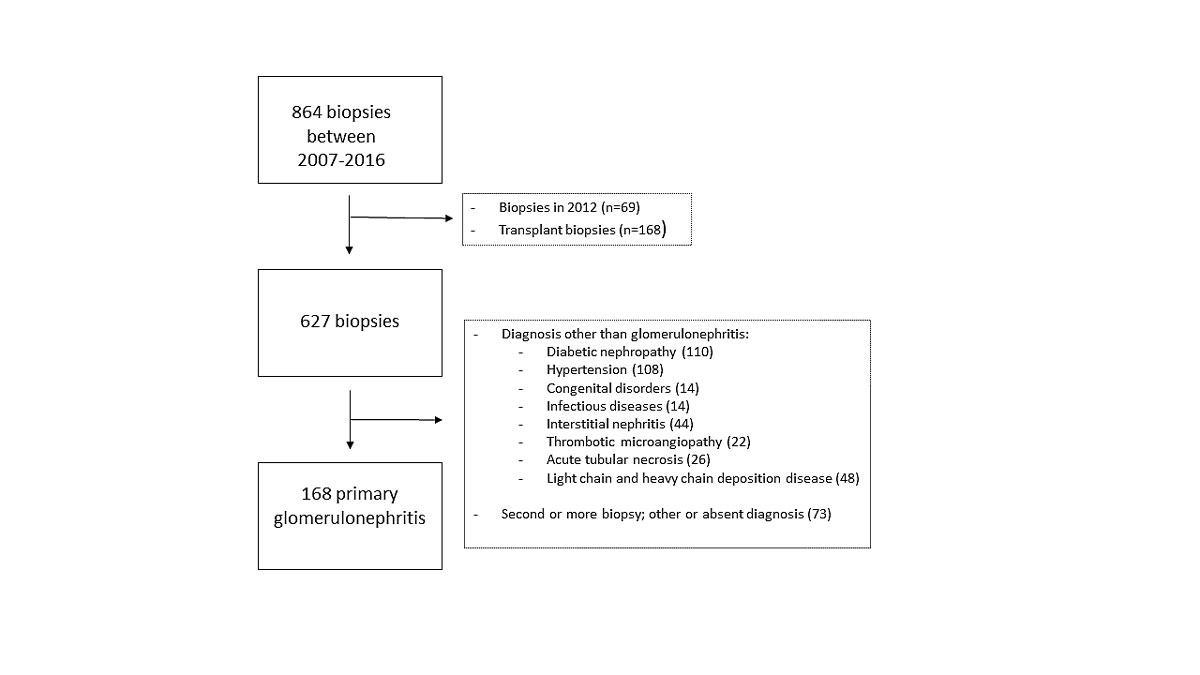
Figure 1 Flow-diagram of biopsy selection.
DOI: https://doi.org/10.4414/smw.2020.20353
Glomerulonephritis is a group of kidney diseases characterised by inflammation of the glomeruli, the filtering units of the kidneys. These inflamed glomeruli can lead to the pathological leak of protein and blood into the urine. Left untreated, this chronic inflammation can lead to scarring and loss of kidney function. Glomerular disease is classified according to histopathological features on renal biopsy as well as some immunological characteristics. Glomerulonephritis can be either primary or secondary to a systemic disease. The clinical presentation is variable, ranging from asymptomatic microhaematuria with or without proteinuria to rapidly progressive renal failure. The symptomatology is typically described as nephrotic syndrome or nephritic syndrome, the former comprising oedema, hypoalbuminaemia, proteinuria and dyslipidaemia and the latter hypertension, renal dysfunction and glomerular haematuria [1].
Glomerulonephritis is rare, with an estimated worldwide incidence of 0.5–2.5 per 100,000 patients per year depending on the specific type [2]. In contrast, the risk of progression to end-stage renal disease (ESRD) is high, explaining why glomerulonephritis accounts for 14–20% of cases of ESRD [3, 4]. The risk of progression to ESRD depends on the disease subtype. For example, an American retrospective study reported an incidence of ESRD of 8.72 per 100 person-years for focal segmental glomerulosclerosis and 1.67 per 100 person-years for minimal change disease [5]. Besides, incidence and prevalence depend on sex, age and geographical location [2].
