Immunosuppression management in renal transplant recipients with normal-immunological risk: 10-year results from the Swiss Transplant Cohort Study
DOI: https://doi.org/10.4414/smw.2020.20354
Andreas
Krisla, Susanne
Stampfab, Dimitri
Haurib, Isabelle
Binetbc, Thomas
Muellerbd, Daniel
Sidlerbe, Karine
Hadayabf, Déla
Golshayanbg, Manuel
Pascualbg, Michael
Kollerab*, Michael
Dickenmannab*, , the Swiss Transplant Cohort Study (STCS)
a Division of Transplantation Immunology and Nephrology, Basel University Hospital, Switzerland
b Swiss Transplant Cohort Study (STCS)
c Nephrology/Transplantation Medicine, Kantonsspital St. Gallen, Switzerland
d Division of Nephrology, University Hospital Zurich, Switzerland
e Division of Nephrology and Hypertension, Inselspital, Bern University Hospital, Switzerland
f Department of Surgery, Nephrology and Transplantation Divisions, Geneva University Hospitals, Switzerland
g Transplantation Centre, Lausanne University Hospital and University of Lausanne, Switzerland
Summary
AIMS OF THE STUDY
Primary maintenance immunosuppressive therapies for renal transplant recipients underwent significant changes in recent years. We aimed to assess time trends and the impact of immunosuppressive regimens in first renal transplant recipients without immunological risk (blood group incompatibility, pre-existing donor-specific antibodies, positive B/T cell cross-match) in a prospective national multicentre cohort.
METHODS
The Swiss Transplant Cohort Study (STCS) prospectively enrols all patients receiving solid organ transplants in Switzerland since 2008 and systematically collects high quality clinical and laboratory data using standardised definitions. The current STCS nested study enrolled all adult transplant-naïve normal-immunological risk renal transplant recipients up to the end of 2017 and investigated different immunosuppressive strategies across a variety of transplantation relevant outcomes.
RESULTS
Of 1191 recipients enrolled at six transplant centres, 115 (10%) died with a functioning allograft and 92 (8%) lost their allograft during a median follow-up time of 5.8 years. The predominant immunosuppressive therapy comprised tacrolimus, mycophenolate mofetil and prednisone (73.7%), whereas 24.3% were treated with ciclosporin instead of tacrolimus. Primary immunosuppression with an mTOR inhibitor (1.1%) or other immunosuppressive combinations (0.8%) was rare. In the years following 2011, ciclosporin-based immunosuppression decreased significantly. The incidence of graft loss was significantly higher in patients with ciclosporin-based than with tacrolimus-based immunosuppression (adjusted hazard ratio [HR] 1.66, 95% confidence interval [CI] 1.29–2.14; p <0.01), but the occurrence of acute transplant rejections did not differ significantly (adjusted HR 1.48, 95% CI 0.82–2.65; p = 0.19). The longitudinal course of the renal allograft function was significantly better (p = 0.013) in recipients of tacrolimus-based immunosuppressive therapy. Graft failure-free survival was higher (HR 1.25, 95% CI 0.97– 1.6; p = 0.08) with tacrolimus-based than with ciclosporin-based immunosuppression. Cytomegalovirus infections occurred more frequently with ciclosporin-based immunosuppression (9.7% vs 6.4% after 1 year), whereas the incidence of BK virus infections was similar in both groups. The median time to prednisone discontinuation was 1.9 years and did not differ between the two groups. Eleven cases of post-transplantation lymphoproliferative disorder were observed during the follow-up period (1 with ciclosporin-based and 10 with tacrolimus-based immunosuppression).
CONCLUSIONS
The available data show that primary maintenance immunosuppression with tacrolimus has displaced ciclosporin-based therapies. The tacrolimus-based immunosuppression therapy showed consistently better results across almost all assessed clinically relevant outcomes. (ClinicalTrials.gov Number: NCT01204944)
Introduction
Kidney transplantation is the most frequently performed organ transplantation worldwide, improving overall life expectancy and quality of life in patients with end-stage renal disease. Alongside the general progress in medical care and surgical procedures, important drivers of improved transplant outcomes are the risk-adapted immunosuppression management and the detection and treatment of allograft rejection.
A major breakthrough that largely improved renal allograft survival was the introduction of the calcineurin inhibitor ciclosporin in the 1980s. In the 1990s, tacrolimus was introduced and after evolving evidence of its superiority to reduce the rate of acute rejection compared with ciclosporin [1–5], the latter was subsequently replaced. Soon after, mycophenolate mofetil replaced azathioprine.
Finally, in the late 1990s, the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus completed the immunosuppressive therapy options [1, 2]. However, since the introduction of these potent new immunosuppressive drugs, the incidence of infectious diseases and malignancies during the post-transplantation period has increased [6–8]. Modern immunosuppressive therapy therefore requires an approach balanced between the prevention of allograft rejection (immunosuppression) and infectious and malignant complications (immunocompetence).
In recent years, immunological risk stratification that is based around donor-specific antigens has become standard practice in Switzerland. It is of interest to comprehensively observe the consequences of different maintenance immunosuppressive therapy approaches across a variety of clinically relevant outcomes on the national level.
