Patiromer and medication optimisation in heart failure with reduced ejection fraction: a Swiss perspective
DOI: https://doi.org/10.4414/smw.2020.20362
Philippe
Meyera, Henri
Lub, Roger
Hullinb
aCardiology Service,
bCardiology, Cardiovascular Department,
Patiromer and medication optimisation in heart failure with reduced ejection fraction: a Swiss perspective
Summary
Despite medical advances, heart failure remains a major public health issue and is associated with considerable morbidity and mortality. Suboptimal use of evidence-based therapies and lack of medication up-titration play important roles in this regard. Hyperkalaemia is a frequent and potentially harmful finding which hinders treatment optimisation in patients with heart failure. In this review, heart failure experts from two Swiss academic hospitals discuss the principles of general pharmacological therapy in heart failure with reduced ejection fraction and the different treatment options for chronic hyperkalaemia, focusing on patiromer, a recently available potassium binder. Patiromer has been accepted for reimbursement since 1 August 2020 in Switzerland, and has been shown in several clinical trials to safely reduce potassium levels over the long term, thereby allowing up-titration or maintenance of renin-angiotensin-aldosterone inhibitors in patients with chronic kidney disease, including those with heart failure. Whether this promising approach improves outcomes in patients with heart failure and reduced ejection fraction is currently under investigation.
Clinical vignette
A 72-year-old patient followed by our heart failure outpatient clinic was seen after an acute heart failure episode triggered by paroxysmal atrial fibrillation, which was converted to sinus rhythm during the hospitalisation. He had a long history of ischaemic heart disease, with multiple percutaneous coronary interventions, but no residual myocardial ischaemia on a recently performed cardiac positron emission computed tomography scan. Left ventricular ejection fraction (LVEF) was calculated at 28% by echocardiography, with mild mitral regurgitation. One year before, a biventricular defibrillator had been implanted. Relevant comorbidities were type 2 diabetes mellitus and stage 3 chronic kidney disease (CKD). During hospitalisation, the patient developed hyperkalaemia up to 6.1 mmol/l, which resolved after spironolactone discontinuation.
At the first consultation, the patient still had New York Heart Association (NYHA) class III dyspnoea. Current medications included: rivaroxaban 15 mg once daily (o.d.), sacubitril-valsartan 200 mg twice daily (b.i.d.), carvedilol 12.5 mg b.i.d., torasemide 10 mg o.d., dapagliflozin 10 mg o.d. and metformin 1000 mg b.i.d. On physical examination, sitting blood pressure was 106/68 mm Hg, standing blood pressure was 103/70 mm Hg standing, and heart rate was 62 bpm. There were no signs of congestion. Laboratory tests gave the following results: sodium 137 mmol/l, potassium (K+) 5.2 mmol/l, serum creatinine 183 µmol/l, estimated glomerular filtration rate (eGFR) 35 ml/min/1.73m2, and N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) 2835 ng/l. An electrocardiogram showed sinus rhythm at 62 bpm with resynchronised ventricular pacing and a QRS duration of 120 ms.
This patient was mildly symptomatic and, despite being euvolaemic, still had elevated NT-pro-BNP levels. Heart failure medication was at the maximum tolerated doses, but did not include a mineralocorticoid receptor antagonist (MRA) because of the past history of hyperkalaemia. We thus decided to reintroduce spironolactone at a dose of 12.5 mg o.d., combined with a novel potassium binder, patiromer, at a dose of 8.4 g o.d. One week later, there was no symptomatic hypotension or deterioration of renal function, and the patient’s K+ level remained at 5.2 mmol/l. Three weeks later, patiromer was increased to 16.8 g o.d. and spironolactone to 25 mg o.d. After three months, the patient was in NYHA class II, NT-proBNP decreased to 1744 ng/l. K+ levels did not exceed 5.4 mmol/l during follow-up.