Apart from the risk of progression to ESRD, the treatment of the different forms of glomerulonephritis is complicated and associated with additional morbidity and costs. Expensive immunosuppressive medication is often necessary and may lead to infectious, malignant or other complications. Consequently, the burden of glomerulonephritis to the healthcare system is substantial. For optimal healthcare planning, detailed epidemiological and demographic data are therefore essential. Unfortunately, up to now and to our knowledge, there are no epidemiological data available for the incidence, prevalence and treatment modalities of glomerulonephritis in Switzerland. The absence of such registries limits the understanding of the natural history of glomerulonephritis. It also hampers the recruitment of glomerulonephritis patients into clinical trials in Switzerland, thus impeding research and evidence-based treatment recommendations.
The aim of this study was therefore to describe the incidence of glomerulonephritis in the western part of Switzerland compared with international data, as well as the evolution of the incidence over time.
This retrospective study was approved by the local ethics committee (CER-VD) and in agreement with the declaration of Helsinki. The study (category A according to the Swiss human research act) was exempted from informed consent.
The pathology department of the University Hospital of Lausanne (CHUV), Switzerland, not only analyses locally obtained biopsies, but is also the reference centre for the regional hospitals and peripheral nephrologists from the cantons Vaud, Fribourg, Valais and Neuchâtel. Data on all biopsies are stored in a database with information on the primary diagnosis, biopsy date and the referring nephrologist. Nephrologists who are not affiliated to a hospital also refer the patients from their practices with an indication for renal biopsy to our university centre or one of the regional hospitals in order for the renal biopsies to take place. As such, the biopsies of all patients with a presumed new diagnosis of glomerulonephritis in these cantons are analysed by the pathology department of the CHUV. Therefore, this dataset is a reliable source for assessment of the incidence and prevalence of glomerulonephritis in the four cantons. All biopsies were analysed by the same experienced renal pathologist (SR).
A first screening included all kidney biopsies analysed and stored in the database at the CHUV between the 1 January 2007 and the 31 December 2016. In a second step, data for 2012 were excluded, as for several months all biopsies had to be sent to other academic centres for analysis because of the temporary absence of our renal pathologist (SR). Transplant biopsies were also excluded.
Thereafter, all written biopsy reports were collected. In order to assess the yearly incidence, only biopsies with a first diagnosis of primary glomerulonephritis in patients aged at least 18 years at the time of biopsy were included in our final analysis. Second or third consecutive biopsies and biopsies from transplanted or post-mortem kidneys were excluded, as well as those with an uncertain diagnosis. The type of glomerulonephritis was classified as minimal change glomerulonephritis, focal segmental glomerulosclerosis, IgA nephropathy, membranous glomerulonephritis, membranoproliferative glomerulonephritis, pauci-immune glomerulonephritis, anti-glomerular basement membrane glomerulonephritis, lupus nephritis or fibrillary glomerulonephritis. The classification of the different types of primary glomerulonephritis was based on currently used diagnostic criteria [6–15].
Data on the number of inhabitants in the different cantons were retrieved from the Federal Office of Statistics. For each year, the incidence was calculated as the number of patients newly diagnosed with glomerulonephritis divided by the number of inhabitants of all the above-mentioned cantons.
Baseline characteristics such as age, sex and biological results including serum creatinine and the level of proteinuria were retrieved from the electronic database of our hospital. The degree of proteinuria was expressed as urinary protein concentration and protein/creatinine ratio. We also documented details of the biopsy procedure, such as the needle size used and the number and size of each sample for each participant. Histological variables included the number of glomeruli per sample, the percentage of glomerular sclerosis and the percentage of interstitial fibrosis, as noted by the pathologist in his final report.
As mentioned above, the main objective of this study was to determine the incidence of glomerulonephritis in the western part of Switzerland. Secondary outcomes were the changes in baseline characteristics over time of the patients with glomerulonephritis and the degree of glomerulosclerosis and interstitial fibrosis, as a proxy of the advancement of the disease at the time of the biopsy, and thus the rapidity of medical diagnosis.
Baseline characteristics were expressed as mean ± standard deviation, median (interquartile range) or percentage, as appropriate. We used Student’s t-test, Wilcoxon test or chi-square tests to compare participants’ results and groups. We used Cuzick’s nonparametric test for trends (nptrend) and linear regression to examine the evolution of biopsy rates and the incidence of glomerulonephritis over time. A p-value below 0.05 was considered significant. All statistical analyses were performed using STATA 14.2 (StataCorp LLC, College Station, Texas, USA).
