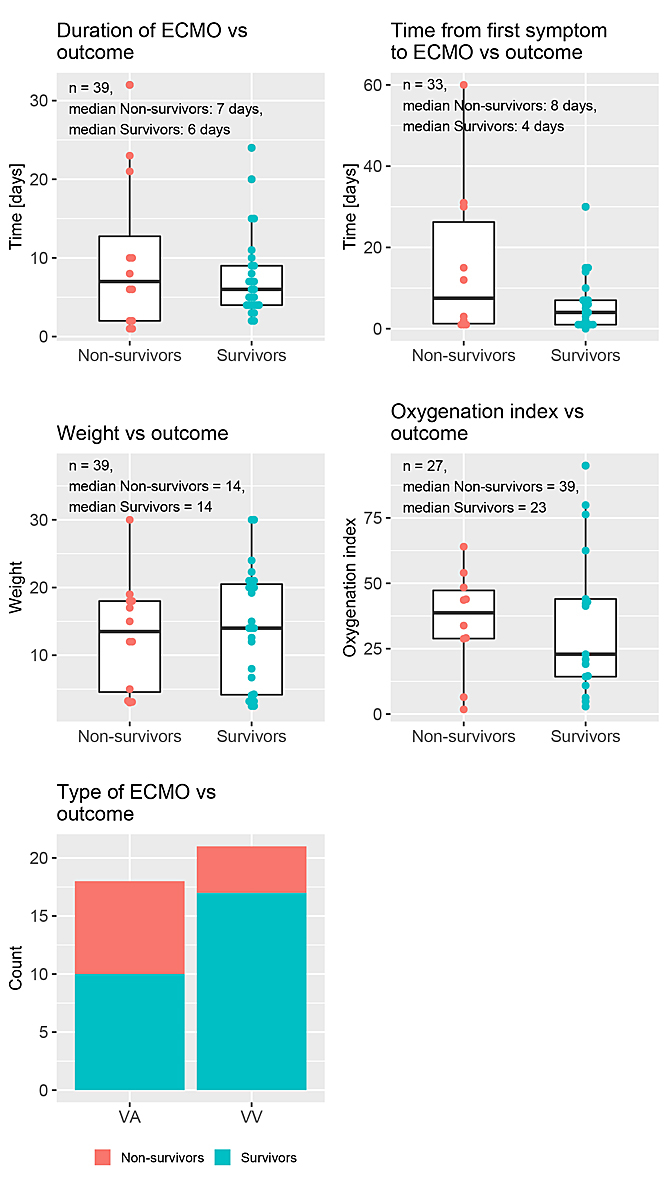
Figure 1 Distribution of the time-independent predictors stratified by outcome.
DOI: https://doi.org/10.4414/smw.2020.20358
Extracorporeal membrane oxygenation (ECMO) is a life-saving tool and a substantial part of every mechanical circulatory support programme. The use of ECMO has grown constantly and substantially [1, 2], with an increasing number of centres and increased applications for its use. Indications include pulmonary and/or cardiac decompensation, elective post-cardiotomy situations and emergency extracorporeal cardiopulmonary resuscitation (ECPR) scenarios. ECMO is a highly invasive therapy with substantial risks of haemorrhagic and thromboembolic complications. The morbidity and mortality associated with ECMO are due to both the native disease processes that lead to ECMO and the complications of ECMO itself. ECMO therapy involves enormous resources, such as a specialised team of trained therapists, necessary space in the intensive care unit, blood products, and the high costs of the machines and disposables. As an efficient life-saving tool, it has become an essential round-the-clock part of every CPR drill. Increased ECMO demand may lead to strain on intensive care resources, occasionally necessitating delays in treatment and the diversion/airlifting of critical patients to distant tertiary centres. Under these circumstances, developing objective parameters which help decision making in order to optimise ECMO availability is imperative [3]. To find biomarkers or a series of values that could determine the use and allocation of ECMO is an ambitious target. However, such studies could stimulate research into thresholds which could guide physicians and care takers in determining prognosis. Serum lactate is one such commonly used marker of tissue perfusion. Elevated lactate levels and the dynamics of lactate washout could provide insights into the recovery of critical patients [3]. Prior studies in neonatal and paediatric patients undergoing cardiac ECMO have shown that elevated lactate (>24–72 hours post-cannulation) is associated with increased mortality [4]. This study evaluated the dynamics of serum lactate and serum pH over time and assessed their possible associations with outcome.
Permission from the Cantonal ethic commission, Zurich for the study of ECMO in children was sought and awarded (No.2019-00184, dated 19 march 2019).
All paediatric patients (0–18 years) who underwent ECMO for pulmonary indications at the University Children’s Hospital Zurich from 2008 to 2018 formed the subject cohort of this exploratory study. This collection included 39 ECMO runs performed in 38 patients. One patient was subjected to two ECMO runs; these were considered as two separate ECMO runs.
