Conference report: dementia research and care and its impact in Switzerland
DOI: https://doi.org/10.4414/smw.2020.20376
Thomas
Leyhea, Mathias
Juckerb, Tobias
Nefc, Marc
Sollbergerd, Florian
Riesee, José
Haba-Rubiof, Henk
Verloog, Regula
Lüthih, Stefanie
Beckeri, Julius
Poppj
a University of Basel, Geriatric Psychiatry, University Department of Geriatric Medicine Felix Platter, and Centre of Old Age Psychiatry, Psychiatric University Hospital, Basel, Switzerland
b Hertie Institute for Clinical Brain Research, University of Tübingen, and German Center for Neurodegenerative Diseases (DZNE), Tübingen, Germany
c ARTORG Centre for Biomedical Engineering Research, University of Bern, Switzerland
d Memory Clinic, Geriatric Psychiatry, University Department of Geriatric Medicine Felix Platter, and Department of Neurology, University Hospital Basel, Switzerland
e University Hospital of Psychiatry Zurich, Department of Geriatric Psychiatry, Zurich, Switzerland
f Centre du Sommeil de Florimont and Centre for Investigation and Research on Sleep/CHUV, Lausanne, Switzerland
g Service of Old Age Psychiatry, Lausanne University Hospital, Switzerland, and School of Health Sciences, Switzerland
h Psychiatric University Hospital, Basel, Switzerland
i Alzheimer Switzerland, Bern, Switzerland
j Old Age Psychiatry, Department of Psychiatry, University Hospital of Lausanne, and University of Zürich, Department of Geriatric Psychiatry, University Hospital of Zürich, Switzerland
Summary
In October 2019, a Swiss panel of experts met for the Dementia Summit in Brunnen, Switzerland, to discuss the latest scientific findings on basic and clinical research, as well as practical and political approaches to the challenges of dementia disorders in Switzerland. Here, we present the conference summary.
To study pathophysiological changes, as well as the underlying mechanism of fluid biomarker changes, excellent experimental approaches, including transgenic mouse models, are available. Current knowledge about presymptomatic disease progression is largely derived from the longitudinal study of individuals with autosomal dominant mutations (Dominantly Inherited Alzheimer Network).
Importantly, more than one third of identified dementia risk factors can be modified. For example, sleep disturbances are not only associated with dementia and neurodegeneration in specific brain regions, but also precede cognitive decline and contribute to the development of brain pathology.
Regarding the neuropsychological examination of dementia disorders, standardised tests of social cognition, one of the six cognitive domains that must be assessed according to the fifth edition of the Diagnostic and Statistical Manual for Mental Disorders, are missing, but now under development.
The most important new therapeutic approach in the treatment of Alzheimer’s disease is the current attempt to prevent β-amyloid accumulation. While until now clinical studies have failed because of side effects or insufficient clinical effectiveness, Biogen recently announced positive results of high doses of aducanumab, a monoclonal antibody against β-amyloid. Other approaches also show promise. In China, sodium oligomannate has been approved to treat Alzheimer's disease. The substance suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression.
Assistive technologies for dementia patients can help identify relevant information for care and nursing, as well as measurements for clinical interventions.
Dementia patients have a high risk of developing delirium, even in the home environment. Therefore, it is necessary to use and further develop multi-disciplinary and systematic detection and prevention strategies.
Homecare models for dementia patients with multidisciplinary teams have been established and evaluated and should be expanded.
Dementia is the third-leading cause of death in Switzerland. In palliative care for severe dementia, the improvement of quality of life is of primary importance.
The goals of the National Dementia Strategy, to increase the quality of life in those affected and to reduce taboos surrounding the disease, are still unrealised. The need for further national and regional engagement in order to implement the different findings of the strategy has largely been acknowledged, and these implementations have become the core tasks of a national dementia platform.
Introduction
Cognitive impairment and dementia constitute a growing public health concern, with severe consequences for patients and their relatives, as well as for healthcare systems and societies worldwide. Despite intensive research efforts and major recent progress, the causes and mechanisms involved in cognitive impairment and in dementias such as Alzheimer’s disease (AD) are only partially understood [1].
The increase in chronic, non-communicable diseases such as dementia disorders is also one of the central challenges for the Swiss healthcare system. Dementia diseases are one of the most common diseases in old age, are considered the most common reason for long-term care of older people, and are the third most common cause of death in people above the age of 85 after cardiovascular diseases and cancer [2]. Around the world, one person is diagnosed with dementia every three seconds [3], and in Switzerland there are around 29,500 new diagnoses a year. Alzheimer's Switzerland estimates that around 155,000 people with dementia live in Switzerland, of whom around 7400 are under the age of 65 [4].
Since old age is the greatest risk factor for dementia, the number of cases is expected to continue to rise, despite the declining rate of new cases, due to increasing life expectancy and good medical care. No curative therapies are available to date, and the failures of pharmacological research so far leave only modest hopes for a corresponding drug.
Facing this situation in October 2019, a Swiss panel of experts met for the Dementia Summit in Brunnen, Switzerland, to discuss the latest scientific findings from basic and clinical research, as well as practical and political approaches to the challenges of dementia disorders in Switzerland.
Translational research (Mathias Jucker)
Although animals do not develop the typical symptoms of dementia, transgenic mouse models are excellent models for studying pathophysiological disease mechanisms and have proven their usefulness for biomarker research in neurodegenerative diseases.
It has been shown that the intracerebral injection of β-amyloid-containing brain extracts can induce cerebral β-amyloidosis and associated pathologies in susceptible β-amyloid precursor protein (APP) transgenic mice. The same is true when the injections are intraperitoneal, albeit the induction occurs only after prolonged incubation times [5]. These and other experiments suggest that the induction and spreading of β-amyloid (Aβ) aggregation in the brain follows a prion-like mechanism [6, 7] (fig. 1).
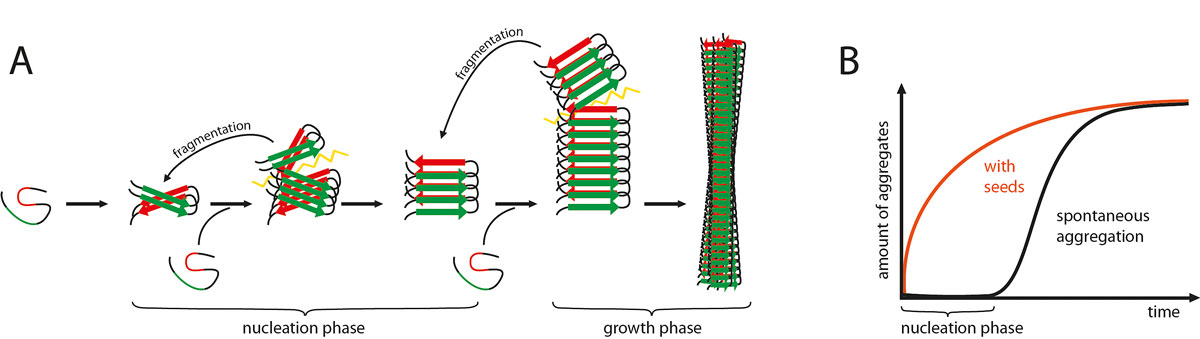
Figure 1
A framework to explain the prion-like induction and spreading of Aβ aggregation. (A) Amyloidogenic proteins such as Aβ typically have β-strands (red and green parts) that interact and mediate the aggregation of Aβ into long and unbranched amyloid fibrils. Such Aβ aggregation starts with a slow nucleation phase that may go through a series of intermediate states until the initial segment of the amyloid fibril is formed. Monomers are then added to the ends of the initial amyloid seed by conformational conversion. With increasing length, the growing amyloid fibril will eventually break up (spontaneously or actively through cellular processes). Thus, amyloid formation becomes self-propagating through the generation, release and spread of new amyloid seeds. (B) The nucleation phase can be greatly shortened by the addition of exogenous seeds (modified from [7]).