The current study is a nested project of the multicentre Swiss Transplant Cohort Study (STCS) [9]. The study aims to describe and compare patterns and consequences of modern maintenance immunosuppressive therapies, biopsy performance and occurrence of transplant relevant outcomes in patients with a normal immunological risk who underwent renal transplantation in Switzerland.
Materials and methods
Study design and data source
We performed a STCS nested study [9]. The STCS is a prospective, nationwide, observational cohort enrolling patients undergoing solid organ transplantation at all six transplant centres in Switzerland (Basel, Bern, Geneva, Lausanne, St Gallen, and Zurich) since May 2008. In the STCS, clinical and laboratory data are prospectively collected via electronic case report forms using standard definitions [10–12]. Transplant physicians regularly see the patients at the time of transplantation (baseline), 6 and 12 months, and yearly thereafter. Transplant biopsies are performed at each centre when deemed indicated as part of routine practice. Patients provided written informed consent to the STCS and the ethics committee affiliated with each transplantation centre approved the STCS and the current nested study.
Study participants
For this study, we considered all adult transplantation-naïve single and double (dual) renal transplant recipients at any Swiss transplantation centre between May 2008 and the end of 2017. Follow-ups were considered until the end of 2019 (censoring). For comparability and clinical significance, the current study focuses on the largest normal-immunological risk stratum. The description of the study population with specific exclusion criteria is provided in supplementary figure S1 (appendix 1). The final study population consists of 1191 renal transplant recipients. Specific exclusion criteria were primary non-function of the graft, a positive B and/or T cell cross-match test, ABO incompatibility, human leucocyte antigen identical match and the presence of donor-specific antibodies at the time of transplantation. Immunological high-risk patients who had anti-thymocyte globulin, thymoglobulin, rituximab, intravenous immunoglobulin or plasmapheresis as induction therapy administered up to the first 10 days after the transplantation procedure were excluded (appendix 1).
Definitions
We defined four immunosuppressive regimen classes. “Tacrolimus-based immunosuppression” was defined as the combination of prednisone, tacrolimus and mycophenolate mofetil. “Ciclosporin-based immunosuppression” was defined as the combination of ciclosporin, prednisone and mycophenolate mofetil. “mTOR-based immunosuppression” was defined as the combination of everolimus or sirolimus, any calcineurin inhibitor and prednisone, optionally with mycophenolate mofetil or azathioprine. Any remaining immunosuppressive drug combination was summarised as “other immunosuppression”.
Graft loss was defined as return to dialysis post-transplantation or re-transplantation without prior dialysis. We considered only biopsy-proven allograft rejection episodes and classified them as acute humoral (capillaritis, glomerulitis, C4d positivity), acute cellular, and acute mixed. We considered only clinical rejections and disregarded subclinical rejections diagnosed by screening biopsies. A patient was defined as being free from prednisone treatment in the case of uninterrupted discontinuation of at least 6 months. We estimated renal allograft function by using the CKD-EPI equation [13]. Occurrence of cytomegalovirus and BK virus infections were defined according to the STCS Infectious Diseases working group [14]. We considered proven and probable infections, as well as viral infectious syndromes. Any occurrence of post-transplantation lymphoproliferative disorder (PTLD) was considered [15].
Statistical analysis
We performed a descriptive analysis of the different immunosuppressive drug combinations prescribed at the time of hospital discharge and displayed the temporal changes in prescription practice over the last 10 years. Additionally, we examined the age trends among donors and recipients since 2008 using non-parametric time-series methods with locally estimated scatterplot smoothing.
The effect of immunosuppressive therapy regimens on time to event outcomes was investigated using the Kaplan-Meier method and cause-specific Cox proportional-hazards models using robust variance estimation [16]. The cumulative incidence function method was used to estimate the probability of graft failure occurrence treating patients’ death as competing event [17–19]. We estimated graft failure-free survival, defined as the time from transplantation until the composite endpoint of graft loss, death, dropout, or censoring, whichever occurred first. Analogously, we chose the composite endpoint prednisone discontinuation, graft failure or death to display the effect of immunosuppression treatment on steroid weaning. Where necessary, we chose composite endpoint definitions to prevent competing risks issues. Moreover, we applied the cause-specific Cox model to assess the direct effect of immunosuppression treatments on the time to prednisone discontinuation [16].
We performed a longitudinal analysis within patient repeated measurements of the estimated glomerular filtration rate (eGFR) values [13] across all available cohort visit time points. Subsequently, we derived model-based estimations for the 1-, 3-, and 5-year time points using a generalised estimating equation (GEE) model [20]. Due to probably diverse time courses of eGFR in patients in different immunosuppressive therapy classes, an interaction between time and immunosuppressive therapy was included into the model. The longitudinal data structure was accounted for using an autocorrelation structure of first order. Values of eGFR over time are illustrated additionally with box plots.
Due to low numbers, recipients treated with mTOR-based immunosuppression or other immunosuppression were disregarded in model-based analyses.
We computed descriptive summaries for the 1-year post-transplantation occurrence of cytomegalovirus and BK virus. Numbers of PTLD cases were reported. Finally, the number of renal transplant recipients who had at least one biopsy in the first year post-transplantation or within the entire observation period were derived and associated with the number of acute rejections in the same time window for the given immunosuppressive therapy groups.