The time-independent variables collected included the patient’s age, weight, pre-ECMO oxygenation index (OI), indication for ECMO, type of ECMO (veno-venous [VV] or veno-arterial [VA]), time from the first symptom to ECMO (in days) and duration of ECMO (in days). The time-dependent variables were pH and lactate, which were measured at the beginning of and several times during the ECMO therapy.
The primary outcome was the survival of the patient (survivor/non-survivor) at hospital discharge.
Statistical analysis was performed using the software R (version 3.5.2). Continuous variables were summarised as medians (interquartile range [IQR]), categorical variables were summarised as frequencies (%). The explanatory p-values in table 1 below were computed using the Kruskal-Wallis rank sum test for the continuous (non-normal) variables and Pearson’s chi-square test (with continuity correction) for the categorical variables. Time-independent variables were analysed using univariate and multivariate Firth's logistic regression analyses. The time-independent variables with distributions skewed to the right (time from the first symptom to ECMO and duration of ECMO) were log-transformed to reduce the influence of extreme values. Time-dependent variables were analysed using univariate and multivariate time-dependent Cox proportional hazards regression models. Since lactate had a skewed distribution and pH had a very small standard deviation, we log-transformed these variables to reduce the influence of extreme values and scaled them to avoid unreasonable estimates and standard errors. The proportional hazards assumption was checked by inspecting the Schoenfeld residuals and a test for proportional hazards assumption by Grambsch and Therneau [5]. The jackknife method was used to examine the influence of individual observations on the Cox model estimates. A threshold analysis for lactate was performed to find a cut-off value with which to classify patients as at low or high risk of death. This was done using the 'minimum p-value approach' [6], i.e., we fitted a Cox regression model with Firth’s penalised likelihood. The p-values were corrected for multiple testing using the method described by Schumacher et al. [6]. The estimated hazard ratio (HR) and its confidence interval (CI) were corrected with a heuristic shrinkage factor [7]. As a sensitivity analysis for the heuristic shrinkage factor, we used the unadjusted and adjusted p-values to estimate a shrinkage factor for correcting the HR and its CI. Missing values in the regression analyses were treated with 'complete case analysis'. No adjustment for multiple testing was done, except for the threshold analysis.