Fluid biomarker research in mice started with the seminal publication of Maia et al. [8]. Aβ and Tau in the cerebrospinal fluid (CSF) of two well-characterised APP transgenic mouse models were measured. Both mouse models exhibited Aβ deposition in the brain, but with different onset and progression trajectories. An age-related decrease in Aβ42 peptide in mouse CSF of 50–80% and a smaller decrease in Aβ40 were found, both inversely correlated with the brain Aβ load. Strikingly, the same mice showed a threefold increase in total endogenous murine Tau in CSF at stages when Aβ pathology was prominent. This observation suggested for the first time that the increase of Tau in the CSF in AD is caused by the Aβ deposition and not by neurofibrillary tangles, since tangles do not occur in APP transgenic mice [8]. In another study, robust increases in neurofilament light chain (NfL) in CSF and blood in murine models of alpha-synucleinopathies, tauopathy and β-amyloidosis were reported [9]. Blood and CSF NfL levels were strongly correlated, and NfL increases coincided with the onset and progression of the corresponding proteopathic lesions in the brain. Experimental induction of alpha-synuclein lesions increased CSF and blood NfL levels, while blocking Aβ lesions attenuated the NfL increase. NfL increases were also found consistently in the CSF and blood of human patients with α-synucleinopathies, tauopathies and AD [9]. Thus, NfL appears to be a promising readout for Aβ- (and other proteopathic lesion)-targeting clinical trials.
The Dominantly Inherited Alzheimer Network (DIAN) study focuses on the preclinical stages of AD. In this study, individuals with autosomal dominant AD mutations are observed longitudinally. It was found that the first changes in the brain occur at least 10–20 years before dementia symptoms appear [10]. Using an ultrasensitive immunoassay technology, it was found that NfL levels in the CSF and serum are correlated with each other and are already elevated at presymptomatic stages in DIAN participants. Longitudinal, within-person analysis of serum NfL confirmed this elevation and revealed that the rate of change of NfL was even more sensitive and could discriminate AD mutation carriers from non-mutation carriers (on a group level) almost a decade earlier than cross-sectional absolute NfL levels (that is, 16.2 vs 6.8 years before the estimated symptom onset). Serum NfL rate of change peaked in participants converting from the presymptomatic to the symptomatic stage and was associated with cortical thinning (assessed by magnetic resonance imaging), but less so with Aβ deposition or glucose metabolism (assessed by positron emission tomography). Serum NfL was predictive for both the rate of cortical thinning and cognitive changes as assessed by the Mini-Mental State Examination and the Logical Memory test. It was concluded that NfL dynamics in serum predict disease progression and brain neurodegeneration even at early presymptomatic stages of familial AD, which supports its potential as a clinically useful AD biomarker [11].
Sleep and cognition (José Haba-Rubio)
One third of the risk factors for dementia, such as hearing loss, hypertension, social isolation and depression, can be influenced [12].
Sleep is an indispensable biological function thought to be essential for brain restoration and memory consolidation [13]. As life progresses, there are significant changes in sleep quantity and architecture, and there is also an increased susceptibility to sleep disorders. Sleep disturbances are particularly frequent in subjects with cognitive deficits such as mild cognitive impairment and dementia [14].
These sleep disturbances have often been dismissed as consequences of the disease process, reflecting the degeneration of the neural pathways that regulate sleep-wake patterns and sleep architecture, or as consequences of its related somatic and psychiatric co-morbidities. Nevertheless, accumulating evidence suggests that sleep disturbances occur very early in the cognitive decline process, and different studies have shown that sleep duration, sleep fragmentation and sleep pathologies can play a role in the pathogenic process leading to cognitive impairment.
In a recent study, the subjective (evaluated using questionnaires) and objective characteristics of sleep (measured by polysomnography) of 580 elderly participants (>65 years) of the population-based CoLaus/PsyCoLaus study (Lausanne, Switzerland) were compared between those with cognitive impairment (measured by the Clinical Dementia Rating Scale score), and those with normal cognition. Sleep-disordered breathing (SDB) was more severe in participants with cognitive impairment, and after adjustments for confounding variables, the apnea/hypopnea index and the oxygen desaturation index were independently associated with cognitive impairment [15] (table 1).
Table 1 Association of polysomnographic variables with a Clinical Dementia Rating Scale > 0, multivariate analysis.
| |
Odds ratio*
|
95% confidence interval
|
p value
|
| Total sleep time, min |
1.00 |
0.97–1.02 |
0.884 |
| Stage N1, min |
1.04 |
0.97–1.10 |
0.246 |
| Stage N2, min |
0.99 |
0.96–1.02 |
0.576 |
| Slow wave sleep (stage N3), min |
1.01 |
0.95–1.07 |
0.792 |
| REM sleep, min |
0.98 |
0.92–1.04 |
0.548 |
| Sleep onset latency, min |
0.97 |
0.91–1.04 |
0.466 |
| Sleep efficiency, % |
0.97 |
0.82–1.13 |
0.675 |
| Wake after sleep onset, min |
1.00 |
0.97–1.03 |
0.832 |
| REM latency, min |
0.99 |
0.96–1.01 |
0.309 |
| Number of stage shifts |
1.01 |
0.97–1.04 |
0.720 |
| Apnoea/hypopnoea index, n/h |
1.15 |
1.00–1.31 |
0.043
|
| Mean SaO2, % |
0.73 |
0.22–2.38 |
0.602 |
| Lowest SaO2, % |
0.79 |
0.56–1.12 |
0.184 |
| Oxygen desaturation index ≥3%, n/h |
1.09 |
0.96–1.22 |
0.173 |
| Oxygen desaturation index ≥4%, n/h |
1.17 |
1.01–1.36 |
0.033
|
| Oxygen desaturation index ≥6%, n/h |
1.33 |
1.03–1.72 |
0.029
|
| Arousal index, n/h |
1.00 |
0.86–1.16 |
0.993 |
| PLMS index, n/h |
1.00 |
0.95–1.06 |
0.885 |
In addition, by analysing data from this large-scale cohort from the general population, the association between markers of sleep-related hypoxemia and brain anatomy in 775 participants who underwent full polysomnography and brain magnetic resonance imaging were investigated. It was found that a lower mean SaO2 was correlated with reduced volumes in the hippocampus-amygdala complex, thalamus, basal ganglia and frontoparietal cortex, suggesting a vulnerability of these regions to nocturnal hypoxemia that might provide a possible explanation for SDB-associated neuropsychological deficits [16].
Finally, available but still limited data also suggest that the treatment of sleep disorders, and particularly the treatment of SDB, is associated with slower cognitive decline in patients with dementia [17].
In summary, sleep plays an important role in cognitive processes and subjects with cognitive impairment have more disturbed sleep. SDB, and in particular nocturnal hypoxemia, is associated with the presence of cognitive impairment and a decrease in the volume of the hippocampus and the amygdala. Prospective and interventional studies are needed to determine the impact of treatment of sleep disorders such as apnoea syndrome on cognitive impairment.
Assessment of social cognitive dysfunction – the development of the Basel version of the Awareness of Social Inference Test (BASIT) (Marc Sollberger)
Social cognition is about cognitive and emotional processes ranging from emotion recognition, the ability to infer other people’s thoughts and feelings, through to planning socially adequate actions [18, 19]. Consequently, social cognitive functions are essential for our quality of life [20, 21].
According to the fifth edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5), social cognition is one of the six cognitive domains that must be assessed for the diagnosis of a neurocognitive disorder. However, in contrast to the other cognitive domains, such as memory or language, tests that assess social cognitive functions reliably and in an ecologically valid manner within a reasonable timeframe are scarce [22]. Since social cognitive deficits are observed to some degree in most brain disorders, including neurodevelopmental, neurovascular, neuroinflammatory, neurodegenerative and psychiatric disorders [19, 22], the development of tests for use in clinical populations is highly important.
According to a recent review article and a further literature review on the clinical assessment of social cognition in clinical populations [22, 23], “The Awareness of Social Inference Test (TASIT)” [24] is probably one of the most comprehensive instruments to date. TASIT comprises video vignettes of actors depicting emotional and social signals in a realistic manner. It has been shown to be valid and reliable [25], displaying deficits in social perception in patients with different brain disorders [26]. However, TASIT is limited by several factors such as (i) the high socio-emotional intensities portrayed by the actors, reducing its sensitivity in detecting social cognitive deficits, (ii) the long administration time, preventing its utility in clinical routine, (iii) quite simple paradigms (only forced-choice labeling tasks), which limit its accuracy in assessing social cognitive functions, and (iv) the limited quality and consistency of the film clips [10, 11]. To overcome these limitations, we adapted several parts of the test and renamed it the “Basel version of the Awareness of Social Inference Test (BASIT)” [27, 28].