Introduction
It is estimated that approximately 200,000 patients suffer from heart failure in Switzerland, based on surveys in neighbouring countries [1]. Due to the general aging of the population and the improving survival of patients with cardiovascular diseases and especially ischaemic heart disease, its prevalence seems likely to increase in our country [2]. Heart failure has become the most important cause of hospitalisation in elderly patients in Switzerland [3], as in other developed countries, and it remains associated with a 5-year mortality rate of approximately 50% despite recent medical advances [4]. Another main issue with heart failure is recurrent hospitalisations, which not only impact on the quality of life and the overall prognosis of the individual patient, but also represent an enormous healthcare burden at the population level [5]. Among the underlying precipitant factors behind readmissions, suboptimal use of evidence-based therapies in heart failure with reduced LVEF (HFrEF) seems to play a major role [5]. This review will discuss the role of hyperkalaemia as a limiting factor for optimising guideline-directed medical therapy in HFrEF and the potential benefits of strategies aimed at reducing K+ levels in this context, focusing on the newly reimbursable potassium binder patiromer.
Pharmacological treatment and up-titration in heart failure with reduced ejection fraction
Over the last four decades, many different pharmacological therapies have been shown, in large-scale randomised clinical trials (RCTs), to improve outcomes including symptom burden, quality of life, functional capacity and, most importantly, hospitalisations and mortality in HFrEF. This therapeutic armamentarium mainly acts by inhibiting the renin-angiotensin-aldosterone system (RAAS) and the sympathetic system, which are overstimulated in HFrEF, and these therapies are therefore often referred to as neurohormonal antagonists. Current guidelines recommend the stepwise introduction of neurohormonal antagonists, namely beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-II receptor blockers (ARBs) or angiotensin receptor neprilysin inhibitors (ARNIs), and MRAs [6]. Each RCT testing these molecules was based on forced up-titration protocols aiming to achieve specified target doses. Therefore, current guidelines recommend progressively increasing the dosages of all neurohormonal antagonists to the maximum tolerated doses in HFrEF [6]. This principle is reflected in the well-known clinicians’ motto “start low, go slow, aim high”.
However, neurohormonal antagonists are rarely used at target doses in clinical practice. European observational data indicate that only 29% and 18% of study participants were on target doses of ACEIs and beta-blockers, respectively [7]. In an analysis of 3518 HFrEF patients from 150 primary care and cardiology practices in the United States, the percentages of patients not treated with ACEIs/ARBs/ARNIs, beta-blockers or MRAs and without documented contraindications were 26.2%, 32.9% and 65.9%, respectively, whereas the percentages of patients on target doses for these drug classes were 16.8%, 27.5% and 76.6%, respectively [8]. Reasons for the non-prescription of guideline-recommended medications have been well investigated in QUALIFY, an international, prospective, observational, longitudinal survey of 7092 outpatients with HFrEF [9]. Hyperkalaemia was the reason for the non-prescription of ACEIs, ARBs and MRAs in 3.9%, 5.5% and 31.4% of the patients, respectively. Other barriers to heart failure treatment implementation have been specifically addressed in a recent Swiss consensus paper [10].
This gap between recommendations and practice translates into worse outcomes. Two RCTs have found that high doses of ACEIs and ARBs are superior to lower doses with regard to mortality outcomes [11, 12]. In the European prospective observational study BIOSTAT-CHF, patients on less than 50% of the recommended doses of ACEIs/ARBs and beta-blockers had an increased risk of death and/or heart failure hospitalisation compared to those on higher doses, even after adjustment for potential confounders [13]. This association of low doses of RAAS inhibitors with poor outcomes was consistent across all the whole age spectrum, according to a recent analysis of the same study [14]. All measures able to overcome barriers to drug up-titration are therefore of great importance. In routine clinical practice, much time is spent convincing patients and their general practitioners that medication up-titration is important despite an apparently stable clinical condition.