The baseline characteristics of the patients stratified by the outcome of interest (survival) are summarised in table 1. Of the 39 ECMO runs in 38 patients, 27 (69%) could be weaned, while 12 runs (31%) in 11 patients could not be weaned. The latter included one patient who had two ECMO runs. Age, weight and duration of ECMO showed similar medians and overlapping IQRs and did not differ between survivors and non-survivors. Figure 1 depicts box and bar plots of various variables stratified by outcome. There were five (12.8%) missing values for the variable “time (1st symptom to ECMO)”, 12 (30.8%) for OI, 14 (35.9%) for height and 14 (35.9%) for hospital stay.
Table 1 Descriptive analysis of the variables.
| Overall | Non-Survivors | Survivors | p-value | |
|---|---|---|---|---|
| N | 39 runs (38 patients) | 11 | 27 | |
| Age (years) | 3.01 (0.17–4.81) |
2.94 (0.19–4.30) |
3.01 (0.17–4.95) |
0.939 |
| Weight (kg) | 14.00 (4.17–20.00) |
13.50 (4.56–18.00) |
14.00 (4.17–20.50) |
0.583 |
| Height (cm) | 100.00 (88.00–117.00) |
95.50 (84.00–117.25) |
102.00 (88.00–114.00) |
0.726 |
| Time from 1st symptom to ECMO (days) | 4.00 (1.00–12.00) |
7.50 (1.25–26.25) |
4.00 (1.00–7.00) |
0.320 |
| Oxygenation index | 33.83 (14.44–46.20) |
38.72 (28.88–47.27) |
22.88 (14.30–44.00) |
0.651 |
| Respiratory indication, n (%) | 0.219 | |||
| – ARDS | 18 (46.2) | 8 (66.7) | 10 (37.0) | |
| – Pneumonia / respiratory syncytial virus / viral infection | 7 (17.9) | 0 (0.0) | 7 (25.9) | |
| – Drowning | 2 (5.1) | 1 (8.3) | 1 (3.7) | |
| – Meconium aspiration | 3 (7.7) | 1 (8.3) | 2 (7.4) | |
| – Pulmonary hypertension | 5 (12.8) | 2 (16.7) | 3 (11.1) | |
| – Others | 4 (10.3) | 0 (0.0) | 4 (14.8) | |
| Type of ECMO, n (%) | 0.172 | |||
| – VV | 21 (53.8) | 4 (33.3) | 17 (63.0) | |
| – VA | 18 (46.2) | 8 (66.7) | 10 (37.0) | |
| Duration of ECMO (days) | 6.00 (4.00–10.00) |
7.00 (2.00–12.75) |
6.00 (4.00–9.00) |
0.795 |
| Lowest pH before ECMO | 7.06 (6.89–7.14) |
7.01 (6.89–7.15) |
7.08 (6.89–7.14) |
0.808 |
| Highest lactate before ECMO | 7.40 (2.50–11.25) |
10.25 (2.12–13.23) |
7.00 (2.85–8.90) |
0.553 |
| Time to normalisation* of pH (hours) | 10.88 (2.65–16.66) |
4.55 (0.68–14.97) |
11.48 (3.12–18.12) |
0.176 |
| Time to normalisation* of lactate (hours) | 12.52 (2.42–30.35) |
18.55 (0.00–24.77) |
11.37 (2.79–32.38) |
0.621 |
| Hospital stay (days) | 26.00 (11.00–40.00) |
11.00 (2.00–32.00) |
34.50 (20.75–44.50) |
0.047 |
ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; VA = veno-arterial; VV = veno-venous All continuous variables are summarised as medians (interquartile range) and all categorical variables are summarised as frequencies (%). The exploratory p-values were computed using the Kruskal-Wallis rank sum test for the continuous (non-normal) variables and Pearson’s chi-square test (with continuity correction) for the categorical variables. * Lactate >2 mmol/l and pH <7.34 were considered abnormal.