We retrospectively collected all kidney biopsy reports between 2007 and 2016 (n = 867), from which we removed transplant biopsies and all biopsies performed in 2012. Thereafter, we excluded 386 biopsies with other aetiologies such as diabetic nephropathy, vascular/ischaemic nephropathy or congenital disorders, biopsies with no diagnosis or an uncertain diagnosis, and biopsies in patients with glomerulonephritis performed for the second, third or fourth time. The selection process is detailed in figure 1. A total of 168 met the inclusion criteria, with a mean of 18.7 biopsies per year for nine consecutive years. During this time period, 52.4% of biopsied patients were men. The mean age was 50 years.

Figure 1 Flow-diagram of biopsy selection.
The mean overall incidence, for all forms of glomerulonephritis combined over the whole 9-year period, was 1.3/100,000/year. The incidence remained stable over time, as illustrated in figure 2. The most common primary glomerulonephritis was IgA nephropathy with 32.7% of cases, followed by lupus nephritis (29.8%) and pauci-immune glomerulonephritis (11.9%). The number of patients and the year-by-year incidence for each subtype of glomerulonephritis is shown in table 1. The linear regression analysis of the incidence of each subtype of glomerulonephritis showed that the yearly incidence of none of these subtypes changed over time.
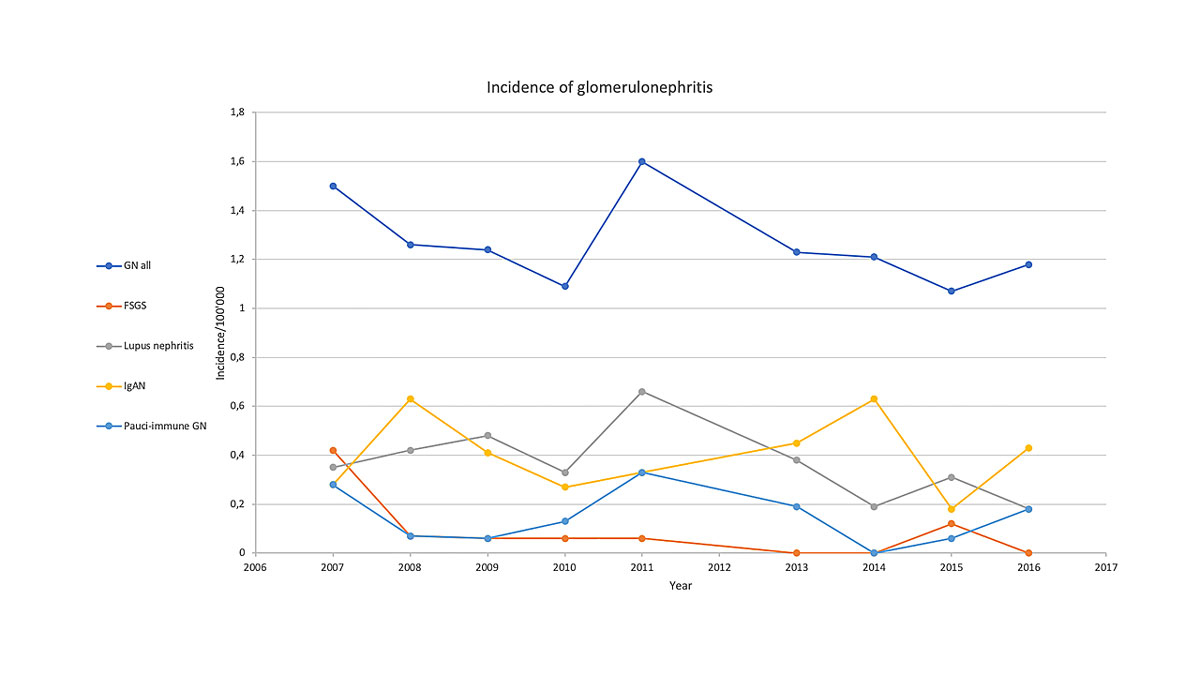
Figure 2 Evolution of the local incidence of glomerulonephritis over time: all causes combined as well as the most frequent forms of primary glomerulonephritis. FSGS = focal segmental glomerulosclerosis; GN = glomerulonephritis; IgAN = IgA nephropathy
Table 1 Absolute number of patients and incidence of the different forms of primary glomerulonephritis between 2007 and 2016.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2013 | 2014 | 2015 | 2016 | Entire period | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n* | I† | n* | I† | n* | I† | n* | I† | n* | I† | n* | I† | n* | I† | n* | I† | n* | I† | n* | I† | |
| MCD | 0 | 0 | 0 | 0 | 1 | 0.07 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.06 | 1 | 0.06 | 3 | 0.02 |
| FSGS | 6 | 0.42 | 1 | 0.07 | 1 | 0.06 | 1 | 0.06 | 1 | 0.06 | 0 | 0 | 0 | 0 | 2 | 0.12 | 0 | 0 | 12 | 0.09 |
| IgAN | 4 | 0.28 | 9 | 0.63 | 6 | 0.41 | 4 | 0.27 | 5 | 0.33 | 7 | 0.45 | 10 | 0.63 | 3 | 0.18 | 7 | 0.43 | 55 | 0.41 |
| MG | 0 | 0 | 1 | 0.07 | 2 | 0.13 | 1 | 0.06 | 3 | 0.2 | 0 | 0 | 1 | 0.06 | 1 | 0.06 | 1 | 0.06 | 10 | 0.07 |
| Pauci-immune GN | 4 | 0.28 | 1 | 0.07 | 1 | 0.06 | 2 | 0.13 | 5 | 0.33 | 3 | 0.19 | 0 | 0 | 1 | 0.06 | 3 | 0.18 | 20 | 0.15 |
