7%) (table 2). STSS occurred in 40.5% of the necrotising fasciitis cases and bacteraemia occurred in 26.2%. There was no significant difference in median age (44 vs 42 years; p = 0.282) or gender distribution (45% vs 43.2% male; p = 0.146) between patients with and without necrotising fasciitis (table 2).
CRP levels were significantly higher in patients with necrotising fasciitis (median 244, IQR 163–322 mg/l) than in patients without necrotising fasciitis (median 134.5, IQR 69–212.5 mg/l; p = 0.001). Median leucocyte counts (necrotising fasciitis group: 15.2 g/L, IQR 10.4–22.7; no necrotising fasciitis group: 13.5 g/l, IQR 9.6–17.8; p = 0.248) and median neutrophil counts (necrotising fasciitis group: 13.8 g/L, IQR 9.6–19.6; no necrotising fasciitis group: 11.4 g/l, IQR 8.1–15.5; p = 0.085) did not differ significantly (table 2, fig.2).
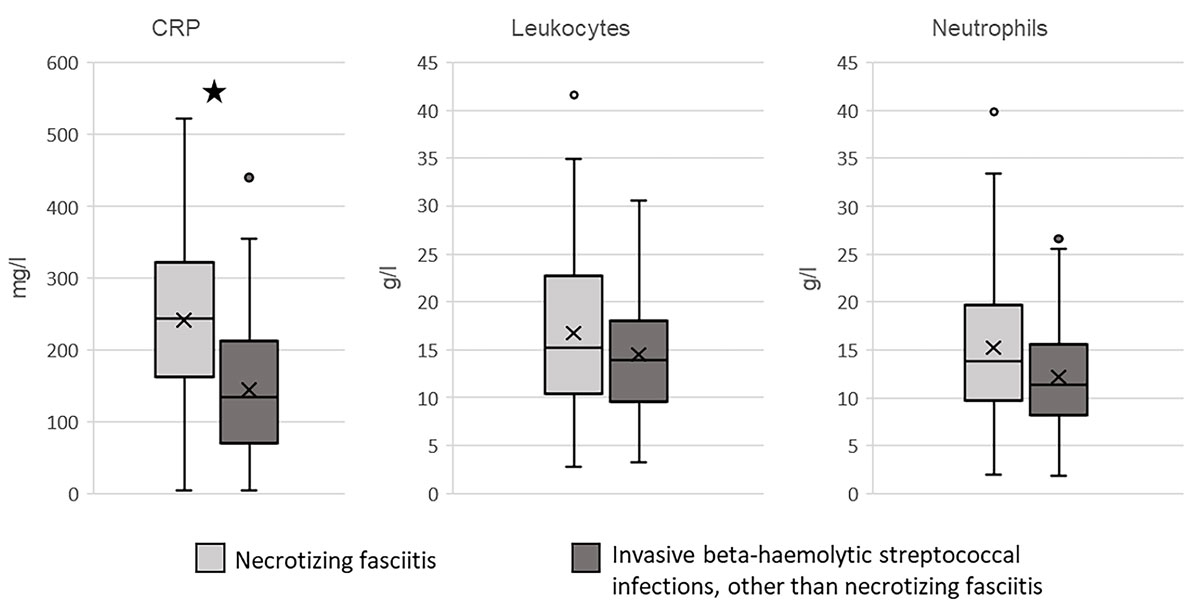
Figure 2 Laboratory values at admission. The box plots represent the C-reactive protein (CRP) levels, leucocyte counts and neutrophil values at admission in patients with (light grey) and without (dark grey) necrotising fasciitis. * Significant difference between patients with and without necrotising fasciitis; p = 0.001 (Mann–Whitney U test).
We found more acute skin lesions in patients with than in patients without necrotising fasciitis (33.3% vs 22.7%; p = 1.000), as well as more trauma in the necrotising fasciitis group (14.3% vs 6.8%; p = 0.303), but neither difference was significant. We did not observe significant differences in the presence of immunosuppression, chronic skin lesions or previous hospitalisation (table 2).
Patients with necrotising fasciitis had significantly more distributive shock (57.1% vs 36.4%; p = 0.041) and acute renal failure (52.4% vs 20.5%; p = 0.001) than those without necrotising fasciitis (table 2). The rates of ARDS, disseminated intravascular coagulation and liver abnormalities were comparable between the two groups (table 2). Necrotising fasciitis patients had a higher case fatality rate at 30 days post admission compared to patients without necrotising fasciitis (11.9% vs 4.5%), but no statistical significance could be detected (table 2).
Patients with and without necrotising fasciitis received β-lactam antibiotics (100% vs 91%; p = 0.241) (table 2). A significantly higher number of patients in the necrotising fasciitis group received a PSI (97.6% vs 43.2%; p <0.001). Clindamycin was the most frequently used PSI (97.6% vs 29.5%; p <0.001), and was mostly given in combination (β-lactam and clindamycin: 97.6% vs 25%; p <0.001). Additional antibiotics (categorised as “others”) were given in 28% of cases with necrotising fasciitis and in 41% of no necrotising fasciitis cases. Early IVIG treatment was given significantly more often to patients with necrotising fasciitis than to those without (33.3% vs 4.5%; p <0.001). There was no difference in the proportions of patients administered IVIG more than 24 hours post admission. All patients with necrotising fasciitis and 28 patients without necrotising fasciitis (63.6%) underwent surgery (p <0.001).