Figure 1 Distribution of the time-independent predictors stratified by outcome.
Longitudinal measurements of serum pH and lactate were analysed. The median number of blood measurements for each patient was 96 (IQR 58.0 to 128.2) and the median time between any two measurements was two hours (IQR 0.7 to 2.6). One patient had a gap of more than 12 days between two blood measurements. This gap occurred because the patient had to be airlifted to another tertiary centre after the ECMO implantation because of a lack of intensive care bed capacity. This patient returned after 12 days. We removed the 12-day interval of that patient from the survival analysis to avoid assuming constant measurements for such a long period. Figure 2 depicts the consecutive pH and lactate values of the patients. Survivors are represented by blue dotted lines, non-survivors by red dotted lines. The lactate values ranged from 0.1 to 22 and the pH values from 6.4 to 7.9.
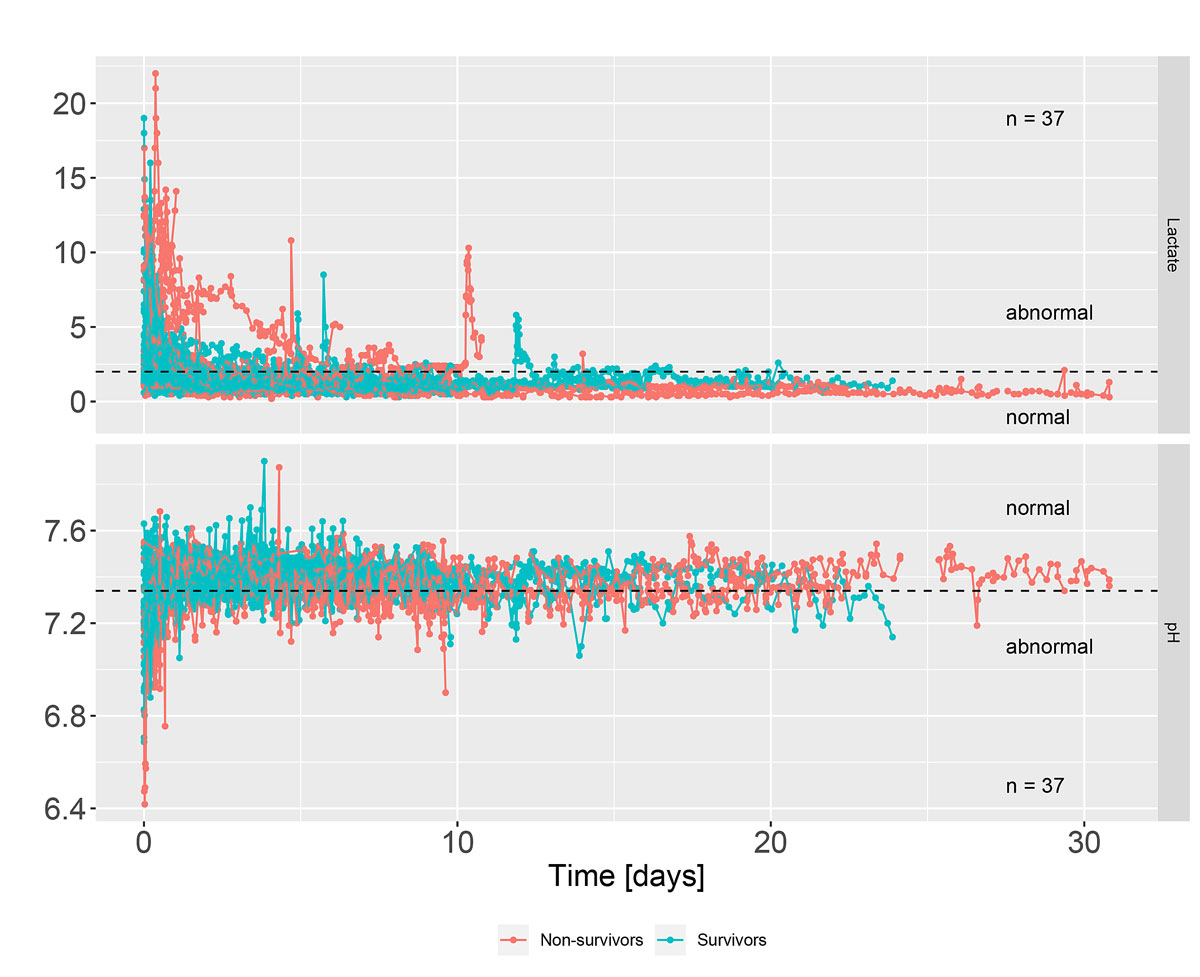
Figure 2 Lactate and pH curves during intensive therapy (survivors / non-survivors). Lactate >2 mmol/l and pH <7.34 were considered abnormal.
The following time-independent variables were included in the univariate analyses to investigate factors associated with survival at discharge:
A multivariate model with time from the first symptom to ECMO and duration of ECMO as explanatory variables found no evidence of an association of these variables with survival (time from the first symptom to ECMO: odds ratio 0.65, 95% CI 0.35 to 1.22, p = 0.18; duration of ECMO: odds ratio 1.71, 95% CI 0.67 to 4.36, p = 0.26).
Longitudinal measurements of serum pH and lactate were included in time-dependent Cox proportional hazards models. The results are shown in figure 3. While the univariate analysis showed weak evidence of an association between log(pH) and survival (HR 0.48, 95% CI 0.22 to 1.02, p = 0.055), this effect vanished when log(lactate) was also included in the model (HR 0.99, 95% CI 0.43 to 2.26, p = 0.98). The univariate model provided very strong evidence of an effect of log(lactate) on survival (HR 4.48, 95% CI 1.92 to 10.48, p = 0.0005). The multivariate model provided strong evidence of an effect of log(lactate) (HR 4.44 (95% CI 1.65 to 11.95, p = 0.003) when log(pH) was also included. The estimated concordance index was 0.646 (standard error [SE] 0.113) for the univariate model with log(pH), 0.817 (SE 0.075) for the univariate model with log(lactate) and 0.825 (SE 0.07) for the multivariate model.
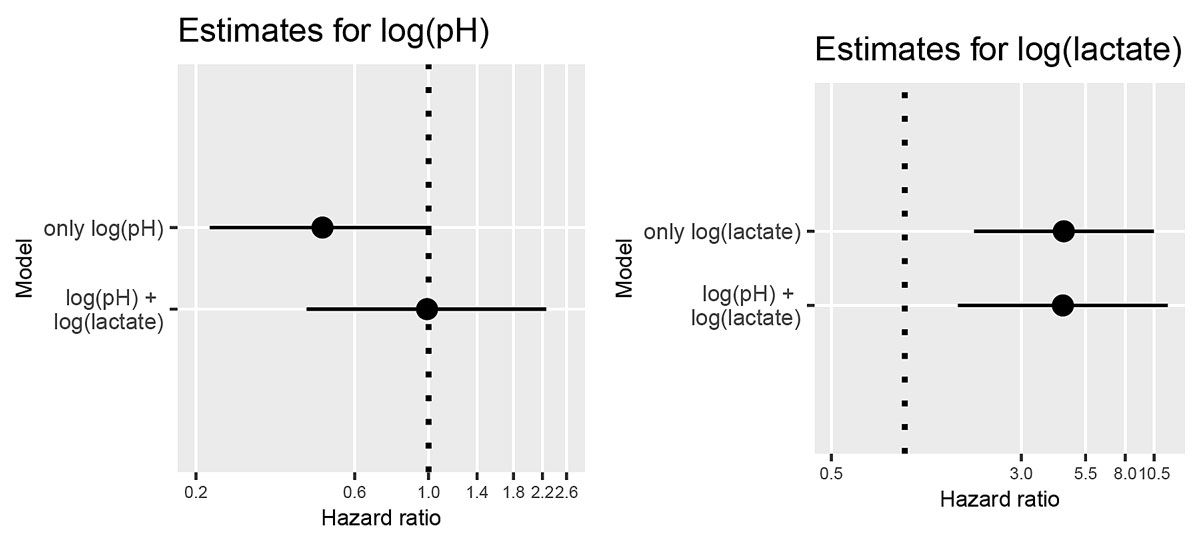
Figure 3 Confidence intervals of estimated hazard ratios of pH and lactate from univariate and multivariate models.
