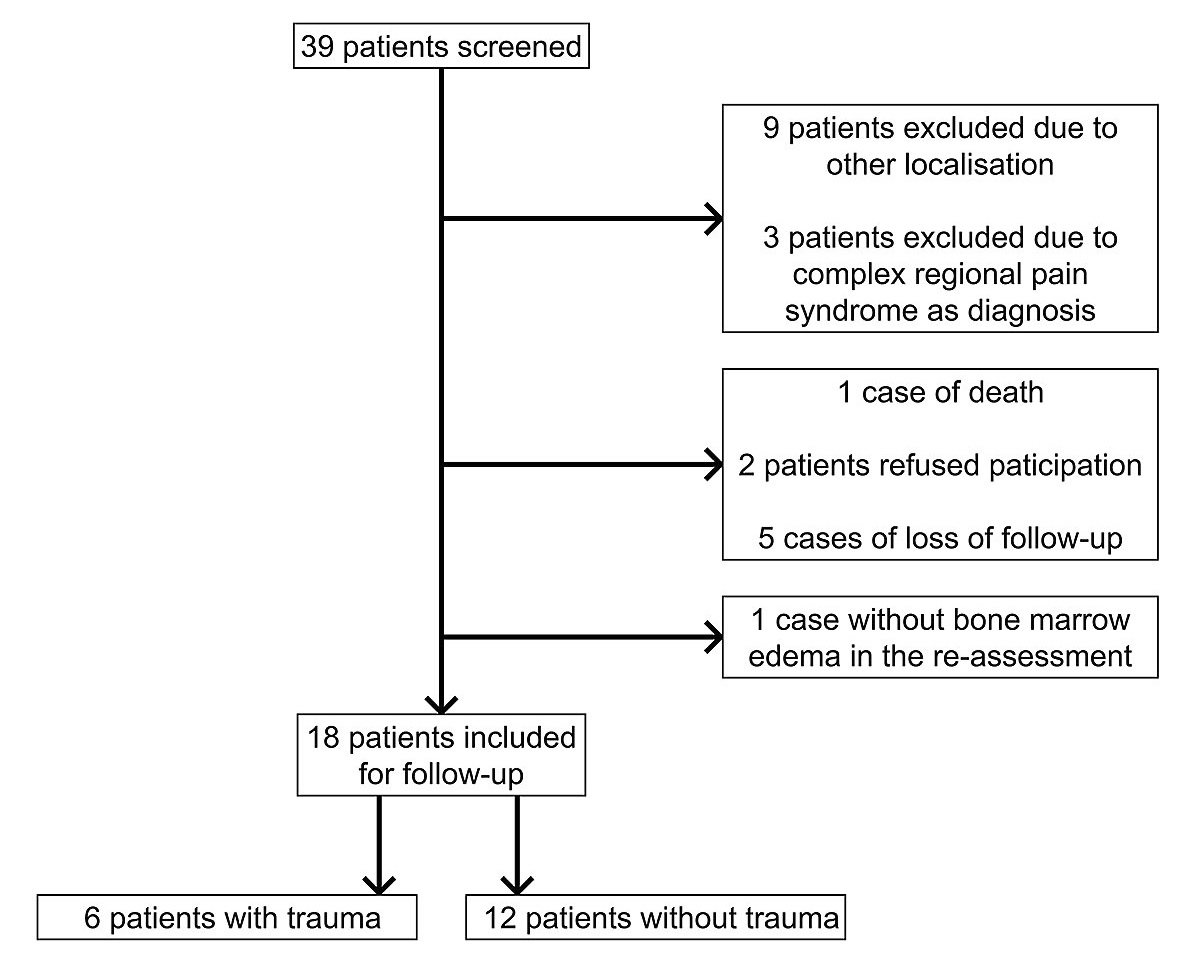
Figure 1 Patient recruitment. This flow-chart shows the patient recruitment
DOI: https://doi.org/10.4414/smw.2020.20384
The reason for joint pain often remained unclear before magnetic resonance imaging (MRI) was available, because the findings on X-ray and computed tomography (CT) scans were unremarkable. With the increasing availability of MRI, multiple unspecific alterations can now be detected. These subchondral changes appear with low signal intensity on T1-weighted (T1w) images, and with high signal intensity on fat-suppressed T2-weighted (T2w) or proton density-weighted (PDw) images [1–5], and are called ‘bone marrow oedema’ [4].
Initially, bone marrow oedema syndrome (synonym: ‘local transient osteoporosis’) was suspected to be a reversible early stage of avascular osteonecrosis, which was later disproven [2, 6]. Nowadays it is considered as a distinct entity, but the aetiology remains unexplained [7]. A bone marrow oedema can occur in isolation, or in the context of other musculoskeletal conditions (e.g., complex regional pain syndrome, which was previously known as ‘algodystrophy’ or ‘reflex sympathetic dystrophy’) [2, 8].
