Immune checkpoint inhibitor therapy-associated encephalitis: a case series and review of the literature
DOI: https://doi.org/10.4414/smw.2020.20377
Johann
Stubya, Thomas
Herrenb, Guido
Schwegler Naumburgerc, Claudia
Papetd, Alain
Rudigera
a Internal Medicine, Department II, Limmattal Hospital Zurich, Schlieren, Switzerland
b Cardiology, Department II, Limmattal Hospital Zurich, Schlieren, Switzerland
c Neurology, Department II, Limmattal Hospital Zurich, Schlieren, Switzerland
d Oncology/Haematology, Department II, Limmattal Hospital Zurich, Schlieren, Switzerland
Summary
BACKGROUND
Immune checkpoint inhibitors (ICIs) can cause a wide spectrum of immune-related adverse events, including encephalitis. To date, no prospective randomised controlled trials examining the patient characteristics, treatment and outcomes of ICI-associated encephalitis have been published. Therefore, we aimed to review case reports and to provide recommendations for the management of ICI-associated encephalitis.
METHODS
A literature search using Google Scholar and PubMed was performed in December 2019. Published case reports and case series of ICI-associated encephalitis were reviewed, and a case series from the Limmattal Hospital in Schlieren, Switzerland was added. The results are presented as numbers and medians (ranges).
RESULTS
Five different ICIs caused encephalitis in the 47 patients included in this case series. Nivolumab was the most frequently involved drug (27/47, 57%). The median time between treatment and onset of symptoms was 65 (4–630) days. Patients presented with rapidly evolving confusion, reduced level of consciousness, headache, seizures and focal neurological deficits. A total of 19 out of the 44 (43%) magnetic resonance imaging (MRI) scans performed revealed findings suggestive of encephalitis. No specific electroencephalogram (EEG) pattern consistent with encephalitis was found, but epileptiform discharges were detected in 7/20 (35%) of all tested patients. Typical findings of cerebrospinal fluid (CSF) analysis were pleocytosis, elevated protein levels and normal glucose concentrations. Forty-four out of 47 (94%) patients received corticosteroids. Intravenous immunoglobulins (IVIG), rituximab and plasma exchange therapy were less frequently prescribed. Nine out of 47 (19%) patients died during the index hospitalisation.
CONCLUSIONS
Encephalitis should be suspected in patients treated with ICIs who present with rapidly evolving confusion. Blood tests, CSF analysis, cerebral MRI and an EEG should be performed. Therapy with intravenous corticosteroids is recommended. Steroid unresponsiveness is rare and should lead to a review of the diagnosis. Alternative treatment options are IVIG, plasma exchange therapy and rituximab.
Abbreviations
- ANCA
-
anti-neutrophil cytoplasmic antibody
- AGNA
-
anti-glia nuclear antibody, SOX1
- Anti-AchR antibody
-
anti-acetylcholine receptor antibody
- Anti-AMPAR 1 antibody
-
anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor 1 antibody
- Anti-AMPAR 2 antibody
-
anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor 2 antibody
- Anti-CASPR2 antibody
-
anti-contactin-associated protein-like 2 antibody
- Anti-CRMP5 antibody
-
anti-collapsin response mediator protein 5 antibody, CV2
- Anti-DPPX antibody
-
anti-dipeptidyl-peptidase-like protein 6 antibody
- Anti-GABAR antibody
-
anti-gamma aminobutyric acid B receptor antibody
- Anti-GAD65 antibody
-
anti-glutamate decarboxylase 65 kDa isoform antibody
- Anti-Gly antibody
-
anti-glycine antibody
- Anti-Hu antibody
-
anti-neuronal nuclear antibody-1, ANNA1
- Anti-LGI1 antibody
-
anti-leucine-rich glioma-inactivated 1 antibody
- Anti-NMDAR antibody
-
anti-N-methyl-D-aspartate receptor antibody
- Anti-Ri antibody
-
anti-neuronal nuclear antibody-2, ANNA2
- Anti-TG antibody
-
anti-thyroglobulin antibody
- Anti-TPO antibody
-
anti-thyroid peroxidase antibody
- Anti-TR antibody
-
anti-human thrombin receptor antibody
- Anti-Yo antibody
-
anti-Purkinje cell cytoplasmic antibody type 1, PCA-1
- Anti-VGKC antibody
-
anti-voltage-gated potassium channel antibody
- Anti-VGCC antibody
-
anti-voltage-gated calcium channel antibody
- Anti-ZIC4 antibody
-
anti-zic family member 4 antibody
- EEG
-
electroencephalogram
- CSF
-
cerebrospinal fluid
- CTLA-4
-
cytotoxic T-lymphocyte-associated antigen 4
- FLAIR
-
fluid-attenuated inversion recovery
- HSV-1
-
herpes simplex virus type 1
- HSV-2
-
herpes simplex virus type 2
- ICI
-
immune checkpoint inhibitor
- ICU
-
intensive care unit
- IV
-
intravenous
- IVIG
-
intravenous immunoglobulins
- MRI
-
magnetic resonance imaging
- NAEs
-
neurological adverse events
- NSCLC
-
non-small cell lung cancer
- PCR
-
polymerase chain reaction
- PD-1
-
programmed cell death protein 1
- PRES
-
posterior reversible encephalopathy syndrome
- SCLC
-
small cell lung cancer
- VZV
-
varicella zoster virus
Introduction
Immune checkpoint inhibitors (ICIs) re-establish the antitumour activity of T-lymphocytes by blocking immune inhibitory receptors such as programmed cell death protein 1 (PD-1, e.g., nivolumab, pembrolizumab and lambrolizumab), programmed cell death ligand 1 (e.g., atezolizumab, durvalumab and avelumab) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4, e.g., ipilimumab) [1–3]. They are effective in patients with melanoma, lung cancer, renal cell cancer, urothelial cancer and other tumour types [4–6].
ICIs have been associated with various neurological immune-related adverse events, including peripheral neuropathies, Guillain-Barré syndrome, myasthenia gravis, Tolosa-Hunt syndrome and autoimmune encephalitis. According to the literature, less than 1% of patients will develop autoimmune encephalitis when treated with ICIs [4, 6, 7], with higher risks during concurrent or sequential ICI use [6].
In the absence of prospective randomised controlled trials examining the patient characteristics, treatment and outcomes of ICI-associated encephalitis, we reviewed the published case reports of ICI-associated encephalitis and added our own five cases. Recommendations for the management of ICI-associated encephalitis are presented.
Methods
A literature search using Google Scholar and PubMed and focusing on publications in English was performed in December 2019. The following search terms were used: “immune checkpoint inhibitors”, “checkpoint inhibitor therapy”, “checkpoint inhibitor treatment”, “ipilimumab”, “pembrolizumab”, “atezolizumab”, “nivolumab”, “durvalumab”, “avelumab”, “encephalitis”, “encephalopathy”, “case report” and “case series”. Additionally, the bibliographies of the retrieved publications were screened. The patient characteristics, treatments and outcome parameters were entered into a Microsoft Excel® (2011) worksheet.
Five patients with ICI-associated encephalitis treated at the Limmattal Hospital in Schlieren, Switzerland were added to this case series. Written consent was obtained from the patients or their next of kin. Data were collected from electronic medical records.
Results
Five cases of ICI-associated encephalitis were diagnosed in the Limmattal Hospital in Schlieren, Switzerland from 2016 to 2020. One case was previously described by Schneider et al. [6]. Detailed descriptions of the four remaining patients are provided in appendix 1. All five patients were included in the case series. The literature search identified 29 published cases. A bibliography screening revealed 13 additional publications. Overall, 47 cases were analysed.
Five different ICIs were implicated in the pathogenesis of autoimmune encephalitis, either as monotherapy or as concurrent or sequential combination therapy. These five drugs were atezolizumab (n = 4), ipilimumab (n = 14), lambrolizumab (n = 1), nivolumab (n = 27) and pembrolizumab (n = 10). No incidences of avelumab- or durvalumab-associated encephalitis were reported. The median (range) delay between the start of ICI treatment and the onset of symptoms was 65 (4–630) days.
The median (range) patient age was 63 (18–83) years. Patients presented with reduced levels of consciousness, confusion, headache, fever, seizures and/or focal neurological deficits (e.g., motor deficits, paraesthesia, aphasia or ataxia). Other symptoms or signs included asterixis, myoclonus, memory loss, nausea, vomiting and abnormal behaviours (e.g., inappropriate laughter). The patient characteristics are summarised in table 1.