Hyperkalaemia in heart failure with reduced ejection fraction
According to a recent consensus document, hyperkalaemia is defined as a K+ level >5 mmol/l and is classified as mild (>5.0 to <5.5 mmol/l), moderate (5.5 to 6.0 mmol/l) or severe (>6.0 mmol/l) [15]. Hyperkalaemia is a frequent finding in patients with HFrEF. In recent large RCTs involving RAAS inhibitors, the incidences of hyperkalaemia >5.5 mmol/l for enalapril, sacubitril/valsartan and eplerenone were 17.3%, 16.1% and 12%, respectively, while hyperkalaemia >6.0 mmol/l was observed in 5.6%, 4.3% and 2.5% of cases, respectively [16, 17]. These numbers are quite significant in view of the carefully selected and monitored trial populations. A recent population-based cohort study from Denmark reported incidence rates of hyperkalaemia >5.0 mmol/l and >5.5 mmol/l of 39% and 20%, respectively, in 31,649 patients with newly diagnosed heart failure during a mean follow-up of 2.2 years [18]. Predictors of hyperkalaemia were CKD, diabetes mellitus and use of MRAs. Swiss data on hyperkalaemia are available from a secondary analysis of the TIME-CHF trial in 566 elderly heart failure patients, mostly with reduced LVEF, who received optimal medical therapy [19]. Over a follow-up of 18 months, 13.4% of the patients presented with moderate hyperkalaemia (>5.5 mmol/l) and 4.9% with severe hyperkalaemia (>6.0 mmol/l). High baseline K+, gout, CKD, higher NYHA class, spironolactone baseline dose and up-titration were identified as independent predictors of hyperkalaemia.
Abnormal K+ levels have a profound effect on the membrane excitability of cardiomyocytes which may result in life-threatening arrhythmias and conduction disturbances. This may explain the increased mortality rate in HFrEF patients with hyperkalaemia, especially hyperkalaemia above 5.5 mmol/l, reported in post-hoc analyses of the RALES and EMPHASIS-heart failure RCTs, which tested spironolactone and eplerenone, respectively [20, 21] However, in both studies the benefits of MRAs compared to a placebo were maintained across the whole spectrum of K+ levels. The magnitude of the impact of hyperkalaemia was further investigated in a large Danish registry of 19,549 HFrEF patients, where K+ levels between 5.1 and 5.5 mmol/l and between 5.6 and 7.4 mmol/l were associated with 1.6 and 3.3 times higher short-term mortality risks (90 days), respectively, compared to normal K+ levels (4.2 to 4.4 mmol/l) [22]. These results are consistent with other observational data from Denmark reporting a 3.3 times higher mortality risk at 6 months in patients with hyperkalaemia >5.0 mmol/l compared to matched patients without hyperkalaemia [18]. Interestingly, more recent observational data from 9222 chronic heart failure patients of the ESC-HFA-EORP heart failure long-term registry suggest that hyperkalaemia may be a risk marker for the discontinuation of RAAS inhibitors rather than an independent predictor of mortality in patients with heart failure [23].
Several strategies to treat chronic hyperkalaemia are currently available (fig. 1
). A simple first approach is restricting dietary potassium intake. For example, salt substitutes with a high potassium content should be avoided. However, there are no studies demonstrating the benefits of low-potassium diets in chronic hyperkalaemia. In contrast, many observational studies in patients with CKD report an association of low dietary potassium intake with worse outcomes and higher mortality [24]. This may be explained by the fact that healthy diets, such as a typical Mediterranean diet, are rich in fruit and vegetables, and consequently in potassium. Loop diuretics represent another option for reducing K+ levels, since these drugs increase urinary potassium excretion. Yet guidelines for chronic stable heart failure recommend the application of loop diuretics at the lowest doses necessary to maintain fluid balance, based on the absence of benefits on cardiovascular outcomes and the risk of negative effects on renal function and electrolytes [6]. Finally, lowering the dosage of drugs impairing potassium excretion may also reduce K+ levels. All drugs with nephrotoxic potential should be avoided. For example, non-steroidal anti-inflammatory drugs are contraindicated in heart failure since renal dysfunction is almost always associated with hyperkalaemia. Current heart failure guidelines recommend halving RAAS inhibitor doses if K+ levels increase to >5.5 mmol/l, monitoring K+ levels closely, and stopping RAAS inhibitor dosing in the case of hyperkalaemia >6 mmol/l [6]. However, this constitutes a real potassium dilemma in clinical practice, since patients developing hyperkalaemia are often those who could potentially benefit the most from maximum neurohormonal blockade. Switching ACEIs or ARBs to ARNIs may also be an option, not only to improve patients’ outcomes, but also to reduce severe hyperkalaemia events, as suggested by a secondary analysis of the PARADIGM-HF trial [25]. Finally, dapagliflozin, a potent therapy newly available for patients with HFrEF in Switzerland, does not interfere with the RAAS and thus may be favoured in patients with HFrEF and hyperkalaemia [26]. Notably, these last two strategies have not yet been studied specifically in patients with HFrEF and hyperkalaemia.