All statistical analyses were performed using R statistical software version 4.0.0 [21].
Role of the funding source
The funders of the study had no role in the study design, data collection, data analyses, data interpretation, or writing of the report. The corresponding author had full access to the data access used for the study and the final responsibility for the decision to submit for publication.
Results
Of 1191 renal transplant recipients enrolled at all six transplant centres, 143 (12%) died during the follow up. 115 (80%) died with a functioning allograft and 92 (8%) lost their allograft during a median follow-up time of 5.8 years. Only 16 patients (1.3%) were lost to follow-up. Primary tacrolimus-based immunosuppression was prescribed most frequently (73.7%), followed by a ciclosporin-based immunosuppression (24.3%) (table 1). Only a minority of renal transplant providers were prescribed mTOR-based immunosuppression (1.1%) or other immunosuppression therapies (0.8%). Tacrolimus-based immunosuppression has been the dominating strategy over the last 10 years with an increasing displacement of a ciclosporin-based regimens since 2011 (fig. 1). mTOR-based immunosuppression or other immunosuppression therapies remained constantly marginal during the reported 10-year period.
Table 1 Patient baseline characteristics in the final study population, overall and by immunosuppressive therapy classes.
| |
Total
|
Immunosuppressive therapy
|
|
TAC-based
|
CsA-based
|
mTOR-based
|
Other
|
| Number of patients, n (% of total) |
1191 (100%) |
878 (73.7%) |
290 (24.3%) |
13 (1.1%) |
10 (0.8%) |
|
Recipient
|
| Female, n (%) |
373 (31.3%) |
282 (32.1%) |
87 (30%) |
1 (7.7%) |
3 (30%) |
| Ethnicity: Caucasian, n (%) |
1098 (92.2%) |
807 (91.9%) |
268 (92.4%) |
13 (100%) |
10 (100%) |
| Single renal transplant, n (%) |
1166 (97.9%) |
855 (97.4%) |
288 (99.3%) |
13 (100%) |
10 (100%) |
| Median age at TX in years, (IQR) |
55.8
(44.6–64.4) |
56.3
(44.7–64.8) |
55.1
(44.1–63.1) |
54.3
(46.6–63.2) |
55.2
(48.3–63.2) |
| Median follow-up time in years, (IQR) |
5.8
(3.5–8.8) |
5.4
(3.4–8.1) |
7.8
(4.3–9.7) |
9.9
(5.6–10.8) |
5.1
(3.3–9.4) |
|
Donor
|
| Living donation, n (%)*
|
507 (42.6%) |
385 (43.8%) |
111 (38.3%) |
9 (69.2%) |
2 (20%) |
| Median age at donation in years, (IQR) |
56
(46–64) |
56
(47–65) |
56
(44.2–64) |
49
(44–59) |
54
(44–67.8) |
|
Allograft
|
| Dialysis beyond post-transplant day seven, n (%) |
127 (10.7%) |
82 (9.3%) |
41 (14.1%) |
1 (7.7%) |
3 (33.0%) |
| Cold ischaemia time in hours, median (IQR) |
6.2
(1.5–10.2) |
6.1
(1.5–10.1) |
6.3
(1.5–10.1) |
2.4
(1.5–6.8) |
7.9
(4.8–11.8) |
|
CMV status, n (%)
|
| R+ |
690 (57.9%) |
523 (59.6%) |
154 (53.1%) |
6 (46.2%) |
7 (70%) |
| D−/R− |
249 (20.9%) |
180 (20.5%) |
63 (21.7%) |
5 (38.5%) |
1 (10%) |
| D+/R− |
240 (20.2%) |
164 (18.7%) |
72 (24.8%) |
2 (15.4%) |
2 (20%) |
| Missing CMV information |
12 (1%) |
11 (1.3%) |
1 (0.3%) |
0 (0.0%) |
0 (0.0%) |
|
Induction therapy, n (%)
|
| Basiliximab |
1090 (91.5%) |
842 (95.9%) |
226 (77.9%) |
12 (92.3%) |
10 (100%) |

Figure 1 Illustration of time trends for the prescription of different immunosuppressive (IS) regimens in normal-risk renal transplant (TX) recipients between 2008 and 2017.
“TAC-based” was defined as the combination of prednisone, tacrolimus and mycophenolate mofetil. “CsA-based” corresponds to the combination of ciclosporin, prednisone and mycophenolate mofetil. “mTOR-based” corresponds to the combination of any calcineurin inhibitor, everolimus and prednisone, with or without mycophenolate mofetil. All other immunosuppressive drug combinations and therapies were summarised as “Other” immunosuppression.
Renal transplant recipients were similar regarding age, gender and follow-up time, except for those treated with mTOR-based immunosuppression. Patients treated with ciclosporin-based immunosuppression had fewer living donations (table 1). Early allograft dysfunction occurred less frequently in tacrolimus-based immunosuppression recipients, whereas cold ischaemia time was similar in both dominating groups.