Table 1 Patient characteristics.
|
Case report
|
Year of publication
|
Country
|
Age
|
Sex
|
Cancer type
|
Brain metastases
|
Cancer therapy
|
Steroid dose*
|
Hospital survival
|
|
Limmattal Hospital
|
| See appendix 1
|
n/a |
CH |
74 |
F |
Pulmonary adenocarcinoma |
No |
Pembrolizumab |
125 |
Yes |
| See appendix 1
|
n/a |
CH |
58 |
F |
Carcinosarcoma of the uterus |
Yes |
Pembrolizumab |
80 |
No |
| See appendix 1
|
n/a |
CH |
63 |
F |
Pulmonary adenocarcinoma |
No |
Nivolumab |
125 |
Yes |
| Schneider et al. [6] |
2017 |
CH |
78 |
M |
Pulmonary squamous cell carcinoma |
No†
|
Nivolumab |
80 |
Yes |
| See appendix 1
|
n/a |
CH |
79 |
M |
Merkel cell carcinoma |
Yes |
Nivolumab + Ipilimumab |
125 |
Yes |
|
Literature search
|
| Larkin et al. [4] |
2017 |
USA |
53 |
F |
Malignant melanoma |
No |
Nivolumab + Ipilimumab |
200 |
Yes |
| Larkin et al. [4] |
2017 |
USA |
61 |
M |
Malignant melanoma |
No |
Nivolumab + Ipilimumab → Nivolumab alone |
1000 |
Yes |
| Larkin et al. [4] |
2017 |
USA |
57 |
M |
Malignant melanoma |
No |
Nivolumab → Ipilimumab → Nivolumab |
n.r. |
Yes |
| Larkin et al. [4] |
2017 |
USA |
83 |
M |
Malignant melanoma |
No |
Nivolumab |
24 |
No |
| Larkin et al. [4] |
2017 |
USA |
58 |
F |
Malignant melanoma |
Yes |
Nivolumab |
1000 |
Yes |
| Shah et al. [8] |
2018 |
USA |
66 |
F |
Pulmonary adenocarcinoma |
No |
Nivolumab |
1000 |
No |
| Shah et al. [8] |
2018 |
USA |
44 |
F |
Pulmonary adenocarcinoma |
Yes |
Nivolumab |
1000 |
Yes |
| Williams et al. [9] |
2016 |
USA |
55 |
F |
Malignant melanoma |
Yes |
Nivolumab + Ipilimumab |
1000 |
Yes |
| Williams et al. [9] |
2016 |
USA |
65 |
M |
Small cell lung cancer |
Yes |
Nivolumab + Ipilimumab |
48 |
Yes |
| Niki et al. [10] |
2016 |
Japan |
51 |
M |
Pulmonary squamous cell carcinoma |
Yes |
Pembrolizumab |
2 mg/kg |
Yes |
| Cook et al. [11] |
2017 |
USA |
72 |
M |
Malignant melanoma |
Yes |
Pembrolizumab |
High dose |
No |
| Kim et al. [12] |
2019 |
Korea |
49 |
M |
Urothelial carcinoma of the bladder |
n.r. |
Atezolizumab |
1000 |
Yes |
| Mandel et al. [13] |
2014 |
USA |
66 |
M |
Malignant melanoma |
No |
Lambrolizumab |
None |
Yes |
| Laserna et al. [14] |
2018 |
USA |
53 |
F |
Cervical squamous cell carcinoma |
No |
Atezolizumab |
90 |
Yes |
| Levine et al. [15] |
2017 |
USA |
59 |
F |
Urothelial carcinoma of the bladder |
Yes |
Atezolizumab |
200 |
Yes |
| Bossart et al. [7] |
2017 |
CH |
60 |
F |
Malignant melanoma |
Yes |
Ipilimumab + Pembrolizumab |
None |
No |
| Arakawa et al. [16] |
2019 |
Japan |
78 |
M |
Pulmonary adenocarcinoma |
n.r. |
Atezolizumab |
1000 |
Yes |
| Salam et al. [17] |
2016 |
UK |
64 |
M |
Malignant melanoma |
No |
Pembrolizumab |
High dose |
Yes |
| Ito et al. [18] |
2017 |
Japan |
75 |
M |
Small cell lung cancer |
Yes |
Nivolumab + Ipilimumab |
500 |
Yes |
| Conry et al. [19] |
2015 |
USA |
41 |
M |
Malignant melanoma |
No |
Ipilimumab |
160 |
Yes |
| Burke et al. [20] |
2018 |
USA |
64 |
F |
Ovarian clear cell carcinoma |
No |
Nivolumab |
12,000 |
Yes |
| Boyd et al. [21] |
2015 |
UK |
71 |
M |
Malignant melanoma |
No |
Ipilimumab |
1000 |
Yes |
| Voskens et al. [22] |
2013 |
DE |
50 |
M |
Malignant melanoma |
No |
Ipilimumab |
High dose |
Yes |
| Cao et al. [23] |
2016 |
USA |
76 |
F |
Malignant melanoma |
Yes |
Ipilimumab |
High dose |
Yes |
| Khoja et al. [24] |
2016 |
Canada |
51 |
F |
Malignant melanoma |
No |
Pembrolizumab |
1000 |
Yes |
| Kazandjian et al. [25] |
2016 |
USA |
70 |
F |
Non-small cell lung cancer |
n.r. |
Nivolumab |
2–4 mg/g |
No |
| Carl et al. [26] |
2015 |
DE |
64 |
M |
Prostate cancer |
No |
Ipilimumab |
1000 |
Yes |
| Richard et al. [27] |
2017 |
USA |
74 |
M |
Pulmonary squamous cell carcinoma |
Yes |
Nivolumab |
n.r. |
Yes |
| Matsuo et al. [28] |
2018 |
Japan |
60 |
M |
Pulmonary pleomorphic carcinoma |
n.r. |
Nivolumab |
High dose |
No |
| Brown et al. [1] |
2017 |
Australia |
67 |
M |
Malignant melanoma |
n.r. |
Pembrolizumab |
High dose |
Yes |
| Feng et al. [29] |
2017 |
Australia |
66 |
M |
Pulmonary adenocarcinoma |
Yes |
Pembrolizumab |
1000 |
Yes |
| Strik et al. [30] |
2017 |
DE |
53 |
M |
Non-Hodgkin lymphoma |
No |
Nivolumab |
1000 |
Yes |
| Chaucer et al. [31] |
2018 |
USA |
44 |
M |
Renal cell carcinoma |
No |
Nivolumab |
n.r. |
Yes |
| Leitinger et al. [32] |
2018 |
Austria |
67 |
F |
Pulmonary squamous cell carcinoma |
n.r. |
Nivolumab |
1000 |
No |
| Zurko et al. [33] |
2018 |
USA |
20 |
M |
Hodgkin lymphoma |
No |
Nivolumab |
160 |
Yes |
| Kopecký et al. [34] |
2018 |
Czech Republic |
63 |
M |
Renal cell carcinoma |
No |
Nivolumab |
2 mg/kg |
Yes |
| De la Hoz et al. [35] |
2018 |
USA |
28 |
F |
Hodgkin lymphoma |
n.r. |
Nivolumab |
1 mg/kg |
Yes |
| Shibaki et al. [36] |
2019 |
Japan |
78 |
M |
Malignant pleural mesothelioma |
No |
Nivolumab |
n.r. |
Yes |
| Zafar et al. [37] |
2019 |
USA |
59 |
F |
Laryngeal squamous cell carcinoma |
n.r. |
Nivolumab |
1000 |
Yes |
| Gill et al. [38] |
2019 |
USA |
68 |
F |
Merkel cell carcinoma |
No |
Nivolumab |
1000 |
No |
| Hottinger et al. [39] |
2018 |
CH |
71 |
F |
Small cell lung cancer |
n.r. |
Nivolumab + Ipilimumab |
1000 |
Yes |
| Gill et al. [38] |
2019 |
USA |
71 |
F |
Pulmonary adenocarcinoma |
n.r. |
Pembrolizumab |
n.r. |
Yes |
Cerebral magnetic resonance imaging (MRI) was performed in 44/47 (94%) patients. In 25/44 (57%) patients, cerebral MRI was normal or showed nonspecific abnormalities. In 19/44 (43%) patients, cerebral MRI findings were consistent with encephalitis. These findings included leptomeningeal enhancement, bilateral hyperintensities in the mesial temporal area or in the basal ganglia, and diffuse encephalitis resembling disseminated demyelination.
An electroencephalogram (EEG) was obtained in 20/47 (43%) patients. No specific EEG pattern was found. Epileptic activity was recorded in 7/20 (35%) patients.
The findings of the cerebral MRIs and EEGs are summarised in table 2.
