Electrolyte disorders in stable renal allograft recipients
DOI: https://doi.org/10.4414/smw.2020.20366
Georg
Beilhacka, Gregor
Lindnerb, Georg-Christian
Funkc, Rossella
Montefortea, Christoph
Schwarzd
aDepartment of Nephrology and Dialysis,
bDepartment of Internal and Emergency Medicine, Buergerspital Solothurn,
cKarl-Landsteiner-Institute for Lung Research and Pulmonary Oncology, Wilheminenspital,
dDepartment of Internal Medicine I, Pyhrn-Eisenwurzenklinikum,
Electrolyte disorders in stable renal allograft recipients
Summary
BACKGROUND
Acid base and electrolyte disorders are frequently reported in the early period after renal transplantation. No comprehensive data exist on the prevalence and patterns of, and contributing factors to, electrolyte disturbances in patients with stable long-term allograft function.
METHODS
We analysed 576 renal transplant recipients (serum creatinine level <2.0 mg/dl) in a cross-sectional study to evaluate the prevalence of electrolyte disorders and the risk factors associated with their occurrence.
RESULTS
A total of 369 patients (64%) of all allograft recipients (n = 576) showed at least one electrolyte and acid base disorder. The most abundant disorder was hypomagnesaemia (25%, n = 143), followed by hyperkalaemia (12.8%, n = 74), hypercalcaemia (12%, n = 69), hypophosphataemia (11.6%, n = 67), metabolic acidosis (11.1%, n = 61) and hyponatraemia (9%, n = 52). All other electrolyte disorders were rare (<6%). In most cases the electrolyte disorders could be classified as mild. Forty percent of the cases had a combined disorder, but without a preferential pattern of combinations. In a multivariate logistic regression analysis, the most important factors contributing significantly to the occurrence of electrolyte disorders were renal function and concomitant medications.
CONCLUSION
Acid base and electrolyte disorders are frequently observed in stable renal allograft recipients, but are usually mild. A combination of two or more electrolyte abnormalities often occurs, although no predominant pattern of a unique combination of electrolyte disorder is recognizable.
Introduction
Normal acid base and electrolyte homeostasis can be managed by a single kidney (i.e. after nephrectomy for organ donation) or by a renal allograft [1–3]. An adequate response of the renin-angiotensin-aldosterone system (RAAS) to different stimuli, as well as the capacity to dilute or concentrate urine, is preserved in stable renal allografts under steroid and azathioprine immunosuppression [4]. Furthermore, potassium, magnesium and phosphate elimination can be maintained with a renal allograft if the glomerular filtration rate is not severely impaired [4]. Earlier studies on renal allograft electrolyte handling were performed in the pre-calcineurin inhibitor era and primarily described the situation in the immediate post-transplant setting. Early after kidney transplantation renal electrolyte handling is often impaired owing to ischaemic tubular damage or persistent secondary hyperparathyroidism, but is generally re-established after 1 year [5]. In the later transplant period different factors such as the immunosuppressive regimen or rejection episodes possibly influence renal acid base and electrolyte handling [6]. Also, concomitant medications were assumed to influence calcium-phosphate, magnesium, potassium and acid base homeostasis [7–9].
Disorders of potassium, magnesium or calcium are of special interest for the clinician since they can result in a variety of adverse events ranging from muscular dysfunction to cardiac arrhythmias [10]. They are further associated with increased mortality [11–13].
Most of the current available studies described the prevalence of single acid base and electrolyte disorders; however, the whole pattern and possible interaction of different disturbances is unknown.
The aim of this study was to describe the prevalence of single and combined electrolyte disorders in a long-term renal transplant population with good excretory allograft function. Additionally, we identified independent risk factors associated with electrolyte disorders.
Subjects and methods
This cross-sectional study was performed at the outpatient clinic of the Department of Nephrology of the Medical University of Vienna between September and December 2004. A single morning fasting blood collection and examination of a 24-hour urine collection was performed on the study day. All allograft recipients who had their transplant for longer than 1 year and with a serum creatinine level <2.0 mg/dl (n = 589) were included in the study. This study design should exclude most of the electrolyte disorders, which are only seen in the first months after transplantation as a result of acute tubular necrosis or high levels of parathormone (PTH). Exclusion criteria were: acute graft rejection (≥ type I, according to the Banff classification) during the past 6 months, chronic diarrhoea (n = 2) or urine draining via a urinary diversion (n = 3). Five patients had to be excluded from the study because of the inability to collect urine over 24 hours. In total, data of 576 patients were available for this analysis. Thirty-two patients were not available for the analysis of combined acid base and electrolyte disorders because of incomplete data.
Patients were screened for acid base and electrolyte disorders by simultaneous measurement of serum (sodium, potassium, phosphate, magnesium, calcium, pH, bicarbonate) and urine (sodium, potassium, phosphate, calcium, pH, volume, protein) parameters. Analysis of magnesium in urine was not available. The blood sample was obtained from a large peripheral vein without use of a tourniquet. Intact serum parathormone (iPTH) levels, and tacrolimus, ciclosporin or rapamycin trough levels were measured additionally. Urine collection was started on the previous day and was continued for 24 hours until the day of examination. In the morning, the collected urine was mixed and a specimen was taken to the study visit. The volume of the urine was measured by the patients themselves and was recorded in 100 ml steps.
Electrolytes in serum and urine were measured by standard laboratory means.
Analysis of metabolic acidosis and renal tubular acidosis in the renal allograft recipients was published earlier [9]. The total urine content of sodium, potassium and calcium was calculated by measuring the concentration of the ions and multiplying by the urine volume. Fractional excretion (Fe) of sodium, potassium and chloride were calculated from laboratory values:
Fe X (%) = (X(urine) × creatinine(Serum)/X(Serum) × creatinine(urine)) × 100
Where X stands for either sodium, potassium or chloride.
We calculated electrolyte free water clearance (EFWC) according to the following formula [14]:
EFWC (l/d) = volume(Urine) ×{1 − (Na + K)Urine/(Na)Serum}
Renal phosphate handling was estimated by calculation of the transport maximum of phosphate in relation to the creatinine clearance (TmP/GFR) if the tubular absorption of phosphate was <80% [15].