Bone marrow oedema syndrome is characterised by the acute onset of severe, disabling pain, with or without prior trauma, and an impaired quality of life. Most frequently, it affects the lower extremity joints (especially hip and knee), and commonly afflicts young to middle-aged men [6, 7, 9]. It is important to exclude other differential diagnoses (e.g., stress fracture, osteoarthritis, osteomyelitis, malignancy, septic arthritis) by performing X-ray and CT scans, bone scintigraphy, laboratory testing, or even arthroscopy, to avoid unnecessary or false treatment. The diagnosis may be challenging, as the symptoms are unspecific.
The term ‘bone marrow oedema’ describes the alterations seen on MRI, not thea etiology, which ranges from traumatic to ischemic, reactive, degenerative, iatrogenic, or idiopathic causes [2, 3, 10]. Histological findings suggest that osteoclasts and the secretion of cytokines play a central role in the pathogenesis of bone marrow oedema, with irritation of the nearby nerve fibres provoking severe pain [2, 3].
Even though bone marrow oedema syndrome is a self-limiting disease (mean period 3–18 months [8], up to 24 months [6, 9]), patients suffer from severe and disabling bone pain with a relevant impairment in daily life. Studies showed that both conservative therapy regimens – such as non-steroidal analgesics [10] or reduction of weight-bearing – and operative management with core-decompression did not resolve the pain [10]. Therefore, alternative therapy options were investigated, such as the use of bisphosphonates or prostacyclins [6–8, 11–13]. The results of both bisphosphonates and prostacyclins are promising, but prostacyclins have a wider range of contraindications (e.g., for patients with coronary heart disease, cardiac failure, or high risk of bleeding), and are more complicated in terms of application [8].
Bisphosphonates reduce bone resorption by inhibiting osteoclast recruitment, differentiation, and action, and by inducing osteoclast apoptosis [14]. In addition, they have a beneficial effect on osteoblasts by preventing their apoptosis and inhibiting mineralisation [15]. Ibandronate is a highly potent bisphosphonate belonging to the group of nitrogen-containing bisphosphonates [15, 16]. Although it can be administered orally, the intravenous application is preferable because it results in better resorption [15]. The most frequent undesirable side effects of ibandronate are transient musculoskeletal pain, headache, flu-like symptoms, gastrointestinal symptoms, and osteonecrosis of the jaw [17]. Caution is required in patients suffering from renal failure or uncorrected hypocalcaemia [18].
Because of their pharmacological property, bisphosphonates are likely to help in cases of a bone marrow oedema. Several studies investigating this topic showed promising results: bisphosphonates have a beneficial effect independent of the aetiology of this condition, rarely have serious side effects, and are mostly safe in application [3, 6, 8, 9, 11, 12, 19]. Regardless, the use of bisphosphonates to treat bone marrow oedema is still off-label [6, 8, 19].
The aim of this study was to investigate if ibandronate may relieve pain in patients with bone marrow oedema syndrome of the knee.
This study is a single-centre, retrospective analysis approved by the local ethics committee of the Canton of Zurich (BASEC-Nr. 2017-00398). Patients included were over 18 years old and agreed to participate. Exclusion criteria were joints other than the knee (e.g., the hip or the ankle) affected by a bone marrow, or the source of the pain being a complex regional pain syndrome.
Between April 2012 and February 2016, 39 patients were screened who had received ibandronate intravenously because of a bone marrow oedema. Twelve patients had to be excluded (nine patients due to other localisations, three patients due to complex regional pain syndrome). Six patients had to be excluded due to loss of follow-up (one death happened three years after our treatment, due to a cardiac event not related to the ibandronate administration; five patients did not respond to the study invitation), and two patients refused the participation. After the re-assessment of the MRI, one patient had to be excluded because no bone marrow oedema was present. Thus, we included 18 participants in total (fig. 1).

Figure 1 Patient recruitment. This flow-chart shows the patient recruitment
Patients were also informed about other treatment options (e.g., analgesics, physiotherapy), and every patient signed an informed consent form including the off-label use. All patients received ibandronate 3 mg intravenously, varying from one to three administrations (with an interval of at least one month), depending on the level of pain and the treatment response.
We extracted the clinical information from the electronic health record of the Kantonsspital Graubünden. In addition, every patient was contacted (first by letter, and then in cases where there was no response, also by phone) and asked to fill in a questionnaire (see appendix published as a separate pdf file for downloading) regarding the beginning of the symptoms, the intensity of pain (measured by the visual analogue scale [VAS] from zero to ten points), the disability in daily life (movement-induced/load-dependent pain, pain at rest or during the night), and the further clinical course after ibandronate administration (including the time until pain reduction was noticed). Furthermore, we asked about prior trauma (any trauma of the affected knee), use of analgesics, operation or infiltration (before and after ibandronate administration) of the affected knee, and pre-existing diseases. Documented side effects were classified as mild (no treatment necessary), moderate (outpatient treatment utilised), and severe (hospitalisation, death, invalidity related to the ibandronate treatment). All this information was anonymised to protect the identity of all participants.
Our primary endpoint was an intra-individual comparison of the intensity of pain (recorded by VAS), the disability in daily life (grade 0 = none; grade 1 = movement-induced/load-dependent pain; grade 2 = pain at rest/during the night; grade 3 = both), and the use of analgesics before and after the administration of bisphosphonates. Secondary endpoints were the number of administrations of bisphosphonates until a decrease of pain was observed, and the identification of clinical and radiological criteria that correlated with a better response to bisphosphonates.