Figure 1 Strategies currently available to treat chronic hyperkalaemia and their limitations.
CPS = calcium polystyrene sulphonate; CKD = chronic kidney disease; HF = heart failure; K+ = potassium; RAAS = renin-angiotensin-aldosterone system; SPS = sodium polystyrene sulphonate.
Potassium binders
The first available potassium binders were synthetic cation-exchange resins. These have been used since 1950 and were approved by the US Food and Drug Administration (FDA) in 1958 to treat hyperkalaemia [27]. Two potassium-binding resins are currently available in Switzerland: calcium polystyrene sulphonate (CPS, Sorbisterit®) and sodium polystyrene sulphonate (SPS, Resonium®). These are insoluble polymers containing acidic structural units that can exchange cations on contact with a solution. Although they have been used for decades, their efficacy and safety have only ever been studied in two RCTs, one of 97 patients with CKD and one of 33 patients with hyperkalaemia, with limited follow-ups of 3 days and 7 days, respectively [28, 29]. Therefore, evidence for the efficacy of these drugs for long-term treatment of hyperkalaemia is lacking, and safety is a major concern since severe upper and lower gastrointestinal injuries, such as necrosis, ulcerations and perforations, have been associated with SPS use [30]. Caution is also advised in patients with severe heart failure due to the relatively high sodium content of SPS (approximately 100 mg sodium per gram of SPS) [31].
Sodium zirconium cyclosilicate is another potassium binder that has been developed more recently [32]. It has been tested in two phase III RCTs and is now approved by the FDA and the European Medicines Agency, but is not currently available in Switzerland and will therefore not be further discussed in the present review.
Patiromer
Patiromer is a sodium-free, organic, non-absorbed polymer. It is synthesised as 100 µm beads and binds potassium in exchange for calcium, predominantly in the colon, where potassium concentration is highest (fig. 2). It therefore increases faecal excretion of potassium and reduces K+ levels. It is usually administrated once daily as an oral powder with a neutral taste. It should be mixed with 80 ml of water or with apple or cranberry juice (low potassium content). The recommended starting daily dose is 8.4 g, and this can be adjusted on a weekly basis to a maximum dose of 25.2 g according to K+ levels and targets. The absorption of ciprofloxacin, levothyroxine, quinidine and metformin can be reduced when these are administered concomitantly with patiromer, but not if taken ≥3 hours apart. Patiromer was approved by the FDA in 2015 and by the European Medicines Agency in 2017. The number of patients treated by patiromer (Veltassa®) in Switzerland is not available, but more than 120,000 patients are currently treated worldwide. Since 1 August 2020, it has been accepted for reimbursement by Swissmedic. The cost of 30 8.4 g or 16.8 g sachets corresponds to CHF 255.