TmP/GFR = phosphate(Serum) − (phosphate(urine) × creatinine(serum)/creatinine(urine))
If the tubular resorption was >80% it was calculated by the nomogram of Walton and Bijovet [16]. To examine kidney calcium handling we calculated the urine calcium/creatinine ratio [17].
Excretory renal function was measured by calculation of the glomerular filtration rate (GFR), using the modified modification of diet in renal disease (MDRD) formula [18]:
GFR (ml/min/1.73m2) = 186 × (serum creatinine)(−1.154) × (age)(−0.203) × (0.742, if female).
All patients included in the study were of Caucasian origin. Known limitations of the estimated GFR are lack of precision (random error) and accuracy (systematic error).
Electrolyte disorders were defined according to the normal range references of the laboratory. Also, patients were grouped according to following definitions. Serum sodium (normal range: 135–145 mmol/l), hyponatraemia: serum sodium <136 mmol/l, hypernatraemia: serum sodium >145 mmol/l; serum potassium (normal range: 3.5–5.0 mmol/l), hypokalaemia: serum potassium <3.5 mmol/l, hyperkalaemia: serum potassium >5.0 mmol/l; serum phosphate (normal range: 0.8–1.6 mmol/l), hypophosphataemia: serum phosphate <0.8 mmol/l, hyperphosphataemia: serum phosphate >1.6 mmol/l; serum calcium (normal range: 2.2-2.6 mmol/l), hypocalcaemia: serum calcium <2.2 mmol/l, hypercalcaemia: serum calcium >2.6 mmol/l (mild); serum magnesium (normal range: 0.7–1.0 mmol/l), hypomagnesaemia: serum magnesium <0.7 mmol/l, hypermagnesaemia: serum magnesium >1.0 mmol/l. Metabolic acidosis HCO3 <20 mmol/l; metabolic alkalosis HCO3 >26 mmol/l. Because of the known association between serum chloride and serum bicarbonate, chloride was not included in the study of combined disturbances [19].
The following parameters that might influence the occurrence of electrolyte disorders were examined from the patient reports and used for further analysis: immunosuppressive therapy (ciclosporin, tacrolimus, mycophenolate mofetil, rapamycin, prednisolone and azathioprine), angiotensin converting-enzyme (ACE) inhibitor or angiotensin receptor (AT2) blocker therapy, use of diuretics (furosemide, hydrochlorothiazide), treatment with statins, allopurinol, proton pump inhibitors, beta-blockers, potassium binding agents (sodium polystyrene sulphonate) or potassium, magnesium or calcium supplements, and treatment with vitamin D. Donor age and source, recipient age and sex, time since transplantation, rejection episodes and diagnosis of diabetes were also included in our analysis.
The study was performed according to the STROBE guidelines for observational studies in epidemiology [20].
Since blood and urine samples were part of the routine work up for patients with renal transplants, written informed consent was not required. The study was approved by the local institutional review board (ECS 1828/2013) and performed according to the declaration of Helsinki of 1975 (revised 2000).
Statistical methods
Statistical analyses were performed using SPSS version 15.0 software (SPSS Inc., Chicago, Illinois). Normally distributed scale variables are presented as mean ± standard deviation; non-normally distributed scale variables and ordinal variables are presented as median (1st to 3rd quartiles), categorical data are shown as counts and percentages. The Mann-Whitney U-test was used for comparison of continuous variables. Fisher’s exact test was used to compare categorical variables between two groups. Correlation between ordinal or interval data was determined by Kendall’s rank correlation. A two-sided p-value 0.05 was considered statistically significant.
Multiple logistic regression models were used to determine the independent predictors of the electrolyte disorders. Only the most abundant electrolyte disorders were used in the regression analysis (hypomagnesaemia, hyperkalaemia, hypercalcaemia, hypophosphataemia and hyponatraemia). The following variables were included in the analysis: serum creatinine, GFR-MDRD, sodium, potassium, chloride, calcium, magnesium, bicarbonate, pH, pCO2, ciclosporin, sirolimus and tacrolimus trough-level, iPTH.
Urine 24 hour collection of: sodium, potassium, calcium, phosphate, protein, albumin, volume.
Calculations: fractional excretion of sodium, chloride, potassium, TmP/GFR, urine calcium/creatinine ratio, electrolyte free water clearance.
Comedications: ciclosporin, mycophenolate, prednisolone, tacrolimus, azathioprine, furosemide, thiazide diuretics, ACE or AT2 inhibitor, magnesium, calcium or potassium supplementation, allopurinol, beta-blockers, potassium-binding agents, proton-pump-inhibitors, statins.
Demographics: recipient and donor age, recipient sex, history of diabetes, number or renal transplantations, rejection episodes, donor source, time point of data collection (years after renal transplantation). Only statistically significant factors are shown in table 3.
Presence vs absence of the respective electrolyte disorder was used as the dependent variable. In order to fulfil the normality assumption independent variables were logarithm transformed or dichotomized. We sequentially added covariates to the regression model if they were significant in the univariable tests (p <0.2). The sequence of considered covariates was based upon the strength of the univariable association. Variables remained in the model if they had a significant independent effect (p <0.05). The final model was verified by both forward and backward variable selection algorithms using all the variables that were significant in the univariable tests (p <0.2). Owing to the high quality of the data only few cases had missing data and were excluded from the analysis.
Results
The demographic data of the 576 participants show that our population consisted of mostly long-term transplant recipients with good renal function (78% of the recipients MDRD-GFR 45 ml/min) (table 1). Overall, 89.9% of the patients received a kidney of a deceased donor. Sixty-four percent of the patients (n = 369) had at least one kind of acid base or electrolyte disorder. Hypomagnesaemia was the most common disorder (24.8%, n = 143). Hyperkalaemia (12.8%, n = 74), hypercalcaemia (12%, n = 69) and hypophosphataemia (11.6%, n = 67) were observed with less frequency (table 2). Most of the serum electrolyte deviations from the normal range can be classified as mild (fig. 1).