Table 2 depicts the means of the most recent lactate values at different times of survivors and of non-survivors, the differences between these means, the 95% CIs of those differences and the corresponding p-values. The p-values were computed using Welch two sample t-tests. Serum lactate at 24 hours was lower in survivors than in non-survivors (difference 2.78, 95% CI −5.36 to −0.20, p = 0.037). Serum lactate during the first 24 hours of intensive therapy in survivors and non-survivors is shown in figure 4.
Table 2 Evolution of serum lactate during the first 48 hours of intensive therapy.
| Last lactate | Mean, survivors |
Mean,
non-survivors |
Difference | Confidence interval | p-Value |
|---|---|---|---|---|---|
| At 6 hours | 3.55 | 4.50 | −0.95 | −3.53 to 1.63 | 0.448 |
| At 12 hours | 2.50 | 5.02 | −2.52 | −5.07 to 0.04 | 0.053 |
| At 24 hours | 1.80 | 4.58 | −2.78 | −5.36 to −0.20 | 0.037 |
| At 48 hours | 1.45 | 3.68 | −2.23 | −4.78 to 0.33 | 0.081 |
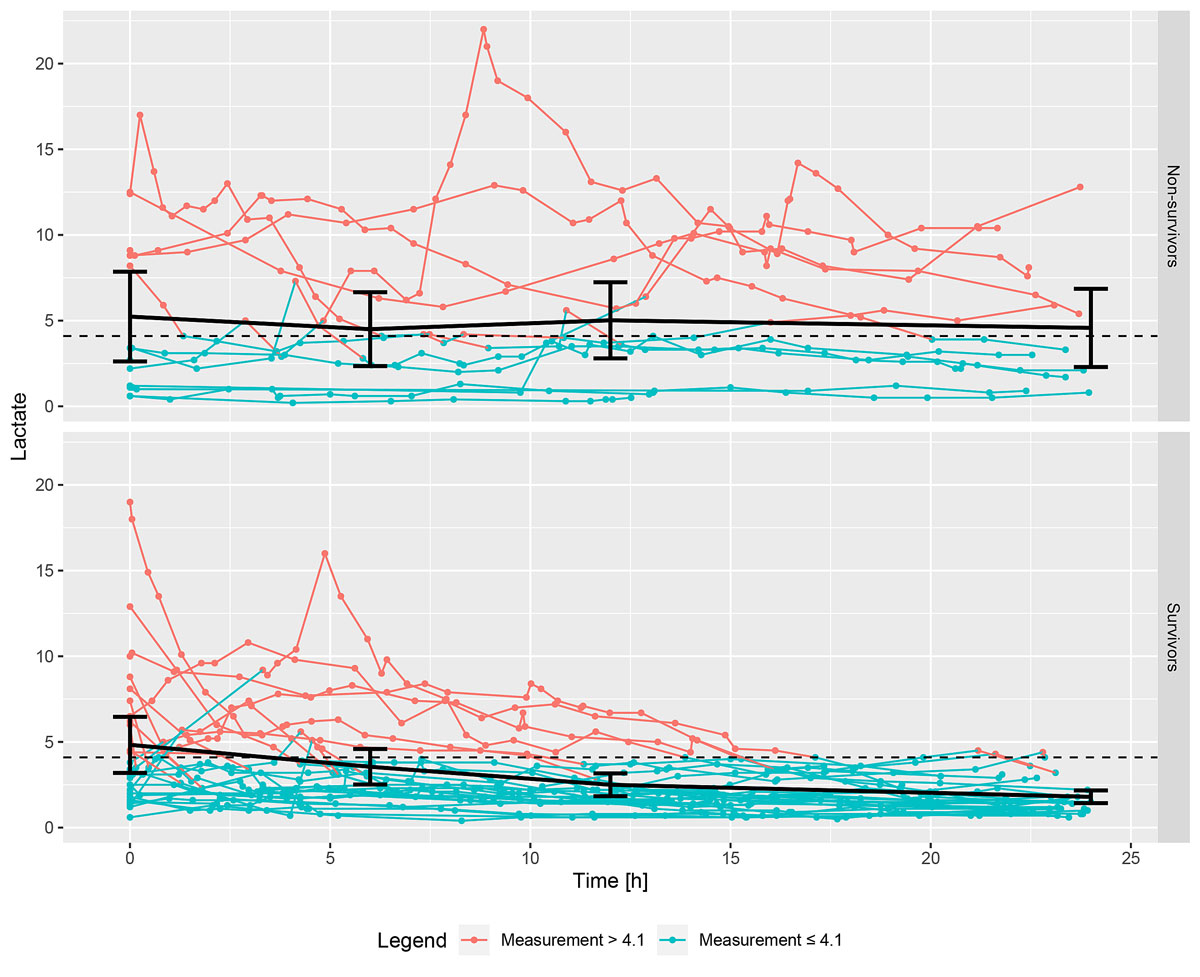
Figure 4 Serum lactate during the first 24 hours of intensive therapy in non-survivors (above) and survivors (below). The black dots and the line correspond to the means and 95% CIs of the first lactate values, at time 0, and to the latest lactate values before 6, 12 and 24 hours after the start of ECMO. Of note, if a line starts, for example, below 4.1 and goes over the threshold of 4.1, it is still displayed as blue, since the measurement was considered constant from this time point onwards until the next measurement.