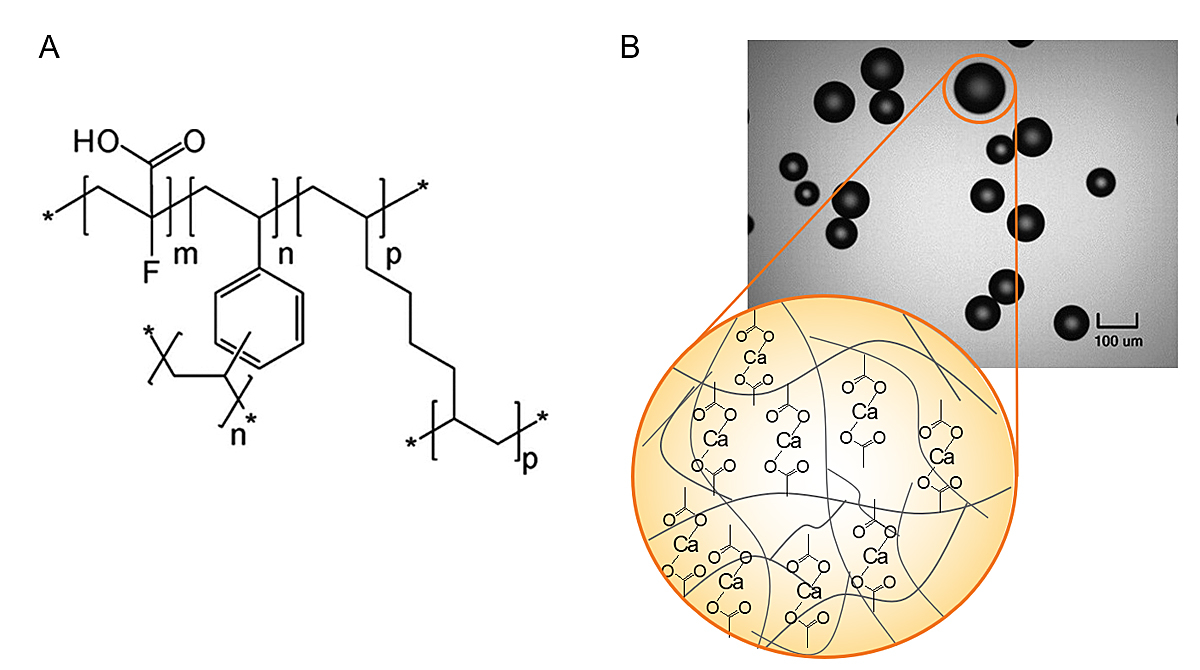
Figure 2 Structure of the novel potassium binder patiromer.
A. Chemical structure of patiromer anion (m indicates number of 2-fluoro-2-propenoic acid groups; n, p, indicate number of cross-linking groups. *An extended polymeric network.
B. Light microscopy images (×100 magnification) of patiromer. Adapted with the permission of SAGE from: Li L, et al. J Cardiovasc Pharmacol Ther. 2016;21(5):456–65.
Existing data with patiromer
Since 2011, patiromer has been tested in four multicentre RCTs, which are summarised in table 1 [33–36]. These trials mainly addressed patients with CKD, with the exception of the PEARL-HF study, which was conducted specifically in patients with heart failure [33]. Sub-analyses of the other three RCTs have been performed for heart failure patients, but these populations were not as well characterised as in heart failure trials [37–39].
Table 1 Summary of the four main clinical trials demonstrating the efficacy and safety of patiromer.
|
Study
|
Duration and design
|
Patient population
|
Baseline treatments
|
Outcomes
|
Adverse events
|
|
Pat
|
Pl
|
PEARL-HF
Pitt B, et al. Eur Heart J. 2011;32(7):820–8 [33] |
4 weeks
Randomised, double-blind, Pat (30 g/day) vs Pl |
105 normokalaemic patients with HF (including 11 with HFpEF, mean LVEF: 40%), indication to initiate spironolactone therapy and
– history of hyperkalaemia causing discontinuation of RAASi/BB,
or
CKD (eGFR <60 ml/min) and were receiving one or more HF therapies (ACEI, ARB, beta-blocker) |
ACEI or ARB or BB |
24% |
18% |
Pat decreased serum K+ levels (difference between groups of -0.45 mmol/l; p <0.001), lowered the incidence of hyperkalaemia (7 vs 25%), and enabled up-titration of patients’ spironolactone dose (91 vs 74%; p = 0.019). |
Mainly gastrointestinal: flatulence (7%), diarrhoea (5%), constipation (5%) and vomiting (4%), and mild or moderate in severity. |
| ACEI or ARB + BB |
73% |
76% |
| ACEI + ARB + BB |
4% |
2% |
| Diuretic |
75% |
74% |
OPAL-HK
Weir MR, et al. N Engl J Med. 2015; 372(3):211–21 [34] |
Phase A (treatment phase – 4 weeks): single-blind, single-arm
Phase B (withdrawal phase – 8 weeks): randomised, placebo-controlled, single-blind |
237 patients with CKD and hyperkalaemia who were on RAASi.