All magnetic resonance images were re-evaluated by an experienced radiologist with specialisation in musculoskeletal radiology, and the bone marrow oedema was graded as previously described by Bartl et al. [6]:
We used GraphPad Prism Version 6 for statistical analysis. Groups were compared by the non-parametric Wilcoxon signed-rank test for paired samples. A p-value of <0.05 was considered statistically significant.
Half of our study population were male, with a median age of 57.5 years overall (range 29–76 years). Six of the 18 participants had a trauma in their history, of whom two had a trauma from a previous knee operation. In the non-trauma group, the causes remained unclear in eight of the patients and the other four participants had mixed aetiological factors (table 1). In more than half of our study population, the right knee was affected.
Table 1 Baseline characteristics of the study population (n = 18).
| Age (years) | Median | 57.5 |
| Range | 29–76 | |
| Male sex | 9 (50%) | |
| Pre-existing diseases* | None | 9 (50%) |
| Obesity | 3 (16.7%) | |
| Malignancy† | 2 (11.1%) | |
| Diabetes mellitus | 1 (5.5%) | |
| Gout | 1 (5.5%) | |
| Osteoarthritis | 1 (5.5%) | |
| Autoimmune disorder‡ | 1 (5.5%) | |
| Operation of the knee prior to current issue | 2 (11.1%) | |
| Aetiology* | Traumatic | 6 (33.3%) |
| Degenerative | 6 (33.3%) | |
| Iatrogenic | 3 (16.7%) | |
| Others | 1 (5.5%) | |
| Unclear | 8 (44.4%) | |
| Systemic steroids in history | 3 (16.7%) | |
| Affected knee | Right | 10 (55.6%) |
| Left | 8 (44.4%) | |
| Duration from first symptoms until diagnosis (days) | Median | 77 |
| Range | 18–907 | |
| Previous treatment* | Trial of conservative therapy | 17 (94.4%) |
| Operation of the knee§ | 3 (16.7%) | |
| Infiltration of the knee | 4 (22.2%) | |
| Pain at baseline (VAS) | Mean | 7.4 |
| Median | 7.75 | |
| Range | 3.5–10 | |
| Disability at baseline | None | 0 |
| Movement-induced / load-dependent pain | 8 (44.4%) | |
| Pain at rest / during night | 2 (11.1%) | |
| Both | 7 (38.9%) | |
| No statement | 1 (5.6%) | |
| * Multiple mentions possible † One had a resection of a basalioma and a neurofibroma, the other had an ovarectomy due to a teratoma. ‡ One had the diagnosis of a sarcoidosis of the lymphonodular system with cutaneous and intrathoracal manifestation. § Two had a partial meniscectomy, one had a total meniscectomy. |
||
Relevant concomitant diagnoses (obesity, malignancy, gout, diabetes mellitus, osteoarthritis, and autoimmune disease) were only present in the personal history of nine participants. Three participants had received systemic steroids in their prior history, one because of sarcoidosis; the indication in the other two cases remained unclear. All other participants (83.3%) had never received systemic steroids before. Relevant hypocalcaemia or renal insufficiency (as contraindications for the use of bisphosphonates) did not occur in any of our participants.
In our study population, the median time until correct diagnosis was 77 days overall (range 18–907 days). In one case, the patient had gonarthritis of the affected knee treated with arthroscopy, and only intermittently suffered from pain, leading to a period of 907 days until correct diagnosis.
During the time until diagnosis, almost half of the participants suffered from a relevant limitation in daily life activities, with movement-induced/load-dependent pain. 11% of the participants reported pain only during rest or at night, and more than one third suffered from both. Pain intensity was assessed by VAS and reached a mean value of 7.4 before treatment, thereby emphasising the high level of disability.
All the participants had tried other treatments before, without benefit; four of them had even tried different treatment options. 94% took analgesics regularly and reduced their weight bearing. In four participants, a local infiltration with analgesics and steroids was performed, and three participants underwent arthroscopy (two had partial meniscectomy, and one had a total meniscectomy).
The median time from diagnosis of bone marrow oedema until the first administration of bisphosphonate was 4.5 days (range 0–161 days). Six participants received the first dose on the same day the bone marrow oedema was diagnosed. In the case of one patient, it took 161 days until ibandronate was administered for the first time; this delay is most probably due to the fact that the bone marrow oedema was diagnosed by a specialist, and the participants had to be referred to the Kantonsspital Graubünden for the therapy with bisphosphonates.
Ten participants (55.6%) had two administrations with a mean interval of 31.4 days (range 23–43 days). The other eight participants received one dose of ibandronate; in two of these cases, no improvement could be monitored. None of the participants received three doses of ibandronate.
Mild side effects (myalgias) were documented for only one participant and no severe adverse events during or after therapy were documented.
