Surgical management of vascular Ehlers-Danlos syndrome and its challenges: a case report
DOI: https://doi.org/10.4414/smw.2020.20379
Francisco da Rocha
de Sousaa, Nicola
Colucciab, Arnaud
Dupuisa, Christian
Tosoa, Nicolas C.
Buchsa, Ziad
Abbassia
a Department of Surgery, Division of Visceral Surgery, Geneva University Hospitals, Geneva, Switzerland
b Department of Clinical-Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Italy
Summary
BACKGROUND
Ehlers-Danlos syndrome (EDS) is a heterogeneous group of rare inherited diseases involving connective tissue. Vascular EDS (vEDS) is associated with abnormal type III collagen, which is an essential component of skin, hollow organs and arterial walls, and causes potentially fatal visceral and arterial complications. The surgical management of these patients is challenging and should be limited to life-saving procedures.
CASE DESCRIPTION
We report a case of a 42-year-old male who presented a hemorrhagic shock due to spontaneous rupture of an ascending branch of the left colic artery. The coexisting presence of multiple abdominal vascular abnormalities suggested the diagnosis of vEDS, later confirmed by the discovery of a new missense mutation in the COL3A1 gene with pathogenic significance. The post-operative course was marked by a mechanical ileus caused by an ischemic stenosis of the descending colon. Failure of conservative management and the well-known risk of colonic perforation in these patients led to the decision to perform a diverting ileostomy.
CONCLUSION
The management of these patients is difficult, and risk-benefit assessments must be made on a case-by-case basis. Less invasive procedures should be considered whenever possible.
Background
Ehlers-Danlos syndrome (EDS) is a heterogeneous group of rare inherited diseases affecting connective tissue. The pathogenesis implies mutations to the genes responsible for the production of several extracellular matrix proteins [1–4]. Particular genetic alterations lead to distinct EDS subtypes [1, 2]. Although the clinical spectrum of EDS is wide, they are often characterized by hyperextensibility of the skin, joint hypermobility and tissue fragility [1–3]. According to the 2017 international classification, there are 13 subtypes of EDS, each with different complications and outcomes [1].
Vascular Ehlers-Danlos syndrome (vEDS), formerly known as type IV, has an estimated prevalence of approximately 1 per 50,000 individuals [5]. This condition is caused by specific mutations in the COL3A1 gene and follows an autosomal dominant mode of inheritance. The COL3A1 gene encodes the pro-alpha-1 chains of type III collagen, which is an essential component of the arterial walls, joints, skin, gastrointestinal tract and uterus [6, 7]. The protein’s stability, and therefore its function, is closely related to the preservation of its repetitive primary structure, the first amino acid of which is invariably a glycine. The majority of identified pathogenic variants result from a single amino acid substitution for glycine in the Gly-X-Y repeat of the triple helical region of the type III collagen molecule, which induces drastic qualitative abnormality [5, 7].
The diagnosis of vEDS is established by identifying a heterozygous pathogenic variant in COL3A1 with molecular genetic testing or by finding abnormalities in the synthesis and mobility of type III collagen chains through biochemical analysis [5–7].
Patients with vEDS are susceptible to potentially fatal visceral (e.g., spontaneous bowel or uterine perforation, diffuse diverticula, megaduodenum, spontaneous pneumothorax) and vascular complications (e.g., dissections, aneurysms, spontaneous ruptures, arteriovenous fistulas) [5–7]. These events strongly impact patients’ median life expectancy, which is around 48 years for non-treated patients. The first major complication may occur by the age of 20, and patients have an 80% risk of EDS-related complications by the age of 40. The mortalities associated with visceral and arterial rupture are 20% and 45% respectively [6].
Surgical procedures are generally not recommended for these patients because of high peri- and postoperative morbidity [2, 5, 6]. In typical cases, vEDS leads to extreme fragility of tissues, which tear apart “like blotting paper”. Bowel walls are fragile, major intraperitoneal adhesions are frequent, creating anastomoses is arduous and achieving perfect hemostasis is difficult [8, 9]. Several authors recommend avoiding surgical or endoscopic management as often as possible: invasive procedures are dangerous and can be the starting point for a chain of complications. Unless a life-saving procedure is needed, these approaches should be avoided [2, 9–13].
We report a case of a patient with undiagnosed vEDS who presented to our hospital with life-threatening intraperitoneal bleeding requiring surgical management after endovascular embolisation failure. The post-operative course was marked by a mechanical ileus caused by an ischemic colonic stenosis. After an unsuccessful attempt at conservative treatment, an endoscopic or surgical approach was mandatory despite the risks involved. With no similar cases reported in the medical literature, we elected to treat the stenosis with the less-invasive surgical approach in order to reduce morbidity.