The minimal p-value was reached at a cut-off value of 4.1 for lactate. Having a lactate value >4.1 instead of ≤4.1 increased the risk of death by a factor of 32.7 (95% CI 4.8 to 221.7, p = 0.0002). The unadjusted HR was 43.0 (95% CI 8.23 to 427.7, p = 0.000007). The sensitivity analysis provided an adjusted HR of 8.6 (95% CI 2.6 to 70.7).
ECMO therapy has come a long way since it was first developed by Gibbon and Lillehei in the 1950s [8]. Having initially been used for respiratory support in adults, it has expanded and established itself as having a critical life-saving role in all age groups; for cardiac, pulmonary and metabolic recovery; and in elective, urgent and ECPR scenarios [9, 10]. Due to improving results and greater availability of ECMO therapy, the demand for ECMO has been steadily increasing [11].
The primary goal of this therapy is to salvage all patients. Survival to discharge in the presented series is 71%. This is comparable to a survival of 70.6% out of a total of 36,705 respiratory ECMO runs in children reported in the Extracorporeal Life Support Organization (ELSO) registry [1]. The question is whether we can analyse other variables and find modifiable factors which may help salvage some of the remaining 30% of patients.
The indications for ECMO involved six diagnostic categories. The small cohort size distributed across six categories made this study underpowered to demonstrate differences in outcome. Despite this, it appears that ARDS and pulmonary hypertension were more frequent in non-survivors. Larger study cohorts would be necessary to decipher the associations of these variables with survival.
Median time from the first symptom to ECMO was almost double in non-survivors compared to in survivors. Although the study failed to provide evidence for this association, this finding may stimulate debate and further studies to deduce whether early ECMO, before the onset of multi-organ dysfunction or irreversible damage to lung parenchyma, could improve survival.
OI was greater in non-survivors than in survivors (38.7, IQR 28.9 to 47.3, versus 22.9, IQR 14.3 to 44.0), without evidence that it differed significantly between the two groups. Whether the lack of evidence is a consequence of the study being underpowered or otherwise, this may point to a beneficial effect of implementing ECMO early, when the OI is still low.
Univariate analysis of our cohort showed greater odds of survival with VV ECMO (OR 0.29, 95% CI 0.07 to 1.23, p = 0.09). While this may just reflect a selection bias (VA ECMO is necessary in sicker patients), a discussion about the pathophysiology is warranted. VV ECMO is theoretically meant to provide only oxygenation and ventilatory support, while depending on autogenous cardiac function for haemodynamic stability. However, patients with lung pathologies, who are subjected to ECMO after a lengthy high-pressure ventilatory therapy, invariably suffer from inadequate left heart preload, thus resulting in a need for high ionotropic support. We have observed that in many cases subjected to VV ECMO, perfusion of oxygenated blood into the lungs and lung rest ventilation lead to better pass-through across the lungs and immediate decongestion of the central venous system. This results in rapid weaning of ionotropic support. In addition, VV ECMO reduces the risk of systemic embolisation and is often performed through a less invasive double-lumen jugular venous cannulation.
In our sample, non-survivors were more likely to have a diagnosis of ARDS, a longer time from the first symptom to ECMO (median 8 days in non-survivors versus 4 days in survivors) and to have had prolonged ventilator therapy resulting in a high OI (median 39 in non-survivors versus 23 in survivors), suggesting certain actionable factors [12]. It may be argued that earlier ECMO support for fulminating infectious-inflammatory pathologies could prevent irreversible lung destruction and lead to better outcomes. Early ECMO may even allow many of these patients to be treated with VV ECMO, with relatively less morbidity. ELSO data tend to support this hypothesis, with time to ECMO >7–14 days, (OR 0.32, p <0.001), presence of cardiac arrest (OR 0.56, p = 0.001), pH per 0.1 unit increase (OR 1.15, p <0.001), higher OI per 10 unit increase (OR 0.95, p = 0.002) and diagnosis of sepsis being associated with unfavourable outcomes [13]. Bayrakci et al. [12] showed that an OI of 33.2 is a suitable cut-off value for ECMO initiation, correlating with high sensitivity and specificity. They further concluded that an OI >40 is associated with a high risk of chronic lung disease when not supported by ECMO.