Table 2 MRI and EEG findings.
|
Case report
|
Year of Publication
|
Brain MRI
|
EEG
|
|
Limmattal Hospital
|
| See appendix 1
|
n/a |
No signs of encephalitis or meningitis. |
Moderate background slowing, focal slowing over the right temporal region, some epileptiform activity with bilateral frontal sharp waves. |
| See appendix 1
|
n/a |
25 × 24 mm metastatic lesion subcortically located in the left medial frontal gyrus. Otherwise, normal MRI. |
n.r. |
| See appendix 1
|
n/a |
Diffuse white matter changes, most likely due to whole brain radiation. |
n.r. |
| Schneider et al. [6] |
2017 |
No signs of encephalitis, carcinomatous meningitis, metastases or stroke. |
Moderate background slowing and focal delta slowing over the left temporal region with singular sharp waves in this region. |
| See appendix 1
|
n/a |
No signs of encephalitis, hypophysitis or ischaemia. |
Mild background slowing, moderate focal slowing over the left temporal region. No epileptiform activity. |
|
Literature search
|
| Larkin et al. [4] |
2017 |
No signs of metastasis or stroke. |
Diffuse, marked cerebral slowing. |
| Larkin et al. [4] |
2017 |
Abnormally high fluid-attenuated inversion recovery (FLAIR) signal without evidence of brain metastasis. |
Epileptic activity. |
| Larkin et al. [4] |
2017 |
No signs of brain metastasis. |
Diffuse slowing and triphasic waves and phase reversal in the right frontal region. No sign of seizure activity. |
| Larkin et al. [4] |
2017 |
Some areas of hypersignal on the supratentorial white matter and FLAIR without a break in the haematocephalic barrier, probably caused by microangiopathy. |
n.r. |
| Larkin et al. [4] |
2017 |
No signs of stroke, brain metastases unchanged, no new lesions. |
n.r. |
| Shah et al. [8] |
2018 |
Symmetric T2 hyperintense and T1 hypointense basal ganglia abnormalities. A repeat brain MRI three weeks later re-demonstrated symmetric T2 hyperintense basal ganglia but with a transition to T1 hyperintensities in the same location. |
n.r. |
| Shah et al. [8] |
2018 |
T2 signal hyperintensities of the bilateral mesial temporal lobes compatible with limbic encephalitis. Additionally, there were two enhancing foci within the left occipital and right temporal lobes, concerning for metastatic disease. |
n.r. |
| Williams et al. [9] |
2016 |
Stable encephalomalacia at sites of prior radiosurgery with no additional metastases. No changes were noted at previously irradiated tumour sites. |
Serial EEG showed intermittent bilateral slowing, then a subclinical seizure of left temporo-occipital origin. Continuous EEG monitoring showed intermittent periods of rhythmic epileptiform activity in the left temporal lobe without clinical correlations. |
| Williams et al. [9] |
2016 |
New nonspecific T2 hyperintensities in the right mesial temporal lobe. |
n.r. |
| Niki et al. [10] |
2016 |
No abnormality other than the previous surgical resection. |
Slow wave in the right frontal lobe, which indicated encephalitis or the aftermath of the previous brain metastasis. |
| Cook et al. [11] |
2017 |
Restricted diffusion in the basal ganglia and right temporal lobe. |
n.r. |
| Kim et al. [12] |
2019 |
Diffuse leptomeningeal enhancement. |
Status epilepticus. |
| Mandel et al. [13] |
2014 |
Unremarkable but limited due to motion artefact. A follow-up brain MRI scan showed stable T2 FLAIR hyperintensities in the right frontal and left occipital lobes, with improvement in the signal originating from both external capsules. |
Periodic epileptiform discharges. |
| Laserna et al. [14] |
2018 |
Diffuse leptomeningeal enhancement. |
No acute abnormalities. The EEG was repeated and showed non-convulsive status epilepticus. |
| Levine et al. [15] |
2017 |
1.0 × 0.9 cm mildly enhancing lesion in the left frontal lobe with no vasogenic oedema or mass effect. |
n.r. |
| Bossart et al. [7] |
2017 |
n.r. |
n.r. |
| Arakawa et al. [16] |
2019 |
No abnormal findings other than the left temporal lobectomy. |
n.r. |
| Salam et al. [17] |
2016 |
Bilateral symmetrical T2 signal change with atrophy within the hippocampi, extending into the anterior temporal lobe and insula. This was more extensive on the left side, with significant volume loss. Previous MRI performed at the referring hospital in November 2014 was reviewed and showed a bilateral high T2 signal affecting the limbic structures, again predominantly on the left, though without volume loss. |
No epileptic activity. |
| Ito et al. [18] |
2017 |
No recurrence of brain metastasis, haemorrhage or infarction. |
No epileptic activity. |
| Conry et al. [19] |
2015 |
New area of restricted diffusion in the posterior splenium of the corpus callosum, with corresponding T2 hyperintensity on FLAIR and no abnormal enhancement or evidence of melanoma metastasis. |
n.r. |
| Burke et al. [20] |
2018 |
No signs of encephalitis or metastasis. |
n.r. |
| Boyd et al. [21] |
2015 |
Unchanged. |
n.r. |
| Voskens et al. [22] |
2013 |
Enhancement in both trigeminal nerves and, additionally, three parenchymal lesions with no indication of meningeosis carcinomatosa or pituitary enlargement. |
n.r. |
| Cao et al. [23] |
2016 |
New signal abnormalities in the left optic nerve, left inferior frontal lobe, and splenium of the corpus callosum, which extended into the parietal lobe and bordered on the stereotactic radiosurgery-treated lesion that by now was no longer enhancing. |
n.r. |
| Khoja et al. [24] |
2016 |
Hyperintense white matter foci in both subcortical (enhancing on FLAIR) and periventricular areas (non-enhancing). There were ovoid lesions perpendicular to the ventricles and lesions involving the corpus callosum. All lesions showed restricted diffusion. The findings were consistent with either demyelination or an ischemic process. A repeat brain MRI after 10 days of steroid treatment showed increased enhancement in the previously noted lesions and lesions involving the cortex, changes more suggestive of multiple infarctions and possibly a vascular process. |
n.r. |
| Kazandjian et al. [25] |
2016 |
n.r. |
n.r. |
| Carl et al. [26] |
2015 |
Mild microangiopathic changes, an old lacunar infarction in the right thalamus and a normal pituitary gland. |
Generalised slowing with prevailing slow theta and delta waves. |
| Richard et al. [27] |
2017 |
n.r. |
Mild slowing and no evidence of seizure activity. |
| Matsuo et al. [28] |
2018 |
High-intensity areas in the inner aspect of the temporal lobe, thalamus, cerebral aqueduct and spinal cord. |
n.r. |
| Brown et al. [1] |
2017 |
T2 hyperintensity of the medial temporal lobes bilaterally with contrast enhancement. |
Background slowing (6–7 Hz) with intermittent delta slowing. |
| Feng et al. [29] |
2017 |
FLAIR hyperintensity adjacent to the resection site, which was consistent with the postoperative changes. |
Generalised, intermittent slowing consistent with mild diffuse encephalopathy. |
| Strik et al. [30] |
2017 |
Small, scattered, T2 and FLAIR hyperintense contrast-enhancing lesions dorsal to the left lateral ventricle and in the midbrain and brain stem. |
n.r. |
| Chaucer et al. [31] |
2018 |
No evidence of metastases or lymphoreticular disorder. |
n.r. |
| Leitinger et al. [32] |
2018 |
Multiple and confluent cortical and subcortical FLAIR hyperintensities within both cerebral hemispheres. |
Moderate slowing. |
| Zurko et al. [33] |
2018 |
Diffusely oedematous cerebellum with patchy enhancement, signs of early tonsillar herniation, and early hydrocephalus. |
n.r. |
| Kopecký et al. [34] |
2018 |
No signs of any tumour lesion. MRI revealed a symmetrical, pathologically increased signal within the basal ganglia consistent with possible inflammatory involvement of these structures. |
n.r. |
| De la Hoz et al. [35] |
2018 |
No abnormalities. |
n.r. |
| Shibaki et al. [36] |
2019 |
T2 high signal intensity in the mesencephalon and medial thalami (typical findings in anti-Ma2-associated encephalitis). |
n.r. |
| Zafar et al. [37] |
2019 |
Multifocal cerebral demyelination, primarily involving the parietal lobe: multiple hyperintense T2 FLAIR signal white matter lesions, primarily in the parietal lobes but also involving the posterior frontal lobes, corpus callosum and right brachium ponti. None of these lesions were enhanced following contrast administration. No restricted diffusion was present. No significant mass effect or midline shift was identified. These findings were suggestive of acute demyelinating encephalomyelitis. |
Diffuse, generalised slowing with practically no significant reactivity to external stimuli. |
| Gill et al. [38] |
2019 |
T2/FLAIR hyperintensities bilaterally in the medial temporal lobes. |
No seizure activity. |
| Hottinger et al. [39] |
2018 |
Severe abnormalities in both hippocampi with contrast-enhancing lesions. |
n.r. |
| Gill et al. [38] |
2019 |
No signal abnormality or enhancement. |
n.r. |
Cerebrospinal fluid (CSF) was analysed in 37/47 (79%) patients. Pleocytosis was found in 30/37 (81%) patients. An increased leucocyte count was found in 24/37 (65%) patients, of whom 16/37 (43%) had lymphocytosis, 2/37 (5%) had monocytosis and 1/37 (3%) had an increased proportion of neutrophils. In 5/37 (14%) patients, the leukocytes were not further differentiated. Protein levels were increased in 24/32 patients (75%, 270 to >6,000 mg/l), but glucose levels were normal in the majority of patients (19/24 patients, 79%). The detailed results of the CSF analyses are provided in table 3.