Case description
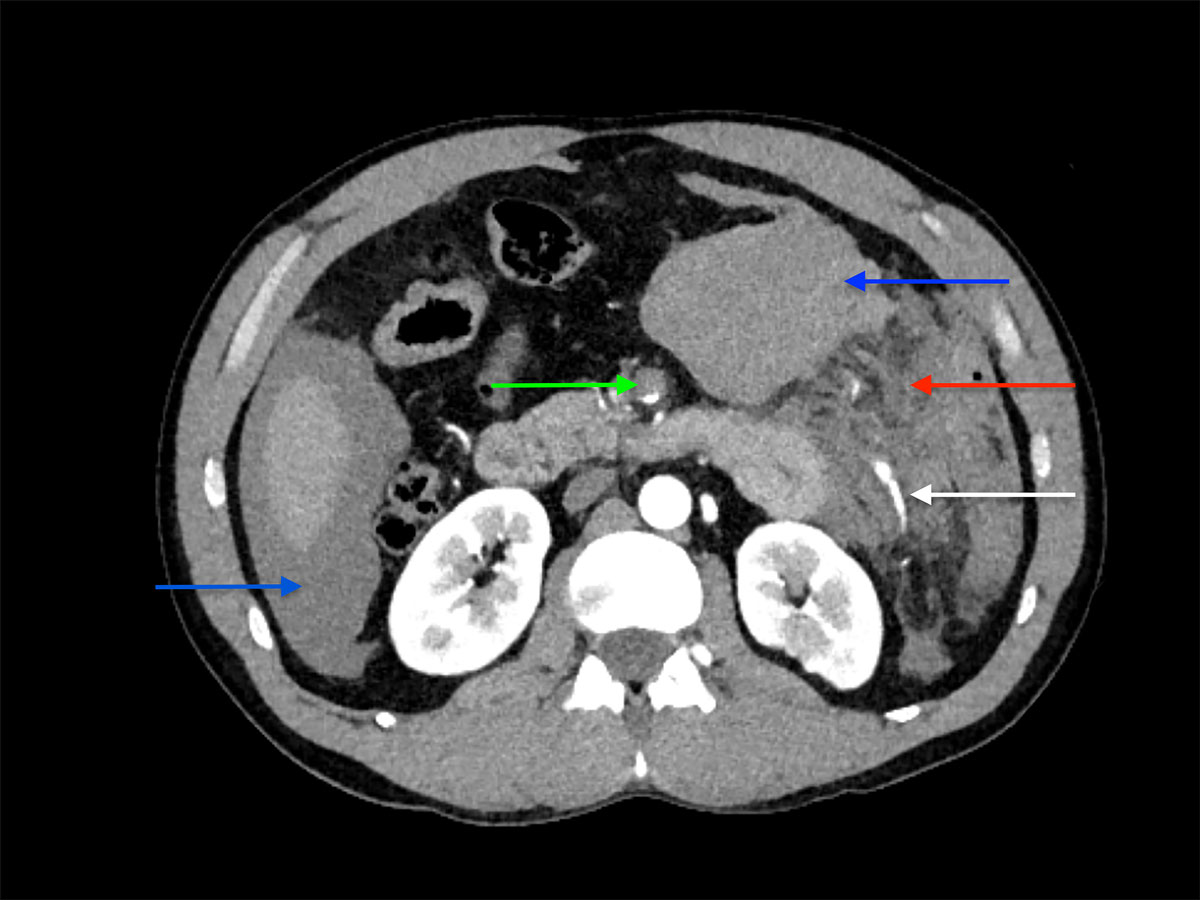
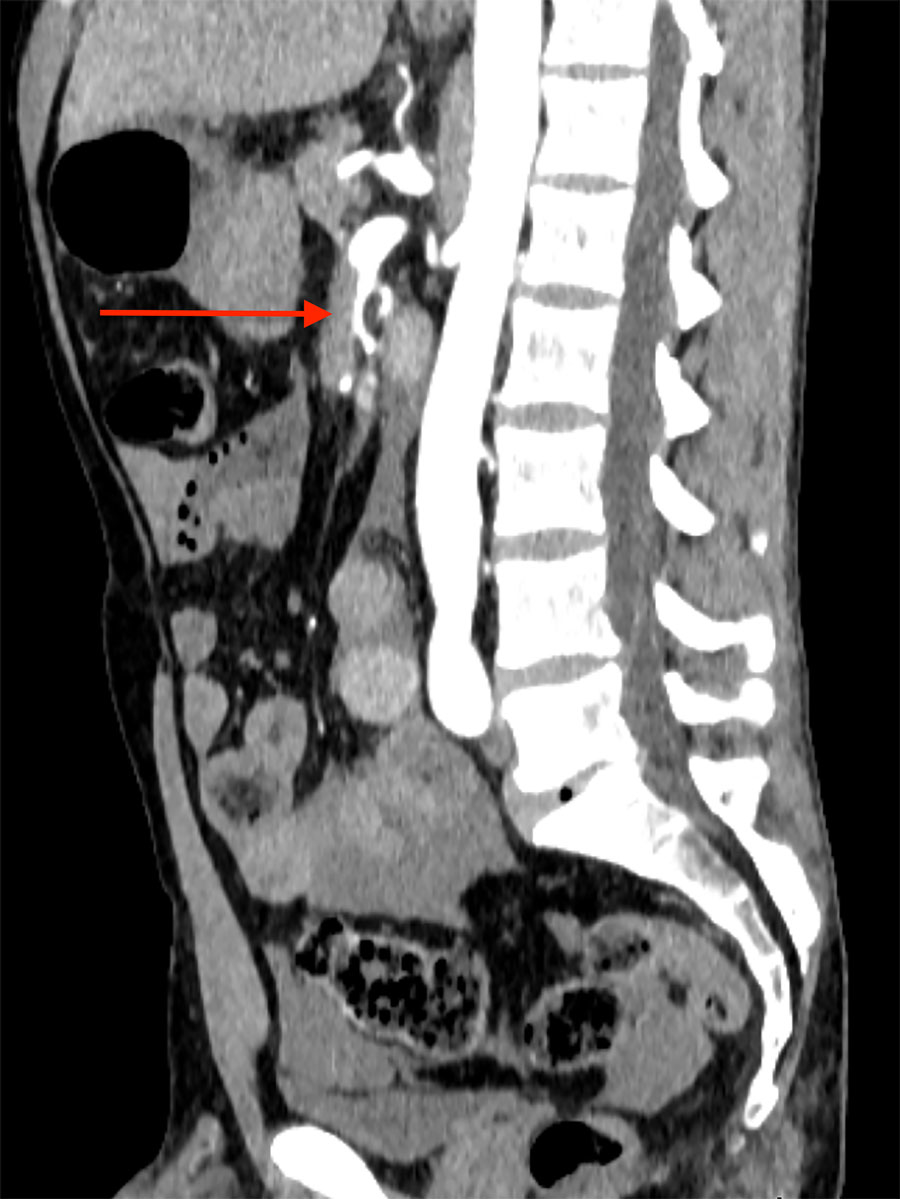
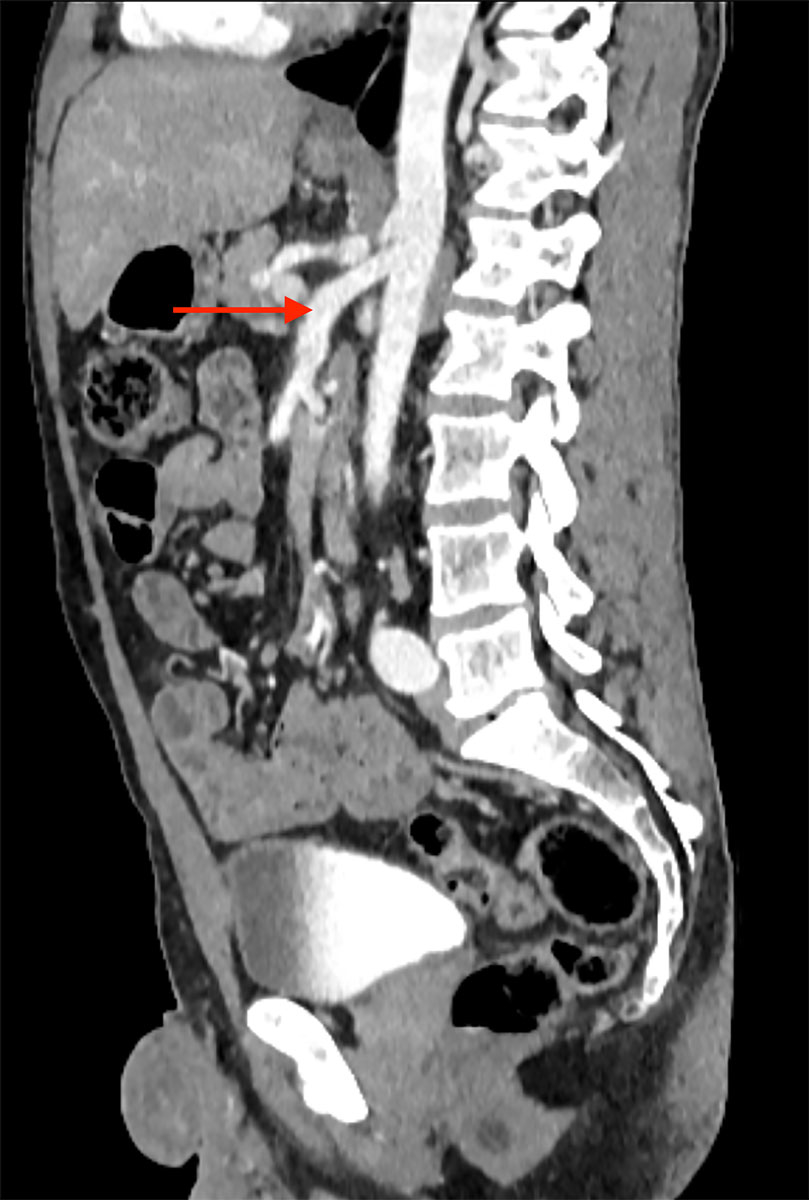
A 42-year-old male was admitted to our emergency department with acute abdominal pain, lipothymia and nausea. The patient was tachycardiac and hypotensive. Physical examination revealed signs of peritonitis. His bloods showed elevated lactates, at 4 mmol/l, while haemoglobin level was normal. The abdominal computed tomography (CT) angiography revealed a massive haemoperitoneum from a ruptured aneurysm of a distal branch of the ascending left colonic artery (fig. 1). Moreover, several aneurysms and strictures of the main intra-abdominal