| Lupus nephritis | 5 | 0.35 | 6 | 0.42 | 7 | 0.48 | 5 | 0.33 | 10 | 0.66 | 6 | 0.38 | 3 | 0.19 | 5 | 0.31 | 3 | 0.18 | 50 | 0.37 |
| Anti-GBM GN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.06 | 0 | 0 | 2 | 0.12 | 2 | 0.12 | 5 | 0.04 |
| MPG | 1 | 0.07 | 0 | 0 | 0 | 0 | 3 | 0.2 | 0 | 0 | 2 | 0.12 | 2 | 0.12 | 2 | 0.12 | 2 | 0.12 | 12 | 0.09 |
| ITG | 1 | 0.07 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.007 |
| All GN | 21 | 1.5 | 18 | 1.26 | 18 | 1.24 | 16 | 1.09 | 24 | 1.6 | 19 | 1.23 | 16 | 1.21 | 17 | 1.07 | 19 | 1.18 | 168 | 1.3 |
| Population (million) | 1.4 | 1.427 | 1.45 | 1.471 | 1.496 | 1.544 | 1.567 | 1.588 | 1.607 | 13.55 | ||||||||||
Anti-GBM GN = anti-glomerular basement membrane glomerulonephritis; FSGS = focal segmental glomerulosclerosis; IgAN = IgA nephropathy; ITG = immunotactoid glomerulopathy; MCD = minimal change disease; MG = membranous glomerulonephritis; MPG = membranoproliferative glomerulonephritis Population: in the cantons of Vaud, Fribourg, Neuchâtel and Valais * Absolute number; † Incidence/100,000 persons/year
The number of biopsies performed each year per million inhabitants increased between 2007 and 2016, as shown in table 2. The analysis of the annual rate of all biopsies in native kidneys, including biopsies with diagnoses other than glomerulonephritis and repeat biopsies in the same patient, but excluding transplant biopsies, revealed a significant increase (p = 0.007).
Table 2 Annual kidney biopsy rate between 2007 and 2016 per million inhabitants. There was a significant increase (p = 0.007) in the total number of kidney biopsies performed in the western region of Switzerland (all diagnoses confounded, and excluding transplant biopsies).
| Year | Number of biopsies | Inhabitants (million) | Annual biopsy rate (per million) |
|---|---|---|---|
| 2007 | 61 | 1.400 | 43.6 |
| 2008 | 62 | 1.427 | 43.4 |
| 2009 | 54 | 1.450 | 37.2 |
| 2010 | 44 | 1.470 | 29.9 |
| 2011 | 60 | 1.496 | 40.1 |
| 2013 | 71 | 1.544 | 46.0 |
| 2014 | 84 | 1.567 | 53.6 |
| 2015 | 88 | 1.588 | 55.4 |
| 2016 | 103 | 1.607 | 64.1 |
Clinical characteristics of patients with a first diagnosis of glomerulonephritis were compared year by year between 2007 and 2016, as detailed in table 3. There was no significant change in the gender distribution and age of the patients. However, creatinine values and proteinuria at the time of the biopsy did increase significantly over the years. Regarding histological characteristics, there was a slight increase in fibrosis and no change in glomerulosclerosis.
