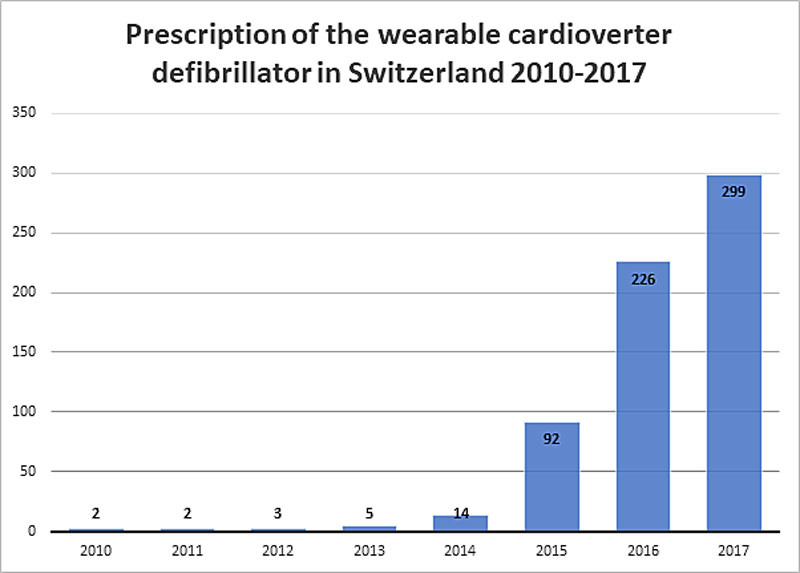
Figure 1 Prescription of the wearable cardioverter defibrillator in Switzerland 2010–2017.
DOI: https://doi.org/10.4414/smw.2020.20343
Cardiovascular mortality remains the number one cause of death in Switzerland. Of the 66,971 persons deceased in 2017 in Switzerland, 31.4% died from cardiovascular causes [1]. Sudden cardiac death is a disastrous manifestation of heart disease, most commonly due to malignant arrhythmia. After the first successful implantation of an implantable cardioverter defibrillator (ICD) in 1980, the ICD has established itself in the early 2000s after several landmark studies for secondary and primary prevention of SCD in patients with severely impaired left ventricular systolic function (EF). The wearable cardioverter defibrillator (WCD), however, emerged later. Auricchio and colleagues first demonstrated its clinical efficacy in the treatment of malignant arrhythmias such as ventricular fibrillation and tachycardia (VF and VT) [2].
In Switzerland, off-label WCD use began in 2011, and was officially approved in 2014 [3]. Current reimbursement in Switzerland is generally for 30 days with the possibility of an extension upon request. Its use is accepted, as in current European guidelines, for temporary protection from sudden cardiac death in selected patients [4, 5]. Despite one randomised controlled trial and several registries investigating the use of the WCD, questions remain regarding its true benefit and patterns of use.
The aim of the Swiss WCD Registry is to provide information on the prescription practices and outcome of patients with WCD in Switzerland and to put these into context with previously published data.
The Swiss WCD Registry is a multicentre, retrospective observational registry including 12 hospitals prescribing WCDs in Switzerland. The WCD (LifeVest, ZOLL, Pittsburgh, Pennsylvania, USA) has previously been described [6, 7]. Participating hospitals are University Hospital Zurich (USZ), University Hospital Basel (USB), University Hospital Bern (Inselspital), University Hospital Geneva (HUG), University Hospital Lausanne (CHUV), Cantonal Hospital Winterthur (KSW), Cantonal Hospital St Gallen (KSSG), Triemli Hospital Zurich (Triemli), Cantonal Hospital Lucerne (LUKS), Cantonal Hospital Aarau (KSA), Cantonal Hospital Graubünden (KSGR), and GZO Regional Healthcare Centre Wetzikon (GZO). The observation period was between the time of the approval and first prescription of a WCD in Switzerland, December 2011, and February 2018. All patients with WCD use were included. Patients who refused to participate or patients with incomplete data, including those patients still wearing the WCD, were excluded. The study was approved by the responsible ethics committee and conforms to the Declaration of Helsinki as revised in 2013.
Baseline characteristics and outcome data were collected from the respective electronic patient charts of each hospital using REDCap Software (Vanderbilt, Nashville, TN, USA). WCD prescription practices for all patients (including the patients treated at the 12 hospitals) in Switzerland was provided by the manufacturer ZOLL. Patient-level WCD data from the LifeVest Network (average daily wear-time, automatic recordings, etc.) were available to all treating physicians; the manufacturer, however, provided a comprehensive pseudonymised database. Underlying heart disease was categorised as ischaemic cardiomyopathy (ICM), nonischaemic cardiomyopathy (NICM) and inherited/congenital heart disease according the final diagnosis at each institution. Indications for WCD use were categorised as follows [6]:
The yearly prescription of WCD since its first use in Switzerland was provided by the manufacturer. No further involvement of the manufacturer in data collection, statistical analysis or writing of the manuscript took place.
A descriptive statistical analysis was performed on the available data set. Categorical variables are reported as frequencies (percentage), continuous variables as means (± standard deviation) or as medians (IQR, range). The follow-up time was also calculated in person-months (number of patients multiplied by the mean wear duration in days for the total study population divided by 30.4 – the calculated average length of a month). The incident appropriate treatment rate (or treatment incidence) was calculated as treatments/person-months and multiplied by three to provide 3-month rate, as previously described [8]. Prolonged WCD use was separately analysed. Exploratory statistical analysis was performed by comparing continuous data using Student’s t-test, the Mann-Whitney U-test or Kruskal-Wallis test as appropriate depending on data distribution and number of samples compared. Categorical data was analysed using the chi-square test. Correlation was calculated using Pearson’s coefficient. A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 25 (IBM Corp., Armonk, NY, USA). This study was conducted according to the STROBE statement.