arteries were found, including dissections of both the superior mesenteric and the left common iliac arteries (figs 2 and 3
).
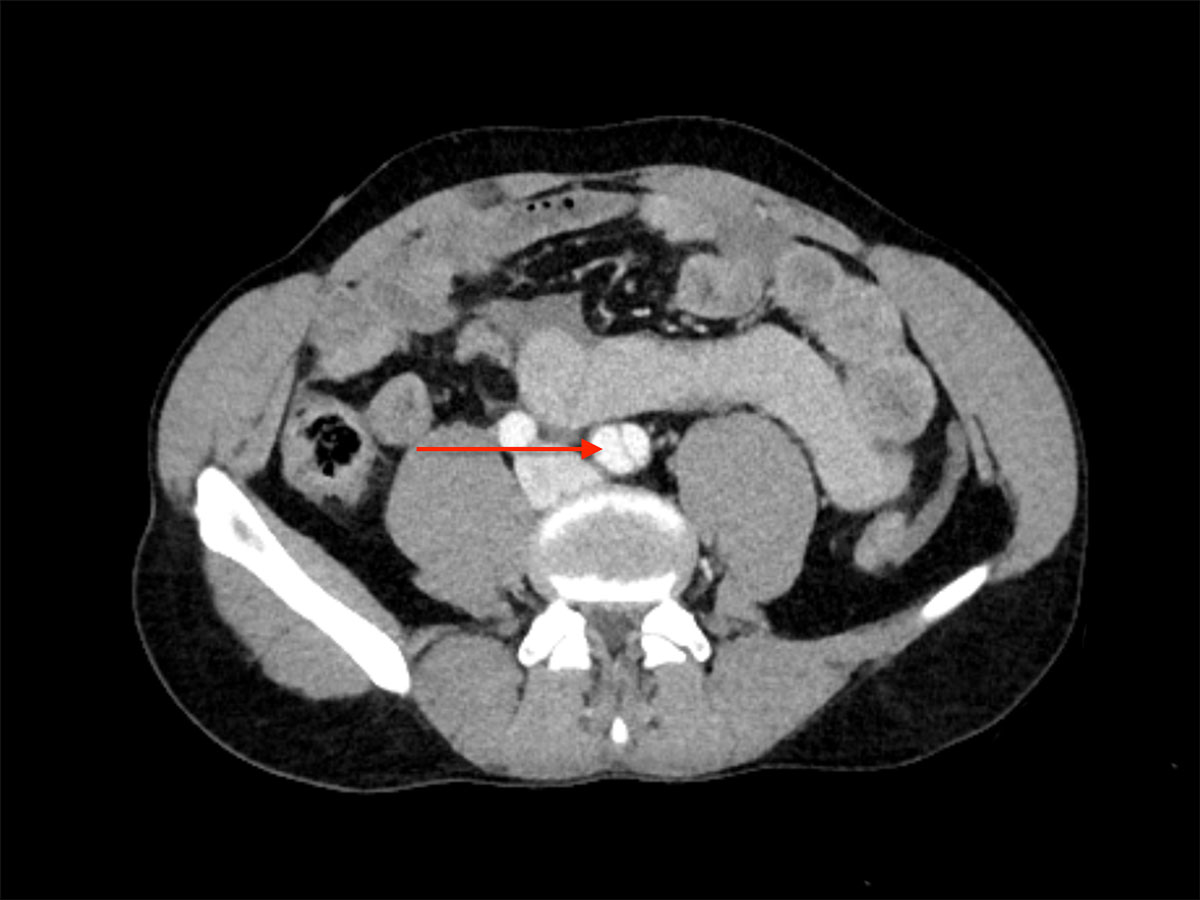
Figure 3 Emergency abdominal CT scan displaying dissection of the left common iliac artery (arrow).
The patient’s medical history was significant for a left renal infarction with a left segmental renal artery dissection and a splenic artery aneurysm found incidentally during an abdominal imaging. Fibromuscular dysplasia was suspected but ruled out after further investigations. Furthermore, the patient had no relevant surgical or vascular family history.