Table 1 Demographic data of the transplant population.
| Male/female |
342/234 (59%/41%) |
| Time since renal transplantation (y) |
8.2 ± 5.5 |
| CSA/Tac/Rapa (%) |
70/21/6 |
| CSA/Tac/Rapa (n) |
403/119/36 |
| Pred/MMF/AZA (%) |
62/54/20 |
| Pred/MMF/AZA (n) |
360/309/116 |
| No CSA/Tac (n) |
50 |
| Donor living (%) |
10.1 |
| Donor age (y) |
42 ± 15 |
| Recipient age (y) |
55 ± 13 |
| Recipient diabetes (%) |
23 |
| Rejection episode patients (%) |
40.8 |
| Furosemide / hydrochlorothiazide (%) |
12/19 |
| ACE inhibitor or AT2 receptor antagonist (%) |
42 |
| Proton pump inhibitors (%) |
20 |
| First renal transplantation (%) |
82 |
| Renal function (GFR-MDRD) |
|
| – <30 ml/min |
<1% |
| – 30–45 ml/min |
22% |
| – 45–60 ml/min |
37% |
| – >60 ml/min |
41% |
| Proteinuria (%) |
33 |
Table 2 Proportional distribution of acid-base and electrolyte disorders in the study population.
|
Electrolyte disorder (definition)
|
No. cases (%)
|
| Hypomagnesaemia (<0.7 mmol/l) |
143 (25%) |
| Hyperkalaemia (>5.0 mmol/l) |
74 (12.8%) |
| Hypercalcaemia (>2.6 mmol/l) |
69 (12%) |
| Hypophosphataemia (<0.8 mmol/l) |
67 (11.6%) |
| Metabolic acidosis (HCO3 <20 mmol/l) |
61 (11.1%) |
| Hyponatraemia (<136 mmol/l) |
52 (9%) |
| Hypocalcaemia (<2.2 mmol/l) |
32 (5.6%) |
| Metabolic alkalosis (HCO3 >26 mmol/l) |
28 (5.1%) |
| Hypermagnesaemia (>1.0 mmol/l) |
4 (0.7%) |
| Hypokalaemia (<3.5 mmol/l) |
3 (0.5%) |
| Hyperphosphataemia (>1.6 mmol/l) |
2 (0.3%) |

Figure 1 Distribution of serum electrolyte levels. The grey area in each figure represents the normal range of serum electrolytes, except in figure “serum-bicarbonate” the lighter grey marks the range of 22–26 mmol/l, the darker grey the area between 20–26 mmol/l, as used in the study.
Combined acid base and electrolyte disorders
In 544 patients we searched for single or combined acid base and electrolyte disorders (fig. 2). In total, 338 of 544 patients (62%) had at least one disorder; 204 (60%) had a single, 100 (30%) a double and 23 (7%) a triple electrolyte disturbance. Hyponatraemia and metabolic acidosis occurred more often in combination with other disorders (79% and 69%, respectively), whereas hypocalcaemia and hypomagnesaemia were mostly seen as a single disorder (53% and 47%, respectively). As can be seen in figure 2, each specific combination of electrolyte disorders was rare (<3%). The most prevalent combinations were hypomagnesaemia with hyponatraemia, hyperkalaemia with hypercalcaemia, hypophosphataemia with hypomagnesaemia, as well as metabolic acidosis with hypomagnesaemia.
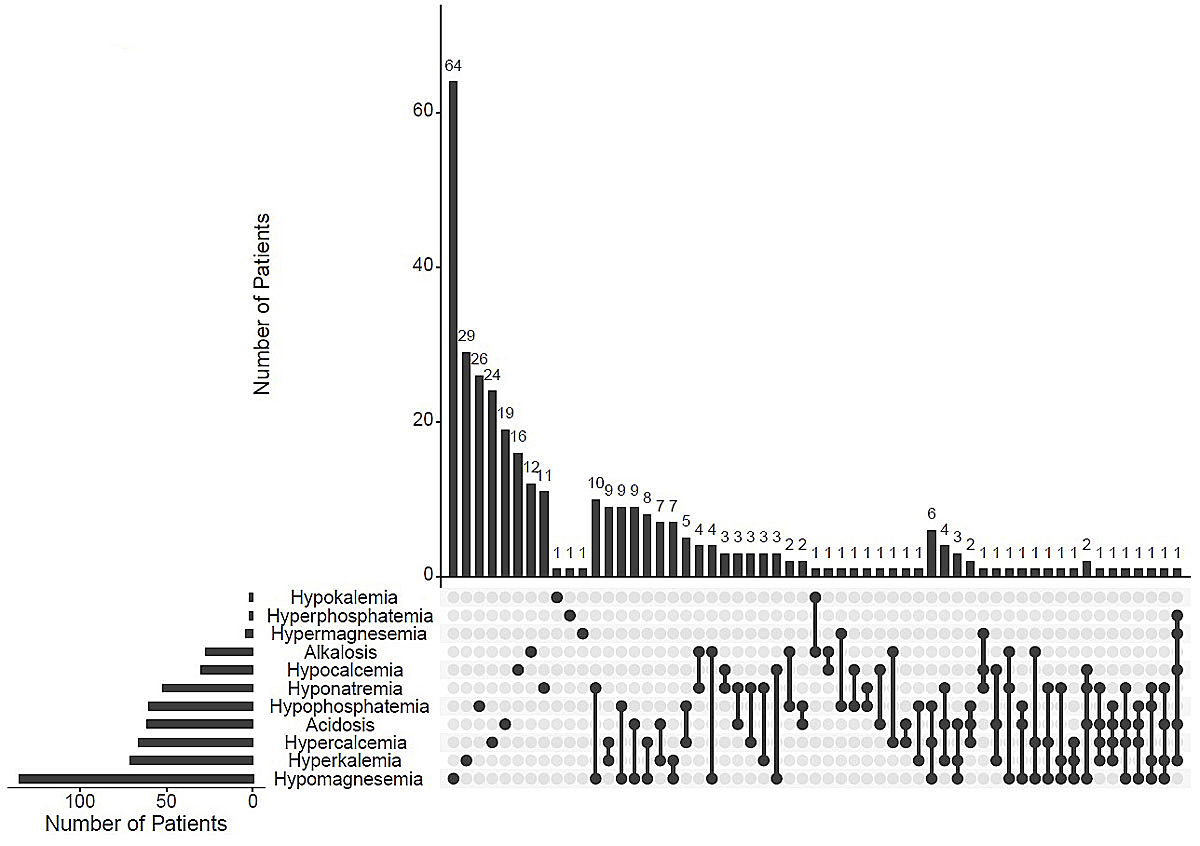
Figure 2 UpSet plot of combinations of acid base and electrolyte disorders. The bar chart on the left displays the total number of patients for each electrolyte disorder. The upper bar chart shows the number of patients with one, two, three or more combinations of acid base and electrolyte disorders. On the lower panel single dark dots indicate a single electrolyte disorder; dark connected dots indicate combinations of two or more disorders.