Between 1 December 2011 and 18 February 2018, 28 prescribing healthcare centres (hospitals and private cardiology practices) prescribed a WCD for a total of 679 patients in Switzerland. The yearly prescription rate rose significantly from its first use in 2010 after its approval by the MiGeL and increased yearly until the end of the study period. The highest prescription rate was in 2017 (fig. 1). The highest monthly prescription rate was 34 in one month in May 2017.

Figure 1 Prescription of the wearable cardioverter defibrillator in Switzerland 2010–2017.
Of all patients, 562 (82.7%) were prescribed a WCD at one of the 12 participating hospitals. After applying the exclusion criteria, 456 patients (81.1% of all patients at participating hospitals) were included in the Swiss WCD Registry (fig. 2). Baseline characteristics were available for all included patients (n = 456, table 1).
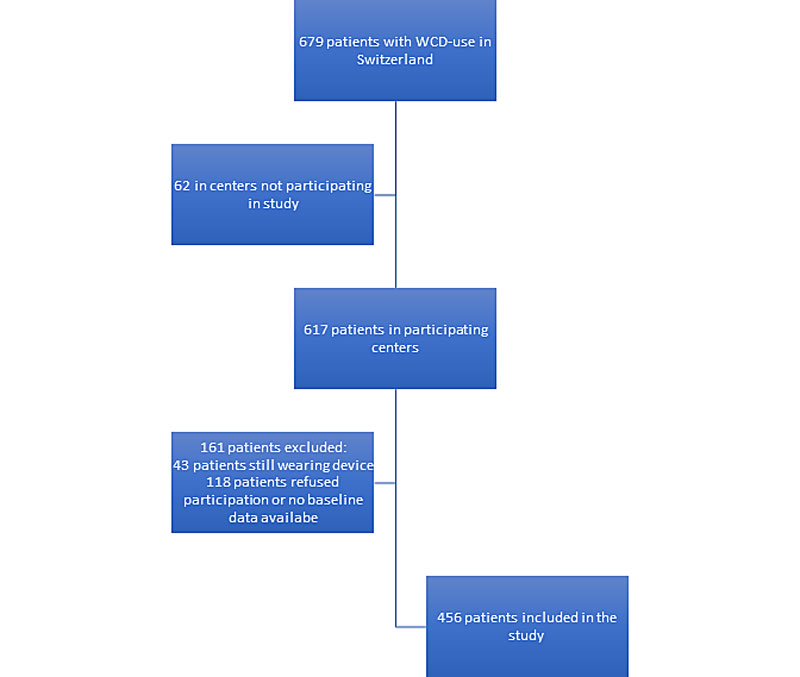
Figure 2 Flow chart of the study population.
Table 1 Baseline characteristics of the study population.
| Hospital | USZ | USB | KSW | HUG | KSSG | Triemli | CHUV | KSA | Inselspital | GZO | LUKS | KSGR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number* | 192 (42) | 43 (9) | 38 (8) | 33 (7) | 27 (6) | 26 (6) | 22 (5) | 21 (5) | 20 (5) | 15 (3) | 13 (3) | 6 (1) |
| Age (years) | 58 ± 13 | 58 ± 14 | 60 ± 17 | 59 ± 14 | 54 ± 15 | 59 ± 12 | 58 ± 14 | 52 ± 15 | 50 ± 17 | 62 ± 14 | 53 ± 12 | 51 ± 15 |
| Female* | 26 (14) | 5 (12) | 7 (18) | 5 (15) | 7 (26) | 3 (12) | 10 (46) | 6 (29) | 5 (25) | 3 (20) | 3 (23) | 1 (17) |
| BMI (kg/m2) | 27 ± 6 | 27 ± 5 | 26 ± 3 | 25 ± 4 | 27 ± 6 | 26 ± 4 | 26 ± 8 | 26 ± 5 | 25 ± 4 | 28 ± 6 | 27 ± 6 | 26 ± 2 |
| EF before WCD use (%)* | 32 ± 13 | 27 ± 11 | 31 ± 15 | 29 ± 13 | 33 ± 16 | 29 ± 10 | 36 ± 17 | 34 ± 10 | 33 ± 14 | 32 ± 7 | 33 ± 15 | 38 ± 12 |
| Atrial fibrillation | 55 (29) | 12 (28) | 9 (28) | 6 (18) | 4 (15) | 8 (31) | 2 (9) | 2 (10) | 4 (20) | 4 (28) | 2 (15) | 0 (0) |
| Indication | ||||||||||||
| – ICM (LVEF <35%) | 82 (43) | 22 (51) | 17 (45) | 14 (42) | 12 (44) | 17 (65) | 8 (36) | 11 (52) | 10 (50) | 8 (53) | 3 (23) | 2 (33) |
| – NICM (LVEF <35%) | 53 (27) | 9 (21) | 13 (34) | 13 (40) | 7 (26) | 5 (19) | 4 (18) | 2 (10) | 1 (5) | 2 (13) | 5 (38) | 1 (17) |
| – Congenital/inherited heart disease | 7 (4) | 0 (0) | 1 (3) | 1 (3) | 2 (7) | 1 (4) | 2 (9) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| – Bridging to ICD implant or HTX | 18 (9) | 6 (14) | 0 (0) | 1 (3) | 1 (4) | 1 (4) | 3 (14) | 1 (5) | 5 (25) | 0 (0) | 1 (8) | 0 (0) |
| – Unknown arrhythmic risk | 32 (17) | 6 (14) | 7 (18) | 4 (12) | 5 (19) | 2 (8) | 5 (23) | 7 (33) | 3 (15) | 5 (34) | 4 (31) | 3 (50) |
| Baseline medication | ||||||||||||
| – Beta-blocker | 170 (89) | 39 (91) | 36 (95) | 30 (91) | 23 (85) | 24 (92) | 22 (100) | 21 (100) | 18 (90) | 15 (100) | 10 (77) | 6 (100) |
| – ACEI/ATIIB/ Sacubitril/valsartan |
169 (88) | 40 (93) | 33 (87) | 27 (82) | 22 (82) | 24 (92) | 15 (68) | 20 (95) | 18 (90) | 15 (100) | 11 (85) | 6 (100) |
| – Aldosterone antagonist* |
105 (55) | 34 (79) | 25 (66) | 16 (49) | 13 (48) | 23 (89) | 14 (64) | 13 (62) | 12 (60) | 9 (60) | 10 (77) | 5 (83) |
| – Amiodarone | 43 (22) | 6 (14) | 3 (8) | 6 (18) | 6 (22) | 6 (23) | 0 (0) | 2 (10) | 4 (20) | 2 (13) | 2 (15) | 0 (0) |
EF = ejection fraction; ICM = ischaemic heart disease; NICM = nonischaemic heart disease; HTX = heart transplant; ACEI = ACE inhibitor; ATIIB = angiotensin II receptor antagonist. Results are reported as frequencies (percentage) or mean ± standard deviation. * Significant difference between prescribing hospitals detected (p <0.05).