Selective embolisation was attempted as first-line therapy. Nevertheless, angiography failed to detect active haemorrhage in the territory of the inferior mesenteric artery. A balloon angioplasty of the superior mesenteric artery dissection was possible, however. This angiography confirmed the pathological aspect of the splanchnic arteries, suggesting a systemic vascular condition (fig. 4).
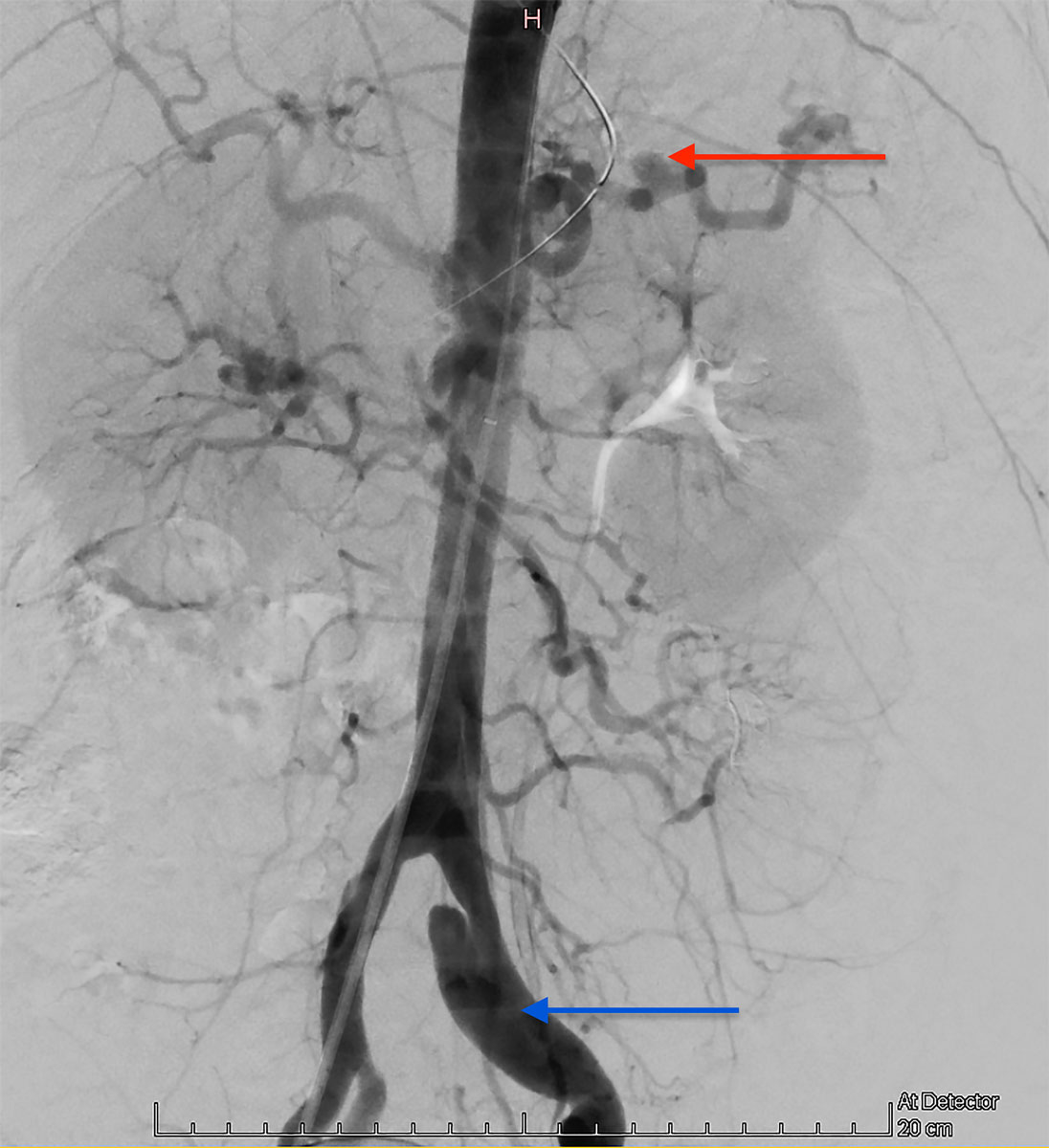
Figure 4 Emergency angiography showing a pathological aspect of the splanchnic arteries, suggesting a systemic vascular condition (splenic artery aneurysm – red arrow – and left common iliac artery dissection – blue arrow). No active haemorrhage in the territory of the inferior mesenteric artery was found.