The typical diagnostic finding of a bone marrow oedema on a magnetic resonance image is shown in figure 2. The analysis of all magnetic resonance images showed heterogeneous findings (table 2). Overall, 67% of our participants suffered from a subchondral insufficiency fracture. The medial condyle of the femur and the medial part of the tibia head were involved in all cases and the lateral parts were spared. Two thirds of the participants without trauma also had a subchondral insufficiency fracture, which demonstrates that subchondral insufficiency fractures are not necessarily caused by trauma, but also occur spontaneously.
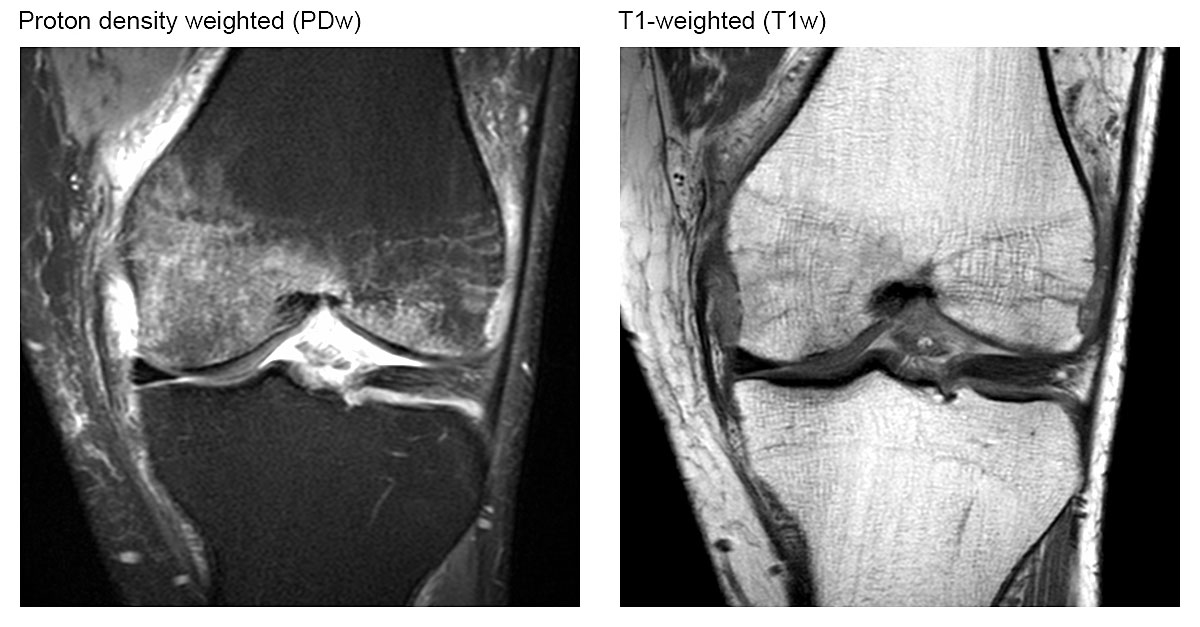
Figure 2 Magnetic resonance imaging appearance of signal changes referred to as bone marrow oedema. Coronal intermediate-weighted MR image with fat suppression illustrating ill-defined high signal intensity in the bone marrow of the medial femoral condyle. Corresponding coronal T1-weighted MR image showing ill-defined hypointensity within the bone marrow. Note: The underlying trabecular structure of cancellous bone is preserved.
Table 2 Radiological findings on magnetic resonance imaging (n = 18)
| Subchondral insufficiency fracture | 12 (66.6%) |
| − Medial femur condyle | 11 (61.1%) |
| − Lateral femur condyle | 0 |
| − Medial head of tibia | 1 (5.5%) |
| − Lateral head of tibia | 0 |
| Only bone marrow oedema | 1 (5.5%) |
| Others* | 6 (33.3%) |
| Severity† | |
| − Grade 1 | 4 (22.2%) |
| − Grade 2 | 5 (27.8%) |
| − Grade 3 | 2 (11.1%) |
| − Grade 4 | 7 (38.9%) |
* Other findings on magnetic resonance images were: contusion (n = 1), osteoarthritis (n = 3), rupture of the meniscus (n = 1), fracture of the cartilage (n = 1) † Grading system according to Bartl et al. [6]
We used the same system as Bartl et al. [6] for grading the severity of changes on magnetic resonance images. More than one third of the participants suffered from a bone marrow oedema of both condyles or of one condyle plus the proximal tibia (grade 4). Grade 1 and 2 concerned almost one quarter of all participants each.
As shown in figure 3, the MRI findings did not correlate with the intensity of pain before bisphosphonate administration, as only three of the nine participants with severe pain (VAS 8 or higher) had grade 4 changes on an MRI scan. On the other hand, the four participants with grade 1 changes in their magnetic resonance images had a mean value of pain of 6.6, which is only slightly lower than in the overall group (mean value of pain overall 7.4).