Table 3 Comparison of baseline characteristics of patients with firstly diagnosed primary glomerulonephritis between 2007 and 2016.
| 2007 | 2008 | 2009 | 2010 | 2011 | 2013 | 2014 | 2015 | 2016 | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Women (%) | 38 | 39 | 50 | 56.3 | 54.2 | 57.9 | 50 | 64.7 | 21.1 | 0.63 |
| Age | 45 ± 17 | 48.4 ± 20 | 47.9 ± 18 | 49.8 ± 18 | 50 ± 13 | 49.2 ± 19 | 58.8 ± 15 | 54.7 ± 20 | 56.8 ± 18 | 0.06 |
| Creatininaemia (µmol/l) | 166 (86–188) | 156 (91–190) | 92 (65–228) | 92 (65 –228) | 110 (68–200) | 130 (89–169) | 245 (119–400) | 107 (70–180) | 160 (128–250) | 0.16 |
| Proteinuria/creatininuria ratio (g/mol) | 233 (127–335) | 244 (58–447) | 188 (96–252) | 190(48–660) | 412 (199–596) | 172 (123–363) | 606 (484–912) | 294 (176–633) | 236 (124–729 | 0.4 |
| Glomerulosclerosis (%) | 16.6 | 22.6 | 16.6 | 25.7 | 22.6 | 27.1 | 21.9 | 16.8 | 21.2 | 0.21 |
| Interstitial fibrosis (%) | 21.9 | 27.7 | 14.3 | 20.7 | 23.7 | 28.1 | 34.8 | 19.3 | 28.4 | 0.22 |
Data are expressed as mean ± standard deviation, median (interquartile range) or percentage, as appropriate.
With respect to the quality of the biopsies, there was a slight decrease in the number of glomeruli per sample, ranging from 37 to 19, although there were still sufficient to ensure interpretation.
Taken together, our study shows that the global incidence of glomerulonephritis in the western part of Switzerland is approximately 1.3/100,000/year; this incidence remained stable between 2007 and 2016. IgA nephropathy had the highest incidence, with 0.40 new cases/100,000 person/ years (32.7% of all cases), followed by lupus nephritis and pauci-immune glomerulonephritis. Of interest, parameters of disease progression increased between the beginning and the end of the study, suggesting that glomerulonephritis was diagnosed at a higher age and more advanced stages in recent times.
The annual biopsy rate in native kidneys increased (np trend p = 0.023), suggesting either a more aggressive biopsy policy, or an increase of kidney disease burden. Our data do not allow us to differentiate between these two possibilities. If the biopsy rate were higher due to a more aggressive biopsy policy, this would further support our conclusion that the incidence of glomerulonephritis (including IgA nephropathy) has not increased and was rather stable in Switzerland over the last decade.
Regarding the stable incidence of glomerulonephritis over time in our study, international studies describe various trends, depending on the country and the underlying type of glomerulonephritis.
For example, a study conducted between 1976 and 2005 in Northern Ireland found an increase in the biopsy rate from 2.02 to 7.08 per hundred thousand population per year. IgA nephropathy was the most common diagnosis (38.8%). The authors showed a significant increase in the proportion of IgA nephropathy and decrease in membranous nephropathy over time [16].
On the other hand, an Italian study reported an increase in all subtypes, but also showed that the increase was in fact only reflecting the better recognition of glomerulonephritis [17].The global increase shown in some studies can indeed be influenced by an increase in the number of biopsies performed [18]. The indication for renal biopsy has widened over time, due in part to the increased safety of the procedure. Another Italian study demonstrated how the biopsy policies evolved, with a significant increase of biopsies performed for the indication of isolated urinary abnormalities [19]. In our population, we also found a significant increase in the number of biopsies carried out per million inhabitants over the study period, consistent with these assumptions. However, the change in biopsy policy was probably less important in Switzerland, owing to the already highly developed healthcare system. Moreover, our data are recent, whereas other studies analysed older data at a time where access to biopsy was probably lower. This could explain why the slight increase in the number of biopsies over time was not accompanied by an increase in the incidence.
Regarding the subtypes of glomerulonephritis, IgA nephropathy was the most frequently diagnosed subtype, with a stable incidence of 0.40/100,000/year. This is lower compared with international results. In Europe, the incidence of IgA nephropathy varies between 0.8 and 3.1 cases/100,000/year, with the lowest reported incidence in Italy and the highest in France [18]. In other registries, the reported incidence was 1.47/year/100,000 in North-western Italy [19], and 4.8 in Finland [18]. In Asia, IgA nephropathy has generally a higher incidence than in Europe, which is not explained just by race differences. For example, an incidence of 4.5/100,000 has been reported in Japan [20] versus 10.5/100,000 in Australia [21].