In collaboration with the film institute East End Film, 99 film clips showing basic emotions (i.e., anger, fear, disgust, happiness, sadness and surprise) and social (i.e., cognitive and affective perspective-taking) signals were shot with eight professional, German-speaking actors. Importantly, the actors were required to portray these emotions and forms of communication at low, medium and high intensities, i.e., three different socio-emotional intensities were generated per scene [27, 28]. These scenes were shown to 240 cognitively and psychically healthy people between 35 and 92 years of age. Each person watched each scene at only one given intensity (for example, participant 1 watched scene 1 at low intensity, scene 2 at medium intensity, and so forth, whereas participant 2 watched scene 1 at high intensity, scene 2 at low intensity, and so forth). Figure 2 shows a screenshot of an example scene (in which anger at low intensity is portrayed). The video clip of this scene can be found online at Vimeo.com. By running Rasch models, graphs that allowed scenes of different levels of difficulty to be selected for use in clinical populations were produced.

Figure 2 Screenshot of a scene in which anger at low intensity is portrayed. The video clip of this scene can be found online at Vimeo.com.
The next step will be the administration of these selected scenes to patients with neurodegenerative and psychiatric disorders to examine their validity and reliability in comparison to other, less ecologically valid tests of social cognition [22].
Drug treatment of Alzheimer’s disease: state of the art and new approaches (Thomas Leyhe)
Four drugs are currently approved to delay progression in AD. These are the three acetylcholinesterase inhibitors donepezil, galantamine and rivastigmine, as well as the N-methyl-d-aspartate antagonist memantine. Furthermore, there is also evidence for the effectiveness of Ginkgo Biloba EGb761 on cognition and non-psychotic behavioural symptoms in patients with mild to moderate Alzheimer’s dementia, so treatment with this preparation can also be considered [29]. The approved drugs are all symptomatically effective. They can delay the course of the disease, but they cannot stop the neurodegenerative process. Therefore, other active substances are urgently needed.
The most important new therapeutic approach in the treatment of AD is the current attempt to prevent Aβ accumulation. Aβ peptides arise from APP. APP can be broken down by an alpha and a gamma secretase into end products that do not form amyloid plaques. But it can also be broken down by the enzyme β-site-APP-cleaving enzyme 1 (BACE-1), a β-secretase and the γ-secretase into amyloid-forming Aβ peptides. Usually, the first route is preferred. A shift towards the second route of degradation is considered the initial factor in AD. The resulting oligomers and intermediate amyloid proteins are particularly toxic to synapses. Finally, they aggregate spontaneously and form the sparingly soluble, neurotoxic amyloid plaques [30].
The most important therapeutic approach is Aβ immunisation. Several large phase III studies of passive immunisation against Aβ have been completed in patients with mild to moderate AD. The active ingredient bapineuzumab has been shown to cause a reduction in the amyloid loading of the brain in patients with Alzheimer's dementia in vivo. This was demonstrated by positron emission tomography using Pittsburgh Compound B ([11C] PiB-PET), a marker of cortical fibrillar Aβ [31]. But neither bapineuzumab [32], solanezumab [33], crenezumab [34] nor gantenerumab [35] showed an effect on the primary endpoints cognition and everyday function.
Another antibody, aducanumab, on the other hand, was not only shown, in a Phase Ib study, to reduce dose-dependent amyloid peptides in the brains of patients with mild Alzheimer's dementia, but also to delay cognitive decline [36]. Aducanumab is a monoclonal antibody (mAb) that binds soluble and insoluble Aβ in the brain and leads to a significant reduction of the neurotoxic peptides. In spring 2019, two Phase III studies of aducanumab (EMERGE and ENGAGE) were stopped after a futility analysis [37]. However, after a reanalysis of its data, the EMERGE study has now shown that the group who received a higher dose (10 mg / kg body weight) of the mAb actually saw an improvement in cognitive function. The effect was not significant at lower doses. Furthermore, a reanalysis of the data from the ENGAGE study showed that the patients in this study who received at least 10 doses of 10 mg / kg mAb also showed a significant improvement in cognitive function. With good safety data, the decision of the US Food and Drug Administration (FDA) on whether aducanumab will receive approval for the treatment of AD is now eagerly awaited [38].
Phase III clinical trials of the mABs gantenerumab and BAN2401 have been launched in AD patients, with results expected in 2022 or 2023. The mAb programmes consider increasing doses and targeting oligomers a potentially appropriate strategy [39].
A further challenge for these therapies is that the pathological processes begin 10–15 years before the onset of symptoms. In at risk groups, such as patients with autosomal dominant AD, who develop symptoms between the ages of 30 and 50 years, mAbs (solanezumab, gantenerumab) have been used 15 years before the onset of the disease (DIAN-TU; ClinicalTrials.gov: NCT01760005). However, a top-line analysis of the first phase II/III clinical trial performed by the DIAN-TU trials platform showed that both of the investigated drugs missed the primary endpoint. That endpoint was a statistically significant difference between drug and placebo on the DIAN Multivariate Cognitive Endpoint, a composite of four cognitive tests developed by DIAN for this stage and type of AD. Additional analyses are ongoing. Given the small sample size and the heterogeneity of disease stage in this trial, some of those analyses will focus on individual trajectories [40].
Another at-risk group is asymptomatic persons who have shown signs of amyloid pathology during screening. The use of solanezumab is being investigated in this group (ClinicalTrials.gov: NCT02008357) and the results are still pending. Unfortunately, another study with a substance that should prevent plaque formation by inhibiting the enzyme BACE-1 in a similar group has even led to a deterioration in cognitive abilities [41]. Other studies with substances targeting BACE-1 or the γ-secretase were also stopped because of ineffectiveness or side effects [42].
Other interventions currently in clinical trials target the tau-related neurological damage. The majority of the substances being investigated are disease-modifying agents [43].
In China, sodium oligomannate has been approved to treat AD. The substance therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit AD progression [44].
The future of Alzheimer’s treatment might be a multi-drug, multi-modal approach akin to chemotherapy. How to combine potential drug treatments, how to select the appropriate subgroups, and in what order they should be selected are all still undecided. The current trends clearly suggest that segmentation and non-amyloid directed methods will fundamentally alter the therapeutic landscape.
Assistive technologies for dementia patients – current state of the art and perspectives (Tobias Nef)
Patients with cognitive impairment often have a strong desire to live at home independently. Given the degenerative nature of the disease, most patients will experience a moment when living independently becomes impossible. Finding the appropriate moment to move to an institutional care setting is a trade-off between the patient’s desire to stay “at home” and the potential risks associated with living independently with impaired cognition (e.g., safety, activities of daily living). In this situation, sensor technology can help supervise the patient’s activities and can share this information with formal and informal caregivers. The basic assumption is that if the caregivers know about the patient’s whereabouts and activities (e.g., cooking, eating, sleeping), this will help them to optimise formal and informal caregiving, allow interventions in emergencies (e.g., falls), and postpone the moment when institutional care is needed (fig. 3).
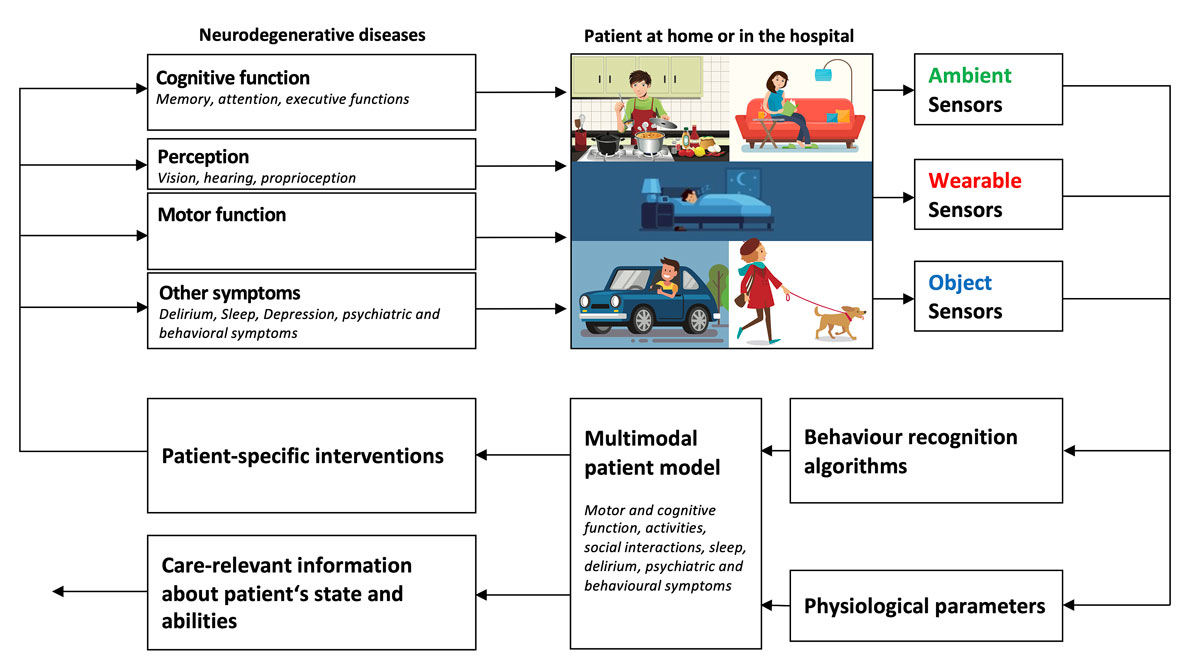
Figure 3 Ambient (contactless), wearable and object-attached sensors measure the behaviour of a patient at home or in the hospital. Machine learning algorithms recognize the patient’s activities and the multimodal patient model combines this information with physiological parameters to produce care-relevant information and/or trigger patient-specific interventions targeting cognitive function, perception, motor function or other symptoms.