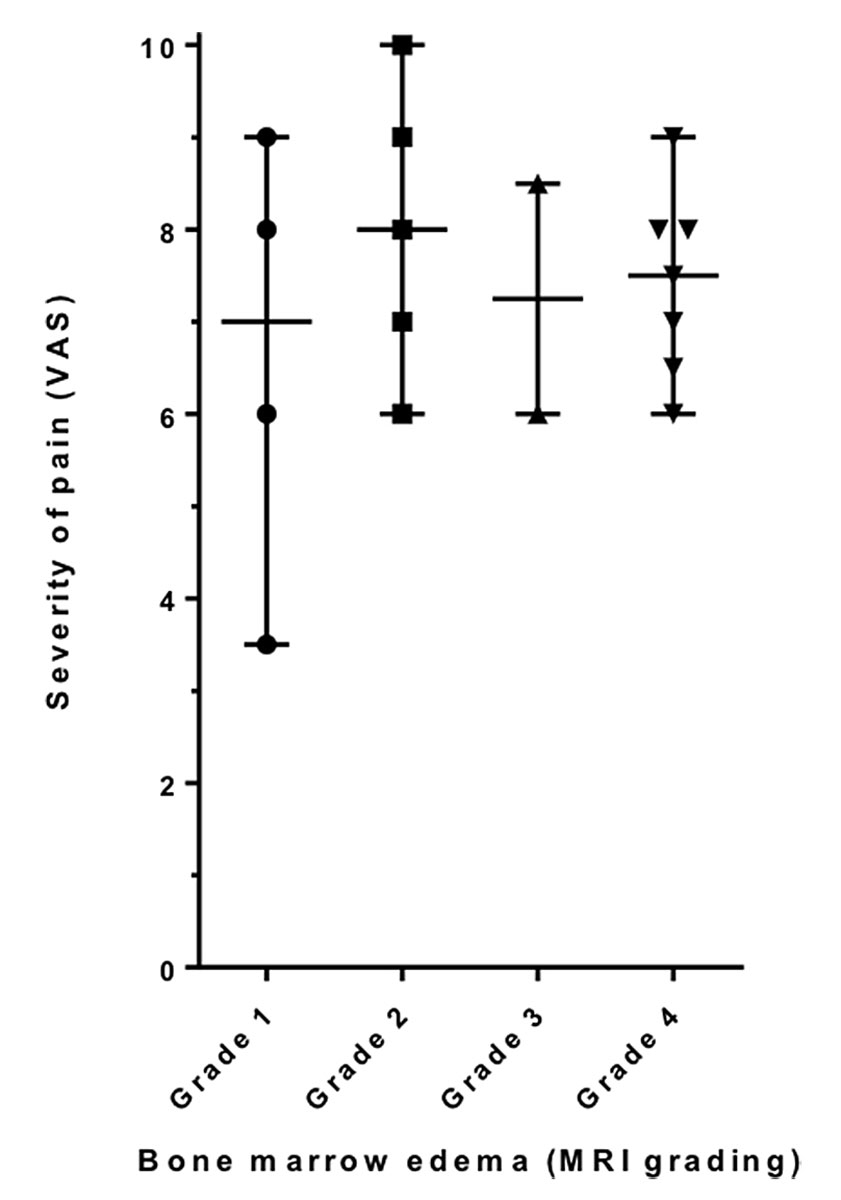
Figure 3 Severity of MRI changes in correlation with pain. MRI findings did not correlate with the intensity of pain before bisphosphonate administration
Grades 1 to 4 describe the severity of changes seen on MRI scan reaching from the affection of one third of one femur condyle or the proximal tibia grade 1) to the involvement of both femur condyles, or one femur condyle plus the proximal tibia (grade 4).
Most participants (72.2%, n = 13) reported improving symptoms. Our results showed a significant reduction of mean pain from 7.4 to 3.8 on VAS (p = 0.0001, fig. 4A). The median period until improvement was 14 days (range 4–90 days). One participant with initial improvement reported a relapse later.