The mean age in the total study population was 57 years (± 14), 81 patients (17.8%) were female, mean body mass index at therapy start was 26.9 kg/m2 (± 5.8) and mean EF at therapy start was 32% (± 13). Atrial fibrillation was diagnosed prior to therapy in 108 patients (24.7%). The most common indications for WCD use were ICM with an EF ≤35% (206 patients, 45.2%), NICM with an EF ≤35% (115 patients, 25.2%), unknown arrhythmic risk (83 patients, 18.2%), bridging to ICD implantation or heart transplant (37 patients, 8.1%) and congenital/inherited heart disease (15 patients, 3.3%). After exploratory analysis, significant differences among prescribing hospitals were found in gender distribution, baseline EF and the proportion of patients treated with a mineralocorticoid antagonists (MRA). This difference for the use of MRA significantly when patients with an EF ≤35% were compared (p = 0.003).
The median wear duration in the total study population was 58 days (IQR 31–94, range 1–455) with a median average daily wear-time of 22.6 hours (IQR 20–23.2, range 0.7–23.8). This observational period sums up to a total of 1005 person-months (table 2). Overall, 217 patients (47.6%) had a wear duration longer than 60 days.
Table 2 Wearable cardioverter defibrillator (WCD) data for the study population.
| Hospital | USZ | USB | KSW | HUG | KSSG | Triemli | CHUV | KSA | Inselspital | GZO | LUKS | KSGR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 192 (42) | 43 (9) | 38 (8) | 33 (7) | 27 (6) | 26 (6) | 22 (5) | 21 (5) | 20 (5) | 15 (3) | 13 (3) | 6 (1) |
| Wear duration (days, IQR) | 63 (35–98) |
71 (18–99) |
54 (28–94) |
40 (23–57) |
42 (15–99) |
73 (38–101) |
44 (21–89) |
76 (45–94) |
47 (18–84) |
57 (41–85) |
77 (50–100) |
43 (10–69) |
| Average daily wear hours (IQR) |
22.6 (19.8–23.2) |
22.8 (19.8–23.4) |
22.6 (18.2–23.3) |
22.5 (19.7–23.1) |
22.3 (20.6–23.3) |
22.6 (20.8–23.3) |
22.4 (21.4–22.9) |
22.9 (19.9–23.2) |
22.3 (18.3–23.1) |
22.8 (22–23.1) |
22.5 (21–23.3) |
21 (17–23.2) |
| Person-months | 423.2 | 94.8 | 83.8 | 72.7 | 59.5 | 57.3 | 48.5 | 46.3 | 44.1 | 33.1 | 28.7 | 13.2 |
| Total WCD treatments |
3 | 4 | 0 | 0 | 2 | 0 | 2 | 0 | 4 | 0 | 0 | 2 |
| WCD treatments (%) | 3 (1.6) | 3 (7) | 0 (0) | 0 (0) | 1 (3.7) | 0 (0) | 1 (4.5) | 0 (0) | 3 (15) | 0 (0) | 0 (0) | 1 (16.7) |
| Treatment incidence per 3 person-months | 2.13 | 12.66 | 0 | 0 | 10.08 | 0 | 12.37 | 0 | 27.22 | 0 | 0 | 45.37 |
Results are reported as frequencies (percentage).
A total of 17 WCD treatments were administered in the total population (treatment rate 3.7%) to a total of 12 patients (2.6% of all patients). The incident appropriate treatment rate per 3 months in the total study population was 5 events per 100 persons over a 3-month wear duration. The range of treatment incidence among the participating hospitals was 0 (50% of all participating hospitals) to 45.37 (table 2). Five patients each received two treatments by the WCD. All delivered treatments were appropriate; there were no inappropriate treatments during the study period (table 3).