Weak, but statistically significant, correlations between the serum levels of different electrolytes could be observed. Figure 3 shows the most relevant correlations between different serum electrolytes. The strongest inverse correlation was between serum potassium and serum bicarbonate (r = 0.34, p <0.001). Additionally, serum sodium and potassium (r = −0.14, p = 0.001), as well as serum potassium and serum calcium (r = 0.11, p = 0.001), showed a weak correlation (data not shown).

Figure 3 Significant correlations between serum electrolyte concentrations.
The multivariable logistic regression analysis showed independent predictors for the different single electrolyte disorders in the 576 allograft recipients (table 3):
Table 3 Independent predictors of electrolyte disorder.
| |
Factors
|
OR
|
95% CI
|
p-value
|
Hypomagnesaemia
Serum magnesium <0.7 mmol/l |
Magnesium supplementation |
3.6 |
1.9–6.8 |
<0.01 |
| β-blocker |
2.6 |
1.6–4.1 |
<0.01 |
| Tacrolimus |
6.9 |
4.1–11.6 |
<0.01 |
| GFR-MDRD (ml/min)*
|
3.6 |
2.0–6.6 |
<0.01 |
| Proton pump inhibitor |
1.7 |
1.001–2.9 |
0.05 |
| Serum sodium (mmol/l) |
0.9 |
0.84–0.9 |
0.02 |
| Number of renal transplants |
1.6 |
1.03–2.5 |
0.04 |
| Proteinuria (yes/no) |
1.5 |
1.1–1.9 |
<0.01 |
| Venous pCO2 (mm Hg) |
0.94 |
0.9–0.98 |
<0.01 |
| Allopurinol (yes/no) |
1.7 |
0.9–3.1 |
0.1 |
Hyperkalaemia
Serum potassium >5 mmol/l |
ACE/AT2 inhibitor |
3.9 |
1.7–8.7 |
<0.01 |
| TmP/GFR |
8.6 |
1.6–46 |
0.01 |
| Serum creatinine (mg/dl) |
15.5 |
4.3–56.5 |
<0.01 |
| Serum calcium (mmol/l) |
77 |
6.9–862.1 |
<0.01 |
| 24-h urine calcium (mmol/d)*
|
0.7 |
0.47–0.91 |
0.01 |
| Prednisolone |
0.4 |
0.2–0.9 |
0.02 |
| Proton pump inhibitor |
0.2 |
0.07–0.77 |
0.02 |
| Furosemide (yes/no) |
0.1 |
0.01–0.75 |
0.01 |
| Potassium binder |
13.9 |
3.8–51.5 |
<0.01 |
Hypercalcaemia
Serum calcium >2.65 mmol/l |
Mycophenolate |
2.1 |
1.2–3.8 |
<0.01 |
| Serum phosphate (mmol/l) |
0.1 |
0.03–0.5 |
<0.01 |
| Calcium/creatinine ratio urine (mmol/mmol) |
3 |
1.1–8.6 |
0.04 |
| Serum potassium (mmol/l) |
3 |
1.6–5.6 |
<0.01 |
| Donor source (living) |
0.09 |
0.01–0.7 |
0.02 |
| Magnesium supplementation |
2.1 |
1.1–4.2 |
0.03 |
| Sex (female) |
1.9 |
1.1–3.3 |
0.02 |
Hypophosphataemia
Serum phosphate <0.8 mmol/l |
MDRD-GFR*
|
18.2 |
5.6–58.9 |
<0.01 |
| Serum chloride (mmol/l) |
1.2 |
1.1–1.4 |
<0.01 |
| TmP/GFR†
|
0.07 |
0.02–0.2 |
<0.01 |
| EFWC (ml/d) |
1 |
0.999–1 |
0.53 |
Hyponatraemia
Serum sodium <136 mmol/l |
Serum phosphate (mmol/l) |
6.1 |
1.1–33.2 |
0.03 |
| Serum magnesium* (mmol/l) |
0.008 |
0.001–0.1 |
<0.01 |
| EFWC (ml/d) |
1.001 |
1.00–1.001 |
<0.01 |
| ACE/AT2 inhibitor |
2.9 |
1.3–6.3 |
<0.01 |
| urine pH |
2.2 |
1.4–3.6 |
<0.01 |
| Serum potassium (mmol/l) |
2.2 |
1.05–4.6 |
0.04 |
| Urinary sodium (mmol/d) |
0.995 |
0.991–1 |
0.04 |
Magnesium
From 143 patients with hypomagnesaemia, only 27% (n = 38) received oral magnesium supplementation. In the group with normal serum magnesium levels (n = 433), 34 patients (13%) were treated with magnesium supplements. The use of tacrolimus enhanced the risk of developing hypomagnesaemia significantly. Also, treatment with proton pump inhibitors and beta-blockers were independent predictors of hypomagnesaemia. The risk of hypomagnesaemia rose with lower serum sodium levels (table 3).
Potassium
Low 24-hour urine potassium excretion is a risk factor of mortality in the general population. The mean (± standard deviation) urine sodium-potassium ratio was 3.8 ± 2 and only 15 patients (3%) of the population had a ratio lower than 1.2. Serum potassium levels did not correlate with 24-hour urine potassium excretion in the whole transplant population. In hyperkalaemic allograft recipients the 24-hour urine potassium excretion was similar to that of normokalaemic patients 72 ± 30 vs 67 ± 32 mmol (FeK 13 ± 4% vs 14 ± 7%). The strongest predictors of hyperkalaemia were renal function estimated from serum creatinine and the therapy with inhibitors of the RAAS (table 3). Additionally, hyperkalaemia was associated with reduced renal phosphate excretion (TmP/GFR), higher serum calcium levels and lower urine calcium output (24-hour urine calcium excretion) (table 3). Concomitant medications such as proton pump inhibitors, prednisolone or furosemide were independently associated with a lower risk of hyperkalaemia (table 3). Although only three patients in our study population had hypokalaemia, 14 patients were treated with oral potassium supplements.