102 (42%) patients with HF (as per the investigators’ judgement).
|
ACEI |
67% |
73% |
Pat decreased K+ levels (mean change at the end of Phase A: −1.01 ± 0.03 mmol/l; p <0.001), reduced the recurrence of hyperkalaemia (15 vs 60%), and enabled more patients to continue their RAASi therapy (94 vs 44%).
Patients with HF: Pat significantly reduced:
– K+ level mean change at the end of Phase A: −1.01 ± 0.03 mmol/l; p <0.001,
– Recurrence of hyperkalaemia (8 vs 52%) at the end of Phase B.
Pat enabled more patients to continue their RAASi therapy (100 vs 55%). |
During phase A, mild-to-moderate constipation was the most common adverse event (11%).
During phase B, the proportion of patients with >1 adverse events was similar in the Pl and Pat groups (50% and 47%, respectively). |
| ARB |
44% |
31% |
| MRA |
7% |
8% |
| Dual RAAS blockade |
18% |
12% |
| Non-RAASi diuretic |
51% |
52% |
AMETHYST-DN
Bakris G, et al. JAMA. 2015; 314:151-61 [35] |
52 weeks
Open-label, with 3 randomised starting doses |
306 patients with type 2 diabetes, CKD, mild to moderate hyperkalaemia, all under RAASi therapy.
105 (35%) patients with HF: LVEF <40%: 26 (8%) patients.
|
ACEI |
49.3% |
Pat starting doses of 4.2 to 16.8 g b.i.d. decreased K+ levels after 4 weeks
– 0.35 (95%CI, 0.22–0.48) mmol/l for 4.2 g;
– 0.51 (95%CI, 0.38–0.64) mmol/l for 8.4 g
– 0.55 (95%CI, 0.42–0.68) mmol/l for 12.6 g, for patients with mild hyperkalaemia;
– 0.87 (95%CI, 0.60–1.14) mmol/l for 8.4 g,
– 0.97 (95%CI, 0.70–1.23) mmol/l the 12.6 g,
– 0.92 (95%CI, 0.67–1.17) mmol/l for 16.8 g for patients with moderate hyperkalaemia.
Patients with HF: At week 4, least squares mean reductions in K+ levels were
– 0.64 mmol/l (mild baseline hyperkalaemia)
– 0.97 mmol/l (moderate baseline hyperkalaemia). |
Hypomagnesaemia (7.2%) was the most common adverse event followed by mild to moderate constipation (4.6%), and diarrhoea (2.7%). |
| ARB |
24.3% |
| MRA |
0.3% |
| Non-RAASi diuretic |
42.8% |
AMBER
Agarwal R, et al. Lancet. 2019; 394(10208):1540–50 [36] |
12 weeks
Randomised, double-blind, placebo-controlled trial |
295 patients with CKD and resistant hypertension (despite >3 antihypertensive drugs), all treated with spironolactone.
132 (45%) patients with HF
– HFrEF: 55 patients
– HFpEF: 54 patients. |
BB |
59% |
58% |
Pat decreased serum K+ levels, reduced the recurrence of hyperkalaemia (p <0.0001), and enabled more patients to remain on spironolactone (86 vs 66%, 95% CI 10·0–29·0; p <0·0001).