Table 3 Patients with treatments by the wearable cardioverter defibrillator.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | USZ | USZ | CHUV | Inselspital | Inselspital | Inselspital | KSGR | KSSG | USB | USB | USZ | USB |
| Age (years) | 71 | 60 | 57 | 53 | 63 | 71 | 46 | 74 | 66 | 82 | 64 | 47 |
| Sex | Male | Male | Female | Male | Male | Male | Male | Male | Female | Male | Male | Male |
| EF before WCD (%) | 27 | 13 | 28 | 35 | 25 | 20 | 25 | 22 | 22 | 25 | 25 | 20 |
| Indication for WCD | Bridging | NICM | ICM | ICM | ICM | ICM | ICM | ICM | ICM | Bridging | Bridging | ICM |
| Atrial fibrillation | No | Yes | No | No | Yes | No | No | Yes | No | No | No | No |
| Wear days | 18 | 72 | 15 | 11 | 14 | 38 | 2 | 5 | 5 | 79 | 40 | 17 |
| Average wear hours | 20.18 | 23.1 | 22.66 | 21.91 | 17.78 | 22.78 | 10.77 | 18.61 | 19.56 | 23.38 | 22.28 | 22.42 |
| Treatments by WCD (n) | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 2 |
| Treated arrhythmia | VT | VF | VT, VT | VT | VT, VT | VF | VT, VF | VT, VT | VT | VT | VT | VF, VF |
| Device implanted | S-ICD | CRT-D | SC-ICD | DC-ICD | DC-ICD | SC-ICD | SC-ICD | SC-ICD | SC-ICD | DC-ICD | SC-ICD | SC-ICD |
| First ICD therapy after implantation | Appropriate shock | none | Appropriate ATP | Appropriate shock | none | Appropriate shock | none | none | none | none | none | none |
CRT = cardiac resynchronisation therapy; DC = dual-chamber; EF = ejection fraction; ICM = ischaemic cardiomyopathy; NICM = nonischaemic cardiomyopathy; S = subcutaneous; SC = single-chamber; WCD = wearable cardioverter defibrillator; VF = ventricular fibrillation; VT = ventricular tachycardia
Thirteen treatments (13/17, 76.5%) were delivered to patients with ICM and EF ≤35%, three treatments (3/17, 17.7%) to patients waiting for ICD implantation or heart transplantation (“bridging”) and one treatment to a patient suffering from NICM with EF ≤35% (1/17, 5.9%). The incident appropriate treatment rate per 100 persons per 3 months was 8.6, 11 and 1.2 for ICM, bridging and NICM, respectively. The mean age of patients with treatments was 63 years old (± 11), 2 of the patients were female (16.7%), they had a mean EF of 24% (± 5) and wore the device for a median of 16 days (IQR 8–39) with an average daily wear time of 22.1 hours (IQR 19.1–22.7). The clinical characteristics of patients with treatments varied among prescribing hospitals (table 3). There was no significant difference between with and without treatments in any of the baseline characteristics including the rate of prior myocardial infarction, any type of revascularisation (percutaneous coronary intervention of aorto-coronary bypass surgery) and coexisting moderate to severe valvulopathy (table S1 in appendix 1).
Of the total study population, 271 patients (47.6%) had a prolonged WCD use (>60 days). There was no significant difference in the rate of prolonged WCD use between hospitals, ranging from 21% (HUG) to 61.5% (LUKS). Of the 12 patients receiving treatments two wore the device longer than 60 days (72 and 79 days).
After discontinuation of WCD use, 212 patients (46.5% of the total study population) were implanted with an ICD, whereas 183 patients (40.1%) no longer had an indication for ICD implantation due to either improvement in EF, disappearance of elevated arrhythmic risk or other reasons (table 4). The remaining patients either refused ICD implantation (20 patients, 4.4%), or had other reasons for no ICD implantation such as end-of-life care, unknown reasons (37 patients, 8.1%), or loss to follow-up (4 patients, 0.8%).
Table 4 Rate of implantable cardioverter defibrillator (ICD) insertion after wearable cardioverter defibrillator (WCD) use.
| Hospital | USZ | USB | KSW | HUG | KSSG | Triemli | CHUV | KSA | Inselspital | GZO | LUKS | KSGR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD implanted | 89 (46.4) | 21 (48.8) | 15 (39.5) | 14 (42.4) | 15 (55.6) | 10 (38.5) | 13 (59.1) | 4 (19) | 13 (65) | 10 (66.7) | 5 (38.5) | 3 (50) |
| No ICD implanted | ||||||||||||
| – Not indicated | 77 (40.1) | 10 (23.3) | 22 (57.9) | 13 (39.4) | 11 (40.7) | 10 (38.5) | 5 (22.7) | 17 (81) | 7 (35) | 5 (33.3) | 4 (30.8) | 2 (33.3) |
| – Patient refusal | 10 (5.2) | 4 (9.3) | 0 | 1 (3) | 1 (3.7) | 2 (7.6) | 0 | 0 | 0 | 0 | 1 (7.7) | 1 (16.7) |
| Other | 14 (7.3) | 7 (16.3) | 1 (2.6) | 5 (15.2) | 0 | 4 (15.4) | 3 (13.6) | 0 | 0 | 0 | 3 (23) | 0 |
| – Lost to follow-up | 2 (1) | 1 (2.3) | 0 | 0 | 0 | 0 | 1 (4.6) | 0 | 0 | 0 | 0 | 0 |
Other reasons included change of therapy to best supportive care, patient deceased, technical difficulties with implantation and unknown reasons. Results are reported as frequencies (percentage).
The WCD has established itself throughout Switzerland for prevention of sudden cardiac death in patients perceived to be at high risk. In this study, for the first time we describe the patient population using the complete Swiss WCD Registry.