Calcium
In long-term allograft recipients a wide range of serum calcium levels, from hypo- to hypercalcaemia could be observed (fig. 1).
All hypercalcaemic patients had inadequate suppression of iPTH (levels >20 pg/ml); and 78% of hypercalcaemic patients had iPTH levels over the upper limit of normal (>65 pg/ml). The use of oral calcium or vitamin D supplementation was not different between patients with hypercalcaemia or with normal serum calcium levels (13% vs 14.3%).
Only 4 of 69 patients with hypercalcaemia showed an elevated urine calcium elimination (defined as urine calcium excretion >7.5 mmol/d), but 25 patients (36%) had hypocalciuria (urine calcium excretion <2.5 mmol/d). Using the urine calcium/creatinine ratio as a parameter of renal calcium handling, hypercalcaemic patients had higher calcium excretion rates than normocalcaemic ones. Also, lower serum phosphate levels were independent predictors for hypercalcaemia (table 3). Mycophenolate treatment and magnesium supplementation doubled the risk for hypercalcaemia. Interestingly, living allograft recipients had a significantly lower prevalence of hypercalcaemia than deceased donor allograft recipients (2% vs 14%, p = 0.02). Furthermore, hypercalcaemia occurred more often in male than in female recipients (15% vs 7%, p = 0.02) and was the only electrolyte disorder differing between men and women.
Hypocalcaemia was a rare electrolyte abnormality (5.6%). Hypocalcaemic patients had higher serum phosphate (1.15 ± 0.2 vs 1.03 ± 0.2 and 0.97 ± 0.2 mmol/l) and TmP/GFR levels (0.89 ± 0.27 vs 0.79 ± 0.2 and 0.72 ± 0.23) compared with normo- and hypercalcaemic recipients (p <0.05). Intact PTH levels did not correlate with serum calcium concentration, and hypocalciuria (<2.5 mmol/d) was very common in the whole study population (55%, n = 293).
Phosphate
Renal phosphate wasting (TmP/GFR <0.8) was the cause for hypophosphataemia in all study patients. A low renal threshold for phosphate and good renal function were the strongest independent predictors of hypophosphataemia (table 3). Additionally, 48% of the patients had impaired renal phosphate handling (TmP/GFR <0.8) without decreased serum phosphate levels. Twenty-five percent of the hypophosphataemic patients had normal iPTH levels.
Sodium
Hyponatraemia was observed in 9% of our transplant population. Higher electrolyte free water clearance and lower urine sodium excretion in 24 hours were associated with an enhanced risk of hyponatraemia. Associated risk factors were higher serum potassium and phosphate levels as well as lower serum magnesium concentration (table 3). Treatment with ACE inhibitors was another independent risk factor for development of hyponatraemia.
Acid base disorders
The details of metabolic acidosis are described previously [9]. Metabolic alkalosis was observed in 5.1% of the recipients. Patients with metabolic alkalosis were older (62 ± 11 vs 49 ± 12 and 55 ± 13 years, p <0.001), had lower serum potassium levels (4.2 ± 0.4 vs 4.8 ± 0.5 and 4.5 ± 0.4 mmol/l, p <0.001) and a higher urine potassium excretion (FeK 19 ± 14 vs 12 ± 5 and 14 ± 6%, p <0.001) compared with patients with metabolic acidosis or without a disturbance of the acid base homeostasis.
Patients without any calcineurin inhibitor therapy (n = 50) had a lower prevalence of hypomagnesaemia, hyperkalaemia and metabolic acidosis compared with calcineurin inhibitor treatment (4% vs 26%; 2% vs 14.1% and 2% vs 11.4%; p <0.01).
Discussion
In this study we describe the prevalence of different electrolyte disorders simultaneously in long-term renal transplant patients with stable allograft function. We found that 40% of the dyselectrolytaemic patients have a combination of two or more electrolyte disorders.
Several studies indicate a great variability in the prevalence of electrolyte disorders in allograft recipients, depending on the time point of evaluation [7, 12, 21, 22]. Recent studies showed an association between serum phosphate, sodium or bicarbonate concentrations in renal allograft recipients and long-term allograft function and mortality, which emphasises that electrolyte disorders have a clinical impact in kidney transplant patients [11–13].
In our study, we confirmed already known risk factors associated with acid base and electrolyte disorders in kidney recipients. The high prevalence of hypomagnesaemia in our transplant population (25%) was also observed by other authors (20%) [23]. Treatment with proton pump inhibitors nearly doubled the risk for hypomagnesaemia in our study (odds ratio 1.7) as decribed by Douwes et al. (odds ratio 2.46) and in a recently published meta-analysis (odds ratio 1.56) [24, 25]. It is hypothesised that proton pump inhibitor-associated hypomagnesaemia after transplantation is mainly due to low intake together with impaired gastrointestinal absorption of magnesium [25]. Tacrolimus impairs renal tubular calcium and magnesium handling, leading to urine calcium and magnesium wasting and hypomagnesaemia [26]. This was confirmed in our study, because tacrolimus treatment was associated with a nearly 7-fold higher risk for developing hypomagnesaemia.
Besides immunosuppressive therapy, other medications contribute to the development of electrolyte disorders: 42% percent of the patients received RAAS inhibitors, which significantly increased the risk of hyperkalaemia and hyponatraemia (odds ratios 3.9 and 2.8, respectively). This finding is in agreement with already published data from transplant patients and the general population [8, 27]. Protective measures against hyperkalaemia are treatment with prednisolone, diuretics and proton pump inhibitors [8]. The use of furosemide 10-fold, proton pump inhibitor 5-fold and prednisolone 2.5-fold reduced the risk of hyperkalaemia in our study.
If good allograft function is established during the first year after engraftment, normalisation of calcium and phosphate homeostasis would be expected. However, a total of 101 (17.5%) patients had a deviation from the normal level of serum calcium or phosphate. This can be explained by different mechanisms. Restoration of hyperparathyroidism is presumed after successful transplantation, but is often incomplete, as described in the literature [28–30]. Only about 35% of our long-term transplanted population had normal iPTH levels.