Ninety-one percent of the clinical isolates were S. pyogenes, 5.8% were S. dysgalactiae ssp. equisimilis and 2.3% were S. anginosus (table 3). Ten percent of patients had a polymicrobial infection, with Staphylococcus aureus being the most common second pathogen isolated (7.0%).
Table 3 Strain identification, monthly occurrence and microbiological characteristics (emm-types, T-types, superantigen) of clinical isolates from 86 patients with invasive beta-haemolytic streptococcal infections.
| n (%) | ||||
|---|---|---|---|---|
| Strain identification | ||||
| Streptococcus pyogenes | 79 (91.9) | |||
| Streptococcus dysgalactiae ssp. equisimilis | 5 (5.8) | |||
| Streptococcus anginosus | 2 (2.3) | |||
| Polymicrobial infection | ||||
| Staphylococcus aureus | 6 (7.0) | |||
| Enterococcus | 1 (1.2) | |||
| Pseudomonas aeruginosa | 1 (1.2) | |||
| Escherichia coli | 1 (1.2) | |||
| Bacteroides fragilis | 1 (1.2) | |||
| Streptococcus ssp. | 2 (2.3) | |||
| Fungi | 3 (3.5) | |||
| Infections per month | n (%) | |||
| January | 10 (11.8) | |||
| February | 13 (15.3) | |||
| March | 9 (10.6) | |||
| April | 10 (11.8) | |||
| May | 6 (7.1) | |||
| June | 8 (9.4) | |||
| July | 4 (4.7) | |||
| August | 6 (7.1) | |||
| September | 5 (5.9) | |||
| October | 2 (2.4) | |||
| November | 4 (4.7) | |||
| December | 8 (9.4) | |||
| Emm-type | n (% of n tested) | |||
| 1 | 24 (44.4) | |||
| 3 | 4 (7.4) | |||
| 12 | 4 (7.4) | |||
| 28 | 3 (5.6) | |||
| 89 | 5 (9.3) | |||
| Other | 14 (25.9) | |||
| T-type | n (% of n tested) | |||
| 1 | 20 (60.6) | |||
| 3 | 4 (12.1) | |||
| 12 | 3 (9.1) | |||
| 28 | 3 (9.1) | |||
| Others | 3 (9.1) | |||
| Superantigens | n (% of n tested) | |||
| SpeG | 39 (92.9) | |||
| smeZ | 37 (88.1) | |||
| SpeA | 25 (59.5) | |||
| SpeJ | 24 (57.1) | |||
| SpeC | 11 (26.2) | |||
| SpeK | 12 (28.6) | |||
| SsA | 4 (9.5) | |||
| SpeH | 4 (9.5) | |||
| SpeI | 3 (7.1) | |||
| SpeM | 1 (2.4) | |||
| SpeL | 1 (2.4) | |||
| Superantigens |
Necrotising fasciitis
n (% of n tested) |
No necrotising fasciitis
n (% of n tested) |
p-value | |
| SpeG | 26 (89.6) | 13 (100) | 0.540 * | |
| smeZ | 24 (82.7) | 13 (100) | 0.302 * | |
| SpeA | 18 (33.3) | 7 (53.8) | 0.616 † | |
| SpeJ | 18 (33.3) | 6 (46.1) | 0.335 † | |
| Emm-type |
Necrotising fasciitis
n (% of n tested) |
No necrotising fasciitis
n (% of n tested) |
p-value | |
| 1 | 18 (54.5) | 6 (28.6) | 0.061† | |
| 3 | 2 (6.1) | 2 (9.5) | 0.638* | |
| 12 | 4 (12.1) | 0 (0) | 0.148* | |
| 28 | 1 (3.0) | 2 (9.5) | 0.553* | |
| 89 | 1 (3.0) | 4 (19.0) | 0.069* | |
Spe = Superantigen; ssp. = subspecies 77 patients suffered from monobacterial infections and 9 from polymicrobial infections. Information was available as follows: monthly occurrence for 85 out of 86 patients, emm-types for 54 out of 86 isolates, T-types for 33 out of 86 isolates, superantigens for 42 out of 86 (necrotising fasciitis group: 29 out of 42; no necrotising fasciitis group: 13 out of 44). * p-value calculated using Fisher’s exact test † p-value calculated using the chi-square test
The most commonly isolated emm-type was emm1 (44.4% of all typed isolates), followed by emm89 (9.3%) (table 3). T-typing was available for 33 isolates: T-type 1 was the most common (60.6%), followed by T-type 3 (12.1%) and T-types 12 and 28 (9.1%) (table 3). Superantigens were assessed for 42 isolates. SpeG was detected in 92.9%, smeZ in 88.1%, speA in 59.5% and SpeJ in 57.1% of cases (table 3). There was no significant difference in superantigens between the necrotising fasciitis and no necrotising fasciitis groups (table 3).