|
Case report
|
Year of publication
|
CSF cell count
|
CSF protein level
|
CSF glucose level
|
|
Limmattal Hospital
|
| See appendix 1
|
n/a |
59/μl |
589 mg/l |
4.1 mmol/l |
| See appendix 1
|
n/a |
n.r. |
n.r. |
n.r. |
| See appendix 1
|
n/a |
n.r. |
n.r. |
n.r. |
| Schneider et al. [6] |
2017 |
16/μl |
1027 mg/l |
1.1 mmol/l |
| See appendix 1
|
n/a |
83/μl |
1065 mg/l |
2.7 mmol/l |
|
Literature search
|
| Larkin et al. [4] |
2017 |
Elevated |
3120 mg/l |
2.4 mmol/l |
| Larkin et al. [4] |
2017 |
14/μl |
850 mg/l |
11.8 mmol/l |
| Larkin et al. [4] |
2017 |
52/μl |
n.r. |
n.r. |
| Larkin et al. [4] |
2017 |
n.r. |
n.r. |
n.r. |
| Larkin et al. [4] |
2017 |
18/μl |
n.r. |
n.r. |
| Shah et al. [8] |
2018 |
Normal |
560 mg/l |
Normal |
| Shah et al. [8] |
2018 |
19/μl |
Normal |
Normal |
| Williams et al. [9] |
2016 |
8/μl |
Normal |
Normal |
| Williams et al. [9] |
2016 |
18/μl |
980 mg/l |
Normal |
| Niki et al. [10] |
2016 |
58/μl |
4460 mg/l |
n.r. |
| Cook et al. [11] |
2017 |
Elevated |
Elevated |
Normal |
| Kim et al. [12] |
2019 |
Elevated |
n.r. |
n.r. |
| Mandel et al. [13] |
2014 |
8/μl |
1030 mg/l |
5.6 mmol/l |
| Laserna et al. [14] |
2018 |
667/μl |
>6000 mg/l |
5.1 mmol/l |
| Levine et al. [15] |
2017 |
9/μl |
1000 mg/l |
4.4 mmol/l |
| Bossart et al. [7] |
2017 |
n.r. |
n.r. |
n.r. |
| Arakawa et al. [16] |
2019 |
139/μl |
1320 mg/l |
Normal |
| Salam et al. [17] |
2016 |
17/μl |
530 mg/l |
n.r. |
| Ito et al. [18] |
2017 |
58/μl |
1280 mg/l |
n.r. |
| Conry et al. [19] |
2015 |
n.r. |
n.r. |
n.r. |
| Burke et al. [20] |
2018 |
Normal |
Normal |
Normal |
| Boyd et al. [21] |
2015 |
Normal |
1370 mg/l |
n.r. |
| Voskens et al. [22] |
2013 |
n.r. |
n.r. |
n.r. |
| Cao et al. [23] |
2016 |
n.r. |
n.r. |
n.r. |
| Khoja et al. [24] |
2016 |
n.r. |
270 mg/l |
4.4 mmol/l |
| Kazandjian et al. [25] |
2016 |
n.r. |
n.r. |
n.r. |
| Carl et al. [26] |
2015 |
Normal |
850 mg/l |
Normal |
| Richard et al. [27] |
2017 |
Normal |
n.r. |
Normal |
| Matsuo et al. [28] |
2018 |
16/μl |
1620 mg/l |
n.r. |
| Brown et al. [1] |
2017 |
Elevated |
n.r. |
n.r. |
| Feng et al. [29] |
2017 |
0/μl |
Normal |
Normal |
| Strik et al. [30] |
2017 |
15/μl |
Normal |
n.r. |
| Chaucer et al. [31] |
2018 |
n.r. |
n.r. |
n.r. |
| Leitinger et al. [32] |
2018 |
30/μl |
560 mg/l |
Normal |
| Zurko et al. [33] |
2018 |
31/μl |
1610 mg/l |
4.1 mmol/l |
| Kopecký et al. [34] |
2018 |
Elevated |
n.r. |
n.r. |
| De la Hoz et al. [35] |
2018 |
136/μl |
600 mg/l |
2.9 mmol/l |
| Shibaki et al. [36] |
2019 |
15/μl |
900 mg/l |
n.r. |
| Zafar et al. [37] |
2019 |
74/μl |
Elevated |
n.r. |
| Gill et al. [38] |
2019 |
3/μl |
330 mg/l |
6.4 mmol/l |
| Hottinger et al. [39] |
2018 |
16/μl |
1145 mg/l |
n.r. |
| Gill et al. [38] |
2019 |
10/μl |
400 mg/l |
n.r. |
One or more antibodies could be detected in 13/25 (52%) patients. The following antibodies were found: anti-CASPR2 (n = 1), anti-GAD65 (n = 2), AGNA (n = 1), anti-Hu (n = 3), anti-NMDAR (n = 2), anti-Ma2 (n = 2), anti-Ri (n = 1), an uncharacterised antibody against Purkinje cells (n = 1) and an unclassified antibody (n = 1). One patient with steroid-responsive encephalopathy associated with autoimmune thyroiditis presented with anti-TPO and anti-TG antibodies. In 12/25 patients (48%), testing for antibodies was negative.
Brain metastases were present in 14/47 (30%) patients. Antibiotics and/or antiviral therapy were initially prescribed in 16/47 (34%) patients. Intravenous treatment with corticosteroids was started in 44/47 (94%) patients. Typically, “high” (>30 mg) and “very high” (>100 mg) daily corticosteroid doses were used [40]. The mean (range) dose was 997 (24–12,000) mg methylprednisolone or equivalent per day. Usually, the intravenous regimen was changed to oral administration after five days and then tapered over several weeks. In 6/47 patients (13%) the corticosteroid dose was simply specified as “high-dose”; in another 4/47 patients (9%), the dose was not specified at all. In addition, twelve patients (26%) received intravenous immunoglobulins (IVIG), six patients (12%) were given rituximab, and four patients (9%) underwent plasma exchange therapy.
Nine out of 47 (19%) patients died during hospitalisation, of whom six patients received a mean (range) corticosteroid dose of 541 (24–1000) mg, two patients received “high-dose” corticosteroids (not further specified), and the remaining patient received no corticosteroid therapy at all.
Discussion
In this study, we analysed five patients treated in our hospital and combined these findings with those of 42 patients described in published case reports and case series. Based on these findings, we provide recommendations for the management of ICI-associated encephalitis.
The ICIs nivolumab, ipilimumab, pembrolizumab, atezolizumab and lambrolizumab were involved in the pathophysiology of ICI-associated encephalitis. Less than half of the MRIs performed revealed findings suggestive of encephalitis. No specific EEG pattern consistent with encephalitis was found, but epileptiform discharges were detected in approximately one third of the EEGs. The majority of patients had CSF pleocytosis, elevated CSF protein levels and normal CSF glucose concentrations. The patients usually received therapy with corticosteroids, whereas IVIG, rituximab and plasma exchanges were less frequently administered.
Risk factors
In general, CTLA-4 inhibitors (e.g., ipilimumab) are associated with a higher incidence of immune-related adverse events than PD-1 inhibitors (e.g., nivolumab, pembrolizumab and lambrolizumab). When two ICIs (e.g., nivolumab + ipilimumab or pembrolizumab + ipilimumab) are given concurrently, the risk seems to be even higher [41–43]. In our review, 29/47 cases (62%) were caused by PD-1 inhibitor monotherapy, and 5/47 cases (11%) were caused by CTLA-4 inhibitor monotherapy. The concurrent or sequential use of ICIs caused encephalitis in 9/47 cases (19%).