The prevalence of hypercalcaemia after transplantation varies, depending on the time of examination, from 5% to 50% [31, 32]. In the long-term setting, Evenepoel described a prevalence of hypercalcaemia (12.4%) in patients 48–60 months after engraftment, similar to our data [28]. In the early period after transplantation hypercalcaemia is associated with lower serum phosphate concentration, higher iPTH levels and high urine calcium excretion [29]. In the long term, the correlation with lower serum phosphate and higher urine calcium excretion (only urine calcium creatinine ratio) persists, but no further correlation between PTH and serum calcium levels is observed [32]. However, inadequate suppression of PTH cannot be excluded as the key reason for hypercalcaemia [7]. The low incidence of hypercalciuria and the lower serum phosphate levels supports the hypothesis that high activity of PTH is the cause of hypercalcaemia [33].
Hypophosphataemia is frequently (up to 93%) observed in the early post-transplant period, mainly because of persistent hyperparathyroidism [29, 34]. In concordance with our data, the prevalence of late post-transplant hypophosphataemia has been reported to be about 12% [35]. It is of interest that renal phosphate wasting and hypophosphataemia persists for more than 1 year after transplantation and remains independent of PTH activity. Beside the above-mentioned factors, donor source, dialysis vintage and the severity of pre-transplant hyperparathyroidism were also associated with hypercalcaemia and hypophosphataemia [7].
Additionally, we analysed electrolytes in 24-hour urine collections. Urine sodium and potassium excretion are potential surrogate markers of cardiovascular mortality. Compared with the Swiss Survey on Salt Intake, our transplant population had a higher mean value of the urine sodium/potassium ratio (3.8 ± 2 vs 2.3) [36]. The treatment with calcineurin inhibitors, which can influence renal sodium and potassium handling, could explain this observation [37]. Urine sodium and potassium content in 24-hour urine collections are associated with higher urine phosphate content, but not with higher urine calcium excretion [38]. Using fractional excretion rates, there is no correlation between the different urine electrolyte excretion rates.
Other observations in our study differ from prior studies. Calcineurin inhibitors potentially impair renal acidification mechanisms, potassium elimination, magnesium absorption and renal concentration and dilution mechanisms [5, 39–42]. In contrast to earlier reports, ciclosporin treatment in our study population was not an independent predictor of any electrolyte disorder (e.g., hyperkalaemia), maybe because of the lower ciclosporin trough levels (85 ± 34 ng/ml). Furthermore, we observed a significantly higher urine calcium excretion rate in patients without calcineurin inhibitor therapy (calcium/creatinine ratio 0.57 ± 2.4 vs 0.21 ± 0.21 mmol/mmol) [26].
Although the prevalence of hyponatraemia was similar to other studies, the reasons are different or not obvious [12, 43]. In our study hyponatraemia was associated with RAAS inhibitor treatment (odds ratio 2.8) and not with calcineurin inhibitors as proposed in the literature [21, 43]. Based on our urine analysis, we observed signs of (ineffective) renal counter regulation against hyponatraemia. The significant correlation of hyponatraemia with the use of RAAS inhibitors and impaired urine water excretion (higher vasopressin secretion) could reflect existing heart failure as the reason for hyponatraemia. Additionally, a high water intake together with a disturbance in renal sodium and water handling may predispose some patients to the development of hyponatraemia.
We further analysed patients without calcineurin inhibitor therapy (n = 50). Earlier studies show that immunosuppressive therapy without calcineurin inhibitors had quite different effects on serum electrolytes [40, 44]. In our study patients without inhibition of calcineurin (either ciclosporin or tacrolimus) had lower incidences of hyponatraemia (2%), hyperkalaemia (8%) and metabolic acidosis (2%), supporting the importance of the type of immunosuppression on the pattern of electrolyte changes. Calcineurin inhibitor-free immunosuppression often includes treatment with mechanistic target of rapamycin (mTOR) inhibitors, which are associated with hypokalaemia and hypophosphataemia, particularly in the early post-transplant period [44, 45]. This was not observed in the present study in long-term renal transplant patients. The association between mycophenolate treatment and hypercalcaemia is not described in the literature and could not be explained by our study data [46].
The cross-sectional study design might have some limitations. Donor age was known in only about half of the patients and could have influenced the results. Our study was based on a single measurement of electrolytes and therefore an analysis of the influence of electrolyte disorders on long-term outcomes was not performed. Recipient body weight and urine magnesium concentration were not available. Also, the small sample size and the different time point of sample collection in relation to transplant date may limit the generalisability of the study.
In conclusion, electrolyte disorders in stable renal allograft recipients are frequent, but usually mild. Risk factors associated with these disorders are concomitant treatment as well as kidney function. We showed that in long-term stable kidney graft patients a combination of two or more electrolyte abnormalities often occurs, although no predominant pattern of a unique combination of electrolyte disorder was recognisable. We speculate that polypharmacy together with polymorbidity and renal function are the reason for such heterogeneity of single and combined acid base and electrolyte disorders. Based on the current literature, some of the disorders (i.e., hyponatraemia or metabolic acidosis) are associated with higher mortality and reduced allograft survival. Therefore, screening for dyselectrolytaemia should be recommended in kidney transplant patients. Further studies are necessary to give recommendations on the treatment (i.e., diet) of electrolyte disorders to improve organ and patient survival.
References
1
Bricker
NS
,
Guild
WR
,
Merrill
JP
,
Reardan
JB
. Studies on the functional capacity of a denervated homotransplanted kidney in an identical twin with parallel observations in the donor. J Clin Invest. 1956;35(12):1364–80 .https://doi.org/10.1172/JCI103393
2
Bricker
NS
,
Straffon
RA
,
Mahoney
EP
,
Merrill
JP
. The functional capacity of the kidney denervated by autotransplantation in the dog. J Clin Invest. 1958;37(2):185–93 .https://doi.org/10.1172/JCI103597
3
Coruzzi
P
,
Musiari
L
,
Mossini
GL
,
Ceriati
R
,
Mutti
A
. The renin-aldosterone system and renal function in kidney transplantation. Clin Nephrol. 1994;41(4):225–9.