Patients with HF: At week 12, 84.1% vs. 68.1% of patients remained on spironolactone (p = 0.0504). |
The most common adverse event was diarrhoea, which occurred in a similar proportion of patients in each treatment group (5 to 6%). |
| RAASi |
100% |
99% |
| Diuretics |
99% |
98% |
| CCB |
73% |
72% |
The randomised, double-blind PEARL-HF trial included 105 chronic heart failure patients with an indication to initiate spironolactone therapy [33]. Eleven patients (six in the patiromer arm and five in the placebo arm) had heart failure with preserved ejection fraction, defined as having an LVEF >50%. Overall, the LVEFs of the two groups were comparable (means of around 40%). The patients had normal baseline K+ levels (4.3–5.1 mmol/l) and either CKD with an eGFR <60 ml/min/1.73m2 or a history of hyperkalaemia that had required the discontinuation of RAAS inhibitors in the last 6 months. Patients were assigned to patiromer or placebo before spironolactone initiation (25 mg/day) and further up-titration (50 mg/day) after two weeks if K+ remained ≤5.1 mmol/l. Spironolactone up-titration was successful in 91% of patients in the patiromer arm compared to 74% in the placebo arm (p = 0.019).
The OPAL-HK study included 237 patients with CKD (eGFR from 15 to <60 ml/min/1.73 m2) and hyperkalaemia (K+ levels from 5.1 to <6.5 mmol/l), all of whom were receiving RAAS inhibitors [34]. In the first, 4-week phase, all patients received patiromer 4.2 g or 8.4 g twice daily. Study participants with moderate to severe baseline hyperkalaemia who reached target K+ levels (3.8 to <5.1 mmol/l) were then randomly assigned to either continuing patiromer or switching to a placebo during a second, 8-week phase. After 4 weeks, the mean reduction in K+ levels was 1.01 mmol/l, with 76% of patients achieving target K+ levels.
A sub-analysis of OPAL-HK was performed in 102 (42%) patients with a clinical diagnosis of heart failure, as per the investigators’ judgement [38]. The mean reduction in K+ from baseline to week four was 1.06 mmol/l, and 76% achieved target K+ levels. At the end of the withdrawal phase, 52% of patients in the placebo arm had at least one hyperkalaemic episode compared to 8% in the continuing patiromer arm. Only 55% of patients in the placebo arm were still on RAAS inhibitors at the end of the study, compared to 100% in the treatment arm.
AMETHYST-DN was a phase II, multicentre, open-label, dose-ranging RCT that included 306 outpatients with type 2 diabetes mellitus, CKD (eGFR from 15 to <60 ml/min/1.73 m2) and hyperkalaemia (>5.0 mmol/l), all of whom were treated by at least one RAAS inhibitor [35]. A 4-week run-in period with optimisation of RAAS inhibition therapy was performed in a subgroup of normokalaemic patients. Then, all hyperkalaemic patients were randomly assigned to different starting doses of patiromer (8.4 g to 33.6 g per day), which could be adjusted to maintain normal K+ levels (3.8 to 5.0 mmol/l) during a total follow-up of 52 weeks. Depending on baseline K+ levels and patiromer dose, least squares mean reductions in K+ levels from baseline to week four ranged from 0.35 to 0.97 mmol/l. The proportion of patients with normal K+ levels at each of the scheduled visits ranged from 77.4% to 95.1%, and adherence during the 52 weeks was high, with >90% of patients continuing patiromer treatment. Over the entire 52-week study, the mean daily patiromer doses were 19.4 ± 9.1 and 27.2 ± 10.8 g/day in patients with mild and moderate hyperkalaemia, respectively.
A sub-analysis of the AMETHYST-DN trial was performed in the 105 (35%) patients with clinical heart failure, 26 of whom had an LVEF <40% [37]. At week four, the least squares mean reductions in K+ levels were 0.64 and 0.97 mmol/l in patients with mild and moderate baseline hyperkalaemia, respectively. From week 12 to week 52, the majority of patients, >88% and >73% in patients with mild and moderate baseline hyperkalaemia, respectively, maintained normal K+ levels at each visit.