Wearable sensors, ambient sensors or a combination of the two can monitor activities in the patient's home. Wearable sensors (e.g., a smartwatch) can measure body movements, recognise activities and measure physiological parameters (e.g., respiration, heartbeat). This sensor type requires the patients’ cooperation and also particular actions (e.g., charging and wearing the sensor). Therefore, this sensor type is not optimally suited to patients with cognitive impairments. Patients often forget to wear the sensor or to recharge the device [45]. This problem can be solved with an ambient sensor system that consists of six to ten matchbox-sized sensor boxes that are distributed throughout the patient's home. The system uses cheap, battery-powered, passive, infrared-based motion recognition sensors that communicate wirelessly and are easy to install in the patient's home. State-of-the-art machine learning algorithms can recognise patients' activities with a sensitivity and specificity both above 90% [46]. Typical information that can be extracted from the sensors are daily sleeping patterns, cooking, eating, and some emergency situations (e.g., falls) [47]. It was shown that these sensors are well accepted by patients and caregivers and that they work reliably in different apartments [48]. Further studies are needed to investigate whether the use of such sensors also fosters independent living and allows institutional care to be delayed.
Another topic discussed in the meeting was the use of computer gaming technology to diagnose cognitive impairments. Previous work has shown that specifically designed computer games are fun to play for patients with cognitive impairments [49, 50]. These games must automatically adjust their difficulty levels to the abilities of the patients to prevent frustration occurring because the game is too difficult or too easy. Depending on the task in the game, different cognitive abilities can be analysed (e.g., memory, selective attention, processing speed). Today, it is clear that computer games can be an easy-to-use and fun addition to a traditional cognitive assessment in the memory clinic [51], but it remains unclear how to link the multiple performance measures in a game to individual cognitive abilities. Also, it is not known how relevant the abilities tested in the games are to daily life.
Prevention of delirium at home: public health issues and avenues for improvement (Henk Verloo)
Switzerland’s public healthcare system is under significant pressure to maintain home-dwelling older adults (HDOAs) in their abodes for as long as possible. Indeed, the majority of HDOAs want to do exactly that, even when they need substantial health care and support [52]. This public healthcare policy approach means that hospitalised older inpatients are quickly discharged home, with their follow-up care being managed by home healthcare services [53]. Their fragile condition, however, puts them at high risk of developing delirium, a disorder linked to high morbidity, poly-medication and high mortality [54] (table 2).
Table 2 Population-based sample (n = 36,792) of elderly persons (65+) returning home from a multisite Swiss public teaching hospital between 2015 and 2017 (based on the principal diagnostic ICD-10 categories F050, F051, F058 and F059).
|
ICD-10
|
Description
|
Frequency
|
| F050 |
Delirium not superimposed on dementia |
14 |
| F051 |
Delirium superimposed on dementia |
619 |
| F058 |
Other form of delirium |
324 |
| F059 |
Delirium, without precision |
78 |
| Total |
1035 |
Delirium is a neuropsychiatric syndrome defined by a disorder of attention or consciousness, accompanied by a worsening of cognitive, perceptive and behavioural functioning. Delirium usually appears acutely, within a few hours or days, and its clinical features fluctuate during the day [55, 56]. Delirium can be characterised by motor subtype, namely as hypoactive, hyperactive or mixed delirium [57]. Understanding of the prognosis of delirium is still evolving, with a growing body of literature exploring associations between the classification of delirium’s aetiologies, its motor subtypes, and the severities of its outcomes [58, 59]. Several authors have documented that the prevalence of delirium among hospitalised older inpatients can reach 60% [60]. Also, the non-detection of an episode is estimated to occur in approximately half of older patients leaving hospital [61]. Better consideration of the high prevalence of post-hospitalisation confusion, its non-detection, and the under-management of silent episodes of delirium may help to reduce the disorder’s negative impacts on the older patients themselves, as well as their informal and formal caregivers [62].
Despite a substantial body of knowledge transfer and evidence-based guidelines, the detection of delirium remains problematic in all healthcare settings [63]. Delirium is common during emergency department (ED) stay and is particularly prevalent among older adults. As many as 7–17% of older adults presenting at the ED meet the diagnostic criteria for delirium. ED professionals frequently miss delirium, perhaps in up to 80% of cases. ED delirium is associated with significantly increased in-hospital, 30-day and 6-month mortality, as well as the loss of independence, accelerated cognitive decline and post-traumatic stress disorder, all of which are troubling to both patients and their families [64].
Strategies for the prevention or early detection of delirium at home are scarce, and their efficiencies hardly investigated. The evidence accumulated in hospitals and the rare clinical studies of interventions for the prevention of physical decline among HDOAs could together serve as the foundation for developing targeted interventions for early detection and treatment [65]. New avenues involving the use of new technologies for the early detection of delirium should be explored [66]. Interventions should also include educational components, supported by well-established clinical pathways at hospital or ED discharge, so that the healthcare professionals involved can quickly detect signs of deterioration among HDOAs and contribute to the development, application and evaluation of effective prevention strategies for older adults who still live at home. There is an urgent need to develop new knowledge to help prevent delirium at home. A rigorous evaluation of interventions must be carried out, investigating both their effectiveness at improving HDOAs' well-being and their efficiency in terms of healthcare spending.
Assertive community treatment for the elderly in the canton of Thurgau (Regula Lüthi)
The project “assertive community treatment for the elderly” began as a one-nurse project, born out of necessity. The psychiatric clinic of Münsterlingen noted that too many older people discharged from inpatient treatment returned to the ward too soon, as their integration back at home was not successful. Exhausted relatives, not enough knowledge about disease management and overwhelmed friends were some reasons. Another was the lack of expertise in psychiatric disorders within domestic care teams at this time (2010).
The newly implemented treatment was successful. However, as costs for outpatient treatment are not covered by the regular health insurance, financial support was supplied by the canton of Thurgau, and for the first three years it had the status of a “pilot project”. After this successful period, it was integrated into routine care – still needing financing from the canton.
The two types of outpatient treatment are transitional treatment and long-term treatment. The transitional treatment is especially helpful for the elderly as it is for a limited time of three months only, but consists of an extensive case management. The treatment is a personalised intervention tailored to the specific needs of patients and their relatives. Mental health nurses, social workers, psychiatrists and psychologists form an interdisciplinary team with a focused psychiatric knowledge base.
Assertive community treatment is nowadays a respected and evidence-based intervention. Several guidelines confirm its impacts on mental health and quality of life [67, 68]. But the costs of this treatment are a problem all over Switzerland. There now exists a new task force at the University of Applied Science SUPSI charged with collecting data about the diverse funding methods and then proposing a new form of financing to all the cantons of Switzerland and to the health insurance companies.
Back to the assertive community treatment for the elderly in the canton of Thurgau: one of the robust results of this new outpatient treatment was a significant reduction in the number of days patients spent in the psychiatric hospitals (fig. 4).
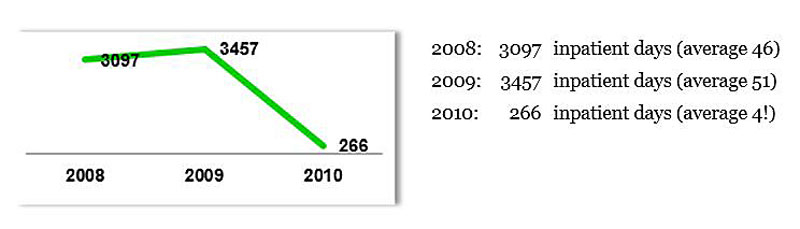
Figure 4 Development of inpatient days for 68 patients receiving transitional assertive community treatment in 2010. This diagram shows a significant reduction in inpatient days for the 68 patients who received the transitional assertive community treatment (Reference source: Presentation Psychiatry Planning – New Nursing Elements in the Provision of Psychiatric Services GDK, Bern 2011).