We report an incident appropriate treatment rate for the total Swiss WCD population of 5 per 100 persons over 3 months. In a meta-analysis, Masri et al. investigated differences in treatment incidences between all studies reporting WCD treatments [8]. They reported a pooled incidence from all included studies of 5 per 100 persons over 3 months, similar to our findings. They noted, however, a large range in treatment incidences and also significant heterogeneity among the included studies regarding study population size and indications for WCD use. We similarly found a large variance for treatment incidence in the hospitals included in our study. This variance could be attributed to the different, and in some cases low, patient numbers leading to frequent numerical outliers, which make the use of deductive statistical analysis impractical. USZ, the hospital with the highest number of WCD prescriptions, reported a low treatment incidence in comparison with the other prescribing hospitals with at least one treatment. Of note, no patients with ICM received a treatment in this subpopulation. Although there was a significant difference in EF in the included centres, the overall guideline-oriented heart failure therapy was established in the vast majority of the total study population without relevant variations between participating centres. Ultimately, no significant difference in any baseline characteristics of patients treated at USZ as compared with all other hospitals explained this difference in treatment incidence.
The significantly higher baseline EF may partially explain the lower treatment incidence observed at USZ compared with other prescribing hospitals with at least one treatment, since in particular patients suffering from ICM with lower EF seemed to benefit more from an ICD, and no patient suffering from ICM received a treatment by the WCD in the USZ subpopulation [9, 10]. Of note, the average LVEF at USZ for this subpopulation was 32% (± 13), which was, however, still higher than the 28% (± 6) reported in the VEST trial. The difference in heart failure medication was also investigated to identify reasons for the difference in treatment incidence. The overall guideline-oriented heart failure therapy, however, was established and optimised in the vast majority of the total study population without relevant differences among participating centres.
Although patients wearing the WCD for bridging had the highest treatment incidence in our study population, these patients have had an established indication for ICD implantation based on previous publications and current guidelines [4, 11–14], hence the term “bridging”. The efficacy of the WCD in this setting has previously been demonstrated, albeit only within the frame of observational studies [15, 16]. Although the largest subpopulation of patients with WCD therapy had NICM in the majority of published observational trials including patients with any indication for therapy [17–22], patients with ICM have been shown to be at higher arrhythmogenic risk even in the early post-myocardial infarction period [10]. In a large observational study investigating patients with ICM using a WCD, the incident-appropriate treatment rate was 7 per 100 persons over 3 months, and in the meta-analysis by Masri et al. 8 per 100 persons over 3 months of follow-up [8, 23]. Our findings were similar.
The only randomised controlled trial investigating WCD use puts our data and previously published observational studies in perspective. The VEST trial included patients with ICM and reduced ejection fraction and investigated whether WCD reduced the rate of sudden cardiac death [24]. The primary endpoint was negative, and they reported a low incident-appropriate treatment rate in the WCD arm (1 per 100 persons over 3 months). This treatment incidence was lower than had previously been reported from observational data and what we found for our population. One major difference between the VEST trial and existing observational data, including this study, is the adherence to therapy. Whereas “real-world” data have consistently shown a high compliance with WCD use [8], Olgin et al. reported a very low adherence to therapy with a mean daily wear-time of the WCD of 14 hours. Furthermore, 5.9% of screened patients died in the VEST trial before inclusion [24], similar to what was reported in the CARISMA study (6.4%), in which the prognostic relevance of arrhythmias in patients with ICM was investigated [25]. These patients most probably might have benefited most from wearing the WCD. On the other hand, the non-significant reduction in sudden cardiac death in the VEST trial probably indicates a competing, non-arrhythmic mortality rate in the investigated study population, similar to the DINAMIT trial [26]. The neutral findings of the VEST trial ultimately do not support routine use of WCD in patients suffering from ICM with reduced EF.
In contrast to the higher treatment incidence in patients suffering from ICM, we saw a lower treatment incidence in patients with NICM in our study population. Only one patient with NICM received a treatment reflecting an incident appropriate treatment rate of 1.2 per 100 persons over 3 months. In previous studies including only patients with NICM this incidence was similar (1–8 per 100 persons per 3 months) [8, 27–29]. There is a controversy regarding ICD indications for patients with NICM, which was further fuelled by the DANISH trial [30]. A recent meta-analysis, however, confirmed an overall benefit from ICD implantation even when the results of the DANISH trial were taken into account [31]. Probably because of the controversy around ICD implantation in NICM, very long wear durations have been reported in such patients. The presumed aim of prolonged WCD use was to avoid unnecessary ICD implantations. Although there have been reports of a decreasing incidence of ICD implantation after prolonged WCD use, during which heart failure therapy was optimised [27], the generally lower incidence of treatment with the WCD makes patient selection difficult. Obviously, data to support a general recommendation of WCD use for these individuals are lacking.
Similar to the high rate of optimal medical therapy of patients with reduced EF, WCD adherence was high in our study population without any significant regional differences and an average daily wear-time of 22.6 hours, which is similar to previous large observational data ranging from 21.7 to 23.1 hours [8, 16, 17, 22, 23]. The Swiss regulatory department responsible for medical devices (MiGeL) generally reimburses 30–60 days of WCD wear duration [3], and accordingly the median wear duration was 58 days in the total study population which is comparable to previous findings [8]. However, 47% of patients wore the WCD for longer than 60 days, and only two of these patients received a treatment (0.9% of all patients with prolonged WCD use). Kutyifa et al reported two patients out of 981 (0.2%) patients wearing the WCD >90 days receiving a treatment compared with 19 out of 1019 patients wearing the WCD ≤90 days [32]. Extended wear duration is commonly reported, but the treatment rate beyond 90 days of wear duration is very low [33]. These findings suggest that WCD wear duration may be further shortened without substantially compromising outcome. In our study population, median wear duration to a treatment was only 13 days in patients with ICM, in line with the findings of the VALIANT trial, which observed an elevated risk during the early post-myocardial infarction period [10]. Reduction in wear days could also lead to a significant reduction in treatment costs. Current pricing according to MiGeL is CHF 124 per day amounting to average costs per patient of CHF 7200, calculated with the median wear duration of 58 days. Nevertheless, independent of the cost of use, if a WCD is prescribed, high wear-compliance is essential to prevent a fatal outcome if ventricular arrhythmias arise [34, 35]. The efficacy of the WCD in patients with higher compliance with therapy was additionally proven in a recently published per protocol analysis of the VEST trial [36].