Paraneoplastic antibodies (e.g., anti-Hu, anti-Yo, anti-Ma2, anti-CRMP5, anti-amphiphysin, anti-Ri, anti-NMDA, anti-VGKC, anti-VGCC and anti-AchR) can develop in tumour patients and are associated with various neurological diseases [44]. The presence of paraneoplastic antibodies may increase the risk of developing ICI-associated encephalitis [38].
In our case series, one or more antibodies could be detected in 52% of the patients tested (anti-CASPR2, anti-GAD65, anti-AGNA, anti-Hu, anti-NMDAR, anti-Ma2, anti-Ri and anti-TPO/TG). Shah et al. [8] reported a patient with pulmonary adenocarcinoma and type 1 diabetes who developed GAD65 antibody-positive encephalitis after treatment with nivolumab. GAD65 is located on pancreatic islet cells as well as on GABAergic neurons of the central nervous system. This patient had a higher risk for ICI-associated encephalitis, as he presumably had pre-existing GAD65 antibodies in association with his diabetes.
Presentation and diagnosis
In our experience, ICI-associated encephalitis should be suspected in patients with rapidly evolving confusion. Other symptoms associated with immune-mediated encephalitis include reduced level of consciousness, headache, seizures and focal neurological deficits. One third of patients presented with fever. The time interval between the start of immune checkpoint blockade and the onset of symptoms was wide, ranging from less than a week to 21 months, with a median of 9 weeks. In the case series of Cuzzubbo et al. [2], the median (range) time of onset was 6 (1–74) weeks.
To establish a diagnosis of ICI-associated encephalitis, potential differential diagnoses must be excluded (table 4). Consultation with a neurologist is advised. Infectious aetiologies (bacterial, fungal or viral meningoencephalitis, especially herpes simplex) and leptomeningeal disease can be ruled out by analysing CSF. Typical CSF findings in patients with ICI-associated encephalitis are elevated white blood cell counts with predominant lymphocytes and elevated protein concentrations. The glucose concentration is usually normal. Polymerase chain reaction assays of CSF for herpes simplex virus type 1, herpes simplex virus type 2 and varicella zoster virus must be ordered. In addition, cytological examination of CSF is recommended.
Table 4 Differential diagnosis of ICI-associated encephalitis.
|
Differential diagnosis
|
Diagnostic tests
|
| Infection (sepsis-induced encephalopathy; bacterial, fungal or viral meningoencephalitis; brain abscess) |
Blood tests including culture, CSF analysis including culture and viral PCR (herpes simplex, varicella zoster), MRI |
| Intracerebral haemorrhage |
MRI |
| Ischaemia |
MRI |
| Leptomeningeal dissemination |
CSF analysis, MRI |
| Metabolic disturbances |
Blood tests*
|
| Nonconvulsive status epilepticus |
EEG |
| Paraneoplastic limbic encephalitis |
Blood tests†, CSF analysis‡, MRI |
| PRES |
MRI |
| Vasculitis |
Blood tests (ANCA), MRI |
A cerebral MRI should be performed to detect brain metastases and to exclude ischemia or intracranial haemorrhage. Nonconvulsive status epilepticus must be ruled out by recording an EEG. Blood and urine cultures must be drawn. A complete blood count, a C-reactive protein test and a comprehensive metabolic panel (including ammonia) should be obtained, and arterial blood gases should be analysed. In selected patients, especially in those not responding to corticosteroid therapy, blood concentrations of vitamins (B1, B12), thyroid-stimulating hormone and cortisol should be measured. Toxicological screening should be performed if an intoxication is suspected. Measurement of the anti-neutrophil cytoplasmic antibody concentration can help to exclude vasculitis [3, 4]. The recommended diagnostic approach is illustrated in figure 1.
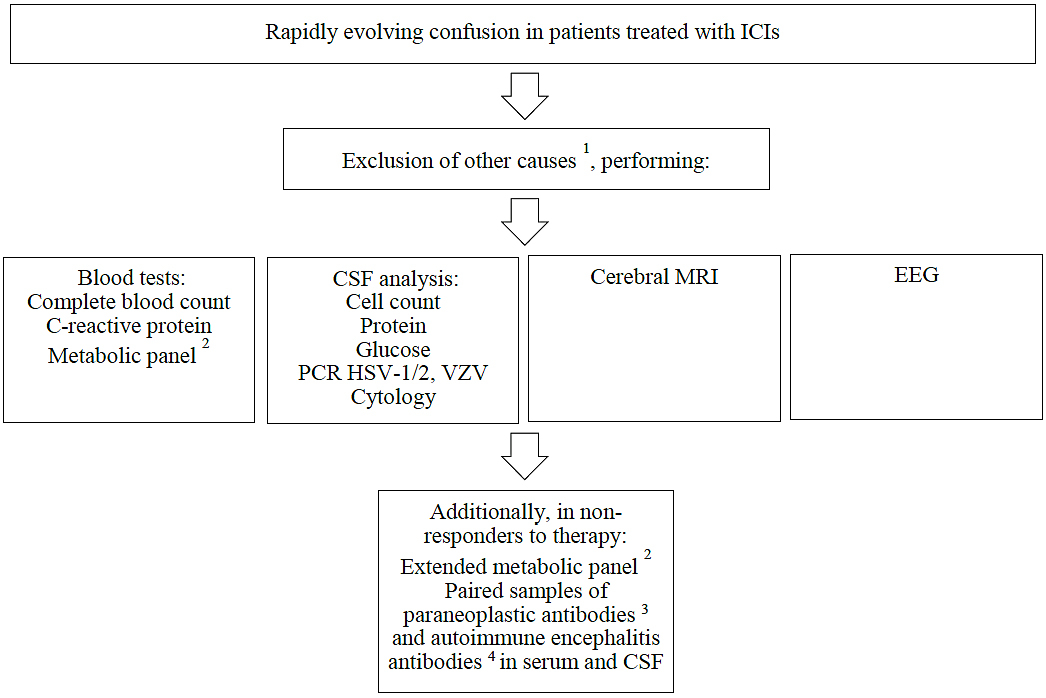
Figure 1 Diagnosis of ICI-associated encephalitis.
1 For differential diagnoses, see table 4.
2
Glucose, creatinine, bilirubin, liver enzymes, ammonia, electrolytes, arterial blood gas analysis; additionally, in non-responders to therapy: vitamins B1/12, thyroid function tests, cortisol concentration, toxicological screening, ANCA
3 Antibodies against AGNA (SOX1), AMPAR1/2, amphiphysin, CRMP5 (CV2), DPPX, GABAR, GAD65, Hu (ANNA1), Ma1, Ma2, Ri (ANNA2), TG, TPO, TR, VGKC (anti-LGI1, anti-CASPR2), Yo (PCA1), ZIC4
4 Antibodies against NMDAR, AMPAR1/2, VGKC (anti-LGI1, anti-CASPR2), Gly, GABAR, etc.
ANCA = anti-neutrophil cytoplasmic antibody; CSF = cerebrospinal fluid; HSV-1 = herpes simplex virus type 1; HSV-2 = herpes simplex virus type 2; ICI = immune checkpoint inhibitor; MRI = magnetic resonance imaging; PCR = polymerase chain reaction; VZV = varicella zoster virus
Management
The severity of neurological adverse events (nAEs) is graded from 1 to 4. In patients with grade 1 nAEs (asymptomatic or mild symptoms), ICI therapy can be continued. However, in patients with grade 2 (moderate symptoms), grade 3 (severe symptoms) and grade 4 (life-threatening symptoms) nAEs, ICIs should be stopped [27, 45].
In our experience, immune-mediated encephalitis is usually graded 3 to 4. Therefore, permanent discontinuation of ICI therapy is advisable, and treatment with corticosteroids should be established. We recommend an initial intravenous dose of 1 to 2 mg/kg body weight/day of methylprednisolone (or equivalent), as suggested in the literature [46, 47]. If symptoms persist, other diagnoses must be entertained, as shown in figure 1. In this case, an extended metabolic panel should be ordered, and paired samples of plasma and CSF should be tested for both paraneoplastic and autoimmune encephalitis antibodies. Autoimmune encephalitis antibodies can be quantified even after an initial treatment with corticosteroids [48]. Data on the time courses of autoimmune encephalitis or paraneoplastic antibodies before and after ICI treatment are not available, however. In the most severe cases, the corticosteroid dose can be increased to ≥1000 mg daily. Due to the long half-life of ICIs, steroids should be tapered slowly over at least four weeks. Potential adverse effects of corticosteroid therapy must be anticipated, including delirium, arterial hypertension, hyperglycaemia and lower resistance to infection. Coadministration of trimethoprim/sulfamethoxazole therapy should be considered, especially if long-term treatment is necessary. In steroid-refractory cases and after careful review of the results of (neuro-)imaging studies, blood tests, CSF analysis, EEG, and titres of paraneoplastic and autoimmune encephalitis antibodies, treatment with IVIG, plasma exchange therapy or rituximab should be considered [4, 45, 46, 49–51]. According to the case report by Hottinger et al. [39], natalizumab, an antibody directed at α4β1 integrin which thus limits lymphocyte recruitment to the CNS, may be considered to treat ICI-associated encephalitis. An algorithm for the management of ICI-associated encephalitis is shown in figure 2.