Being a highly resource intensive, high-end therapy, ECMO puts a strain on resources at the tertiary medical institutions where these patients are treated. This may occasionally lead to the cancellation of planned operations, the transport and airlifting of critically ill patients to other centres, etc. Our hospital practices a three tier (surgeon, assistant, cardiac perfusionist) ECMO alarm system for any reanimation lasting more than five minutes, resulting in approximately 30 ECMO alarms a year. We have three ECMO systems (Levitronix®) at the University Children’s Hospital Zurich, catering to an area with a population of about 4 million. The secondary goal of such high-end therapy is to discern markers of prognosis. Objective, verifiable criteria, similar to those which exist for brain death, are needed when logistics demand that this precious therapy is allocated to those patients most likely to benefit from it. The present coronavirus (COVID-19) pandemic has raised the possibility that even the availability of mechanical ventilators may fall short of the demand, thus vindicating our endeavour. Despite optimal salvage and transport, there may still be patients who are attended too late, when irreversible organ damage has already set in, for example after drowning. Clinical and lab markers suggestive of the point of no return need to be developed so as to enable objective, informed decision making. While our small dataset could not demonstrate a cut-off value of low pH which implies a bad prognosis, ELSO data have shown pH to be a risk factor. Brunner et al [14], studying their ECPR results, showed that the median lowest pH of survivors before ECMO was 7.08 (IQR from 7.1 to 7.3), while that of non-survivors was 6.7 (IQR from 6.6 to 6.9).
Our study found increased serum lactate to be associated with higher mortality (fig. 3). However, potential confounding factors cannot be excluded. A threshold value of >4.1 is associated with a 33-fold (95% CI 4.8 to 221.7, p = 0.0002) greater risk of death compared to patients with serum lactate ≤4.1. However, this result should be interpreted very carefully. The estimated HR seems extremely high and differs from the sensitivity analysis estimate by quite a lot. This could indicate that the corrected estimate is still exaggerated. A known disadvantage of the “minimum p-value approach” is that one cannot know by exactly how much the estimates are overestimated. There are various correction techniques for shrinking the estimate, but these cannot be checked without an independent dataset for validation. In general, this method is known to be very data-dependent, leading to different cut-off points [15]. The lactate threshold of 4.1 should therefore be regarded as a guide for future studies which could then investigate it without the problem of multiple testing. Yang et al. [16] showed that lactate >5 mmol/l was the most significant indicator of 30-day mortality. It is notable that in a post-cardiotomy scenario, Siegel et al. [17] reported lactate >4.5 to be highly predictive of mortality. Buijs et al. [18] [19] have suggested that in addition to static lactate values, dynamic values, i.e., trends over time, may be better predictors of survival in paediatric age groups, but not in neonates. These studies suggest that lactate is a potential marker whose dynamics for different age groups, different indications and different clinical situations should be prospectively studied in order to create evidence-based guidelines.
Our study has the limitations inherent to a retrospective study. The small number of patients in the cohort, the heterogeneity of aetiology and the variability of other factors, in the context of the number of events that occurred, make this study considerably underpowered. Moreover, the small number of events limited the possibility of adjusting the analysis for potential confounders. Various factors such as the operator, era, ECMO flows, unexpected detrimental events, etc., which may have influenced the outcomes, were not studied. A selection bias regarding VV/VA is plausible, and confounding effects cannot be ruled out. These factors should form the basis of future studies.