An exploratory laparotomy was then performed in order to control the haemorrhagic source. After the evacuation of almost two litres of blood, an active bleeding from a ruptured aneurysm of a collateral of the ascending left colonic artery was found and treated by ligation. There were no other abnormalities noted at laparotomy. A near-infrared fluorescence angiography with indocyanine green (PINPOINT® endoscopic fluorescence imaging system, Stryker, Kalamazoo, Michigan, USA) confirmed an excellent splanchnic vascularisation, including the colonic portion near to the previously ligated artery.
Following the procedure, the patient was admitted to the intensive care unit until postoperative day 1. The postoperative course was uneventful until day 14, when the patient developed signs of ileus. A conservative approach, with fasting and nasogastric tube decompression, failed to improve the clinical course. Radiological investigations revealed a left colonic stenosis (fig. 5), probably due to arterial ligation.
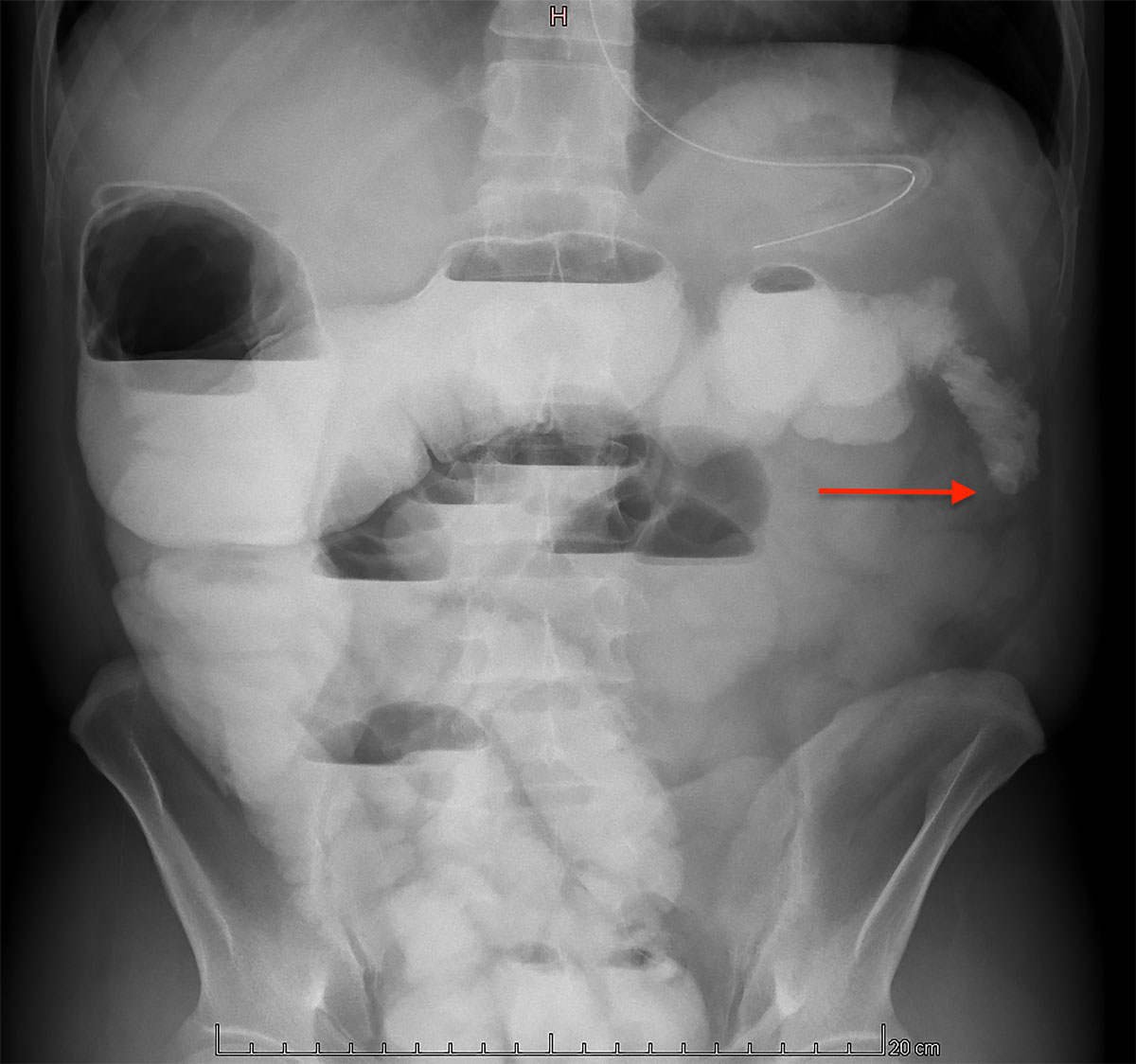
Figure 5 Abdominal radiography with oral contrast showing a left colonic stenosis (arrow).