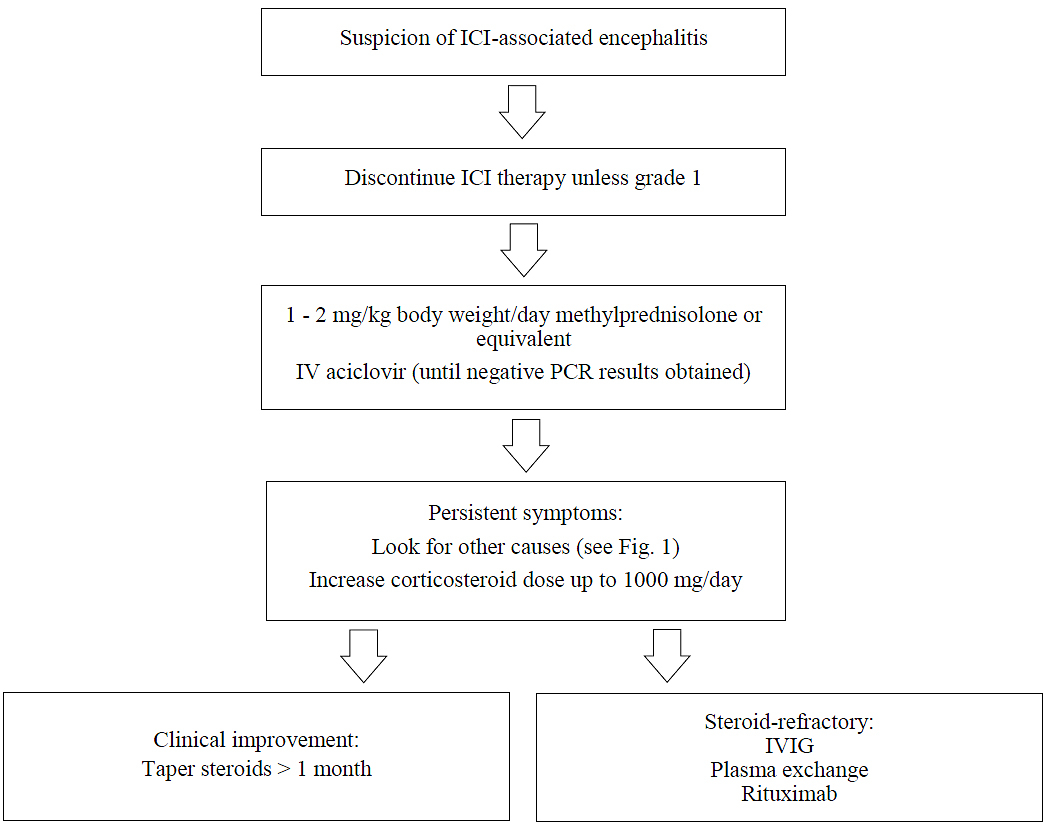
Figure 2 Management of ICI-associated encephalitis.
ICI = immune checkpoint inhibitor; ICU = intensive care unit; IV = intravenous; IVIG = intravenous immunoglobulins; PCR = polymerase chain reaction
Prognosis
Most patients with ICI-associated encephalitis respond to intravenous corticosteroids. Due to early diagnosis and appropriate management, the majority of patients (82%) included in this review improved significantly. However, nine patients (18%) died during their hospital stay.
Limitations of the study
The study design is descriptive and retrospective. As there are no prospective randomised controlled trials examining the diagnosis and therapy of ICI-associated encephalitis, the current data provide the best available basis for treatment recommendations. Prospective studies are needed to identify the optimal treatment.
Implications for further research
New indications for ICI therapy and combinations with other therapeutic strategies (e.g., chemotherapy) are in preclinical and clinical testing. The incidence of and risk factors for ICI-associated encephalitis will be reported accordingly. To date, ICI encephalitis is a diagnosis of exclusion. However, we propose that the diagnosis can be made after clinical evaluation and performing basic laboratory analysis, MRI and EEG. Expanded testing for rare autoimmune diseases of the brain should be reserved for therapy-refractory cases.
It would be interesting to study whether the concomitant administration of ICIs and corticosteroids could prevent the development of encephalitis. It remains unclear whether the presence of paraneoplastic antibodies is a risk factor for ICI-associated encephalitis, whether the antibodies are possibly induced by ICI therapy, or whether they lead to an alternative diagnosis, e.g., paraneoplastic limbic encephalitis. Further studies are needed to determine the role of paraneoplastic antibodies in ICI encephalitis.
Conclusions
The use of ICIs for multiple tumour types is increasing. In patients treated with ICIs presenting with rapidly evolving confusion, encephalitis should be suspected. Blood tests, CSF analysis, cerebral MRI and an EEG should be ordered. The treatment of choice is intravenous corticosteroids. Steroid unresponsiveness is rare and should lead to a review of the diagnosis. Alternative treatment options are IVIG, plasma exchange therapy and rituximab.
Appendix 1 Descriptions of the four unpublished cases
Case 1
A 74-year-old woman with pulmonary adenocarcinoma (cT2a, Nx, cM1c, stage IVB, no brain metastases) presented to the emergency room with sudden onset of fever, confusion and a reduced level of consciousness. First-line therapy with carboplatin, pemetrexed and pembrolizumab was established 17 days before. Due to elevated inflammatory markers and opacities in a chest X-ray, pneumonia was suspected for which antibiotic treatment was started. The patient’s condition continued to deteriorate. Computerised tomography (CT) of the brain, chest and abdomen did not show any signs of infection. Blood cultures were negative. Lumbar puncture revealed leucocytosis (85% monocytes) and increased values of protein (589 mg/l, normal value 150–450 mg/l), glucose (4.1 mmol/l, normal value 2.2–3.9 mmol/l) and lactate (2.9 mmol/l, normal value 1.2–2.1 mmol/L). PCR assays for herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and varicella zoster virus (VZV) were negative. Serological tests showed a positive IgG result and negative IgM result for tick-borne encephalitis (TBE), which was due to a vaccination. Meningoencephalitis was suspected, and the patient was transferred to the intensive care unit (ICU) with a reduced Glasgow Coma Scale (GCS) of 11. Since antibiotic and antiviral treatment (ceftriaxone, amoxicillin, metronidazole, and aciclovir) did not improve the patient’s condition, an electroencephalogram (EEG) was obtained. The EEG showed focal findings in the parieto-temporal region, suggesting encephalopathy. An MRI scan of the brain did not show any signs of encephalitis or meningitis. Nevertheless, a diagnosis of immune-mediated encephalitis due to cancer treatment with pembrolizumab was established. Antimicrobial therapy was stopped, and the patient was treated with intravenous steroids (methylprednisolone, 125 mg daily) and intravenous immunoglobulins (IVIG, 30 g/300 ml). The patient’s condition improved rapidly within five days in the ICU, and she was transferred back to the ward. The corticosteroid therapy was changed to oral prednisone 50 mg daily, followed by a steroid taper over several weeks. The patient was discharged from the hospital after 17 days.
Case 2
A 58-year-old woman with stage IV carcinosarcoma of the uterus received third-line therapy with lenvatinib and pembrolizumab. She was previously treated with carboplatin, paclitaxel and bevacizumab well as with hormonal therapy with medroxyprogesterone acetate and later tamoxifen. A solitary brain metastasis was treated with radiosurgery. She developed diffuse pain, fever, aphasia, agitation and confusion 13 days after starting the new therapeutic regimen with pembrolizumab. She was intubated and transferred to the intensive care unit. A CT scan of the brain did not show signs of intracerebral haemorrhage or another new pathology. Neurology consultation was obtained. Herpes simplex encephalitis or limbic encephalitis were suspected, and treatment with aciclovir and methylprednisolone (80 mg IV) was established. Furthermore, the dose of her seizure prophylaxis with levetiracetam was increased. A brain MRI, electroencephalogram (EEG) and analysis of CSF were not performed. The patient’s condition promptly improved with steroid treatment, and she was transferred back to the ward after 3 days. Due to further progression of the carcinosarcoma with infiltration of the ureters with consecutive severe renal failure, further cancer treatment was stopped, and the regimen changed to palliation. The patient died a couple of days later.