The probability of graft failure was higher among renal transplant recipients treated with ciclosporin-based than with tacrolimus-based immunosuppression. This observation was valid for the entire follow-up period (10 years after transplantation: 15.1% with ciclosporin-based vs 9.4% with tacrolimus-based immunosuppression) (fig. 2). The hazard for graft failure was bigger with ciclosporin-based immunosuppression than tacrolimus-based immunosuppression (adjusted hazard ratio [HR] 1.66, 95% confidence interval [CI] 1.29–2.14; p <0.01) (table 2) and, similarly, the probability of graft failure-free survival was higher with tacrolimus-based than with ciclosporin-based immunosuppression (fig. S3 in appendix 1).

Figure 2 Renal allograft function displayed as estimated glomerular filtration rate (eGFR in ml/min/1.73m2).
TX = transplantation; IS = immunosuppression
“TAC-based” was defined as the combination of prednisone, tacrolimus and mycophenolate mofetil. “CsA-based” corresponds to the combination of ciclosporin, prednisone and mycophenolate mofetil.
Table 2 Effect estimates from cause-specific Cox proportional hazard models.
HR
(95% CI)
[p-value]
|
Cause-specific endpoint
|
|
Graft failure*
|
Acute rejection†
|
|
Number of events
|
86
|
301
|
| |
Univariate
|
Multivariable
|
Univariate
|
Multivariable
|
|
Risk factor
|
|
| CsA-based vs TAC-based IS |
1.97
(1.52–2.55)
[<0.01] |
1.66
(1.29–2.14)
[<0.01] |
1.69
(0.83–3.46)
[0.15] |
1.48
(0.82–2.65)
[0.19] |
| Living vs deceased donor |
0.42
(0.23–0.79)
[0.01] |
0.43
(0.22–0.83)
[0.01] |
0.95
(0.82–1.11)
[0.52] |
0.88
(0.75–1.03)
[0.12] |
| Donor age by 10 years increase |
1.42
(1.15–1.75)
[<0.01] |
1.39
(1.21–1.60)
[<0.01] |
0.97
(0.87–1.08)
[0.52] |
0.99
(0.87–1.13)
[0.91] |
| Female vs male recipient |
0.8
(0.48–1.32)
[0.38] |
0.84
(0.52–1.35)
[0.46] |
0.72
(0.64–0.82)
[<0.01] |
0.73
(0.66–0.81)
[<0.01] |
| Recipients age by 10 years increase |
1.17
(0.98–1.4)
[0.09] |
0.98
(0.82–1.17)
[0.81] |
0.91
(088–0.94)
[<0.01] |
0.9
(0.87–0.94)
[<0.01] |
| TX year (2013–2017) vs (2008–2012) |
0.48
(0.31–0.74)
[<0.01] |
0.53
(0.35–0.78)
[<0.01] |
0.55
(0.32–0.95)
[0.03] |
0.6
(0.39–0.92)
[0.02] |
Although more biopsies were performed in the tacrolimus-based immunosuppression group than in the ciclosporin-based immunosuppression group (particularly at year one: 62.5% vs 54.8%; end of follow-up: 71.1% vs 69%), acute rejection episodes were less frequent in the former (table 3). However, the cause-specific Cox analysis of the acute rejection endpoint showed a nonsignificantly higher hazard with ciclosporin-based than with the tacrolimus-based immunosuppression (table 2, adjusted HR 1.48, 95% CI 0.82–2.65; p = 0.19).
Table 3 Biopsy performance and occurrence of acute rejections in normal-immunological risk patients after renal transplantation.
| |
Total
|
Immunosuppressive therapy
|
|
TAC-based
|
CsA-based
|
mTOR-based
|
Other
|
| Number of patients, n (% of total) |
1191 (100%) |
878 (73.7%) |
290 (24.3%) |
13 (1.1%) |
10 (0.8%) |
|
Renal transplant recipients with at least one biopsy, n (%)
|
| Within 1 year post-TX |
723 (60.7%) |
549 (62.5%) |
159 (54.8%) |
12 (92.3%) |
3 (30.0%) |
| Over entire period |
842 (70.7%) |
624 (71.1%) |
200 (69.0%) |
12 (92.3%) |
6 (60.0%) |
|
Renal transplant recipients with at least one acute rejection episode, n (%)
|
| Within 1 year post-TX |
254 (21.3%) |
168 (19.1%) |
81 (27.9%) |
3 (23.1%) |
2 (20.0%) |
| Over entire period |
312 (26.2%) |
198 (22.6%) |
108 (37.2%) |
3 (23.1%) |
3 (30.0%) |
There was no noticeable difference in time to prednisone discontinuation in renal transplant survivors free of allograft failure (fig. 3), nor did we see a significant effect of immunosuppressive therapy in the cause-specific Cox model (HR 0.82, 95% CI 0.41–1.63; p = 0.57). Overall, the median time to prednisone discontinuation was 1.9 years.

Figure 3 Median age of renal transplant recipients and organ donors between 2008 and 2017.
The longitudinal course of renal allograft function estimated using the CKD-EPI formula showed increasingly larger differences between the two immunosuppression groups over time (fig. 4) with a significant difference in favour of tacrolimus-based immunosuppression (p = 0.013, table 4).