Despite its high incidence, IgA nephropathy is not the most frequent type of glomerulonephritis in developing countries. For example, a single-centre study from India reported that IgA nephropathy accounted for 10.25% of all cases of glomerulonephritis, whereas the most frequent type was minimal change glomerulonephritis (25.42%), followed by focal segmental glomerulosclerosis (22.58%) [22]. The same trend was seen in Africa, with minimal change disease again the most frequent glomerulonephritis (16.5% of all cases), followed by focal segmental glomerulosclerosis (15.9%) and IgA nephropathy (2.8%) [23]. In South America, a Brazilian study found focal segmental glomerulosclerosis to be the most frequent aetiology (28.8%) and only 18.3% were IgA nephropathy [24]. Finally, epidemiological studies in the Middle East reported that focal segmental glomerulosclerosis was the most commonly diagnosed glomerulonephritis (35% of cases) in Iraq, whereas IgA nephropathy accounted for only 7% of cases [25] and in Saudi Arabia focal segmental glomerulosclerosis accounted for 39.8% of cases [26].
It remains an open question why the incidence of IgA nephropathy was lower in Switzerland than in surrounding countries. It could be that screening and biopsy policies differ between Switzerland and other European countries, such as considering biopsy for asymptomatic urinary abnormalities. Wirta et al. demonstrated the importance of biopsy policy in a Finnish study; they compared data from a university hospital with data from surrounding central hospitals. The increased biopsy rate in the university hospital was linked to a significant increase in the reported glomerulonephritis incidence, despite a similar population [18]. Another possible explanation may be that our database is not complete. In this context, one could argue that some patients living in the canton of Vaud were taken care of by another academic hospital in a different canton, such as Geneva or Bern. This occurs in general only if there is a special medical indication, which is unlikely considering the similar levels of medical competence of the different university centres.
For lupus nephritis, we noted a mean incidence of 0.37/100,000/year (29.8% of all glomerulonephritis cases) in our study, with a trend towards a decrease of its incidence in recent years.
Interestingly, we observed a trend towards an increase in the mean age of patients at the time of the biopsy, although this did not reach statistical significance, probably due to the relatively small sample size. Patients with glomerulonephritis diagnosed in 2007 were almost ten years younger than patients newly diagnosed in 2016. This trend is also seen internationally [16, 17, 19, 27]. Whether this is a simple consequence of the worldwide ageing population or linked to the increased safety and availability of renal biopsies leading to a more expanded use in older patients remains an open question. The relatively high degree of glomerulosclerosis and interstitial fibrosis reminds us that glomerulonephritis can be extremely aggressive and is often advanced at the time of diagnosis, underlining the need for rapid diagnostic strategies.
Notably, the proportion of ESRD due to glomerulonephritis is possibly changing. As stated above, glomerulonephritis used to account for up to 20% of cases of ESRD [2]. In the United States renal data system, this percentage is slowly decreasing, with a prevalence of 18% in 1999, 16% in 2006 and 15% in 2016 [28]. This could be explained by improvements in treatment and also the relative increase of diabetes nephropathy, which is actually the leading cause of ESRD and has seen its incidence steadily increase worldwide over the last decades. In Switzerland, data on patients with ESRD have been systematically recorded only since 2013 in the Swiss national dialysis registry (srrqap, Swiss renal registry and quality assessment programme). According to this registry, there was no increase in the proportion of glomerulonephritis amongst dialysis patients in Switzerland between 2015 and 2018 (see table 4A). The proportion of incident ESRD patients with biopsy-proven glomerulonephritis also did not change (table 4B). Clearly, this period of 4 years is too short to draw definite conclusions on trends. Interestingly, in line with the low incidence of patients with newly diagnosed glomerulonephritis in the French-speaking cantons outlined above, the prevalence and incidence of patients with ESRD due to glomerulonephritis is also slightly lower than in other countries.