All tested strains were susceptible to penicillin and clindamycin (table 4). Tetracycline was the only antibiotic against which resistance was found: 11.9% of the 45 tested isolates were resistant, of which 2.4% showed an intermediate resistance phenotype.
Table 4 Antibiotic susceptibility testing for clinical isolates from 86 patients with beta-haemolytic streptococcal infections.
| Antibiotic susceptibility* |
Isolates tested
n tested/n total |
Sensitive
n (% of n tested) |
Intermediate
n (% of n tested) |
Resistant
n (% of n tested) |
|---|---|---|---|---|
| β-lactam antibiotics | ||||
| Penicillin | 45/86 | 45 (100) | 0 (0) | 0 (0) |
| Amoxicillin | 30/86 | 30 (100) | 0 (0) | 0 (0) |
| Ampicillin | 16/86 | 16 (100) | 0 (0) | 0 (0) |
| Ceftriaxone | 16/86 | 16 (100) | 0 (0) | 0 (0) |
| Cefotaxime | 42/86 | 42 (100) | 0 (0) | 0 (0) |
| Piperacillin/tazobactam | 14/86 | 14 (100) | 0 (0) | 0 (0) |
| PSI | ||||
| Erythromycin | 25/86 | 25 (100) | 0 (0) | 0 (0) |
| Clarithromycin | 18/86 | 18 (100) | 0 (0) | 0 (0) |
| Clindamycin | 45/86 | 45 (100) | 0 (0) | 0 (0) |
| Gentamycin | 15/86 | 15 (100) | 0 (0) | 0 (0) |
| Tetracycline | 42/86 | 36 (85.7) | 1 (2.4) | 5 (11.9) |
| Telithromycin | 14/86 | 14 (100) | 0 (0) | 0 (0) |
| Others | ||||
| Levofloxacin | 43/86 | 43 (100) | 0 (0) | 0 (0) |
| Rifampicin | 14/86 | 14 (100) | 0 (0) | 0 (0) |
| Vancomycin | 19/86 | 19 (100) | 0 (0) | 0 (0) |
PSI = protein synthesis inhibitor. * According to EUCAST breakpoints at time of identification (2000–2014).
This is the first analysis of clinical and microbiological characteristics of patients with severe invasive beta-haemolytic streptococcal infections treated in Zurich, Switzerland. Eighty-six patients recruited between May 2000 and May 2014 were enrolled in this study, which provides information on invasive beta-haemolytic streptococcal infections in Switzerland and allows comparisons with other countries, including those enrolled in the Strep-EURO programme. We found yearly incidences of 0.47 cases per 100,000 population for invasive infections and of 0.24 cases per 100,000 population for necrotising fasciitis. The necrotising fasciitis incidence seems to be not considerably underestimated since the additional systematic search found a comparable incidence of 0.33 cases per 100,000 population.
One of the key findings of this study is the low case fatality rate in Zurich compared to other studies (Lamagni et al. 19% overall and 32% for necrotising fasciitis patients, Kaul et al. 34% for necrotising fasciitis patients) [7, 58]. In a retrospective study in Germany, the case fatality rate for patients with invasive GAS infections was 33% for necrotising fasciitis and 46% for STSS [22]. In the United States, a case fatality rate of 26.9% was described for patients with invasive GAS infections associated with necrotising fasciitis [19]. The frequent use of the antibiotic combination of penicillin and clindamycin in almost 61.2% of patients and surgical debridement in 81% of patients are possible explanations for the lower case fatality rate in our study. Furthermore, compared to other studies, our patients were younger (median age 44 vs 61 and 57.5 years [21, 58]). The virulence of our strains might have been lower, since the virulent M3 emm-type was found in only 12% of cases. As mentioned in the limitations, selection biases may also explain the low mortality.
The demographic and clinical data of the patients were similar to other studies. The median age of the patients included in our study was 44 years, similar to results from other national studies [31, 59]. Females and males were equally affected by invasive streptococcal infections, as previously observed in Cyprus, Greece and Italy in patients with severe GAS infections [7]. The invasive streptococcal infections had a seasonal pattern, with a higher number of cases during winter and spring, as reported previously (table 3) [7, 22]. Non-necrotic infections occurred in 27.9% of cases; similar rates were reported in France (30%) [59].
We found a broad range of manifestations of invasive streptococcal infections. The rates of soft tissue infections (76.7%) and severe manifestations such as necrotising fasciitis (48.8%) and STSS (36%) observed in our study were higher than those observed in an active surveillance study of 278 patients with invasive GAS infections carried out in Denmark between 2003 and 2004, where only 26% of patients had soft tissue infections and 6% had necrotising fasciitis [60]. Imöhl et al. reported similar numbers in Germany (necrotising fasciitis in 20.8% and STSS in 16.6% of all patients) [61]. This discrepancy could be due to a selection bias linked to the study design. The fact that only streptococcal infections that met the criteria of having been verified by infectious disease specialists and microbiologists were included in the study led to the selection of the most severe cases. In addition, all patients were admitted at the USZ, a tertiary care hospital treating on average more severely ill patients [31, 59].