Figure 4 Probability of graft failure post renal transplantation, stratified by immunosuppression therapy.
“TAC-based” was defined as the combination of prednisone, tacrolimus and mycophenolate mofetil. “CsA-based” corresponds to the combination of ciclosporin, prednisone and mycophenolate mofetil.
Table 4 Mean estimated glomerular filtration rate according to immunosuppressive therapy at different time points post-transplantation.
|
Estimate (95% CI)*
|
Time since transplantation
|
|
1 year
|
3 years
|
5 years
|
| TAC-based IS |
53.4 (52.2–54.6) |
52.7 (51.5–53.9) |
51.9 (50.6–53.3) |
| CsA-based IS |
50.6 (48.3–52.9) |
49.2 (47.0–51.4) |
47.7 (45.3–50.2) |
The probability of contracting CMV infection within the first-year post-transplantation was noticeably higher in recipients of ciclosporin-based immunosuppression (9.7% vs 6.4%) (table 5). In contrast, tacrolimus-based immunosuppression seemed to increase the probability of BK virus infection within the first year post-transplantation (5.0% vs 4.1% with ciclosporin-based immunosuppression). Overall, PTLD was observed in only 11 renal transplant recipients. All but one case occurred in patients receiving tacrolimus-based immunosuppression. Five of these 11 PTLD cases were observed within the first year after transplantation.
Table 5 Occurrence of CMV and BKV infection at 1 year post transplantation.
| |
|
Immunosuppressive therapy
|
|
Total
|
TAC-based
|
CsA-based
|
mTOR-based
|
Other
|
| Number of patients, n (% of total) |
1191 (100%) |
878 (73.7%) |
290 (24.3%) |
13 (1.1%) |
10 (0.8%) |
|
One-year post-transplantation
|
| CMV, n (%) |
84 (7.1%) |
56 (6.4%) |
28 (9.7%) |
0 (0%) |
0 (0%) |
| BKV, n (%) |
56 (4.7%) |
44 (5%) |
12 (4.1%) |
0 (0%) |
0 (0%) |
Living and deceased donor recipients
In our study, 42.6% were living-donor transplant recipients (LDTRs) (table S1 in appendix 1). There was no relevant evolution of age over the assessed time span, neither for donors nor for recipients (fig. 5). The number of renal transplant recipients with a tacrolimus-based immunosuppression was slightly higher in LDTR than in deceased donor transplant recipients (DDTRs) and the proportion of early allograft dysfunction was clearly higher in DDTRs than in LDTRs. LDTRs had a slightly higher incidence of BK virus infections and a slightly lower number of cytomegalovirus infections compared with DDTR.
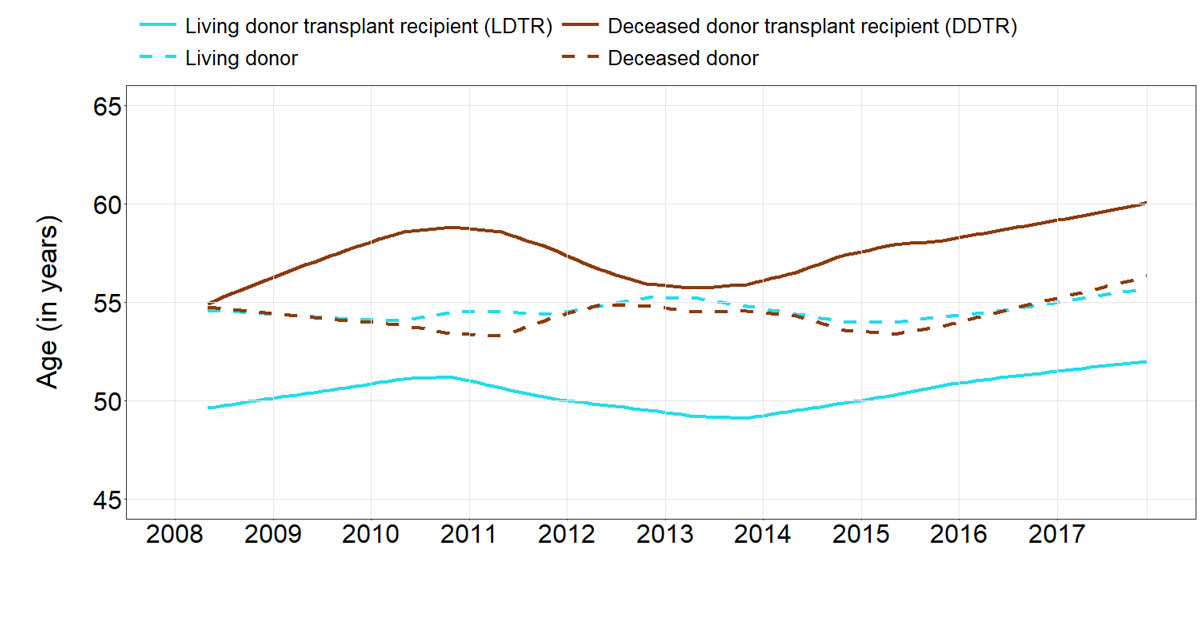
Figure 5 Time to discontinuation of prednisone in renal transplant survivors without graft failure*, stratified by immunosuppression therapy.