Table 4 A: The proportion (percentage) of prevalent patients with biopsy-proven glomerulonephritis between 2015 and 2018.
| Year | Total number of patients with end-stage renal disease on dialysis in Swiss registry | Number of patients without glomerulonephritis | Mean age of patients without glomerulonephritis | Patients with biopsy proven glomerulonephritis | Mean age of patients with biopsy proven glomerulonephritis | Percent of patients with glomerulonephritis |
|---|---|---|---|---|---|---|
| 2015 | 3585 | 3078 | 69.0 ± 14.3 | 507 | 60.9 ± 16.0 | 14.1 |
| 2016 | 3679 | 3187 | 68.8 ± 14.3 | 492 | 60.7 ± 15.7 | 13.4 |
| 2017 | 3682 | 3174 | 68.9 ± 14.4 | 508 | 61.7 ± 15.4 | 13.7 |
| 2018 | 3788 | 3262 | 69.6 ± 14.1 | 526 | 61.7 ± 15.7 | 13.9 |
Table 4B The proportion of incident patients with biopsy-proven glomerulonephritis between 2015 and 2018.
| Year | Total number of incident patients with end-stage renal disease on dialysis in Swiss registry | Patients with biopsy proven glomerulonephritis | Patients with other renal diseases | No information on biopsy | Percent of patients with glomerulonephritis |
|---|---|---|---|---|---|
| 2015 | 799 | 92 | 707 | 1 | 11.5 |
| 2016 | 783 | 73 | 710 | 0 | 9.3 |
| 2017 | 770 | 82 | 688 | 0 | 10.6 |
| 2018 | 818 | 97 | 721 | 0 | 11.9 |
Our results should be interpreted in the light of the strengths and limitations of the study. Firstly, the presented results are based on a small number of cases, analysed over a relatively short period of time. As mentioned, we based our analyses on the population of the cantons that send their biopsies to the pathology department of our university hospital. It is nevertheless possible that some biopsies were sent elsewhere, or that some patients chose to go to a different hospital outside of their home canton. Besides, we excluded about 13% of the biopsy reports because of uncertainty in the diagnosis. This may have slightly lowered the incidence rates.
Despite these limitations, our study provides for the first time data on the regional epidemiology of glomerulonephritis in the French-speaking part of Switzerland. In the future, the analysis could be expanded to all the other tertiary referring centres in Switzerland, in order to increase the number of cases and obtain results representing the whole country.
Overall, our data shows that the incidence of glomerulonephritis in the western part of Switzerland is lower than in surrounding countries, and rather stable over the last decade. The most frequent glomerulonephritis was IgA nephropathy, consistent with international data from developed countries.
MP is supported by a grant from the Swiss National Science Foundation (FN 320030-169191).
No conflict of interest relevant to this article was reported.
1 Halfon M , Pruijm M . Glomérulonéphrites. Swiss Med Forum. 2017;17(13):298–305. doi:.https://doi.org/10.4414/smf.2017.02891
2 McGrogan A , Franssen CF , de Vries CS . The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–30. doi:.https://doi.org/10.1093/ndt/gfq665
3UK Renal Registry. UK Renal Registry 21st Annual Report. Data to 31 December 2017. Bristol, UK: 2019. Available from https://www.renalreg.org/publications-reports/.
4 Saran R , Li Y , Robinson B , Abbott KC , Agodoa LY , Ayanian J , et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3, Suppl 1):S1–305. doi:.https://doi.org/10.1053/j.ajkd.2015.12.014
5 Sim JJ , Bhandari SK , Batech M , Hever A , Harrison TN , Shu YH , et al. End-Stage Renal Disease and Mortality Outcomes Across Different Glomerulonephropathies in a Large Diverse US Population. Mayo Clin Proc. 2018;93(2):167–78. doi:.https://doi.org/10.1016/j.mayocp.2017.10.021
6 Madaio MP , Harrington JT . The diagnosis of glomerular diseases: acute glomerulonephritis and the nephrotic syndrome. Arch Intern Med. 2001;161(1):25–34. doi:.https://doi.org/10.1001/archinte.161.1.25
7 Vivarelli M , Massella L , Ruggiero B , Emma F . Minimal Change Disease. Clin J Am Soc Nephrol. 2017;12(2):332–45. doi:.https://doi.org/10.2215/CJN.05000516
8 Rosenberg AZ , Kopp JB . Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–17. doi:.https://doi.org/10.2215/CJN.05960616
9 Rodrigues JC , Haas M , Reich HN . IgA Nephropathy. Clin J Am Soc Nephrol. 2017;12(4):677–86. doi:.https://doi.org/10.2215/CJN.07420716