Another, qualitative outcome was the easing of the burden on the patients’ relatives. The third outcome was a training programme about psychiatric disorders, recovery orientation, etc. for all nurses working in primary care teams. It is an ongoing and successful treatment, and therefore a small but important improvement in the integrated mental healthcare system.
The end of life with dementia (Florian Riese)
Dementia is a potentially life-limiting condition that leads to unique palliative care needs (fig. 5).
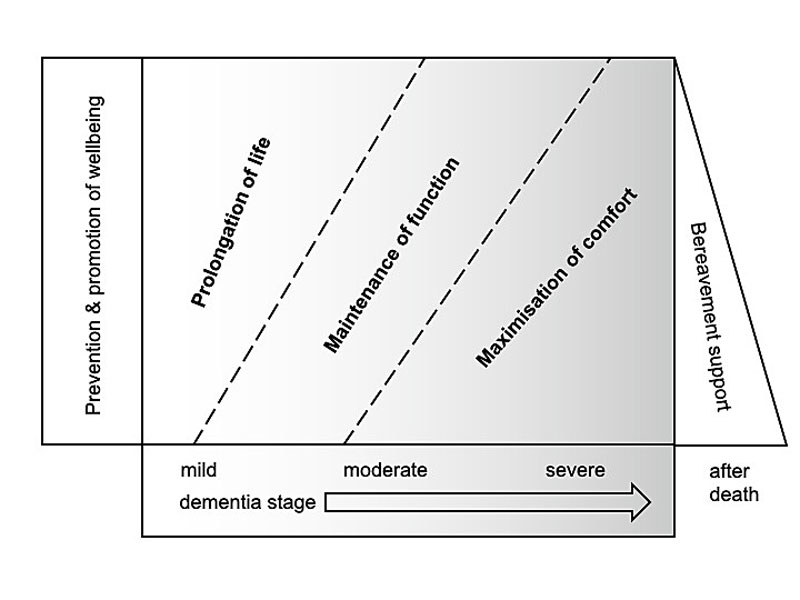
Figure 5 Goals of care by dementia stage (modified from [69]).
After cardiovascular disease and cancer, dementia has become the third most frequent cause of death in Switzerland, accounting for almost 10% of all deaths in 2017 [2]. People who die from dementia as an underlying condition mostly die from dehydration, cachexia and infections as immediate causes [70]. Many more die not from dementia, but with dementia, i.e., while suffering from varying degrees of dementia as a comorbidity. International data suggest that most people with dementia – at least in the advanced stages – die either in hospital or in long-term care [71].
Even though dementia is often (mis-)understood as mainly a cognitive or behavioural condition, there is no indication that physical symptom burden is lower at the end of life in patients with dementia than in those without [72]. The behavioural and psychological symptoms of dementia (BPSD) often complicate end-of-life care. Agitation remains frequent, even in the final months before death [73], but more longitudinal studies are needed to elucidate the course of BPSD in advanced dementia [74]. Treatment of BPSD is best achieved by adopting a systematic approach and frequently involves both non-pharmacological and pharmacological measures [75].
In its advanced stages, dementia is frequently associated with reduced intake of food and liquids, as well as with an increased risk of infections, particularly pneumonia. Therefore, the potential use of tube feeding, artificial hydration and antibiotics in (repeated) episodes of pneumonia are key questions in dementia care. Based on the available evidence, the current guidelines of the Swiss Academy of Medical Sciences advise against the use of percutaneous endoscopic gastroscopy feeding tubes in advanced dementia [76]. The available evidence for the use of artificial hydration and antibiotic pneumonia treatment in advanced dementia is very limited, so decisions are mostly based on the individual circumstances. However, the potential benefits of these interventions on patients’ survival and symptom burden may be only transient [77].
In the terminal phase of dementia, continuous deep sedation (CDS) may be discussed as a palliative care option. In a recent Swiss study, about half of the decision-makers included were open to CDS [78]. However, international data indicate that CDS in advanced dementia does not guarantee a dying process free from struggle or agony [79]. In Switzerland, assisted suicide is incompatible with advanced dementia due to the associated loss of decision-making capacity [80].
Decisions in advanced dementia – including those for health care – are usually not made by the patients themselves, but by their healthcare proxies, unless an advance directive has been established. Clarifying the goals of any care with the decision-makers, i.e., whether prolongation of life span, maintaining or regaining functional abilities, or comfort and well-being are the leading objectives, helps to limit burdensome medical interventions in advanced dementia [81]. In recent years, decision aids (e.g., evidence-based brochures or videos) that support the shared decision-making process have become available [77, 82]. In order to provide optimal end-of-life care for persons with advanced dementia, close cooperation between all the parties involved is required. Future research and increasing clinical experience will guide this process.
National Dementia Strategy Switzerland: lessons learnt? (Stefanie Becker)
In order to respond to the growing challenges of dementia disorders, the federal government and the cantons adopted the “National Dementia Strategy 2014–2017” at the end of November 2013 [83]. The preparatory work was initiated by a manifesto that the patient and family organisation Alzheimer Switzerland had published back in 2008. In November 2016, the strategy was extended to 2019 because the original time period was not – despite the great commitment of the actors involved – sufficient to fully achieve the project’s goals (fig. 6).
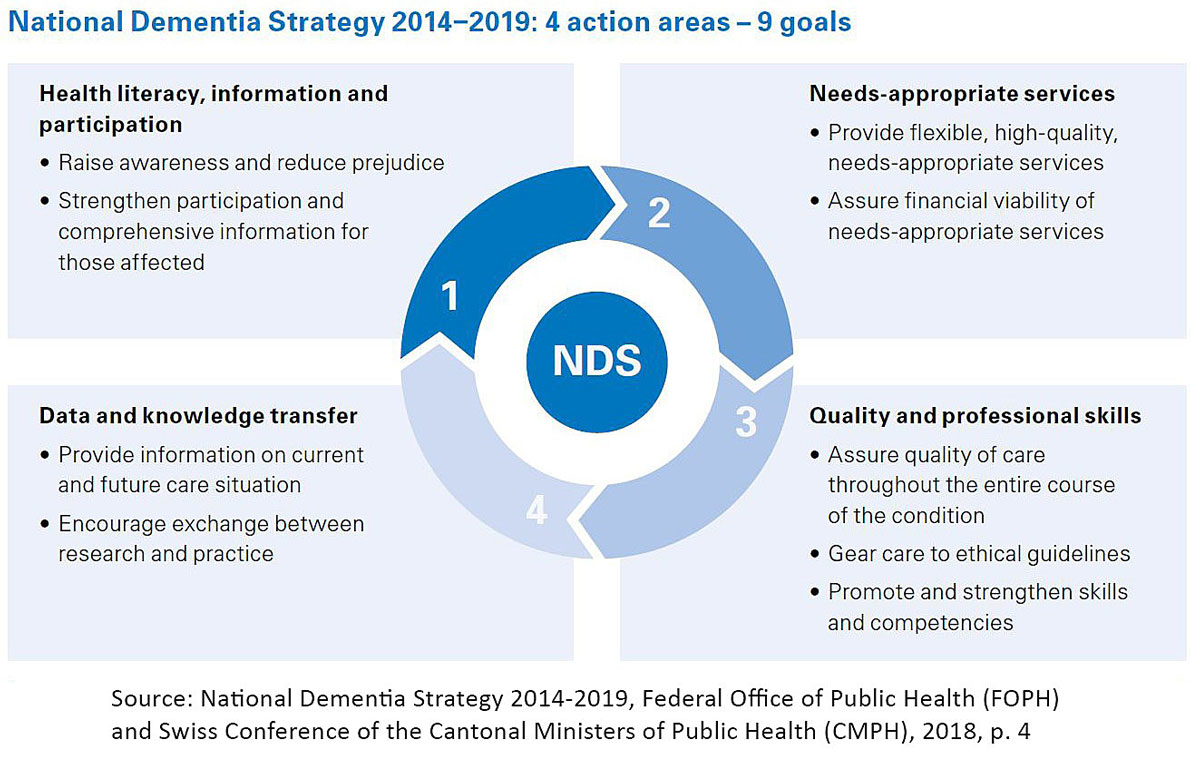
Figure 6 Action areas and goals of the National Dementia Strategy 2014–2019.