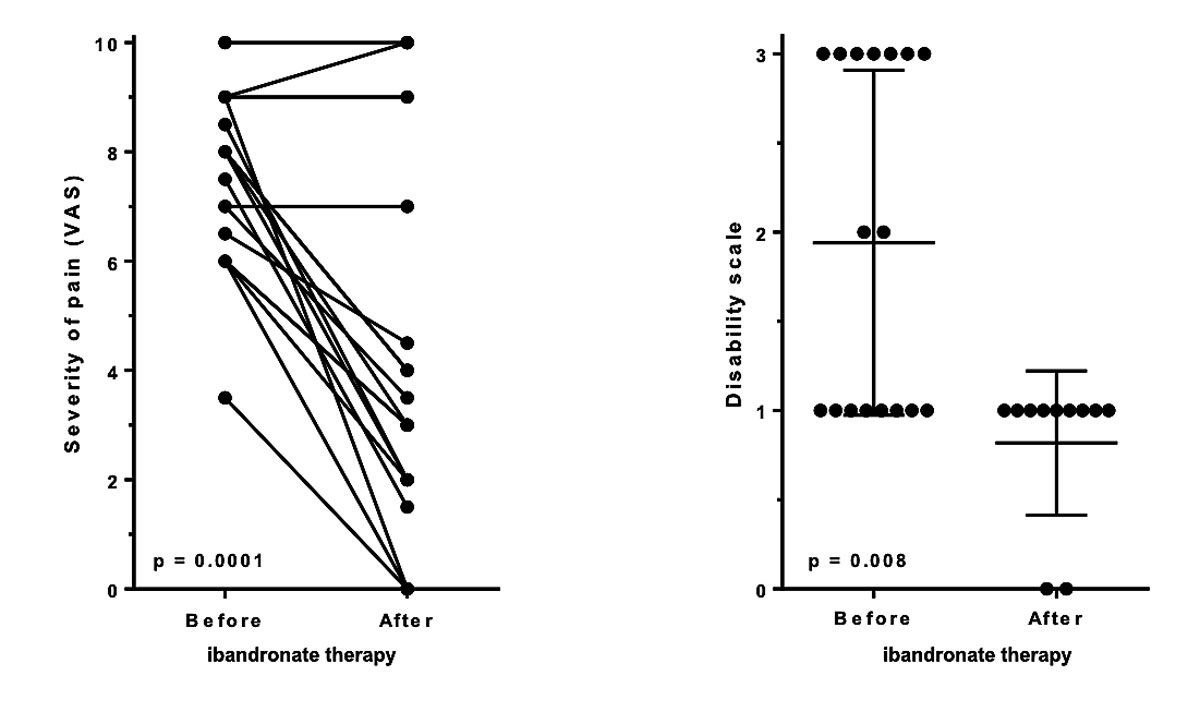
Figure 4 Change in pain (VAS) and disability in daily life, before and after ibandronate therapy. Significant reduction of pain (panel A) and significant decrease of disability in daily life (less movement-induced/load-dependent pain, pain at rest or during the night: panel B) after ibandronate therapy
Disability grading: 0 = none, 1 = movement-induced / load-dependent pain, 2 = pain at rest / during night, 3 = both
Only four of the 18 participants reported little or no improvement; interestingly, all of these participants suffered from moderate to severe pain.
Furthermore, the disability in daily life was also significantly improved (p = 0.008, fig. 4B): no participant reported pain during rest or at night after completing bisphosphonate therapy, which resulted in an improvement of quality of life.
Our analysis showed that participants who received two dosages of ibandronate profited more, as their pain relief after completed therapy was greater.
No significant correlation was found between therapy response and the duration between occurrence of symptoms, timing of diagnosis, and beginning of ibandronate administration.
The evaluation after administration of ibandronate in our study is limited since only ten participants received an MRI scan after completed bisphosphonate therapy. Five of these MRI scans showed an improvement of the bone marrow oedema, three showed a stable situation, and two showed a further aggravation after completed bisphosphonate administration (fig. 5).
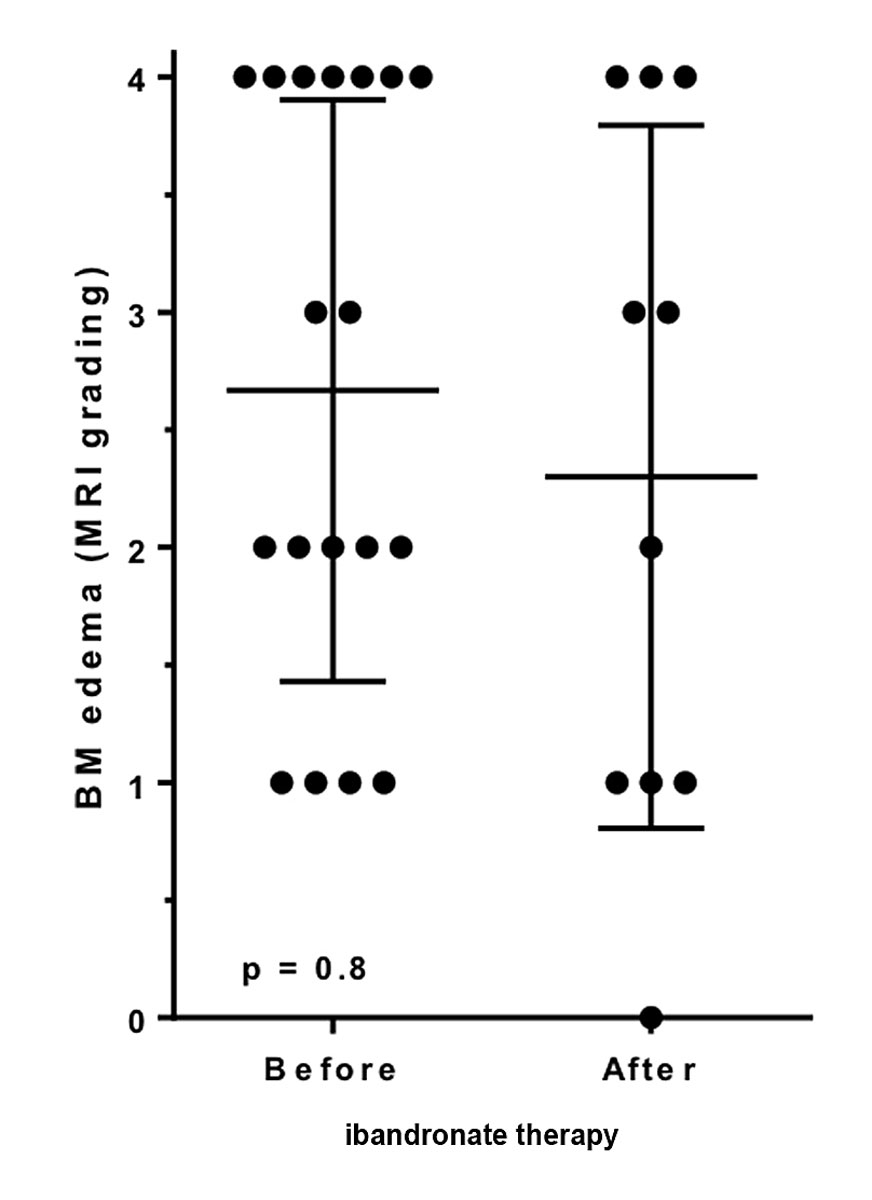
Figure 5 Severity of MRI changes before and after ibandronate therapy. The changes on MRI scan did not significantly differ before or after ibandronate therapy
Even though all participants with pre-treatment grade 4 changes in the MRI scan had a VAS scale value above six points – which corresponds to severe pain – the mean VAS value was, at 7.4, equal to the overall mean VAS value. Therefore, a significant and relevant correlation between the changes in the MRI scans and the severity of pain could not be established either before or after administration of ibandronate.