The purpose of the WCD is to prevent sudden cardiac death in patients at risk, but also to decrease the rate of unnecessary ICD implantations, especially in the presence of transient risk factors. We report an ICD implantation rate of 47% after termination of WCD use. Previously, smaller studies reported rates of between 32% and 55% [20, 27, 28, 37, 38]. The currently recruiting observational HF-Opt trial (NCT03016754) is investigating the possible use of the WCD to enable maximal up-titration of heart failure medication to further reduce the ICD implantation rate. In addition to the prevention of possibly unnecessary ICD implantations, this strategy may also be interesting in view of cost-effectiveness. Until hard clinical endpoint data using this approach are available, however, indications for ICD implantation should follow current guidelines [4].
The major limitation of our study is its retrospective and observational nature. Furthermore, the heterogeneity and relatively small patient numbers limit the impact of comparative statistical analysis. Nevertheless, our data provide useful real-life data on WCD use and efficacy in patients at risk for sudden cardiac death in Switzerland.
In the reported study period, the use of WCD was increasing in Switzerland with results comparable to previously published registry data. In light of the paucity of randomised controlled data supporting its use, careful and individualised patient selection is crucial, and standard operating procedures at prescribing hospitals may be of value.
Table S1 Comparison of patients with and without wearable cardioverter defibrillator treatment.
| Treatment by WCD | Yes | No |
|---|---|---|
| Age at therapy start (years) | 63 (11) | 57 (14) |
| Sex (female) | 2 (17) | 79 (18) |
| BMI | 27.7 (5.7) | 26.8 (5.8) |
| Indication for WCD use | ||
| – ICM and LVEF <35% | 8 (66.7) | 198 (44.6) |
| – NICM and LVEF <35% | 1 (8.3) | 114 (25.7) |
| – Congenital or inherited heart disease | 0 (0) | 15 (3.4) |
| – Bridging to ICD implant or HTX | 3 (25) | 34 (7.7) |
| – Risk stratification with LVEF >35% | 0 (0) | 83 (18.7) |
| LVEF before WCD (%) | 24 (5) | 32 (13) |
| Atrial fibrillation | 4 (33.3) | 104 (23.4) |
| Wear days of WCD | 26 (26) | 69 (53) |
| Average wear hours of WCD | 20.5 (3.6) | 20.8 (4.2) |
HTX = heart transplantation; ICM = ischaemic cardiomyopathy; LVEF = left ventricular ejection fraction; NICM = nonischaemic cardiomyopathy; WCD = wearable cardioverter defibrillator Values are reported as mean (± standard deviation) or number (percentage).
The authors thank ZOLL for providing data from the LifeVest Network. No further involvement of the manufacturer in data collection, statistical analysis or writing of the manuscript took place. The authors thank Carine Stettler for her assistance in the data collection.
No external funding was received for this study.
SR: educational grants from Abbott, Amgen, Bayer, Biotronik, Bristol-Myers Squibb, Daiichi Sankyo, Merck/MSD, Novartis, Sanofi-Aventis, Servier. CS: consultant and/or speaker fees from Abbott, Biosense Webster, Biotronik, Boston-Scientific, Medtronic, Microport and Zoll; research granting from Biotronik, Medtronic, Boston Scientific. BS: speaker’s fees from Zoll and Medtronic. AMS: educational grants from Abbott, Bayer, Biotronik, Biosense Webster, Boston Scientific, Medtronic, and Pfizer BMS; he owns stock from Gilead Sciences. TR: consultant and or speaker fees from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Medtronic, Pfizer-BMS and Roche, all for work unrelated to the submitted study; received support for his institution from Abbott/SJM, Biosense-Webster, Biotronik, Boston-Scientific and Medtronic for work unrelated to the submitted study. LR: consultant and/or speaker fees from Abbott and Medtronic. UE: received fellowship and training support from Boston Scientific, Medtronic, Abbott/St. Jude Medical, and Biosense-Webster. Speaker and/or consultant honoraria from Novartis, AstraZeneca, Daiichi Sankyo, Boehringer, Menarini, and Biotronik, as well as institutional research grants from MicroPort, Biotronik, Boston-Scientific; all unrelated to this work. ASM.: received fellowship and training support from Biotronik, Boston Scientific, Medtronik, Abbott/St. Jude Medical, and Biosense Webster and speaker and/or consultant honoraria from Biosense Webster, Medtronik, Abbott/St. Jude Medical, AstraZeneca, Daiichi Sankyo, Biotronik, MicroPort; all unrelated to this work. JS: consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Bayer, Berlin Chemie / Menarini, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston-Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, WebMD, and Zoll; he reports ownership of CorXL; grant support through his institution from Abbott, Bayer Healthcare, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, and Medtronic. FD: institutional grant support from Abbott, Amgen, Astra-Zeneca, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis and Zoll. LH reports grants from Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Biosense Webster, Biotronik, Boston Scientific, Bracco, B. Braun, Edwards Lifesciences, MicroPort, Novartis, Vascular Medical, Zoll, grants and personal fees from Daiichi-Sankyo and Medtronic, unrelated to the submitted work.
1Todesursachenstatistik 2017, Bundesamt für Statistik, 16.12.2019. Available from https://www.bfs.admin.ch/bfsstatic/dam/assets/11227248/master. 2019;1–2.