The superordinate goals were a better understanding of dementia, its destigmatisation and the acceptance of those affected by society. The strategy aimed to optimise the interaction with, quality of treatment of and care for people with dementia and to improve the quality of life of those affected by dementia. This also includes providing dementia-appropriate, integrated care throughout the course of the disease, from early diagnosis to palliative care.
To achieve these goals, four fields of action and nine goals were defined. The implementation was carried out using a so-called multiplier approach, which was based on the involvement of both national umbrella organisations and specialist organisations (as multipliers of the results obtained). However, public funds were not available for the implementation of the strategy, and research into understanding the disease mechanistically was not part of the National Dementia Strategy.
The evaluation showed that even though the strategy’s medical and political importance was generally highly valued, its goals could not be achieved as desired [84]. For many of the organisations involved, the strategy provided an important legitimisation for the investment of resources in the subject area. However, the chosen implementation concept and the lack of funding have both slowed the implementation and led to the a-symmetrical participation of mainly larger organisations. So far, those affected have hardly benefited from the results. Undesired effects such as the confusing proliferation of offers must now be countered with control and coordination to meet individual needs.
In order to counter this criticism and to ensure that the resources previously used have a lasting impact, a national platform was launched after the strategy was completed. It is intended to coordinate on-going and new activities under one national umbrella, and thus contribute to the sustainable impact of the resources previously used. In addition to the previous stakeholders, cantons, cities and municipalities are now more involved. It remains to be seen whether this structure will achieve noticeable results for people with dementia and their relatives, even if there is still no funding available.
Conclusions
While great progress has been made in basic and translational research into dementia diseases in recent years, drug studies have so far failed. Now, after reanalysing two phase III study results, a request for the approval of aducanumab, a mAb against Aβ, is in the process of being submitted to the FDA. In China, sodium oligomannate has been approved to treat AD. The substance therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit AD progression. It remains unclear whether these new and potential future treatment options can significantly impact the increasing number of people with dementia in Switzerland. Such uncertainties call for substantial funding of basic and clinical dementia research, as well as research translation.
Prevention remains an essential prerequisite to reducing the incidence of dementia, as one third of dementia’s risk factors can be modified. Preventing and treating sleep disturbances may help to protect against dementia.
The development of clinical tests to assess social cognitive dysfunction reliably, in an ecologically valid manner and within a reasonable timeframe in order to improve the early diagnosis and monitoring of the different dementia disorders is very important.
A great challenge, both worldwide and in Switzerland, is the improvement of care for people with dementia. Successful assertive community treatment for the elderly has been developed, and assistive technologies can help to maintain home-dwelling. Awareness for delirium has improved, but it remains under-recognised and undertreated.
Dementia is life-limiting and needs unique palliative care. In order to provide optimal end-of-life care for persons with advanced dementia, close cooperation between all the parties involved is required.
In order to respond to the growing challenges of dementia disorders, the National Dementia Strategy is an important, but not a sufficient, instrument of health politics. National funding and the inclusion of multiple stakeholders as well as people with dementia add essential value to the developmental process and to the expected outcomes. Questions of how, who and where to implement the results must be a central part of a national strategy in order to achieve a sustainable impact for those living with the condition.
It can be concluded that we will only be able to meet the complex challenge of dementia through the interaction of consultants, carers, researchers, politicians, and patient and family associations, as well as business experts, patrons and artists.
References
1National Academies of Sciences, Engineering, and Medicine, Division of Behavioral National Academies of Sciences, Engineering, and Medicine, Division of Behavioral and Social Sciences and Education, Board on Behavioral, Cognitive, and Sensory Sciences, Committee on Developing a Behavioral and Social Science Research Agenda on Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias. In: Winters T, editor. Alzheimer’s Disease and related dementias: Experience and caregiving, epidemiology, and models of care: Proceedings of a workshop - in brief. Washington (DC): National Academies Press (US); 2020.
2World Health Organization. Global health estimates: Deaths by case, age, sex, by country and by region. Geneva: World Health Orgaization; 2018.
3Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report: The Global Impact of Dementia. London: Alzheimer’s Disease International; 2017.
4Alzheimer Schweiz. Demenz in der Schweiz: 2019, Zahlen und Fakten. https://www.alzheimer-schweiz.ch/de/publikationen-produkte/produkt/demenz-in-der-schweiz-2019-zahlen-und-fakten-1/. (2020).
5
Eisele
YS
,
Obermüller
U
,
Heilbronner
G
,
Baumann
F
,
Kaeser
SA
,
Wolburg
H
, et al.
Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330(6006):980–2. doi:.https://doi.org/10.1126/science.1194516
6
Prusiner
SB
. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336(6088):1511–3. doi:.https://doi.org/10.1126/science.1222951
7
Jucker
M
,
Walker
LC
. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. doi:.https://doi.org/10.1038/nature12481
8
Maia
LF
,
Kaeser
SA
,
Reichwald
J
,
Hruscha
M
,
Martus
P
,
Staufenbiel
M
, et al.
Changes in amyloid-β and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med. 2013;5(194):194re2. doi:.https://doi.org/10.1126/scitranslmed.3006446
9
Bacioglu
M
,
Maia
LF
,
Preische
O
,
Schelle
J
,
Apel
A
,
Kaeser
SA
, et al.
Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91(1):56–66. doi:.https://doi.org/10.1016/j.neuron.2016.05.018
10
Bateman
RJ
,
Xiong
C
,
Benzinger
TL
,
Fagan
AM
,
Goate
A
,
Fox
NC
, et al.; Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi:.https://doi.org/10.1056/NEJMoa1202753
11
Preische
O
,
Schultz
SA
,
Apel
A
,
Kuhle
J
,
Kaeser
SA
,
Barro
C
, et al.; Dominantly Inherited Alzheimer Network. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019;25(2):277–83. doi:.https://doi.org/10.1038/s41591-018-0304-3
12
Livingston
G
,
Sommerlad
A
,
Orgeta
V
,
Costafreda
SG
,
Huntley
J
,
Ames
D
, et al.
Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. doi:.https://doi.org/10.1016/S0140-6736(17)31363-6
13
Diekelmann
S
,
Born
J
. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–26. doi:.https://doi.org/10.1038/nrn2762
14
Moran
M
,
Lynch
CA
,
Walsh
C
,
Coen
R
,
Coakley
D
,
Lawlor
BA
. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6(4):347–52. doi:.https://doi.org/10.1016/j.sleep.2004.12.005
15
Haba-Rubio
J
,
Marti-Soler
H
,
Tobback
N
,
Andries
D
,
Marques-Vidal
P
,
Waeber
G
, et al.
Sleep characteristics and cognitive impairment in the general population: The HypnoLaus study. Neurology. 2017;88(5):463–9. doi:.https://doi.org/10.1212/WNL.0000000000003557
16
Marchi
NA
,
Ramponi
C
,
Hirotsu
C
,
Haba-Rubio
J
,
Lutti
A
,
Preisig
M
, et al.
Mean oxygen saturation during sleep is related to specific brain atrophy pattern. Ann Neurol. 2020;87(6):921–30. doi:.https://doi.org/10.1002/ana.25728
17
Cooke
JR
,
Ayalon
L
,
Palmer
BW
,
Loredo
JS
,
Corey-Bloom
J
,
Natarajan
L
, et al.
Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5(4):305–9. doi:.https://doi.org/10.5664/jcsm.27538
18
Arioli
M
,
Crespi
C
,
Canessa
N
. Social Cognition through the lens of cognitive and clinical neuroscience. BioMed Res Int. 2018;2018:4283427. doi:.https://doi.org/10.1155/2018/4283427
19
Sollberger
M
,
Rankin
KP
,
Miller
BL
. Social cognition. Continuum (Minneap Minn). 2010;16(4 Behavioral Neurology):69–85. doi:.https://doi.org/10.1212/01.CON.0000368261.15544.7c
20
Yogarajah
M
,
Mula
M
. Social cognition, psychiatric comorbidities, and quality of life in adults with epilepsy. Epilepsy Behav. 2019;100(Pt B):106321. doi:.https://doi.org/10.1016/j.yebeh.2019.05.017
21
Maat
A
,
Fett
AK
,
Derks
E
; GROUP Investigators. Social cognition and quality of life in schizophrenia. Schizophr Res. 2012;137(1-3):212–8. doi:.https://doi.org/10.1016/j.schres.2012.02.017
22
Henry
JD
,
von Hippel
W
,
Molenberghs
P
,
Lee
T
,
Sachdev
PS
. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. 2016;12(1):28–39. doi:.https://doi.org/10.1038/nrneurol.2015.229
23De Roche S, Sollberger M. Soziale Kognition bei neurodegenerativen Krankheiten im DSM-5. Fakultät für Psychologie UB, editor. 2015.