The follow-up was performed by a questionnaire 19 to 64 months (median 41.5 months) after the last administration of ibandronate. More than half of the participants (55.6%) stated to be pain-free in the long-term follow-up, and the same group did not use alternative therapies (e.g., regular use of analgesics, operation, or local infiltration). After completing therapy, only four participants (22.2%) had to take analgesics regularly. Five participants underwent an operation (one had an arthroscopic partial meniscectomy, three had an implantation of a total knee prosthesis and one had an implantation of a unicompartimental knee prosthesis). For three participants, a local infiltration with analgesics and steroids was performed.
Six participants suffered from light pain (VAS 1–3), and only one participant still had moderate pain (VAS 4–6). None of the participants reported severe pain (VAS 7–10) after completing therapy. The mean decrease of pain was 4.4 points on the visual analogue scale (from 7.4 before therapy to 3.8 after therapy).
Our results show that patients with bone marrow oedema of the knee profit from administration of ibandronate after less than a month. These patients reported not only pain relief, but also an increase of quality of life and reduced use of analgesics, which were the primary endpoints of this study. These results support the study of Bartl et al. [6], which showed a significant improvement of pain score (VAS) after treatment with intravenous ibandronate in comparison with standard therapy (analgesic and reduced weight bearing). Though bone marrow oedema syndrome is a self-limiting disease, bisphosphonate therapy can positively influence its natural course, and potentially prevent operative management. Varenna et al. [11] and Ringe et al. [9] have also showed promising results with intravenous bisphosphonates and comparable sample size, but they investigated different joints of the lower extremity, and the mean age of their study group was notably lower compared to our collective. Our results are more representative of the general population.
Felson et al. described a correlation between pain and proven bone marrow oedema on an MRI scan [20]. A prediction regarding the response to bisphosphonate therapy by grading the bone marrow oedema on an MRI scan was not possible in our study, as the study population was too small. Independently of the severity on an MRI scan, the participants profited from bisphosphonate therapy. It remains unclear whether the lack of response in certain participants was due to an irreversibility of the alterations in the knee joint. Korompilias et al. [7] described a strong predictive value for irreversible lesions if a low signal intensity of at least 4 mm thickness on either T2- or contrast-enhanced T1-weighted images was present. We could not find an explicit correlation between the changes in the MRI scans and the severity of pain, neither before nor after administration of bisphosphonates. Further studies are needed to investigate radiologic criteria, to distinguish between reversible and irreversible changes, and to investigate if they differ in the response to bisphosphonate therapy.
One strength of this study was that an experienced radiologist with specialisation in musculoskeletal radiology reassessed all magnetic resonance images. Furthermore, it was a single-centre study with no changes of the study team during follow-up, and therefore patient management was highly standardised.
Two limitations of this study are the small number of participants, and the lack of a comparison group. Further prospective studies with a comparison group are needed to distinguish between the natural course of the disease and the beneficial effects of bisphosphonates, as well as to identify radiological and clinical criteria that predict the greatest benefit of bisphosphonates in patients with bone marrow oedema. With this information, a statement regarding the disease-related costs would be possible.
Another limitation of our study is its potential recall bias, as we collected data regarding disabilities and co-medications after completed treatment with ibandronate.
In conclusion, patients with bone marrow oedema of the knee profited from administration of ibandronate independently of the severity showed on MRI scans. If the first administration leads to an insufficient control of pain, the administration of a second dose is recommended.
We thank the patients for approving the use of their data for scientific and educational purposes.
The authors acknowledge PD Dr. med. Christoph Schäffeler of the Institute of Radiology, Kantonsspital Graubünden, for the support with the MRI analyses in this project.
No financial support or other potential conflict of interest relevant to this article were reported.