After closely examining the safety and efficiency of each therapeutic option (colonic resection, diverting stoma or endoscopic colonic dilatation), the decision to perform a lateral diverting ileostomy, favouring the less invasive procedure, was taken on a multidisciplinary basis. The surgery was uncomplicated and the recovery uneventful.
In addition to surgical management, genetic and vascular assessments were done. Molecular genetic testing confirmed the vEDS diagnosis by detecting a missense mutation in the COL3A1 gene: c.2321G>A, heterozygous, p.(Gly774Asp). Although this variant was not linked to vEDS in several gene mutation databases (Leiden Open Variation Database, ClinVar, Human Gene Mutation Database), we performed some prediction algorithms to help evaluate its clinical significance (SIFT, Polyphen2, MutationTaster, CADD13, dbscSNV11). This assessment suggested that the likely pathogenicity of the variant was caused by it affecting one of the structural glycines of the triple helix of the type III collagen. This, in combination with typical clinical presentation, allowed us to confirm the diagnosis.
Extensive vascular imaging (cerebral, cervical and thoracic) showed pathological bilateral carotid axis with pseudoaneurysm and dissection sequelae. Treatment with low doses of celiprolol (Celectol®) was thus introduced, and this was progressively increased to the target dose of 400 mg once daily.
The patient was discharged from the hospital 11 days after the second surgery and 1 month after his admission.
A follow-up consultation 1 month later confirmed an excellent clinical evolution, with no evidence of abdominal pain, bowel disorders or poor wound healing. Eight weeks later, a barium enema was performed, and no signs of stenosis were found (fig. 6). Six months following the creation of the ostomy, restoration continuity was performed using the former ileostomy site. The postoperative period was simple and the patient was discharged home nine days later.
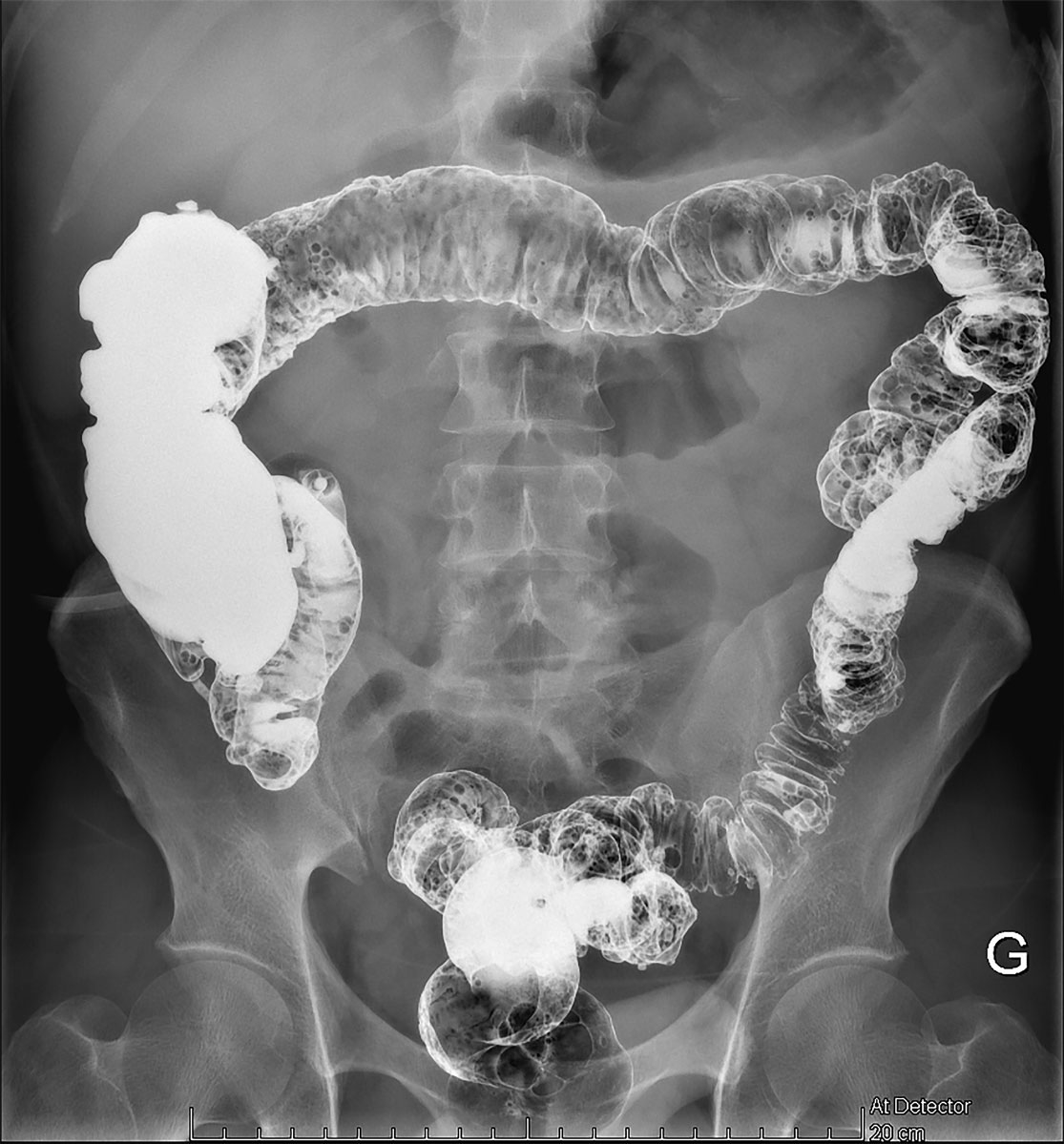
Figure 6 Abdominal radiography with barium enema through the ostomy showing no signs of stenosis
Currently, the patient is recovering well from the latest surgery and is gaining weight. An abdominal CT angiography was recently performed and showed a good blood flow after the superior mesenteric artery angioplasty (fig. 7). Celiprolol treatment is well tolerated and blood pressure is strictly monitored by self-measurements and regularly managed by his general practitioner. Finally, a dedicated rare disease team in our hospital follows the patient closely.