Case 3
A 63-year-old woman with advanced pulmonary adenocarcinoma (cT1, cN2, M1c, UICC stage IV without brain metastases) that had progressed despite chemotherapy with carboplatin and pemetrexed was treated with nivolumab. The patient developed muscle weakness and dysarthria after approximately 10 months. She was admitted to the emergency room with a reduced level of consciousness but without fever. An MRI scan of the brain that was performed in the rehabilitation clinic prior to hospitalisation did not reveal signs of ischaemia, encephalitis or meningitis. An electroneuromyography (ENMG) study did not show signs of myositis or demyelinating polyneuropathy. An EEG was not obtained, and CSF was not analysed. Clinically, autoimmune encephalitis was suspected, and the patient received intravenous steroids (125 mg of methylprednisolone daily), which led to a clear improvement of her condition. Steroid treatment was changed to oral prednisone, and the patient was discharged home after 11 days in the inpatient ward with a steroid taper regimen.
Case 4
A 79-year-old man with stage IV Merkel-cell carcinoma was brought to the emergency room with aphasia, visual disturbance and memory loss. There was no sign of intracerebral haemorrhage, aneurysm or vascular stenosis in the CT scan of the brain. Brain MRI did not detect encephalitis, hypophysitis, or ischaemia. An EEG showed mild background slowing with moderate focal slowing over the left temporal region but no epileptiform activity. CSF analysis revealed an increased cell count (83/μl, normal value <5/μl) with 90% monocytes, an increased level of protein (1065 mg/l, normal value 150–450 mg/l) and a normal concentration of glucose (2.7 mmol/l, normal value 2.2–3.9 mmol/l). The patient was treated with fourth-line therapy with ipilimumab and nivolumab, which was started 12 days prior to admission. Previously, he received cisplatin/etoposide, avelumab and talimogene laherparepvec. Due to a clinical diagnosis of encephalitis, intravenous immunosuppressive therapy with 125 mg methylprednisolone was initiated, and the patient was transferred to the ICU. Additionally, the patient received aciclovir until the PCR assays for HSV-1, HSV-2 and VZV were negative. Given the rapid improvement of his clinical condition, intravenous methylprednisolone was changed to oral prednisone and tapered over a few weeks. The patient was discharged from the ICU and the hospital after three and six days, respectively.
References
1
Brown
MP
,
Hissaria
P
,
Hsieh
AH
,
Kneebone
C
,
Vallat
W
. Autoimmune limbic encephalitis with anti-contactin-associated protein-like 2 antibody secondary to pembrolizumab therapy. J Neuroimmunol. 2017;305:16–8. doi:.https://doi.org/10.1016/j.jneuroim.2016.12.016
2
Cuzzubbo
S
,
Javeri
F
,
Tissier
M
,
Roumi
A
,
Barlog
C
,
Doridam
J
, et al.
Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer. 2017;73:1–8. doi:.https://doi.org/10.1016/j.ejca.2016.12.001
3
Astaras
C
,
de Micheli
R
,
Moura
B
,
Hundsberger
T
,
Hottinger
AF
. Neurological adverse events associated with immune checkpoint inhibitors: diagnosis and management. Curr Neurol Neurosci Rep. 2018;18(1):3. doi:.https://doi.org/10.1007/s11910-018-0810-1
4
Larkin
J
,
Chmielowski
B
,
Lao
CD
,
Hodi
FS
,
Sharfman
W
,
Weber
J
, et al.
Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017;22(6):709–18. doi:.https://doi.org/10.1634/theoncologist.2016-0487
5
Gandhi
L
,
Rodríguez-Abreu
D
,
Gadgeel
S
,
Esteban
E
,
Felip
E
,
De Angelis
F
, et al.; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. doi:.https://doi.org/10.1056/NEJMoa1801005
6
Schneider
S
,
Potthast
S
,
Komminoth
P
,
Schwegler
G
,
Böhm
S
. PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep Oncol. 2017;10(2):473–8. doi:.https://doi.org/10.1159/000477162
7
Bossart
S
,
Thurneysen
S
,
Rushing
E
,
Frontzek
K
,
Leske
H
,
Mihic-Probst
D
, et al.
Case report: encephalitis, with brainstem involvement, following checkpoint inhibitor therapy in metastatic melanoma. Oncologist. 2017;22(6):749–53. doi:.https://doi.org/10.1634/theoncologist.2016-0366
8
Shah
S
,
Dunn-Pirio
A
,
Luedke
M
,
Morgenlander
J
,
Skeen
M
,
Eckstein
C
. Nivolumab-induced autoimmune encephalitis in two patients with lung adenocarcinoma. Case Rep Neurol Med. 2018;2018:2548528. doi:.https://doi.org/10.1155/2018/2548528
9
Williams
TJ
,
Benavides
DR
,
Patrice
K-A
,
Dalmau
JO
,
de Ávila
ALR
,
Le
DT
, et al.
Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73(8):928–33. doi:.https://doi.org/10.1001/jamaneurol.2016.1399
10
Niki
M
,
Nakaya
A
,
Kurata
T
,
Nakahama
K
,
Yoshioka
H
,
Kaneda
T
, et al.
Pembrolizumab-induced autoimmune encephalitis in a patient with advanced non-small cell lung cancer: A case report. Mol Clin Oncol. 2019;10(2):267–9.
11Cook C, McKown AC, Brummel NE. A Rare Case Of Pembrolizumab-Associated Autoimmune Limbic Encephalitis Following Treatment Of Malignant Melanoma. A58 Critical Care Case Reports: Neuro-Critical Care: American Thoracic Society; 2017. p. A2005-A.
12
Kim
A
,
Keam
B
,
Cheun
H
,
Lee
S-T
,
Gook
HS
,
Han
M-K
. Immune-Checkpoint-Inhibitor-Induced Severe Autoimmune Encephalitis Treated by Steroid and Intravenous Immunoglobulin. J Clin Neurol. 2019;15(2):259–61. doi:.https://doi.org/10.3988/jcn.2019.15.2.259
13
Mandel
JJ
,
Olar
A
,
Aldape
KD
,
Tremont-Lukats
IW
. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci. 2014;344(1-2):229–31. doi:.https://doi.org/10.1016/j.jns.2014.06.023
14
Laserna
A
,
Tummala
S
,
Patel
N
,
El Hamouda
DEM
,
Gutiérrez
C
. Atezolizumab-related encephalitis in the intensive care unit: Case report and review of the literature. SAGE Open Med Case Rep. 2018;6:X18792422. doi:.https://doi.org/10.1177/2050313X18792422
15
Levine
JJ
,
Somer
RA
,
Hosoya
H
,
Squillante
C
. Atezolizumab-induced encephalitis in metastatic bladder cancer: a case report and review of the literature. Clin Genitourin Cancer. 2017;15(5):e847–9. doi:.https://doi.org/10.1016/j.clgc.2017.03.001
16
Arakawa
M
,
Yamazaki
M
,
Toda
Y
,
Saito
R
,
Ozawa
A
,
Kosaihira
S
, et al.
Atezolizumab-induced encephalitis in metastatic lung cancer: a case report and literature review. eNeurologicalSci. 2019;14:49–50. doi:.https://doi.org/10.1016/j.ensci.2018.12.001
17
Salam
S
,
Lavin
T
,
Turan
A
. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep. 2016;2016:bcr2016215012. doi:.https://doi.org/10.1136/bcr-2016-215012
18
Ito
M
,
Fujiwara
S
,
Fujimoto
D
,
Mori
R
,
Yoshimura
H
,
Hata
A
, et al.
Rituximab for nivolumab plus ipilimumab-induced encephalitis in a small-cell lung cancer patient. Ann Oncol. 2017;28(9):2318–9. doi:.https://doi.org/10.1093/annonc/mdx252
19
Conry
RM
,
Sullivan
JC
,
Nabors
LB, 3rd
. Ipilimumab-induced encephalopathy with a reversible splenial lesion. Cancer Immunol Res. 2015;3(6):598–601. doi:.https://doi.org/10.1158/2326-6066.CIR-15-0035
20
Burke
M
,
Hardesty
M
,
Downs
W
. A case of severe encephalitis while on PD-1 immunotherapy for recurrent clear cell ovarian cancer. Gynecol Oncol Rep. 2018;24:51–3. doi:.https://doi.org/10.1016/j.gore.2018.03.007
21
Boyd
K
,
Kalladka
D
,
Overell
J
,
Waterston
A
. Ipilimumab induced encephalitis: a case report. Immunome Res. 2015;11(2):1.