1 Roemer FW , Frobell R , Hunter DJ , Crema MD , Fischer W , Bohndorf K , et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17(9):1115–31. doi:.https://doi.org/10.1016/j.joca.2009.03.012
2 Eriksen EF . Treatment of bone marrow lesions (bone marrow edema). Bonekey Rep. 2015;4:755. doi:.https://doi.org/10.1038/bonekey.2015.124
3 Bartl R. Knochenmarködem: Pathogenese, Diagnostik und Therapie. Orthopädie & Rheuma. 2011;14(10).
4 Wilson AJ , Murphy WA , Hardy DC , Totty WG . Transient osteoporosis: transient bone marrow edema? Radiology. 1988;167(3):757–60. doi:.https://doi.org/10.1148/radiology.167.3.3363136
5 Blum A , Roch D , Loeuille D , Louis M , Batch T , Lecocq S , et al. [Bone marrow edema: definition, diagnostic value and prognostic value]. J Radiol. 2009;90(12):1789–811. doi:.https://doi.org/10.1016/S0221-0363(09)73586-3
6 Bartl C , Imhoff A , Bartl R . Treatment of bone marrow edema syndrome with intravenous ibandronate. Arch Orthop Trauma Surg. 2012;132(12):1781–8. doi:.https://doi.org/10.1007/s00402-012-1617-1
7 Korompilias AV , Karantanas AH , Lykissas MG , Beris AE . Bone marrow edema syndrome. Skeletal Radiol. 2009;38(5):425–36. doi:.https://doi.org/10.1007/s00256-008-0529-1
8 Baier C , Schaumburger J , Götz J , Heers G , Schmidt T , Grifka J , et al. Bisphosphonates or prostacyclin in the treatment of bone-marrow oedema syndrome of the knee and foot. Rheumatol Int. 2013;33(6):1397–402. doi:.https://doi.org/10.1007/s00296-012-2584-0
9 Ringe JD , Dorst A , Faber H . Effective and rapid treatment of painful localized transient osteoporosis (bone marrow edema) with intravenous ibandronate. Osteoporos Int. 2005;16(12):2063–8. doi:.https://doi.org/10.1007/s00198-005-2001-6
10 Hofmann S , Kramer J , Breitenseher M , Pietsch M , Aigner N . Knochenmarködem im Kniegelenk. Differenzialdiagnostik und therapeutische möglichkeiten. Orthopade. 2006;35(4):463–77. doi:.https://doi.org/10.1007/s00132-006-0952-8
11 Varenna M , Zucchi F , Binelli L , Failoni S , Gallazzi M , Sinigaglia L . Intravenous pamidronate in the treatment of transient osteoporosis of the hip. Bone. 2002;31(1):96–101. doi:.https://doi.org/10.1016/S8756-3282(02)00812-8
12 Ringe JD , Body JJ . A review of bone pain relief with ibandronate and other bisphosphonates in disorders of increased bone turnover. Clin Exp Rheumatol. 2007;25(5):766–74.
13 Meizer R , Radda C , Stolz G , Kotsaris S , Petje G , Krasny C , et al. MRI-controlled analysis of 104 patients with painful bone marrow edema in different joint localizations treated with the prostacyclin analogue iloprost. Wien Klin Wochenschr. 2005;117(7-8):278–86. doi:.https://doi.org/10.1007/s00508-005-0326-y
14 Rogers MJ , Watts DJ , Russell RG . Overview of bisphosphonates. Cancer. 1997;80(8, Suppl):1652–60. doi:.https://doi.org/10.1002/(SICI)1097-0142(19971015)80:8+<1652::AID-CNCR15>3.0.CO;2-Z
15Harold B, Rosen M. Pharmacology of bisphosphonates. UpToDate. 2017. https://www.uptodate.com/contents/pharmacology-of-bisphosphonates.
16 Mühlbauer RC , Bauss F , Schenk R , Janner M , Bosies E , Strein K , et al. BM 21.0955, a potent new bisphosphonate to inhibit bone resorption. J Bone Miner Res. 1991;6(9):1003–11. doi:.https://doi.org/10.1002/jbmr.5650060915
17Rosen HN. Risks of bisphosphonate therapy in patients with osteoporosis. UpToDate. 2017. https://www.uptodate.com/contents/risks-of-bisphosphonate-therapy-in-patients-with-osteoporosis.
18Rosen HN. The use of bisphosphonates in postmenopausal women with osteoporosis. UpToDate. 2018. https://www.uptodate.com/contents/the-use-of-bisphosphonates-in-postmenopausal-women-with-osteoporosis.
19 Simon MJK , Barvencik F , Luttke M , Amling M , Mueller-Wohlfahrt HW , Ueblacker P . Intravenous bisphosphonates and vitamin D in the treatment of bone marrow oedema in professional athletes. Injury. 2014;45(6):981–7. doi:.https://doi.org/10.1016/j.injury.2014.01.023
20 Felson DT , Chaisson CE , Hill CL , Totterman SM , Gale ME , Skinner KM , et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi:.https://doi.org/10.7326/0003-4819-134-7-200104030-00007
No financial support or other potential conflict of interest relevant to this article were reported.