24
McDonald
S
,
Flanagan
S
,
Rollins
J
,
Kinch
J
. TASIT: A new clinical tool for assessing social perception after traumatic brain injury. J Head Trauma Rehabil. 2003;18(3):219–38. doi:.https://doi.org/10.1097/00001199-200305000-00001
25
McDonald
S
,
Bornhofen
C
,
Shum
D
,
Long
E
,
Saunders
C
,
Neulinger
K
. Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disabil Rehabil. 2006;28(24):1529–42. doi:.https://doi.org/10.1080/09638280600646185
26
McDonald
S
. New frontiers in neuropsychological assessment: Assessing social perception using a standardised instrument, The Awareness of Social Inference Test. Aust Psychol. 2012;47(1):39–48. doi:.https://doi.org/10.1111/j.1742-9544.2011.00054.x
27Jarsch M, Sollberger M. Entwicklung der Basler Version des The Awareness of Social Inference Test (BASIT) - mit Schwerpunkt Perspektivenübernahme. Fakultät für Psychologie UB, editor. 2017.
28Ryff I, Sollberger M. Entwicklung der Basler Version des The Awareness of Social Inference Test (BASIT) - mit Schwerpunkt Emotionserkennung. Fakultät für Psychologie UB, editor. 2017.
29Deuschl G, Maier W, et al. S3-Leitlinie Demenzen. In: Deutsche Gesellschaft für Neurologie, Hrsg. Leitlinien für Diagnostik und Therapie in der Neurologie. Available at: www.dgn.org/leitlinien. 2016.
30
Querfurth
HW
,
LaFerla
FM
. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–44. doi:.https://doi.org/10.1056/NEJMra0909142
31
Rinne
JO
,
Brooks
DJ
,
Rossor
MN
,
Fox
NC
,
Bullock
R
,
Klunk
WE
, et al.
11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–72. doi:.https://doi.org/10.1016/S1474-4422(10)70043-0
32
Salloway
S
,
Sperling
R
,
Fox
NC
,
Blennow
K
,
Klunk
W
,
Raskind
M
, et al.; Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–33. doi:.https://doi.org/10.1056/NEJMoa1304839
33
Doody
RS
,
Thomas
RG
,
Farlow
M
,
Iwatsubo
T
,
Vellas
B
,
Joffe
S
, et al.; Alzheimer’s Disease Cooperative Study Steering Committee; Solanezumab Study Group. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311–21. doi:.https://doi.org/10.1056/NEJMoa1312889
34
Cummings
JL
,
Cohen
S
,
van Dyck
CH
,
Brody
M
,
Curtis
C
,
Cho
W
, et al.
ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90(21):e1889–97. doi:.https://doi.org/10.1212/WNL.0000000000005550
35
Ostrowitzki
S
,
Lasser
RA
,
Dorflinger
E
,
Scheltens
P
,
Barkhof
F
,
Nikolcheva
T
, et al.; SCarlet RoAD Investigators. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):95. doi:.https://doi.org/10.1186/s13195-017-0318-y
36
Sevigny
J
,
Chiao
P
,
Bussière
T
,
Weinreb
PH
,
Williams
L
,
Maier
M
, et al.
The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6. doi:.https://doi.org/10.1038/nature19323
37
Beyond amyloid: New approaches to Alzheimer’s disease treatment. EBioMedicine. 2020;51:102648. doi:.https://doi.org/10.1016/j.ebiom.2020.102648
38Cure Alzheimer’s Fund. Biogen announces intention to file with FDA for approval for new Alzheimer’s drug aducanumab. 2020. Available at: https://curealz.org/news-and-events/aducanumab/.
39
Sabbagh
MN
. Alzheimer’s disease drug development pipeline 2020. J Prev Alzheimers Dis. 2020;7(2):66–7.
40DIAN-TU Phase 3 Clinical Trials, Topline Results [news release]. Chicago, Ill: Alzheimer’s Association. Available at: alz.org/news/2020/dian-tu-phase-3-clinical-trials-topline-results [Accessed 2020 February 10].
41AlzForum. Cognitive decline trips up API trials of BACE inhibitor. https://www.alzforum.org/news/research-news/cognitive-decline-trips-api-trials-bace-inhibitor. 2019.
42
Huang
LK
,
Chao
SP
,
Hu
CJ
. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020;27(1):18. doi:.https://doi.org/10.1186/s12929-019-0609-7
43
Cummings
J
,
Lee
G
,
Ritter
A
,
Sabbagh
M
,
Zhong
K
. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y). 2019;5(1):272–93. doi:.https://doi.org/10.1016/j.trci.2019.05.008
44
Wang
X
,
Sun
G
,
Feng
T
,
Zhang
J
,
Huang
X
,
Wang
T
, et al.
Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29(10):787–803. doi:.https://doi.org/10.1038/s41422-019-0216-x
45
Schütz
N
,
Saner
H
,
Rudin
B
,
Botros
A
,
Pais
B
,
Santschi
V
, et al.
Validity of pervasive computing based continuous physical activity assessment in community-dwelling old and oldest-old. Sci Rep. 2019;9(1):9662. doi:.https://doi.org/10.1038/s41598-019-45733-8
46
Stucki
RA
,
Urwyler
P
,
Rampa
L
,
Müri
R
,
Mosimann
UP
,
Nef
T
. A web-based non-intrusive ambient system to measure and classify activities of daily living. J Med Internet Res. 2014;16(7):e175. doi:.https://doi.org/10.2196/jmir.3465
47
Urwyler
P
,
Stucki
R
,
Rampa
L
,
Müri
R
,
Mosimann
UP
,
Nef
T
. Cognitive impairment categorized in community-dwelling older adults with and without dementia using in-home sensors that recognise activities of daily living. Sci Rep. 2017;7(1):42084. doi:.https://doi.org/10.1038/srep42084
48
Lenouvel
E
,
Novak
L
,
Nef
T
,
Klöppel
S
. Advances in Sensor Monitoring Effectiveness and Applicability: A Systematic Review and Update. Gerontologist. 2020;60(4):e299–308. doi:.https://doi.org/10.1093/geront/gnz049
49
Nef
T
,
Chesham
A
,
Schütz
N
,
Botros
AA
,
Vanbellingen
T
,
Burgunder
JM
, et al.
Development and Evaluation of Maze-Like Puzzle Games to Assess Cognitive and Motor Function in Aging and Neurodegenerative Diseases. Front Aging Neurosci. 2020;12:87. doi:.https://doi.org/10.3389/fnagi.2020.00087
50
Vallejo
V
,
Wyss
P
,
Rampa
L
,
Mitache
AV
,
Müri
RM
,
Mosimann
UP
, et al.
Evaluation of a novel Serious Game based assessment tool for patients with Alzheimer’s disease. PLoS One. 2017;12(5):e0175999. doi:.https://doi.org/10.1371/journal.pone.0175999
51
Chesham
A
,
Wyss
P
,
Müri
RM
,
Mosimann
UP
,
Nef
T
. What older people like to play: Genre preferences and acceptance of casual games. JMIR Serious Games. 2017;5(2):e8. doi:.https://doi.org/10.2196/games.7025
52World Health Organization. Integrated care for older people (ICOPE): guidance for person-centred assessment and pathways in primary care. Geneva: World Health Organization; 2019.
53World Health Organization. Integrated care for older people (ICOPE) implementation framework: guidance for systems and services. Geneva: World Health Organization; 2019.
54
Persico
I
,
Cesari
M
,
Morandi
A
,
Haas
J
,
Mazzola
P
,
Zambon
A
, et al.
Frailty and Delirium in Older Adults: A Systematic Review and Meta-Analysis of the Literature. J Am Geriatr Soc. 2018;66(10):2022–30. doi:.https://doi.org/10.1111/jgs.15503
55
Trzepacz
PT
,
Meagher
DJ
,
Franco
JG
. Comparison of diagnostic classification systems for delirium with new research criteria that incorporate the three core domains. J Psychosom Res. 2016;84:60–8. doi:.https://doi.org/10.1016/j.jpsychores.2016.03.011
56
Tieges
Z
,
Evans
JJ
,
Neufeld
KJ
,
MacLullich
AMJ
. The neuropsychology of delirium: advancing the science of delirium assessment. Int J Geriatr Psychiatry. 2018;33(11):1501–11. doi:.https://doi.org/10.1002/gps.4711
57
Kim
SY
,
Kim
JM
,
Kim
SW
,
Kim
ES
,
Kang
HJ
,
Lee
JY
, et al.