10 Wasserstein AG . Membranous glomerulonephritis. J Am Soc Nephrol. 1997;8(4):664–74.
11 Fakhouri F . Approche clinique des glomérulonéphrites membranoprolifératives primitives [Clinical approach to primary membranoproliferative glomerulonephritis]. Nephrol Ther. 2016;12(Suppl 1):S65–9. doi:.https://doi.org/10.1016/j.nephro.2016.01.008
12 Syed R , Rehman A , Valecha G , El-Sayegh S . Pauci-Immune Crescentic Glomerulonephritis: An ANCA-Associated Vasculitis. BioMed Res Int. 2015;2015:402826. doi:.https://doi.org/10.1155/2015/402826
13 McAdoo SP , Pusey CD . Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2017;12(7):1162–72. doi:.https://doi.org/10.2215/CJN.01380217
14 Almaani S , Meara A , Rovin BH . Update on Lupus Nephritis. Clin J Am Soc Nephrol. 2017;12(5):825–35. doi:.https://doi.org/10.2215/CJN.05780616
15 Schwartz MM , Korbet SM , Lewis EJ . Immunotactoid glomerulopathy. J Am Soc Nephrol. 2002;13(5):1390–7. doi:.https://doi.org/10.1097/01.ASN.0000013397.06964.19
16 Hanko JB , Mullan RN , O’Rourke DM , McNamee PT , Maxwell AP , Courtney AE . The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant. 2009;24(10):3050–4. doi:.https://doi.org/10.1093/ndt/gfp254
17 Zaza G , Bernich P , Lupo A ; ‘Triveneto’ Register of Renal Biopsies (TVRRB). Incidence of primary glomerulonephritis in a large North-Eastern Italian area: a 13-year renal biopsy study. Nephrol Dial Transplant. 2013;28(2):367–72. doi:.https://doi.org/10.1093/ndt/gfs437
18 Wirta O , Mustonen J , Helin H , Pasternack A . Incidence of biopsy-proven glomerulonephritis. Nephrol Dial Transplant. 2008;23(1):193–200. doi:.https://doi.org/10.1093/ndt/gfm564
19 Stratta P , Segoloni GP , Canavese C , Sandri L , Mazzucco G , Roccatello D , et al. Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am J Kidney Dis. 1996;27(5):631–9. doi:.https://doi.org/10.1016/S0272-6386(96)90096-7
20 Schena FP , Nistor I . Epidemiology of IgA Nephropathy: A Global Perspective. Semin Nephrol. 2018;38(5):435–42. doi:.https://doi.org/10.1016/j.semnephrol.2018.05.013
21 Briganti EM , Dowling J , Finlay M , Hill PA , Jones CL , Kincaid-Smith PS , et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16(7):1364–7. doi:.https://doi.org/10.1093/ndt/16.7.1364
22 Golay V , Trivedi M , Abraham A , Roychowdhary A , Pandey R . The spectrum of glomerular diseases in a single center: A clinicopathological correlation. Indian J Nephrol. 2013;23(3):168–75. doi:.https://doi.org/10.4103/0971-4065.111833
23 Okpechi IG , Ameh OI , Bello AK , Ronco P , Swanepoel CR , Kengne AP . Epidemiology of Histologically Proven Glomerulonephritis in Africa: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(3):e0152203. doi:.https://doi.org/10.1371/journal.pone.0152203
24 Machado SGMR , Quadros T , Watanabe Y , Aquino CF , Otoni A , Pinto SW . Most common histopathological patterns of the Minas Gerais Association of the Centers of Nephrology. Rev Assoc Med Bras (1992). 2019;65(3):441–5. doi:.https://doi.org/10.1590/1806-9282.65.3.441
25 Ali AA , Sharif DA , Almukhtar SE , Abd KH , Saleem ZSM , Hughson MD . Incidence of glomerulonephritis and non-diabetic end-stage renal disease in a developing middle-east region near armed conflict. BMC Nephrol. 2018;19(1):257. doi:.https://doi.org/10.1186/s12882-018-1062-7
26 AlFaadhel T , Alsuwaida A , Alsaad K , Almezaini L , Ahmed N , AlHamad MY , et al. Prevalence and 20-year epidemiological trends of glomerular diseases in the adult Saudi population: a multicenter study. Ann Saudi Med. 2019;39(3):155–61. doi:.https://doi.org/10.5144/0256-4947.2019.155
27 Nie P , Chen R , Luo M , Dong C , Chen L , Liu J , et al. Clinical and Pathological Analysis of 4910 Patients Who Received Renal Biopsies at a Single Center in Northeast China. BioMed Res Int. 2019;2019:6869179. doi:.https://doi.org/10.1155/2019/6869179
28USRDS annual data report. Epidemiology of kidney disease in the United States, in National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: United States Renal Data System; 2019
MP is supported by a grant from the Swiss National Science Foundation (FN 320030-169191).
No conflict of interest relevant to this article was reported.