4
Blaufox
MD
,
Lewis
EJ
,
Jagger
P
,
Lauler
D
,
Hickler
R
,
Merrill
JP
. Physiologic responses of the transplanted human kidney. N Engl J Med. 1969;280(2):62–6 .https://doi.org/10.1056/NEJM196901092800202
5
Heering
P
,
Degenhardt
S
,
Grabensee
B
. Tubular dysfunction following kidney transplantation. Nephron. 1996;74(3):501–11 .https://doi.org/10.1159/000189443
6
Velic
A
,
Hirsch
JR
,
Bartel
J
,
Thomas
R
,
Schröter
R
,
Stegemann
H
, et al.
Renal transplantation modulates expression and function of receptors and transporters of rat proximal tubules. J Am Soc Nephrol. 2004;15(4):967–77 .https://doi.org/10.1097/01.ASN.0000117287.74203.89
7
Pochineni
V
,
Rondon-Berrios
H
. Electrolyte and Acid-Base Disorders in the Renal Transplant Recipient. Front Med (Lausanne). 2018;5:261 .https://doi.org/10.3389/fmed.2018.00261
8
Mitterbauer
C
,
Heinze
G
,
Kainz
A
,
Kramar
R
,
Hörl
WH
,
Oberbauer
R
. ACE-inhibitor or AT2-antagonist therapy of renal transplant recipients is associated with an increase in serum potassium concentrations. Nephrol Dial Transplant. 2008;23(5):1742–6 .https://doi.org/10.1093/ndt/gfm864
9
Schwarz
C
,
Benesch
T
,
Kodras
K
,
Oberbauer
R
,
Haas
M
. Complete renal tubular acidosis late after kidney transplantation. Nephrol Dial Transplant. 2006;21(9):2615–20 .https://doi.org/10.1093/ndt/gfl211
10
Gettes
LS
. Electrolyte abnormalities underlying lethal and ventricular arrhythmias. Circulation. 1992;85(1, Suppl):I70–6.
11
Jeon
HJ
,
Kim
YC
,
Park
S
,
Kim
CT
,
Ha
J
,
Han
DJ
, et al.
Association of Serum Phosphorus Concentration with Mortality and Graft Failure among Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2017;12(4):653–62 .https://doi.org/10.2215/CJN.07090716
12
Han
SS
,
Han
M
,
Park
JY
,
An
JN
,
Park
S
,
Park
S-K
, et al.
Posttransplant Hyponatremia Predicts Graft Failure and Mortality in Kidney Transplantation Recipients: A Multicenter Cohort Study in Korea. PLoS One. 2016;11(5):e0156050 .https://doi.org/10.1371/journal.pone.0156050
13
Park
S
,
Kang
E
,
Park
S
,
Kim
YC
,
Han
SS
,
Ha
J
, et al.
Metabolic Acidosis and Long-Term Clinical Outcomes in Kidney Transplant Recipients. J Am Soc Nephrol. 2017;28(6):1886–97 .https://doi.org/10.1681/ASN.2016070793
14
Lindner
G
,
Schwarz
C
. Electrolyte-free water clearance versus modified electrolyte-free water clearance: do the results justify the effort?
Nephron, Physiol. 2012;120(1):1–5 .https://doi.org/10.1159/000336550
15
Payne
RB
. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35(2):201–6 .https://doi.org/10.1177/000456329803500203
16
Walton
RJ
,
Bijvoet
OL
. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;306(7929):309–10 .https://doi.org/10.1016/S0140-6736(75)92736-1
17
Smalcelj
R
,
Kusec
V
. Gestörte Regulation der Calcium Ausscheidung bei Nierentransplantierten [Impaired regulation of calcium excretion in kidney transplant recipients]. Wien Klin Wochenschr. 2011;123(11-12):334–9 .https://doi.org/10.1007/s00508-011-1575-6
18
Masson
I
,
Flamant
M
,
Maillard
N
,
Rule
AD
,
Vrtovsnik
F
,
Peraldi
M-N
, et al.
MDRD versus CKD-EPI equation to estimate glomerular filtration rate in kidney transplant recipients. Transplantation. 2013;95(10):1211–7 .https://doi.org/10.1097/TP.0b013e318288caa6
19
Havlin
J
,
Matousovic
K
,
Schück
O
. Sodium-Chloride Difference as a Simple Parameter for Acid-Base Status Assessment. Am J Kidney Dis. 2017;69(5):707–8 .https://doi.org/10.1053/j.ajkd.2016.12.019
20
von Elm
E
,
Altman
DG
,
Egger
M
,
Pocock
SJ
,
Gøtzsche
PC
,
Vandenbroucke
JP
; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296 .https://doi.org/10.1371/journal.pmed.0040296
21
Higgins
R
,
Ramaiyan
K
,
Dasgupta
T
,
Kanji
H
,
Fletcher
S
,
Lam
F
, et al.
Hyponatraemia and hyperkalaemia are more frequent in renal transplant recipients treated with tacrolimus than with cyclosporin. Further evidence for differences between cyclosporin and tacrolimus nephrotoxicities. Nephrol Dial Transplant. 2004;19(2):444–50 .https://doi.org/10.1093/ndt/gfg515
22
Van de Cauter
J
,
Sennesael
J
,
Haentjens
P
. Long-term evolution of the mineral metabolism after renal transplantation: a prospective, single-center cohort study. Transplant Proc. 2011;43(9):3470–5 .https://doi.org/10.1016/j.transproceed.2011.09.030
23
Van Laecke
S
,
Van Biesen
W
. Hypomagnesaemia in kidney transplantation. Transplant Rev (Orlando). 2015;29(3):154–60 .https://doi.org/10.1016/j.trre.2015.05.002
24
Boonpheng
B
,
Thongprayoon
C
,
Bathini
T
,
Sharma
K
,
Mao
MA
,
Cheungpasitporn
W
. Proton pump inhibitors and adverse effects in kidney transplant recipients: A meta-analysis. World J Transplant. 2019;9(2):35–47 .https://doi.org/10.5500/wjt.v9.i2.35
25
Douwes
RM
,
Gomes-Neto
AW
,
Schutten
JC
,
van den Berg
E
,
de Borst
MH
,
Berger
SP
, et al.