Discussion
This case report outlines the typical presentation and features of vEDS, with a particular emphasis on a surgical perspective.
As far as is known, this is the first case describing a symptomatic ischemic colonic stenosis refractory to medical treatment in a vEDS patient. No clear treatment recommendations are available, except for the imperative to be minimally invasive.
After the case was closely analysed from a multidisciplinary perspective, an endoscopic strategy (dilation of the stenotic segment) was deemed inappropriate even though it is less invasive than others options, due to the high risk of viscus perforation during the procedure [9, 14]. A segmental colonic resection of the stenotic portion (with or without a diverting ostomy) appeared invasive, technically dangerous and related to a high morbidity [5, 12, 13].
Subsequently, performing a diverting ileostomy was judged to be the safest solution. This option allowed the resolution of the bowel obstruction whilst being reasonably minimally invasive. The complete and spontaneous stricture regression a few weeks later permitted an uncomplicated restoration of bowel continuity. By opting for a more conservative approach, a more complex surgery with higher morbidity and mortality was avoided.
Another crucial point is prevention, which is only possible when the diagnosis is established at an early age. Except when complications occur, the diagnosis is often suspected because of family history. However, many cases without any family background, but with de novo mutations, have been described [15]. In this setting, the diagnosis is suggested by clinical or radiological arguments and is confirmed by genetic testing.
In this report, we present a new missense variant of the COL3A1 gene causing vEDS. Its pathogenicity was determined on the basis of the typical clinical presentation and the structural consequence of the glycine to aspartic acid missense mutation. We suggested further genetic investigations in the form of family genetic counselling in order to confirm the interpretation of this variant.
Finally, once the diagnosis is established, patients should be closely monitored, follow tight blood pressure control, with a goal systolic pressure of less than 120 mm Hg, and avoid some situations in order to decrease the likelihood of complications: intense exertion with the potential to cause blood pressure peaks, stretching exercises or carrying heavy loads, any medication or substances with vasopressor or hypertensive effects, and constipation should all be avoided. All invasive procedures, from arterial blood gas to digestive endoscopy, are contraindicated except in the case of life-threatening situations. Surveillance consists of periodic arterial CT imaging to detect new aneurysms or dissections or changes in existing treated ones [16, 17].
Currently, there is no established therapy or real consensus about drug management of vEDS. However, some authors suggest the use of antihypertensive drugs, in particular cardioselective and vasodilatory beta-blockers, to reduce the occurrence of vascular complications. Celiprolol, a beta-2 agonist with vasodilatory properties, has become the main treatment for patients with vEDS in most European countries [18, 19]. Recently, some authors have described an improvement in the biomechanical integrity of the aortic wall, and thereby potentially reduced risk of dissection or rupture, in a vEDS animal model treated with celiprolol. Moreover, their data showed no benefit to using bisoprolol, another, non-vasodilating beta-blocker, or losartan, an angiotensin II receptor type 1 blocker, to improve the integrity of arterial walls [20, 21].
In the United States, the Food and Drug Administration (FDA) has refused to recognise the use of this drug to treat patients with vEDS, declaring that there is no significant evidence for its effectiveness in this pathology, and has called for further investigations. The FDA also considers fluoroquinolones a risk factor for arterial events and advises healthcare professionals to avoid using this antibiotic class in patients at increased risk of arterial events, such as those with vEDS, unless there are no other treatment options available [22].
Gene therapy could provide a solution in the future, by restoring synthesis of type III collagen in deficient fibroblasts [23].
Conclusion
Vascular EDS is a rare condition with potentially fatal complications. Patients affected by vEDS should be followed at a reference centre. Often unknown by physicians, vEDS should be included in differential diagnoses whenever there is an evocative clinical presentation (such as rupture or arterial dissection in a person under 40 years, or history of unexplained sigmoid perforation or spontaneous pneumothorax) or a family history of complex vascular diseases.
Management must be multidisciplinary and based on a case-by-case approach after an in-depth risk-benefit assessment. Treatment should be as conservative as possible: invasive procedures must be used only for life-threatening situations, due to high rates of complications.
Acknowledgments
The authors thank Drs J.L. Blouin and M. Guipponi from the Genetic Medicine Division, Geneva University Hospitals, Geneva, Switzerland, who performed the molecular genetic testing and the interpretation of its results, definitely establishing the diagnosis of vEDS in this patient.
Written informed consent was obtained from the patient for publication.
References
1
Malfait
F
,
Francomano
C
,
Byers
P
,
Belmont
J
,
Berglund
B
,
Black
J
, et al.
The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):8–26. doi:.https://doi.org/10.1002/ajmg.c.31552
2
Malfait
F
,
De Paepe
A
. The Ehlers-Danlos syndrome. Adv Exp Med Biol. 2014;802:129–43. doi:.https://doi.org/10.1007/978-94-007-7893-1_9
3
Pyeritz
RE
. Ehlers-Danlos syndrome. N Engl J Med. 2000;342(10):730–2. doi:.https://doi.org/10.1056/NEJM200003093421009
4
Bénistan
K
. Les syndromes d’Ehlers–Danlos: classification, diagnostics différentiels et traitements. Douleurs: Évaluation-Diagnostic-Traitement. 2018;19(4):161–5. doi:.https://doi.org/10.1016/j.douler.2018.07.010
5Pepin M, Byers P. Ehlers Danlos Syndrome Type IV. In: Gene Reviews (internet). Editors: Pagon, RA, Bird TD, Dolan CR, et al. 2019 edition. Seattle, WA: University of Washington; 2011
6
Pepin
MG
,
Schwarze
U
,
Rice
KM
,
Liu
M
,
Leistritz
D
,
Byers
PH
. Survival is affected by mutation type and molecular mechanism in vascular Ehlers-Danlos syndrome (EDS type IV). Genet Med. 2014;16(12):881–8. doi:.https://doi.org/10.1038/gim.2014.72
7
Frank
M
,
Albuisson
J
,
Ranque
B
,
Golmard
L
,
Mazzella
JM
,
Bal-Theoleyre
L
, et al.