In this study, we mostly observed highly elevated CRP levels (on average 36 times higher than normal levels) and neutrophil counts (on average 1.5 times higher than normal levels). The observed CRP values were significantly higher for the patients with necrotising fasciitis as compared with the results of Wong et al. in Singapore [62].
We moreover observed a high rate of complications, especially distributive shock (in 45.3% of cases) and acute renal failure (in 34.9% of cases). This prevalence is comparable to that of a Canadian prospective study of 77 patients with necrotising fasciitis, which found acute renal failure in 35% of cases [58]. Two other studies found lower rates of distributive shock and renal failure, which can be partially attributed to the more severe condition of patients admitted to reference hospitals such as our tertiary care hospital [7, 61]. Here again, the selection of severe cases due to our study design is probably the main reason behind this discrepancy.
Mehta et al. found that ARDS was associated with invasive GAS infections in 34% of patients [63], while we found less ARDS in the necrotising fasciitis subgroup. One reason for this is that the definition of ARDS has been constantly changing in recent years. Another reason may be the lack of documentation and the fact that the ventilation parameters, which would have revealed the number of unreported cases of ARDS, were not recorded [64]. Only half of patients with invasive streptococcal infections in our study showed risk factors, with acute skin lesions and immunosuppression (27.9% and 22.1%) the most common. Kaul et al. found a lower percentage of immunosuppressed patients in their necrotising fasciitis group (13%), but a higher number of patients with skin lesions (47%) [58].
Patients with necrotising fasciitis were mostly treated with β-lactam antibiotics and clindamycin, and all of them underwent surgery, as advised by international guidelines. IVIG was given to almost half of the necrotising fasciitis patients. The retrospective nature of the study does not allow us to assess the reasons for using (or not using) IVIG or the effect of IVIG. Based on the available literature, adding IVIG to the treatment of patients with severe GAS infections, including those with necrotising fasciitis or STSS, seems advisable, but there is not yet clear evidence of its efficacy [34, 65–67].
In our study, GAS constituted 91.9% of all pathogens isolated from patients with invasive streptococcal infections. Only 5.8% and 2.3% of the pathogens were categorised as S. dysgalactiae ssp. equisimilis and S. anginosus, respectively. This is in contrast to findings from Norway, where almost 50% of invasive streptococcal infections were due to streptococci categorised as Lancefield groups C/G [14]. In a recent publication, a retrospective search of the database of blood cultures from between 2006 and 2015 in Bern, Switzerland, showed 67 positive blood cultures with C/G streptococci isolates [49]. The limited number of patients included in our study might explain the low number of organisms other than GAS. Elliott et al. showed that in 154 out of 182 cases (84%) of necrotising soft tissue infections, more than one organism was cultured [68]. In our study, polymicrobial infections accounted for 10.45% of cases and S. aureus was the most commonly co-isolated bacterium (7% of cases).
In accordance with previous findings, emm-types 1, 3, 12, 28 and 89 were the most frequent serotypes found in this study [21, 60, 69]. Certain emm-types, such as emm1, are isolated more often in specific diseases such as STSS and necrotising fasciitis [21]. emm-types 1, 3, 5 and 43 were shown to correlate with a higher lethality [21, 69]. The most common T-types we detected were types 1, 3 and 12, which agrees with the results obtained by the Strep-EURO research group in 2009 [21]. We did not find a significant correlation between the occurrences of emm-types 1, 3, 12, 28 and 89 in the necrotising fasciitis vs the no necrotising fasciitis groups.
Superantigens were tested in almost half of the clinical isolates. SpeG was the most commonly detected superantigen (92.9%), followed by smeZ, speA and speJ (88.1%, 59.5%, 57.1% respectively). These percentages are similar to those reported by Luca-Harari et al. for streptococcal pyrogenic exotoxin (speG, 99%) and streptococcal superantigen genes (speA, 40%) in invasive GAS infections isolates [60]. A study from Poland and Germany found speG in 83%, smeZ in 77% and speJ in 5% of patients with invasive GAS wound, skin and soft tissue infections [70]. Since no significant difference existed between patients with or without necrotising fasciitis, we assume that superantigens and smeZ are not the major factors affecting the severity of invasive GAS infections. However, due to the low number of isolates analysed, further testing is needed for confirmation.
As reported elsewhere, we detected no resistance to penicillin in the clinical isolates [29, 31]. However, in contrast to reports from France and Italy, our isolates showed no resistance to clindamycin [71, 72]. Clindamycin has been shown to act on non-replicating bacteria, in contrast to penicillin, which only acts on actively replicating bacteria [73]. Clindamycin has been shown to improve clinical outcome in an necrotising fasciitis mouse model [74]. We observed resistance to tetracycline in five strains (11.9%), similar to previous observations [75].