Our study has demonstrated that serum lactate level might provide guidance on prognosis in ECMO therapy performed for pulmonary indications. However, static and dynamic lactate levels, lactate clearance over time, and the influence of age on serum lactate, among others aspects, need to be further studied, with detailed data collection and analysis, in order to provide conclusive guidelines.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Thiagarajan RR . Extracorporeal Membrane Oxygenation for Cardiac Indications in Children. Pediatr Crit Care Med. 2016;17(8, Suppl 1):S155–9. doi:.https://doi.org/10.1097/PCC.0000000000000753
2 Baek MS , Lee SM , Chung CR , Cho WH , Cho YJ , Park S , et al. Improvement in the survival rates of extracorporeal membrane oxygenation-supported respiratory failure patients: a multicenter retrospective study in Korean patients. Crit Care. 2019;23(1):1. doi:.https://doi.org/10.1186/s13054-018-2293-5
3 Lequier L , Joffe AR , Robertson CM , Dinu IA , Wongswadiwat Y , Anton NR , et al.; Western Canadian Complex Pediatric Therapies Program Follow-up Group. Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg. 2008;136(4):976–983.e3. doi:.https://doi.org/10.1016/j.jtcvs.2008.02.009
4 Howard TS , Kalish BT , Wigmore D , Nathan M , Kulik TJ , Kaza AK , et al. Association of Extracorporeal Membrane Oxygenation Support Adequacy and Residual Lesions With Outcomes in Neonates Supported After Cardiac Surgery. Pediatr Crit Care Med. 2016;17(11):1045–54. doi:.https://doi.org/10.1097/PCC.0000000000000943
5 Grambsch PM , Therneau TM . Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81(3):515–26. doi:.https://doi.org/10.1093/biomet/81.3.515
6Crowley J, Hoering A. Handbook of Statistics in Clinical Oncology. Boca Raton, FL: CRC Press; 2012.
7 Van Houwelingen JC , Le Cessie S . Predictive value of statistical models. Stat Med. 1990;9(11):1303–25. doi:.https://doi.org/10.1002/sim.4780091109
8 Mosier JM , Kelsey M , Raz Y , Gunnerson KJ , Meyer R , Hypes CD , et al. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: history, current applications, and future directions. Crit Care. 2015;19(1):431. doi:.https://doi.org/10.1186/s13054-015-1155-7
9 Wilhelm MJ , Inderbitzin DT , Reser D , Halbe M , Van Tillburg K , Albrecht R , et al. Outcome of inter-hospital transfer of patients on extracorporeal membrane oxygenation in Switzerland. Swiss Med Wkly. 2019;149:w20054. doi:.https://doi.org/10.4414/smw.2019.20054
10 Wagner K , Sangolt GK , Risnes I , Karlsen HM , Nilsen JE , Strand T , et al. Transportation of critically ill patients on extracorporeal membrane oxygenation. Perfusion. 2008;23(2):101–6. doi:.https://doi.org/10.1177/0267659108096261
11 Erdil T , Lemme F , Konetzka A , Cavigelli-Brunner A , Niesse O , Dave H , et al. Extracorporeal membrane oxygenation support in pediatrics. Ann Cardiothorac Surg. 2019;8(1):109–15. doi:.https://doi.org/10.21037/acs.2018.09.08
12 Bayrakci B , Josephson C , Fackler J . Oxygenation index for extracorporeal membrane oxygenation: is there predictive significance? J Artif Organs. 2007;10(1):6–9. doi:.https://doi.org/10.1007/s10047-006-0359-7
13 Domico MB , Ridout DA , Bronicki R , Anas NG , Cleary JP , Cappon J , et al. The impact of mechanical ventilation time before initiation of extracorporeal life support on survival in pediatric respiratory failure: a review of the Extracorporeal Life Support Registry. Pediatr Crit Care Med. 2012;13(1):16–21. doi:.https://doi.org/10.1097/PCC.0b013e3182192c66
14 Brunner A , Dubois N , Rimensberger PC , Karam O . Identifying Prognostic Criteria for Survival after Resuscitation Assisted by Extracorporeal Membrane Oxygenation. Crit Care Res Pract. 2016;2016:9521091. doi:.https://doi.org/10.1155/2016/9521091
15 Altman DG , Lausen B , Sauerbrei W , Schumacher M . Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86(11):829–35. doi:.https://doi.org/10.1093/jnci/86.11.829
16 Yang L , Fan Y , Lin R , He W . Blood Lactate as a Reliable Marker for Mortality of Pediatric Refractory Cardiogenic Shock Requiring Extracorporeal Membrane Oxygenation. Pediatr Cardiol. 2019;40(3):602–9. doi:.https://doi.org/10.1007/s00246-018-2033-2
17 Siegel LB , Dalton HJ , Hertzog JH , Hopkins RA , Hannan RL , Hauser GJ . Initial postoperative serum lactate levels predict survival in children after open heart surgery. Intensive Care Med. 1996;22(12):1418–23. doi:.https://doi.org/10.1007/BF01709563
18 Buijs EA , Houmes RJ , Rizopoulos D , Wildschut ED , Reiss IK , Ince C , et al. Arterial lactate for predicting mortality in children requiring extracorporeal membrane oxygenation. Minerva Anestesiol. 2014;80(12):1282–93.
19 Allen M . Lactate and acid base as a hemodynamic monitor and markers of cellular perfusion. Pediatr Crit Care Med. 2011;12(4, Suppl):S43–9. doi:.https://doi.org/10.1097/PCC.0b013e3182211aed
Shared first authorship
No financial support and no other potential conflict of interest relevant to this article was reported.