2 Auricchio A , Klein H , Geller CJ , Reek S , Heilman MS , Szymkiewicz SJ . Clinical efficacy of the wearable cardioverter-defibrillator in acutely terminating episodes of ventricular fibrillation. Am J Cardiol. 1998;81(10):1253–6. doi:.https://doi.org/10.1016/S0002-9149(98)00120-9
3MiGeL yearly report. Available from https://www.bag.admin.ch/bag/de/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Mittel-und-Gegenstaendeliste.html. 2020
4 Priori SG , Blomström-Lundqvist C , Mazzanti A , Blom N , Borggrefe M , Camm J , et al.; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867. doi:.https://doi.org/10.1093/eurheartj/ehv316
5 Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JGF , Coats AJS , et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
6 Kovacs B , Reek S , Saguner AM , Krasniqi N , Eriksson U , Duru F . Outcomes during and after the use of the wearable cardioverter-defibrillator in a tertiary-care and a regional hospital in Switzerland. Swiss Med Wkly. 2019;149:w20136. doi:.https://doi.org/10.4414/smw.2019.20136
7 Kovacs B , Reek S , Krasniqi N , Eriksson U , Duru F . Die tragbare Defibrillatorweste. Swiss Med Forum. 2018;18:509–12. doi:
8 Masri A , Altibi AM , Erqou S , Zmaili MA , Saleh A , Al-Adham R , et al. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol. 2019;5(2):152–61. doi:.https://doi.org/10.1016/j.jacep.2018.11.011
9 Moss AJ , Zareba W , Hall WJ , Klein H , Wilber DJ , Cannom DS , et al.; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi:.https://doi.org/10.1056/NEJMoa013474
10 Solomon SD , Zelenkofske S , McMurray JJV , Finn PV , Velazquez E , Ertl G , et al.; Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352(25):2581–8. doi:.https://doi.org/10.1056/NEJMoa043938
11 Kuck KH , Cappato R , Siebels J , Rüppel R . Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102(7):748–54. doi:.https://doi.org/10.1161/01.CIR.102.7.748
12 Connolly SJ , Gent M , Roberts RS , Dorian P , Roy D , Sheldon RS , et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101(11):1297–302. doi:.https://doi.org/10.1161/01.CIR.101.11.1297
13 Buxton AE , Lee KL , Fisher JD , Josephson ME , Prystowsky EN , Hafley G ; Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341(25):1882–90. doi:.https://doi.org/10.1056/NEJM199912163412503
14 Wyse DG , Friedman PL , Epstein AE ; Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576–84. doi:.https://doi.org/10.1056/NEJM199711273372202
15 Ellenbogen KA , Koneru JN , Sharma PS , Deshpande S , Wan C , Szymkiewicz SJ . Benefit of the Wearable Cardioverter-Defibrillator in Protecting Patients After Implantable-Cardioverter Defibrillator Explant: Results From the National Registry. JACC Clin Electrophysiol. 2017;3(3):243–50. doi:.https://doi.org/10.1016/j.jacep.2016.09.002
16 Zishiri ET , Williams S , Cronin EM , Blackstone EH , Ellis SG , Roselli EE , et al. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013;6(1):117–28. doi:.https://doi.org/10.1161/CIRCEP.112.973552
17 Kutyifa V , Moss AJ , Klein H , Biton Y , McNitt S , MacKecknie B , et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation. 2015;132(17):1613–9. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.015677
18 Chung MK , Szymkiewicz SJ , Shao M , Zishiri E , Niebauer MJ , Lindsay BD , et al. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol. 2010;56(3):194–203. doi:.https://doi.org/10.1016/j.jacc.2010.04.016
19 Opreanu M , Wan C , Singh V , Salehi N , Ahmad J , Szymkiewicz SJ , et al. Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: A national database analysis. J Heart Lung Transplant. 2015;34(10):1305–9. doi:.https://doi.org/10.1016/j.healun.2015.04.004
20 Erath JW , Vamos M , Sirat AS , Hohnloser SH . The wearable cardioverter-defibrillator in a real-world clinical setting: experience in 102 consecutive patients. Clin Res Cardiol. 2017;106(4):300–6. doi:.https://doi.org/10.1007/s00392-016-1054-1
21 Lamichhane M , Gardiner JC , Bianco NR , Szymkiewicz SJ , Thakur RK . National experience with long-term use of the wearable cardioverter defibrillator in patients with cardiomyopathy. J Interv Card Electrophysiol. 2017;48(1):11–9. doi:.https://doi.org/10.1007/s10840-016-0194-6
22 Wäßnig NK , Günther M , Quick S , Pfluecke C , Rottstädt F , Szymkiewicz SJ , et al. Experience With the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation. 2016;134(9):635–43. doi:.https://doi.org/10.1161/CIRCULATIONAHA.115.019124
23 Epstein AE , Abraham WT , Bianco NR , Kern KB , Mirro M , Rao SV , et al. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J Am Coll Cardiol. 2013;62(21):2000–7. doi:.https://doi.org/10.1016/j.jacc.2013.05.086
24 Olgin JE , Pletcher MJ , Vittinghoff E , Wranicz J , Malik R , Morin DP , et al.; VEST Investigators. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N Engl J Med. 2018;379(13):1205–15. doi:.https://doi.org/10.1056/NEJMoa1800781
25 Bloch Thomsen PE , Jons C , Raatikainen MJP , Moerch Joergensen R , Hartikainen J , Virtanen V , et al.; Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation. 2010;122(13):1258–64. doi:.https://doi.org/10.1161/CIRCULATIONAHA.109.902148
26 Hohnloser SH , Kuck KH , Dorian P , Roberts RS , Hampton JR , Hatala R , et al.; DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–8. doi:.https://doi.org/10.1056/NEJMoa041489