The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers-Danlos syndrome. Eur J Hum Genet. 2015;23(12):1657–64. doi:.https://doi.org/10.1038/ejhg.2015.32
8
Bade
MA
,
Queral
LA
,
Mukherjee
D
,
Kong
LS
. Endovascular abdominal aortic aneurysm repair in a patient with Ehlers-Danlos syndrome. J Vasc Surg. 2007;46(2):360–2. doi:.https://doi.org/10.1016/j.jvs.2007.03.045
9
Solomon
JA
,
Abrams
L
,
Lichtenstein
GR
. GI manifestations of Ehlers-Danlos syndrome. Am J Gastroenterol. 1996;91(11):2282–8.
10
Burcharth
J
,
Rosenberg
J
. Gastrointestinal surgery and related complications in patients with Ehlers-Danlos syndrome: a systematic review. Dig Surg. 2012;29(4):349–57. doi:.https://doi.org/10.1159/000343738
11
Byard
RW
,
Keeley
FW
,
Smith
CR
. Type IV Ehlers-Danlos syndrome presenting as sudden infant death. Am J Clin Pathol. 1990;93(4):579–82. doi:.https://doi.org/10.1093/ajcp/93.4.579
12
Freeman
RK
,
Swegle
J
,
Sise
MJ
. The surgical complications of Ehlers-Danlos syndrome. Am Surg. 1996;62(10):869–73.
13
Berney
T
,
La Scala
G
,
Vettorel
D
,
Gumowski
D
,
Hauser
C
,
Frileux
P
, et al.
Surgical pitfalls in a patient with type IV Ehlers-Danlos syndrome and spontaneous colonic rupture. Report of a case. Dis Colon Rectum. 1994;37(10):1038–42. doi:.https://doi.org/10.1007/BF02049321
14
Baichi
MM
,
Arifuddin
RM
,
Mantry
PS
. Gastrointestinal bleeding in a patient with Ehlers-Danlos syndrome: an endoscopic dilemma. Dig Dis Sci. 2005;50(7):1342–3. doi:.https://doi.org/10.1007/s10620-005-2784-5
15
Demirogullari
B
,
Karabulut
R
,
Demirtola
A
,
Karabulut
B
,
Gol
IH
,
Aybay
C
, et al.
A novel mutation in the vascular Ehlers-Danlos syndrome: a case presenting with colonic perforations. J Pediatr Surg. 2006;41(8):e27–30. doi:.https://doi.org/10.1016/j.jpedsurg.2006.04.009
16
Byers
PH
,
Belmont
J
,
Black
J
,
De Backer
J
,
Frank
M
,
Jeunemaitre
X
, et al.
Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175(1):40–7. doi:.https://doi.org/10.1002/ajmg.c.31553
17
Perdu
J
,
Boutouyrie
P
,
Lahlou-Laforêt
K
,
Khau Van Kien
P
,
Denarié
N
,
Mousseaux
E
, et al.
Syndrome d’Ehlers-Danlos vasculaire [Vascular Ehlers-Danlos syndrome]. Presse Med. 2006;35(12 Pt 2):1864–75. Article in French. doi:.https://doi.org/10.1016/S0755-4982(06)74919-3
18
Ong
KT
,
Perdu
J
,
De Backer
J
,
Bozec
E
,
Collignon
P
,
Emmerich
J
, et al.
Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet. 2010;376(9751):1476–84. doi:.. Correction in: Lancet. 2016;388(10044):564.https://doi.org/10.1016/S0140-6736(10)60960-9
19
Frank
M
,
Adham
S
,
Seigle
S
,
Legrand
A
,
Mirault
T
,
Henneton
P
, et al.
Vascular Ehlers-Danlos Syndrome: Long-Term Observational Study. J Am Coll Cardiol. 2019;73(15):1948–57. doi:.https://doi.org/10.1016/j.jacc.2019.01.058
20
Dubacher
N
,
Münger
J
,
Gorosabel
MC
,
Crabb
J
,
Ksiazek
AA
,
Caspar
SM
, et al.
Celiprolol but not losartan improves the biomechanical integrity of the aorta in a mouse model of vascular Ehlers-Danlos syndrome. Cardiovasc Res. 2020;116(2):457–65. doi:.https://doi.org/10.1093/cvr/cvz095
21
Gorosabel
MC
,
Dubacher
N
,
Meienberg
J
,
Matyas
G
. Vascular Ehlers-Danlos syndrome: can the beneficial effect of celiprolol be extrapolated to bisoprolol?
Eur Heart J Cardiovasc Pharmacother. 2020;6(3):199–200. doi:.https://doi.org/10.1093/ehjcvp/pvz067
22U.S. Food and Drug Administration. 2020. FDA Warns About Increased Risk Of Ruptures Or Tears In The Aorta. Blood [online]. Available at: <https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics> [Accessed 2020 October 1].
23
Watanabe
A
,
Wada
T
,
Tei
K
,
Hata
R
,
Fukushima
Y
,
Shimada
T
. A Novel Gene Therapy Strategy for Vascular Ehlers-Danlos Syndrome by the Combination with RNAi Mediated Inhibition of a Mutant Allele and Transcriptional Activation of a Normal Allele. Mol Ther. 2005;11:S240. doi:.https://doi.org/10.1016/j.ymthe.2005.07.158