The present study has several limitations. First, patients included in the analysis were subject to a selection bias. Only patients reported by infectious disease specialists and microbiologists as having an invasive beta-haemolytic streptococcal infection were included. Although this could have led us to overlook patients, the incidence found by the additional systematic search seems to show that we probably identified the majority of cases. Second, treatment guidelines and awareness for microbiological sampling have evolved considerably over the last few decades, which has probably led to a heterogeneity of the collected patient and microbiological data over time. Third, the clinical information used for this study was retrieved from patients’ medical records retrospectively. Thus, the quality of the information and the analysed patient outcomes rely on the accuracy of these medical records, which depends on the knowledge, personal experience and subjective interpretation of the treating physicians. Misinterpretations of the clinical data at the time of diagnosis may explain why the rate of complications is higher than in other prospective studies [7, 31]. Fourth, the retrospective nature of the study does not allow any understanding of the rationale for the medical and/or surgical treatments provided. Also, only univariate analysis was performed, with no corrections for multiple testing.
In conclusion, we provide the first analysis of clinical and microbiological characteristics of patients with severe invasive beta-haemolytic streptococcal infections treated in a tertiary hospital in Zurich, Switzerland. We characterise in detail the sites of infection, the occurrence of necrotising fasciitis, the risk factors for invasive infections and the occurrence of complications. We moreover show relevant differences in the clinical data and current microbiological characteristics of patients with and without necrotising fasciitis. This study provides an extensive clinical, epidemiological and microbiological analysis of cases with an invasive streptococcal infection and enables comparisons with other studies. We call for continuous local surveillance of invasive streptococcal infections in the future, as practiced in various countries including the members of the Strep-EURO programme. This would help to further our understanding of the dynamics of invasive streptococcal infections and would provide a continuous epidemiological description of the causal microorganisms.
We thank the technicians of the Institute of Medical Microbiology of the University of Zürich for their expert help and assistance.
Contributed equally to the study
This research was funded by a Swiss National Foundation grant (grant number 310030_146295/1) to ASZ and a Promedica Foundation grant (1449/M) to SDB.
The authors have no conflict of interest to declare.
1 Bisno AL . Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991;325(11):783–93. doi:.https://doi.org/10.1056/NEJM199109123251106
2 Cone LA , Woodard DR , Schlievert PM , Tomory GS . Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317(3):146–9. doi:.https://doi.org/10.1056/NEJM198707163170305
3 Cunningham MW . Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13(3):470–511. doi:.https://doi.org/10.1128/CMR.13.3.470
4 Waddington CS , Snelling TL , Carapetis JR . Management of invasive group A streptococcal infections. J Infect. 2014;69(Suppl 1):S63–9. doi:.https://doi.org/10.1016/j.jinf.2014.08.005
5 Parks T , Barrett L , Jones N . Invasive streptococcal disease: a review for clinicians. Br Med Bull. 2015;115(1):77–89. doi:.https://doi.org/10.1093/bmb/ldv027
6World Health Organization. The current evidence for the burden of group A streptococcal diseases. Geneva: World Health Organization; 2005.
7 Lamagni TL , Darenberg J , Luca-Harari B , Siljander T , Efstratiou A , Henriques-Normark B , et al.; Strep-EURO Study Group. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46(7):2359–67. doi:.https://doi.org/10.1128/JCM.00422-08
8 Konrad D , Zbinden R , Kuster H , Hunziker UA ; Daniel Konrad, Reinhard Zbinden, Ha. Group G streptococcus sacroilitis with sepsis in a 15-y-old adolescent. Scand J Infect Dis. 1999;31(1):100–2. doi:.https://doi.org/10.1080/00365549950161998
9 Bruun T , Kittang BR , de Hoog BJ , Aardal S , Flaatten HK , Langeland N , et al. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect. 2013;19(12):E545–50. doi:.https://doi.org/10.1111/1469-0691.12276
10 Vartian C , Lerner PI , Shlaes DM , Gopalakrishna KV . Infections due to Lancefield group G streptococci. Medicine (Baltimore). 1985;64(2):75–88. doi:.https://doi.org/10.1097/00005792-198503000-00001
11 Broyles LN , Van Beneden C , Beall B , Facklam R , Shewmaker PL , Malpiedi P , et al. Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009;48(6):706–12. doi:.https://doi.org/10.1086/597035
12 Sylvetsky N , Raveh D , Schlesinger Y , Rudensky B , Yinnon AM . Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622–6. doi:.https://doi.org/10.1016/S0002-9343(02)01117-8
13 Laupland KB , Ross T , Church DL , Gregson DB . Population-based surveillance of invasive pyogenic streptococcal infection in a large Canadian region. Clin Microbiol Infect. 2006;12(3):224–30. doi:.https://doi.org/10.1111/j.1469-0691.2005.01345.x
14 Kittang BR , Bruun T , Langeland N , Mylvaganam H , Glambek M , Skrede S . Invasive group A, C and G streptococcal disease in western Norway: virulence gene profiles, clinical features and outcomes. Clin Microbiol Infect. 2011;17(3):358–64. doi:.https://doi.org/10.1111/j.1469-0691.2010.03253.x
15 Descamps V , Aitken J , Lee MG . Hippocrates on necrotising fasciitis. Lancet. 1994;344(8921):556. doi:.https://doi.org/10.1016/S0140-6736(94)91956-9
16 McCormick JK , Yarwood JM , Schlievert PM . Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55(1):77–104. doi:.https://doi.org/10.1146/annurev.micro.55.1.77
17 Khamnuan P , Chongruksut W , Jearwattanakanok K , Patumanond J , Yodluangfun S , Tantraworasin A . Necrotizing fasciitis: risk factors of mortality. Risk Manag Healthc Policy. 2015;8:1–7.