Do the phenotypes of symptom fluctuation differ among motor subtypes in patients with delirium?
J Pain Symptom Manage. 2018;56(5):667–77. doi:.https://doi.org/10.1016/j.jpainsymman.2018.07.022
58
Girard
TD
,
Thompson
JL
,
Pandharipande
PP
,
Brummel
NE
,
Jackson
JC
,
Patel
MB
, et al.
Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–22. doi:.https://doi.org/10.1016/S2213-2600(18)30062-6
59
Morandi
A
,
Zambon
A
,
Di Santo
SG
,
Mazzone
A
,
Cherubini
A
,
Mossello
E
, et al.; Italian Study Group on Delirium (ISGoD). Understanding factors associated with psychomotor subtypes of delirium in older inpatients with dementia. J Am Med Dir Assoc. 2020;21(4):486–492.e7. doi:.https://doi.org/10.1016/j.jamda.2020.02.013
60
Johansson
YA
,
Bergh
I
,
Ericsson
I
,
Sarenmalm
EK
. Delirium in older hospitalized patients-signs and actions: a retrospective patient record review. BMC Geriatr. 2018;18(1):43. doi:.https://doi.org/10.1186/s12877-018-0731-5
61
Bauernfreund
Y
,
Butler
M
,
Ragavan
S
,
Sampson
EL
. TIME to think about delirium: improving detection and management on the acute medical unit. BMJ Open Qual. 2018;7(3):e000200. doi:.https://doi.org/10.1136/bmjoq-2017-000200
62
Carbone
MK
,
Gugliucci
MR
. Delirium and the family caregiver: The need for evidence-based education interventions. Gerontologist. 2015;55(3):345–52. doi:.https://doi.org/10.1093/geront/gnu035
63
Savaskan
E
,
Baumgartner
M
,
Georgescu
D
,
Hafner
M
,
Hasemann
W
,
Kressig
RW
, et al.
Empfehlungen zur Prävention, Diagnostik und Therapie des Delirs im Alter. Praxis (Bern 1994). 2016;105(16):941–52. doi:.https://doi.org/10.1024/1661-8157/a002433
64
Pérez-Ros
P
,
Martínez-Arnau
FM
. Delirium assessment in older people in emergency departments. A literature review. Diseases. 2019;7(1):14. doi:.https://doi.org/10.3390/diseases7010014
65
Singler
K
,
Thomas
C
. HELP – Hospital Elder Life Program – ein multimodales Interventionsprogramm zur Delirprävention bei älteren Patienten [HELP - Hospital Elder Life Program - multimodal delirium prevention in elderly patients]. Internist (Berl). 2017;58(2):125–31. Article in German. doi:.https://doi.org/10.1007/s00108-016-0181-0
66
Davoudi
A
,
Manini
TM
,
Bihorac
A
,
Rashidi
P
. Role of wearable accelerometer devices in delirium studies: A systematic review. Crit Care Explor. 2019;1(9):e0027. doi:.https://doi.org/10.1097/CCE.0000000000000027
67Schweizerische Gesundheitsdirektorenkonferenz (GDK). Leitfaden zur Psychiatrieplanung. 2008.
68Deutsche Gesellschaft für Psychiatrie und Psychotherapie. Psychosomatik und Nervenheilkunde: S3 Leitlinie Psychosoziale Therapien bei schweren psychischen Erkrankungen. 2. Auflage. 2018.
69
van der Steen
JT
,
Radbruch
L
,
Hertogh
CMPM
,
de Boer
ME
,
Hughes
JC
,
Larkin
P
, et al.; European Association for Palliative Care (EAPC). White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28(3):197–209. doi:.https://doi.org/10.1177/0269216313493685
70
Koopmans
RTCM
,
van der Sterren
KJMA
,
van der Steen
JT
. The ‘natural’ endpoint of dementia: death from cachexia or dehydration following palliative care?
Int J Geriatr Psychiatry. 2007;22(4):350–5. doi:.https://doi.org/10.1002/gps.1680
71
Houttekier
D
,
Cohen
J
,
Bilsen
J
,
Addington-Hall
J
,
Onwuteaka-Philipsen
BD
,
Deliens
L
. Place of death of older persons with dementia. A study in five European countries. J Am Geriatr Soc. 2010;58(4):751–6. doi:.https://doi.org/10.1111/j.1532-5415.2010.02771.x
72
Pautex
S
,
Herrmann
FR
,
Le Lous
P
,
Ghedira
M
,
Zulian
GB
,
Michon
A
, et al.
Symptom relief in the last week of life: is dementia always a limiting factor?
J Am Geriatr Soc. 2007;55(8):1316–7. doi:.https://doi.org/10.1111/j.1532-5415.2007.01267.x
73
Mitchell
SL
,
Teno
JM
,
Kiely
DK
,
Shaffer
ML
,
Jones
RN
,
Prigerson
HG
, et al.
The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–38. doi:.https://doi.org/10.1056/NEJMoa0902234
74
Eicher
S
,
Theill
N
,
Geschwindner
H
,
Moor
C
,
Wettstein
A
,
Bieri-Brüning
G
, et al.
The last phase of life with dementia in Swiss nursing homes: the study protocol of the longitudinal and prospective ZULIDAD study. BMC Palliat Care. 2016;15(1):80. doi:.https://doi.org/10.1186/s12904-016-0151-2
75
Savaskan
E
,
Bopp-Kistler
I
,
Buerge
M
,
Fischlin
R
,
Georgescu
D
,
Giardini
U
, et al.
Empfehlungen zur Diagnostik und Therapie der behavioralen und psychologischen Symptome der Demenz (BPSD) [Recommendations for diagnosis and therapy of behavioral and psychological symptoms in dementia (BPSD)]. Praxis (Bern 1994). 2014;103(3):135–48. Article in German. doi:.https://doi.org/10.1024/1661-8157/a001547
76
Loizeau
AJ
,
Theill
N
,
Cohen
SM
,
Eicher
S
,
Mitchell
SL
,
Meier
S
, et al.
Fact Box decision support tools reduce decisional conflict about antibiotics for pneumonia and artificial hydration in advanced dementia: a randomized controlled trail. Age Ageing. 2019;48(1):67–74. doi:.https://doi.org/10.1093/ageing/afy149
77
Loizeau
AJ
,
Cohen
SM
,
Mitchell
SL
,
Theill
N
,
Eicher
S
,
Martin
M
, et al.
Physician and surrogate agreement with assisted dying and continuous deep sedation in advanced dementia in Switzerland. Neurodegener Dis. 2019;19(1):4–11. doi:.https://doi.org/10.1159/000499113
78
Anquinet
L
,
Rietjens
JA
,
Vandervoort
A
,
van der Steen
JT
,
Vander Stichele
R
,
Deliens
L
, et al.
Continuous deep sedation until death in nursing home residents with dementia: a case series. J Am Geriatr Soc. 2013;61(10):1768–76. doi:.https://doi.org/10.1111/jgs.12447
79
Swiss Academy Of Medical Sciences. Medical-ethical guidelines: Management of dying and death. Swiss Med Wkly. 2018;148:w14664. doi:.https://doi.org/10.4414/smw.2018.14664
80
Loizeau
AJ
,
Shaffer
ML
,
Habtemariam
DA
,
Hanson
LC
,
Volandes
AE
,
Mitchell
SL
. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922–9. doi:.https://doi.org/10.1001/jamainternmed.2018.1413
81
Davies
N
,
Schiowitz
B
,
Rait
G
,
Vickerstaff
V
,
Sampson
EL
. Decision aids to support decision-making in dementia care: a systematic review. Int Psychogeriatr. 2019;31(10):1403–19. doi:.https://doi.org/10.1017/S1041610219000826
82Various. Lebensende mit Demenz. Eicher S, Geschwinder H, Wolf H, Riese F, editors. University of Zurich; 2018. https://www.zfg.uzh.ch/de/publikat/zfg/buecher.html
83Bundesamt für Gesundheit (BAG) und Schwz. Konferenz der kantonalen Gesundheitsrektorinnen und -direktoren (GDK) Nationale Demenzstrategie 2014–2019. 2016
84Frey K, Frey M, Schläpfer B, Suri M. Schlussbericht: Evaluation Nationale Demenzstrategie 2014-2019. B,S,S, und Kek-CDC consultants. Basel/Zürich. 2019.