22
Voskens
CJ
,
Goldinger
SM
,
Loquai
C
,
Robert
C
,
Kaehler
KC
,
Berking
C
, et al.
The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:.https://doi.org/10.1371/journal.pone.0053745
23
Cao
Y
,
Nylander
A
,
Ramanan
S
,
Goods
BA
,
Ponath
G
,
Zabad
R
, et al.
CNS demyelination and enhanced myelin-reactive responses after ipilimumab treatment. Neurology. 2016;86(16):1553–6. doi:.https://doi.org/10.1212/WNL.0000000000002594
24
Khoja
L
,
Maurice
C
,
Chappell
M
,
MacMillan
L
,
Al-Habeeb
AS
,
Al-Faraidy
N
, et al.
Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res. 2016;4(3):175–8. doi:.https://doi.org/10.1158/2326-6066.CIR-15-0186
25
Kazandjian
D
,
Suzman
DL
,
Blumenthal
G
,
Mushti
S
,
He
K
,
Libeg
M
, et al.
FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–42. doi:.https://doi.org/10.1634/theoncologist.2015-0507
26
Carl
D
,
Grüllich
C
,
Hering
S
,
Schabet
M
. Steroid responsive encephalopathy associated with autoimmune thyroiditis following ipilimumab therapy: a case report. BMC Res Notes. 2015;8(1):316. doi:.https://doi.org/10.1186/s13104-015-1283-9
27
Richard
K
,
Weslow
J
,
Porcella
SL
,
Nanjappa
S
. A case report of steroid responsive nivolumab-induced encephalitis. Cancer Contr. 2017;24(5):1073274817729069. doi:.https://doi.org/10.1177/1073274817729069
28
Matsuoka
H
,
Kimura
H
,
Koba
H
,
Tambo
Y
,
Ohkura
N
,
Hara
J
, et al.
Nivolumab-induced limbic encephalitis with anti-Hu antibody in a patient with advanced pleomorphic carcinoma of the lung. Clin Lung Cancer. 2018;19(5):e597–9. doi:.https://doi.org/10.1016/j.cllc.2018.04.009
29
Feng
S
,
Coward
J
,
McCaffrey
E
,
Coucher
J
,
Kalokerinos
P
,
O’Byrne
K
. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol. 2017;12(11):1626–35. doi:.https://doi.org/10.1016/j.jtho.2017.08.007
30
Strik
H
,
Keber
U
,
Hammoud
WA
,
Riera-Knorrenschild
J
,
Carl
B
,
Dodel
R
, et al.
Immune checkpoint inhibitor-associated CNS autoimmune disorder (ICICAD) following nivolumab treatment: A new entity of drug-induced autoimmune encephalitis?
Eur J Cancer. 2017;87:205–8. doi:.https://doi.org/10.1016/j.ejca.2017.09.026
31
Chaucer
B
,
Stone
A
,
Demanes
A
,
Seibert
SM
. Nivolumab-Induced Encephalitis in Hereditary Leiomyomatosis and Renal Cell Cancer Syndrome. Case Rep Oncol Med. 2018;2018:4273231. doi:.https://doi.org/10.1155/2018/4273231
32
Leitinger
M
,
Varosanec
MV
,
Pikija
S
,
Wass
RE
,
Bandke
D
,
Weis
S
, et al.
Fatal necrotizing encephalopathy after treatment with nivolumab for squamous non-small cell lung cancer: case report and review of the literature. Front Immunol. 2018;9:108. doi:.https://doi.org/10.3389/fimmu.2018.00108
33
Zurko
J
,
Mehta
A
. Association of immune-mediated cerebellitis with immune checkpoint inhibitor therapy. Mayo Clin Proc Innov Qual Outcomes. 2018;2(1):74–7. doi:.https://doi.org/10.1016/j.mayocpiqo.2017.12.001
34
Kopecký
J
,
Kubeček
O
,
Geryk
T
,
Slováčková
B
,
Hoffmann
P
,
Žiaran
M
, et al.
Nivolumab induced encephalopathy in a man with metastatic renal cell cancer: a case report. J Med Case Reports. 2018;12(1):262. doi:.https://doi.org/10.1186/s13256-018-1786-9
35
De la Hoz
A
,
Foolad
F
,
Gallegos
C
,
Kornblau
S
,
Kontoyiannis
DP
. Nivolumab-induced encephalitis post allogeneic stem cell transplant in a patient with Hodgkin’s disease. Bone Marrow Transplant. 2019;54(5):749–51. doi:.https://doi.org/10.1038/s41409-018-0363-6
36
Shibaki
R
,
Murakami
S
,
Oki
K
,
Ohe
Y
. Nivolumab-induced autoimmune encephalitis in an anti-neuronal autoantibody-positive patient. Jpn J Clin Oncol. 2019;49(8):793–4. doi:.https://doi.org/10.1093/jjco/hyz087
37
Zafar
Z
,
Vogler
C
,
Hudali
T
,
Bhattarai
M
. Nivolumab-Associated Acute Demyelinating Encephalitis: A Case Report and Literature Review. Clin Med Res. 2019;17(1-2):29–33. doi:.https://doi.org/10.3121/cmr.2019.1417
38
Gill
A
,
Perez
MA
,
Perrone
CM
,
Bae
CJ
,
Pruitt
AA
,
Lancaster
E
. A case series of PD-1 inhibitor-associated paraneoplastic neurologic syndromes. J Neuroimmunol. 2019;334:576980. doi:.https://doi.org/10.1016/j.jneuroim.2019.576980
39
Hottinger
AF
,
de Micheli
R
,
Guido
V
,
Karampera
A
,
Hagmann
P
,
Du Pasquier
R
. Natalizumab may control immune checkpoint inhibitor-induced limbic encephalitis. Neurol Neuroimmunol Neuroinflamm. 2018;5(2):e439. doi:.https://doi.org/10.1212/NXI.0000000000000439
40
Buttgereit
F
,
da Silva
JA
,
Boers
M
,
Burmester
GR
,
Cutolo
M
,
Jacobs
J
, et al.
Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61(8):718–22. doi:.https://doi.org/10.1136/ard.61.8.718
41
Robert
C
,
Schachter
J
,
Long
GV
,
Arance
A
,
Grob
JJ
,
Mortier
L
, et al.; KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. doi:.https://doi.org/10.1056/NEJMoa1503093
42
Larkin
J
,
Chiarion-Sileni
V
,
Gonzalez
R
,
Grob
JJ
,
Cowey
CL
,
Lao
CD
, et al.
Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:.https://doi.org/10.1056/NEJMoa1504030
43
Wolchok
JD
,
Chiarion-Sileni
V
,
Gonzalez
R
,
Rutkowski
P
,
Grob
J-J
,
Cowey
CL
, et al.
Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–56. doi:.https://doi.org/10.1056/NEJMoa1709684
44
Gozzard
P
,
Maddison
P
. Which antibody and which cancer in which paraneoplastic syndromes?
Pract Neurol. 2010;10(5):260–70. doi:.https://doi.org/10.1136/jnnp.2010.224105
45
Connolly
C
,
Bambhania
K
,
Naidoo
J
. Immune-Related Adverse Events: A Case-Based Approach. Front Oncol. 2019;9:530. doi:.https://doi.org/10.3389/fonc.2019.00530
46Oncology NCPGi. Management of Immunotherapy‐Related Toxicities, Version 1.2020. 2019 December 16. Available at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf.
47
Brahmer
JR
,
Lacchetti
C
,
Schneider
BJ
,
Atkins
MB
,
Brassil
KJ
,
Caterino
JM
, et al.; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68. doi:.https://doi.org/10.1200/JCO.2017.77.6385
48
Gresa-Arribas
N
,
Titulaer
MJ
,
Torrents
A
,
Aguilar
E
,
McCracken
L
,
Leypoldt
F
, et al.
Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167–77. doi:.https://doi.org/10.1016/S1474-4422(13)70282-5
49
Postow
MA
,
Sidlow
R
,
Hellmann
MD
. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68. doi:.https://doi.org/10.1056/NEJMra1703481
50
Haanen
J
,
Carbonnel
F
,
Robert
C
,
Kerr
K
,
Peters
S
,
Larkin
J
, et al.
Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–42. doi:.https://doi.org/10.1093/annonc/mdx225
51
Hottinger
AF
. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29(6):806–12. doi:.https://doi.org/10.1097/WCO.0000000000000391