18 O’Loughlin RE , Roberson A , Cieslak PR , Lynfield R , Gershman K , Craig A , et al.; Active Bacterial Core Surveillance Team. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853–62. doi:.https://doi.org/10.1086/521264
19 Nelson GE , Pondo T , Toews KA , Farley MM , Lindegren ML , Lynfield R , et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478–86. doi:.https://doi.org/10.1093/cid/ciw248
20 Factor SH , Levine OS , Schwartz B , Harrison LH , Farley MM , McGeer A , et al. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis. 2003;9(8):970–7. doi:.https://doi.org/10.3201/eid0908.020745
21 Luca-Harari B , Darenberg J , Neal S , Siljander T , Strakova L , Tanna A , et al.; Strep-EURO Study Group. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2009;47(4):1155–65. doi:.https://doi.org/10.1128/JCM.02155-08
22 Wahl RU , Lütticken R , Stanzel S , van der Linden M , Reinert RR . Epidemiology of invasive Streptococcus pyogenes infections in Germany, 1996-2002: results from a voluntary laboratory surveillance system. Clin Microbiol Infect. 2007;13(12):1173–8. doi:.https://doi.org/10.1111/j.1469-0691.2007.01821.x
23 Olafsdottir LB , Erlendsdóttir H , Melo-Cristino J , Weinberger DM , Ramirez M , Kristinsson KG , et al. Invasive infections due to Streptococcus pyogenes: seasonal variation of severity and clinical characteristics, Iceland, 1975 to 2012. Euro Surveill. 2014;19(17):5–14. doi:.https://doi.org/10.2807/1560-7917.ES2014.19.17.20784
24 Astorino T , Genrich I , Macgregor L , Victor CS , Eckhouse DR , Barbour L . Necrotizing fasciitis: early detection may save your patient’s limb. Orthop Nurs. 2009;28(2):70–6, quiz 77–8. doi:.https://doi.org/10.1097/NOR.0b013e318199ecb4
25 Wong CH , Chang HC , Pasupathy S , Khin LW , Tan JL , Low CO . Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454–60. doi:.https://doi.org/10.2106/00004623-200308000-00005
26 Bilton BD , Zibari GB , McMillan RW , Aultman DF , Dunn G , McDonald JC . Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surg. 1998;64(5):397–400, discussion 400–1.
27 Carapetis JR , Jacoby P , Carville K , Ang SJ , Curtis N , Andrews R . Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59(3):358–65. doi:.https://doi.org/10.1093/cid/ciu304
28 Zimbelman J , Palmer A , Todd J . Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096–100. doi:.https://doi.org/10.1097/00006454-199912000-00014
29 Brown SD , Rybak MJ . Antimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001-2002, as part of the PROTEKT US study. J Antimicrob Chemother. 2004;54(Suppl 1):i7–15. doi:.https://doi.org/10.1093/jac/dkh313
30 Russell NE , Pachorek RE . Clindamycin in the treatment of streptococcal and staphylococcal toxic shock syndromes. Ann Pharmacother. 2000;34(7-8):936–9. doi:.https://doi.org/10.1345/aph.19095
31 Imöhl M , Reinert RR , Mutscher C , van der Linden M . Macrolide susceptibility and serotype specific macrolide resistance of invasive isolates of Streptococcus pneumoniae in Germany from 1992 to 2008. BMC Microbiol. 2010;10(1):299. doi:.https://doi.org/10.1186/1471-2180-10-299
32 Stevens DL , Bisno AL , Chambers HF , Dellinger EP , Goldstein EJ , Gorbach SL , et al.; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–52. doi:.https://doi.org/10.1093/cid/ciu296
33 Sriskandan S , Ferguson M , Elliot V , Faulkner L , Cohen J . Human intravenous immunoglobulin for experimental streptococcal toxic shock: bacterial clearance and modulation of inflammation. J Antimicrob Chemother. 2006;58(1):117–24. doi:.https://doi.org/10.1093/jac/dkl173
34 Tarnutzer A , Andreoni F , Keller N , Zürcher C , Norrby-Teglund A , Schüpbach RA , et al. Human polyspecific immunoglobulin attenuates group A streptococcal virulence factor activity and reduces disease severity in a murine necrotizing fasciitis model. Clin Microbiol Infect. 2019;25(4):512.e7–13. doi:.https://doi.org/10.1016/j.cmi.2018.07.007
35 Kadri SS , Swihart BJ , Bonne SL , Hohmann SF , Hennessy LV , Louras P , et al. Impact of Intravenous Immunoglobulin on Survival in Necrotizing Fasciitis With Vasopressor-Dependent Shock: A Propensity Score-Matched Analysis From 130 US Hospitals. Clin Infect Dis. 2017;64(7):877–85.