Proton-Pump Inhibitors and Hypomagnesaemia in Kidney Transplant Recipients. J Clin Med. 2019;8(12):2162 .https://doi.org/10.3390/jcm8122162
26
Nijenhuis
T
,
Hoenderop
JGJ
,
Bindels
RJM
. Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol. 2004;15(3):549–57 .https://doi.org/10.1097/01.ASN.0000113318.56023.B6
27
Palmer
SC
,
Mavridis
D
,
Navarese
E
,
Craig
JC
,
Tonelli
M
,
Salanti
G
, et al.
Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385(9982):2047–56 .https://doi.org/10.1016/S0140-6736(14)62459-4
28
Evenepoel
P
,
Claes
K
,
Kuypers
D
,
Maes
B
,
Bammens
B
,
Vanrenterghem
Y
. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant. 2004;19(5):1281–7 .https://doi.org/10.1093/ndt/gfh128
29
Evenepoel
P
,
Van Den Bergh
B
,
Naesens
M
,
De Jonge
H
,
Bammens
B
,
Claes
K
, et al.
Calcium metabolism in the early posttransplantation period. Clin J Am Soc Nephrol. 2009;4(3):665–72 .https://doi.org/10.2215/CJN.03920808
30
Wolf
M
,
Weir
MR
,
Kopyt
N
,
Mannon
RB
,
Von Visger
J
,
Deng
H
, et al.
A Prospective Cohort Study of Mineral Metabolism After Kidney Transplantation. Transplantation. 2016;100(1):184–93 .https://doi.org/10.1097/TP.0000000000000823
31
Messa
P
,
Cafforio
C
,
Alfieri
C
. Clinical impact of hypercalcemia in kidney transplant. Int J Nephrol. 2011;2011:906832 .https://doi.org/10.4061/2011/906832
32
Reinhardt
W
,
Bartelworth
H
,
Jockenhövel
F
,
Schmidt-Gayk
H
,
Witzke
O
,
Wagner
K
, et al.
Sequential changes of biochemical bone parameters after kidney transplantation. Nephrol Dial Transplant. 1998;13(2):436–42 .https://doi.org/10.1093/oxfordjournals.ndt.a027843
33
Borchhardt
K
,
Sulzbacher
I
,
Benesch
T
,
Födinger
M
,
Sunder-Plassmann
G
,
Haas
M
. Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Transplant. 2007;7(11):2515–21 .https://doi.org/10.1111/j.1600-6143.2007.01950.x
34
Khosroshahi
HT
,
Shoja
MM
,
Azar
SA
,
Tubbs
RS
,
Safa
J
,
Etemadi
J
, et al.
Calcium and phosphorus metabolism in stable renal transplant recipients. Exp Clin Transplant. 2007;5(2):670–2.
35
Evenepoel
P
,
Meijers
BKI
,
de Jonge
H
,
Naesens
M
,
Bammens
B
,
Claes
K
, et al.
Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3(6):1829–36 .https://doi.org/10.2215/CJN.01310308
36
Glatz
N
,
Chappuis
A
,
Conen
D
,
Erne
P
,
Péchère-Bertschi
A
,
Guessous
I
, et al.
Associations of sodium, potassium and protein intake with blood pressure and hypertension in Switzerland. Swiss Med Wkly. 2017;147:w14411.
37
Hoorn
EJ
,
Walsh
SB
,
McCormick
JA
,
Fürstenberg
A
,
Yang
C-L
,
Roeschel
T
, et al.
The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17(10):1304–9 .https://doi.org/10.1038/nm.2497
38
van der Wijst
J
,
Tutakhel
OAZ
,
Bos
C
,
Danser
AHJ
,
Hoorn
EJ
,
Hoenderop
JGJ
, et al.
Effects of a high-sodium/low-potassium diet on renal calcium, magnesium, and phosphate handling. Am J Physiol Renal Physiol. 2018;315(1):F110–22 .https://doi.org/10.1152/ajprenal.00379.2017
39
Bantle
JP
,
Nath
KA
,
Sutherland
DE
,
Najarian
JS
,
Ferris
TF
. Effects of cyclosporine on the renin-angiotensin-aldosterone system and potassium excretion in renal transplant recipients. Arch Intern Med. 1985;145(3):505–8 .https://doi.org/10.1001/archinte.1985.00360030153026
40
Foley
RJ
,
Hamner
RW
,
Weinman
EJ
. Serum potassium concentrations in cyclosporine- and azathioprine-treated renal transplant patients. Nephron. 1985;40(3):280–5 .https://doi.org/10.1159/000183479
41
Heering
PJ
,
Kurschat
C
,
Vo
DT
,
Klein-Vehne
N
,
Fehsel
K
,
Ivens
K
. Aldosterone resistance in kidney transplantation is in part induced by a down-regulation of mineralocorticoid receptor expression. Clin Transplant. 2004;18(2):186–92 .https://doi.org/10.1046/j.1399-0012.2003.00154.x
42
Aguilera
S
,
Deray
G
,
Desjobert
H
,
Benhmida
M
,
Le Hoang
P
,
Jacobs
C
. Effects of cyclosporine on tubular acidification function in patients with idiopathic uveitis. Am J Nephrol. 1992;12(6):425–30 .https://doi.org/10.1159/000168493
43
Musso
CG
,
Castañeda
A
,
Giordani
M
,
Mombelli
C
,
Groppa
S
,
Imperiali
N
, et al.
Hyponatremia in kidney transplant patients: its pathophysiologic mechanisms. Clin Kidney J. 2018;11(4):581–5 .https://doi.org/10.1093/ckj/sfy016
44
Morales
JM
,
Wramner
L
,
Kreis
H
,
Durand
D
,
Campistol
JM
,
Andres
A
, et al.; Sirolimus European Renal Transplant Study Group. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002;2(5):436–42 .https://doi.org/10.1034/j.1600-6143.2002.20507.x
45
Schwarz
C
,
Böhmig
GA
,
Steininger
R
,
Mayer
G
,
Oberbauer
R
. Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol Dial Transplant. 2001;16(2):378–82 .https://doi.org/10.1093/ndt/16.2.378
46
Kamińska
J
,
Sobiak
J
,
Suliburska
JM
,
Duda
G
,
Głyda
M
,
Krejpcio
Z
, et al.
Effect of mycophenolate mofetil on plasma bioelements in renal transplant recipients. Biol Trace Elem Res. 2012;145(2):136–43 .https://doi.org/10.1007/s12011-011-9178-7