“TAC-based” was defined as the combination of prednisone, tacrolimus and mycophenolate mofetil. “CsA-based” corresponds to the combination of ciclosporin, prednisone and mycophenolate mofetil.
* There is no test statistic provided for the difference of the two curves to prevent any causal conclusions drawn about the effect of different IS treatments on time to prednisone discontinuation (for further explanation see fig. S4 in appendix 1).
The probability of graft loss was higher in DDTRs than in LDTRs in the raw comparison (fig. S2 in appendix 1), as well as in the multivariable model (HR 0.43, 95% CI (0.22–0.83; p = 0.01) (table 2). Although the number of biopsies and the number of biopsy-proven rejections was lower in LDTRs than in DDTRs (table S1), donor type was not predictive for the occurrence of acute rejection in the multivariable cause-specific Cox model (HR 0.88, 95% CI 0.75–1.03; p = 0.12) (table 2). Time to prednisone discontinuation was significantly shorter in surviving DDTRs than in LDTRs with a functioning allograft (fig. S5, p <0.01). Average eGFR was significantly lower in DDTRs than in LDTRs at 1, 3, and 5 years post-transplantation (table S2 in appendix 1).
Discussion
We investigated the evolution of immunosuppressive therapies among very strictly defined normal-immunological risk renal transplant recipients in Switzerland from the year 2008 until the end of 2017, considering demographic changes, living and deceased donor transplants, prednisone withdrawal, graft function, graft failure, and the occurrence of the most relevant infectious diseases and PTLD.
Throughout the investigated period, renal transplant recipients were predominantly treated with tacrolimus-based immunosuppression. A proportion of 30–40% were still being treated with ciclosporin-based immunosuppression from 2008 to 2011, with a subsequent decline to below 20%. The choice of immunosuppressive maintenance therapy for Swiss renal transplant recipients was compared with other European countries. A recently published European-wide analysis showed that between 2006 and 2015, about 74.6% of renal transplant recipients had immunosuppressive therapy containing tacrolimus, whereas 20.5% were treated with a regimen containing ciclosporin [22]. Accordingly, 73.7% were receiving tacrolimus-based and 24.3% ciclosporin-based immunosuppression in Switzerland between 2008 and 2017. During the analysed period, mTOR-based immunosuppression and other immunosuppression were scarcely used as primary immunosuppressants.
The frequency of acute rejection episodes has been substantially reduced in recent decades, mainly by more efficacious immunosuppressive drugs and sophisticated allocation strategies. The observed 1-year biopsy proven acute rejection rate of 21.3% fits well with published data [23]. Not surprisingly, the raw overall acute rejection rate for renal transplant recipients treated with the older ciclosporin-based immunosuppression and the less efficient mTOR-based immunosuppression was higher compared with tacrolimus-based immunosuppression. These findings are in accordance with previously conducted trials [1, 2, 24]. However, fitting cause-specific Cox models to our data did not reveal any significant difference in the occurrence of acute rejection with tacrolimus-based than with ciclosporin-based immunosuppression. We only discovered a time-effect, with significantly fewer acute rejections during the transplantation period 2013–2017 as compared with 2008–2012. Nevertheless, centre-specific differences in post-transplantation monitoring (including biopsies) and management may also be important contributing factors.
Acute rejection rates in DDTRs were higher than in LDTRs, which can be explained by longer cold ischaemia time, higher incidence of early graft dysfunction and the higher number of graft biopsies in DDTRs (table S1 in appendix 1).
Glucocorticoids are usually the first agents to be withdrawn or reduced to avoid side effects in renal transplant recipients. In a Spanish study, 23.3% of renal transplant recipients were weaned off glucocorticoids within 12 months after transplantation [25]. In our population, 31% of all recipients were able to discontinue prednisone after 12 months and 60% were free of prednisone 5 years post-transplantation (results not shown). Patients treated with tacrolimus-based immunosuppression had on average a higher hazard for prednisone discontinuation; however, the difference did not reach statistical significance. This result is in line with the observation that patients with ciclosporin-based immunosuppression had a statistically nonsignificant higher risk of acute rejection, which is potentially treated with prednisone. Graft outcome and graft function have gradually improved over recent decades [26], which is mainly attributable to more sophisticated allocation criteria, more efficacious immunosuppressive drugs, and globally better management. Accordingly, our data show an encouragingly low cumulative incidence for graft failure after renal transplantation (fig. 3) and quite stable graft function over the observation period (table 4). Tacrolimus-based immunosuppression led to a further reduction of graft failure compared with ciclosporin-based immunosuppression. This finding is in accordance with previously published studies [1, 2].
If graft loss occurred, however, the mortality rate during subsequent dialysis remained high in our data, which is comparable to a recent STCS publication [27].
Cytomegalovirus and BK virus are common and ubiquitous infections that usually cause latent infections in healthy adults. However, in renal transplant recipients these infections are among the most important pathogens, since they increase the risk of allograft failure, morbidity and death. The rates of cytomegalovirus and BK virus infection were low in our cohort (table 5) and comparable to other data [28–30]. The favourable numbers might indicate the awareness of these complications in the Swiss transplantation community, as well as efficient prophylactic, diagnostic and therapeutic strategies. Furthermore, our analysis excluded renal transplant recipients with high immunological risk, who are prone to more aggressive immunosuppression and therefore exhibit a higher risk for viral infections.