36 Sriskandan S , McKee A , Hall L , Cohen J . Comparative effects of clindamycin and ampicillin on superantigenic activity of Streptococcus pyogenes. J Antimicrob Chemother. 1997;40(2):275–7. doi:.https://doi.org/10.1093/jac/40.2.275
37 Linnér A , Darenberg J , Sjölin J , Henriques-Normark B , Norrby-Teglund A . Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. 2014;59(6):851–7. doi:.https://doi.org/10.1093/cid/ciu449
38 Fischetti VA . Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2(3):285–314. doi:.https://doi.org/10.1128/CMR.2.3.285
39 Beall B , Facklam R , Thompson T . Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34(4):953–8. doi:.https://doi.org/10.1128/JCM.34.4.953-958.1996
40 Darenberg J , Luca-Harari B , Jasir A , Sandgren A , Pettersson H , Schalén C , et al. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis. 2007;45(4):450–8. doi:.https://doi.org/10.1086/519936
41 Chelsom J , Halstensen A , Chelsom J , Haga T , Høiby EA . Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet. 1994;344(8930):1111–5. doi:.https://doi.org/10.1016/S0140-6736(94)90629-7
42 Commons RJ , Smeesters PR , Proft T , Fraser JD , Robins-Browne R , Curtis N . Streptococcal superantigens: categorization and clinical associations. Trends Mol Med. 2014;20(1):48–62. doi:.https://doi.org/10.1016/j.molmed.2013.10.004
43 Watson DW . Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960;111:255–84. doi:.https://doi.org/10.1084/jem.111.2.255
44 Seth A , Stern LJ , Ottenhoff TH , Engel I , Owen MJ , Lamb JR , et al. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature. 1994;369(6478):324–7. doi:.https://doi.org/10.1038/369324a0
45 Dellabona P , Peccoud J , Kappler J , Marrack P , Benoist C , Mathis D . Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990;62(6):1115–21. doi:.https://doi.org/10.1016/0092-8674(90)90388-U
46 Reda KB , Kapur V , Mollick JA , Lamphear JG , Musser JM , Rich RR . Molecular characterization and phylogenetic distribution of the streptococcal superantigen gene (ssa) from Streptococcus pyogenes. Infect Immun. 1994;62(5):1867–74. doi:.https://doi.org/10.1128/IAI.62.5.1867-1874.1994
47 Kuehl R , Tschudin-Sutter S , Siegemund M , Marsch S , Battegay M , Wetterauer C , et al. High Mortality of Non-Fournier Necrotizing Fasciitis With Enterobacteriales: Time to Rethink Classification? Clin Infect Dis. 2019;69(1):147–50. doi:.https://doi.org/10.1093/cid/ciy1011
48 Rieger UM , Gugger CY , Farhadi J , Heider I , Andresen R , Pierer G , et al. Prognostic factors in necrotizing fasciitis and myositis: analysis of 16 consecutive cases at a single institution in Switzerland. Ann Plast Surg. 2007;58(5):523–30. doi:.https://doi.org/10.1097/01.sap.0000244978.27053.08
49 Ruppen C , Rasmussen M , Casanova C , Sendi P . A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: frequent occurrence among female intravenous drug users. Swiss Med Wkly. 2017;147:w14469.
50 Schulthess B , Brodner K , Bloemberg GV , Zbinden R , Böttger EC , Hombach M . Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J Clin Microbiol. 2013;51(6):1834–40. doi:.https://doi.org/10.1128/JCM.02654-12
51 Clarridge JE, 3rd . Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17(4):840–62. doi:.https://doi.org/10.1128/CMR.17.4.840-862.2004
52 Matsuo M , Hiramatsu M , Singh M , Sasaki T , Hishinuma T , Yamamoto N , et al. Genetic and Transcriptomic Analyses of Ciprofloxacin-Tolerant Staphylococcus aureus Isolated by the Replica Plating Tolerance Isolation System (REPTIS). Antimicrob Agents Chemother. 2019;63(2):e02019-18. doi:.https://doi.org/10.1128/AAC.02019-18
53 Moody MD , Padula J , Lizana D , Hall CT . Epidemiologic Characterization of Group a Streptococci by T-Agglutination and M-Precipitation Tests in the Public Health Laboratory. Health Lab Sci. 1965;2:149–62.
54 Lintges M , Arlt S , Uciechowski P , Plümäkers B , Reinert RR , Al-Lahham A , et al. A new closed-tube multiplex real-time PCR to detect eleven superantigens of Streptococcus pyogenes identifies a strain without superantigen activity. Int J Med Microbiol. 2007;297(6):471–8. doi:.https://doi.org/10.1016/j.ijmm.2007.03.015
55 Bauer AW , Kirby WM , Sherris JC , Turck M . Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–6. doi:.https://doi.org/10.1093/ajcp/45.4_ts.493
56CLSI. Performance Standards for Antimicrobial Susceptibility testing. 29th edition. Wayne, PA: Clinical and Laboratory Standards Institute. 2019.
57Bevölkerung in Zahlen. 2020. Available from: https://www.zh.ch/de/soziales/bevoelkerungszahlen.html?keyword=bevoelkerung#/details/127@statistisches-amt-kanton-zuerich [cited 2020 September 9].