27 Duncker D , König T , Hohmann S , Bauersachs J , Veltmann C . Avoiding Untimely Implantable Cardioverter/Defibrillator Implantation by Intensified Heart Failure Therapy Optimization Supported by the Wearable Cardioverter/Defibrillator-The PROLONG Study. J Am Heart Assoc. 2017;6(1):e004512. doi:.https://doi.org/10.1161/JAHA.116.004512
28 Duncker D , König T , Hohmann S , Bauersachs J , Veltmann C . Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin Cardiol. 2017;40(8):586–90. doi:.https://doi.org/10.1002/clc.22706
29 Saltzberg MT , Szymkiewicz S , Bianco NR . Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J Card Fail. 2012;18(1):21–7. doi:.https://doi.org/10.1016/j.cardfail.2011.09.004
30 Køber L , Thune JJ , Nielsen JC , Haarbo J , Videbæk L , Korup E , et al.; DANISH Investigators. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375(13):1221–30. doi:.https://doi.org/10.1056/NEJMoa1608029
31 Shun-Shin MJ , Zheng SL , Cole GD , Howard JP , Whinnett ZI , Francis DP . Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta-analysis of 8567 patients in the 11 trials. Eur Heart J. 2017;38(22):1738–46. doi:.https://doi.org/10.1093/eurheartj/ehx028
32 Kutyifa V , Vermilye K , Daimee UA , McNitt S , Klein H , Moss AJ . Extended use of the wearable cardioverter-defibrillator in patients at risk for sudden cardiac death. Europace. 2018;20(FI2):f225–32. doi:.https://doi.org/10.1093/europace/euy091
33 Kovacs B , Reek S , Krasniqi N , Eriksson U , Duru F . Extended Use of the Wearable Cardioverter-Defibrillator: Which Patients Are Most Likely to Benefit? Cardiol Res Pract. 2018;2018:7373610. doi:.https://doi.org/10.1155/2018/7373610
34 Al-Khatib SM , Pokorney SD . Knowledge and Insights Vested in Us by the VEST. Circulation. 2018;138(24):2735–7. doi:.https://doi.org/10.1161/CIRCULATIONAHA.118.035390
35 Olgin JE , Lee BK , Vittinghoff E , Morin DP , Zweibel S , Rashba E , et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J Cardiovasc Electrophysiol. 2020;31(5):1009–18. doi:.https://doi.org/10.1111/jce.14404
36 Olgin JE , Lee BK , Vittinghoff E , Morin DP , Zweibel S , Rashba E , et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J Cardiovasc Electrophysiol. 2020;31(5):1009–18. doi:.https://doi.org/10.1111/jce.14404
37 Leyton-Mange JS , Hucker WJ , Mihatov N , Reynolds M , Albert C , Lubitz SA , et al. Experience With Wearable Cardioverter-Defibrillators at 2 Academic Medical Centers. JACC Clin Electrophysiol. 2018;4(2):231–9. doi:.https://doi.org/10.1016/j.jacep.2017.09.180
38 Rosenkaimer SL , El-Battrawy I , Dreher TC , Gerhards S , Röger S , Kuschyk J , et al. The Wearable Cardioverter-Defibrillator: Experience in 153 Patients and a Long-Term Follow-Up. J Clin Med. 2020;9(3):893. doi:.https://doi.org/10.3390/jcm9030893
No external funding was received for this study.
SR: educational grants from Abbott, Amgen, Bayer, Biotronik, Bristol-Myers Squibb, Daiichi Sankyo, Merck/MSD, Novartis, Sanofi-Aventis, Servier. CS: consultant and/or speaker fees from Abbott, Biosense Webster, Biotronik, Boston-Scientific, Medtronic, Microport and Zoll; research granting from Biotronik, Medtronic, Boston Scientific. BS: speaker’s fees from Zoll and Medtronic. AMS: educational grants from Abbott, Bayer, Biotronik, Biosense Webster, Boston Scientific, Medtronic, and Pfizer BMS; he owns stock from Gilead Sciences. TR: consultant and or speaker fees from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Medtronic, Pfizer-BMS and Roche, all for work unrelated to the submitted study; received support for his institution from Abbott/SJM, Biosense-Webster, Biotronik, Boston-Scientific and Medtronic for work unrelated to the submitted study. LR: consultant and/or speaker fees from Abbott and Medtronic. UE: received fellowship and training support from Boston Scientific, Medtronic, Abbott/St. Jude Medical, and Biosense-Webster. Speaker and/or consultant honoraria from Novartis, AstraZeneca, Daiichi Sankyo, Boehringer, Menarini, and Biotronik, as well as institutional research grants from MicroPort, Biotronik, Boston-Scientific; all unrelated to this work. ASM.: received fellowship and training support from Biotronik, Boston Scientific, Medtronik, Abbott/St. Jude Medical, and Biosense Webster and speaker and/or consultant honoraria from Biosense Webster, Medtronik, Abbott/St. Jude Medical, AstraZeneca, Daiichi Sankyo, Biotronik, MicroPort; all unrelated to this work. JS: consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Bayer, Berlin Chemie / Menarini, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston-Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, WebMD, and Zoll; he reports ownership of CorXL; grant support through his institution from Abbott, Bayer Healthcare, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, and Medtronic. FD: institutional grant support from Abbott, Amgen, Astra-Zeneca, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis and Zoll. LH reports grants from Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Biosense Webster, Biotronik, Boston Scientific, Bracco, B. Braun, Edwards Lifesciences, MicroPort, Novartis, Vascular Medical, Zoll, grants and personal fees from Daiichi-Sankyo and Medtronic, unrelated to the submitted work.