PTLDs comprise a heterogeneous group of lymphoproliferative disorders after solid organ or haematopoietic transplantation with a high morbidity and mortality. A significant number of PTLDs are associated with Epstein-Barr virus [31]. In our study, the incidence, as compared with the literature, was low (0.92%). Since our patient collective had a normal immunological risk, most of them were treated with an anti-interleukin-2 receptor antibody (e.g., basiliximab) as induction therapy (table 1). For these patients, lower incidences of PTLDs have been described than in patients treated with mono- or polyclonal T-cell depleting antibodies (e.g., OKT3, thymoglobulin) [8].
This real-life cohort study is limited by its lack of a randomised and blinded design and the potential confounder of time, as tacrolimus-based immunosuppression is more recent. Furthermore, we restricted our analyses to normal-immunological risk renal transplant recipients. Therefore, low- and high-immunological risk renal transplant recipients were excluded. A further limitation is the absence of an internationally coherent definition of the immunological risk stratification. In this study, we used the local convention for immunological risk stratification (see appendix 1). Time-dependent switches between different immunosuppressive regimens were not considered.
We conclude that kidney graft recipients with normal immunological risk in Switzerland nowadays are mainly treated with tacrolimus-based immunosuppression that consists of tacrolimus, mycophenolate mofetil and prednisone. Prednisone can be successfully discontinued in up to 60%. Rates of graft failure, long-term graft function and complications are favourable. Thus, this regimen is associated with better renal allograft and patient outcomes.
Appendix 1 Supplementary material
Immunological-risk stratification according to local convention
Figure S1: Selection of the final study population by exclusion criteria, illustrated as flow-chart.
Figure S2: Probability of graft failure post renal transplantation, stratified by donor type.
Figure S3: Graft failure free survival, stratified by immunosuppression therapy.
Figure S4: Graft failure free survival, stratified by donor type.
Figure S5: Time to discontinuation of prednisone in renal transplant survivors without graft failure, stratified by donor type.
Table S1: Patient’s baseline characteristics, biopsy and acute rejection information and occurrence of CMV and BKV infections post renal transplantation in the final study population, overall and by donor type.
Table S2: Mean estimated glomerular filtration rate for donor types at different time points post renal transplantation.
The members of the Swiss Transplant Cohort Study (STCS)
The appendix is available as a separate file at: https://smw.ch/article/doi/smw.2020.2054.
Acknowledgements
This study was conducted in the framework of the Swiss Transplant Cohort Study (STCS). The STCS is funded by the Swiss National Science Foundation (SNSF) Grant 33CS30_177522, Unimedsuisse and the transplant centres.
*
Joint last authors with equal contribution
Authors' contributions
AK and SS contributed equally as lead authors. MK and MD contributed equally as last authors. AK wrote the manuscript, performed research and participated in the study design, SS and DH performed statistical analyses, contributed to the manuscript and the study design, MD and MK reviewed and critically revised the manuscript and contributed to the study design, IB, TM, DS, KH, DG and MP provided critical feedback. All authors have given final approval of the version to be published.
References
1
Webster
AC
,
Woodroffe
RC
,
Taylor
RS
,
Chapman
JR
,
Craig
JC
. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331(7520):810. doi:.https://doi.org/10.1136/bmj.38569.471007.AE
2
Ekberg
H
,
Tedesco-Silva
H
,
Demirbas
A
,
Vítko
S
,
Nashan
B
,
Gürkan
A
, et al.; ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357(25):2562–75. doi:.https://doi.org/10.1056/NEJMoa067411
3
Pirsch
JD
,
Miller
J
,
Deierhoi
MH
,
Vincenti
F
,
Filo
RS
. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation. 1997;63(7):977–83. doi:.https://doi.org/10.1097/00007890-199704150-00013
4
Mayer
AD
,
Dmitrewski
J
,
Squifflet
JP
,
Besse
T
,
Grabensee
B
,
Klein
B
, et al.
Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: a report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64(3):436–43. doi:.https://doi.org/10.1097/00007890-199708150-00012
5
Krämer
BK
,
Montagnino
G
,
Del Castillo
D
,
Margreiter
R
,
Sperschneider
H
,
Olbricht
CJ
, et al.; European Tacrolimus vs Cyclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrol Dial Transplant. 2005;20(5):968–73. doi:.https://doi.org/10.1093/ndt/gfh739
6
Bosmans
JL
,
Verpooten
GA
. Malignancy after kidney transplantation: still a challenge. Kidney Int. 2007;71(12):1197–9. doi:.https://doi.org/10.1038/sj.ki.5002306
7
Penn
I
. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998;63(7):147–58.
8
Wimmer
CD
,
Rentsch
M
,
Crispin
A
,
Illner
WD
,
Arbogast
H
,
Graeb
C
, et al.
The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007;71(12):1271–8. doi:.https://doi.org/10.1038/sj.ki.5002154
9
Koller
MT
,
van Delden
C
,
Müller
NJ
,
Baumann
P
,
Lovis
C
,
Marti
HP
, et al.
Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol. 2013;28(4):347–55. doi:.https://doi.org/10.1007/s10654-012-9754-y
10Swiss Transplant Cohort Study. Study description. 2020; Available from: https://www.stcs.ch/about/study-description.
11Swiss Transplant Cohort Study. Annual Report July 2019; Available from: https://www.stcs.ch/internal/reports/2019july-stcs_annual_report.pdf.
12
Haas
M
,
Loupy
A
,
Lefaucheur
C
,
Roufosse
C
,
Glotz
D
,
Seron
D
, et al.
The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. doi:.https://doi.org/10.1111/ajt.14625
13
Levey
AS
,
Stevens
LA
,
Schmid
CH
,
Zhang
YL
,
Castro
AF, 3rd
,
Feldman
HI
, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi:.https://doi.org/10.7326/0003-4819-150-9-200905050-00006
14
van Delden
C
,
Stampf
S
,
Hirsch
HH
,
Manuel
O
,
Meylan
P
,
Cusini
A
, et al.; Swiss Transplant Cohort Study. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;ciz1113. doi:.https://doi.org/10.1093/cid/ciz1113
15
Steiner
R
,
Kridel
R
,
Giostra
E
,
McKee
T
,
Achermann
R
,
Mueller
N
, et al.; The Swiss Transplant Cohort Study Stcs. Low 5-year cumulative incidence of post-transplant lymphoproliferative disorders after solid organ transplantation in Switzerland. Swiss Med Wkly. 2018;148:w14596.
16
Koller
MT
,
Raatz
H
,
Steyerberg
EW
,
Wolbers
M
. Competing risks and the clinical community: irrelevance or ignorance?
Stat Med. 2012;31(11-12):1089–97. doi:.https://doi.org/10.1002/sim.4384
17
Aalen
O
. Nonparametric estimation of partial transition probabilities in multiple decrement models. Ann Stat. 1978;6(3):534–45. doi:.https://doi.org/10.1214/aos/1176344198
18
Gray
RJ
. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–54. doi:.https://doi.org/10.1214/aos/1176350951
19Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken, NJ: John Wiley & Sons, Inc; 1980.
20Diggle P. Analysis of longitudinal data. Oxford: Oxford University Press; 2013.
21Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Berlin: Springer; 2000.
22
Coemans
M
,
Süsal
C
,
Döhler
B
,
Anglicheau
D
,
Giral
M
,
Bestard
O
, et al.
Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int. 2018;94(5):964–73. doi:.https://doi.org/10.1016/j.kint.2018.05.018
23
Opelz
G
,
Döhler
B
; Collaborative Transplant Study. Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation. 2009;87(6):795–802. doi:.https://doi.org/10.1097/TP.0b013e318199c1c7
24
Kamel
M
,
Kadian
M
,
Srinivas
T
,
Taber
D
,
Posadas Salas
MA
. Tacrolimus confers lower acute rejection rates and better renal allograft survival compared to cyclosporine. World J Transplant. 2016;6(4):697–702. doi:.https://doi.org/10.5500/wjt.v6.i4.697
25
Gonzalez-Molina
M
,
Gentil
MA
,
Burgos
D
,
Cabello
M
,
Cobelo
C
,
Bustamante
J
, et al.
Effect of long-term steroid withdrawal in renal transplant recipients: a retrospective cohort study. NDT Plus. 2010;3(Suppl_2):ii32–6.
26
Wehmeier
C
,
Georgalis
A
,
Hirt-Minkowski
P
,
Amico
P
,
Hoenger
G
,
Voegele
T
, et al.
2222 kidney transplantations at the University Hospital Basel: a story of success and new challenges. Swiss Med Wkly. 2016;146:w14317. doi:.https://doi.org/10.4414/smw.2016.14317
27
Bonani
M
,
Achermann
R
,
Seeger
H
,
Scharfe
M
,
Müller
T
,
Schaub
S
, et al.
Dialysis after graft loss: a Swiss experience. Nephrol Dial Transplant. 2020;gfaa037. doi:.https://doi.org/10.1093/ndt/gfaa037
28
Dharnidharka
VR
,
Cherikh
WS
,
Abbott
KC
. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87(7):1019–26. doi:.https://doi.org/10.1097/TP.0b013e31819cc383
29
Hirsch
HH
,
Brennan
DC
,
Drachenberg
CB
,
Ginevri
F
,
Gordon
J
,
Limaye
AP
, et al.
Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277–86. doi:.https://doi.org/10.1097/01.TP.0000156165.83160.09
30
Santos
CA
,
Brennan
DC
,
Fraser
VJ
,
Olsen
MA
. Delayed-onset cytomegalovirus disease coded during hospital readmission after kidney transplantation. Transplantation. 2014;98(2):187–94. doi:.https://doi.org/10.1097/TP.0000000000000030
31
Evens
AM
,
Roy
R
,
Sterrenberg
D
,
Moll
MZ
,
Chadburn
A
,
Gordon
LI
. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr Oncol Rep. 2010;12(6):383–94. doi:.https://doi.org/10.1007/s11912-010-0132-1