58 Kaul R , McGeer A , Low DE , Green K , Schwartz B , Simor AE . Population-based surveillance for group A streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997;103(1):18–24. doi:.https://doi.org/10.1016/S0002-9343(97)00160-5
59 Lepoutre A , Doloy A , Bidet P , Leblond A , Perrocheau A , Bingen E , et al.; Microbiologists of the Epibac Network. Epidemiology of invasive Streptococcus pyogenes infections in France in 2007. J Clin Microbiol. 2011;49(12):4094–100. doi:.https://doi.org/10.1128/JCM.00070-11
60 Luca-Harari B , Ekelund K , van der Linden M , Staum-Kaltoft M , Hammerum AM , Jasir A . Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J Clin Microbiol. 2008;46(1):79–86. doi:.https://doi.org/10.1128/JCM.01626-07
61 Imöhl M , Reinert RR , Ocklenburg C , van der Linden M . Epidemiology of invasive Streptococcus pyogenes disease in Germany during 2003-2007. FEMS Immunol Med Microbiol. 2010;58(3):389–96. doi:.https://doi.org/10.1111/j.1574-695X.2010.00652.x
62 Wong CH , Khin LW , Heng KS , Tan KC , Low CO . The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–41. doi:.https://doi.org/10.1097/01.CCM.0000129486.35458.7D
63 Mehta S , McGeer A , Low DE , Hallett D , Bowman DJ , Grossman SL , et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest. 2006;130(6):1679–86. doi:.https://doi.org/10.1016/S0012-3692(15)50887-8
64 Ferguson ND , Fan E , Camporota L , Antonelli M , Anzueto A , Beale R , et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–82. doi:.https://doi.org/10.1007/s00134-012-2682-1
65 Parks T , Wilson C , Curtis N , Norrby-Teglund A , Sriskandan S . Polyspecific Intravenous Immunoglobulin in Clindamycin-treated Patients With Streptococcal Toxic Shock Syndrome: A Systematic Review and Meta-analysis. Clin Infect Dis. 2018;67(9):1434–6. doi:.https://doi.org/10.1093/cid/ciy401
66 Madsen MB , Hjortrup PB , Hansen MB , Lange T , Norrby-Teglund A , Hyldegaard O , et al. Immunoglobulin G for patients with necrotising soft tissue infection (INSTINCT): a randomised, blinded, placebo-controlled trial. Intensive Care Med. 2017;43(11):1585–93. doi:.https://doi.org/10.1007/s00134-017-4786-0
67 Andreoni F , Ugolini F , Keller N , Neff A , Nizet V , Hollands A , et al. Immunoglobulin Attenuates Streptokinase-Mediated Virulence in Streptococcus dysgalactiae Subspecies equisimilis Necrotizing Fasciitis. J Infect Dis. 2018;217(2):270–9. doi:.https://doi.org/10.1093/infdis/jix560
68 Elliott D , Kufera JA , Myers RA . The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179(5):361–6. doi:.https://doi.org/10.1016/S0002-9610(00)00360-3
69 Siljander T , Lyytikäinen O , Vähäkuopus S , Snellman M , Jalava J , Vuopio J . Epidemiology, outcome and emm types of invasive group A streptococcal infections in Finland. Eur J Clin Microbiol Infect Dis. 2010;29(10):1229–35. doi:.https://doi.org/10.1007/s10096-010-0989-9
70 Strus M , Heczko PB , Golińska E , Tomusiak A , Chmielarczyk A , Dorycka M , et al. The virulence factors of group A streptococcus strains isolated from invasive and non-invasive infections in Polish and German centres, 2009-2011. Eur J Clin Microbiol Infect Dis. 2017;36(9):1643–9. doi:.https://doi.org/10.1007/s10096-017-2978-8
71 Cornaglia G , Ligozzi M , Mazzariol A , Valentini M , Orefici G , Fontana R ; The Italian Surveillance Group for Antimicrobial Resistance. Rapid increase of resistance to erythromycin and clindamycin in Streptococcus pyogenes in Italy, 1993-1995. Emerg Infect Dis. 1996;2(4):339–42. doi:.https://doi.org/10.3201/eid0204.960410
72 Herruzo R , Chamorro L , García ME , González MC , López AM , Manceñido N , et al. Prevalence and antimicrobial-resistance of S. pneumoniae and S. pyogenes in healthy children in the region of Madrid. Int J Pediatr Otorhinolaryngol. 2002;65(2):117–23. doi:.https://doi.org/10.1016/S0165-5876(02)00145-3
73 Sawai J , Hasegawa T , Kamimura T , Okamoto A , Ohmori D , Nosaka N , et al. Growth phase-dependent effect of clindamycin on production of exoproteins by Streptococcus pyogenes. Antimicrob Agents Chemother. 2007;51(2):461–7. doi:.https://doi.org/10.1128/AAC.00539-06
74 Andreoni F , Zürcher C , Tarnutzer A , Schilcher K , Neff A , Keller N , et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017;215(2):269–77.
75 Imöhl M , van der Linden M . Antimicrobial Susceptibility of Invasive Streptococcus pyogenes Isolates in Germany during 2003-2013. PLoS One. 2015;10(9):e0137313. doi:.https://doi.org/10.1371/journal.pone.0137313
The appendix is available in the PDF version of this article.