The AMBER study was a phase II multicentre, randomised, double-blind, placebo-controlled study that included 295 patients with CKD (eGFR 25 to 45 ml/min/1.73 m2) and resistant hypertension, but normal baseline K+ levels (4.3 to 5.1 mmol/l) [36]. Patients were randomly assigned to receive either placebo or patiromer (8.4 g o.d.) in addition to open-label spironolactone on top of all their baseline blood pressure medications. At the end of the 12-week follow-up, the proportion of patients remaining on spironolactone was 86% in the patiromer group compared to 66% in the placebo group (p <0.0001), with 35% of patients in the patiromer group and 65% of patients in the placebo group having experienced a hyperkalaemia event.
In 2020, a pre-specified subgroup analysis of the AMBER study in patients with a clinical history of heart failure was published [39]. Sixty-three of the 132 heart failure patients were randomised to patiromer and 69 to a placebo. Fifty-five (42%) patients had HFrEF and 54 (41%) had heart failure with preserved LVEF. The remaining patients had either heart failure with midrange LVEF or an unknown LVEF. In the heart failure subgroup, the proportion of patients remaining on spironolactone at week 12 was 84.1% in the patiromer group compared to 68.1% in the placebo group (p = 0.0504), with no significant interaction between the subgroups with and without heart failure (p = 0.8085). Adverse events were also consistent with those of the overall study population.
All these studies demonstrate that patiromer can safely reduce K+ levels over the long term and can improve successful initiation or up-titration of spironolactone in patients with CKD who are being treated with RAAS inhibitors. Notably, with the exception of the PEARL-HF study, the proportion of heart failure patients was relatively small, ranging from 35% in AMETHYST-DN to 45% in AMBER. Furthermore, these populations were not very well characterised with regards to, for example, LVEF. So far, there has been no definitive evidence that adding patiromer in HFrEF patients with hyperkalaemia or who are at risk for hyperkalaemia in order to allow initiation/up-titration of heart failure therapies improves outcomes. This will be the objective of the ongoing DIAMOND study (NCT03888066), a phase IIIb, multicentre, double-blind, placebo-controlled, randomised study which is expected to be completed in March 2022. There will be a 12-week run-in phase on open-label patiromer with heart failure treatment optimisation, followed by a randomised withdrawal of patiromer during the rest of the study. The study plans to recruit 2388 heart failure patients with LVEF <40%, eGFR >30 ml/min/1.73 m2, previous heart failure hospitalisation within the last 12 months, and K+ levels >5.0 mmol/l or a history of hyperkalaemia. The primary endpoint will be time to first occurrence of cardiovascular death or cardiovascular hospitalisation.
Conclusion
In conclusion, patiromer is the first well-tested potassium binder available in Switzerland that has been shown to safely and effectively reduce K+ levels over the long term in various groups of patients at risk of hyperkalaemia, including those with heart failure. It is important to note that in selected patients with symptomatic HFrEF and renal dysfunction who are prone to repeated episodes of hyperkalaemia >5.0 mmol/l, patiromer may not only normalise K+ levels over the long term, but may also allow a higher proportion of patients to reach and maintain target doses of RAAS inhibitors. This approach may solve the “potassium dilemma” and improve outcomes in this high-risk population, but it must still be validated in further trials such as the ongoing DIAMOND RCT.
Author contributions
All authors participated in writing and approved the final manuscript.
References
1
Savarese
G
,
Lund
LH
. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi:.https://doi.org/10.15420/cfr.2016:25:2
2Https://www.Obsan.Admin.Ch/en. 2015
3Www.Health-stat.Admin.Ch.
4
Schmidt
M
,
Ulrichsen
SP
,
Pedersen
L
,
Bøtker
HE
,
Sørensen
HT
. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide cohort study. Eur J Heart Fail. 2016;18(5):490–9. doi:.https://doi.org/10.1002/ejhf.486
5
Ambrosy
AP
,
Fonarow
GC
,
Butler
J
,
Chioncel
O
,
Greene
SJ
,
Vaduganathan
M
, et al.
The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–33. doi:.https://doi.org/10.1016/j.jacc.2013.11.053
6
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
7
Maggioni
AP
,
Anker
SD
,
Dahlström
U
,
Filippatos
G
,
Ponikowski
P
,
Zannad
F
, et al.; Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15(10):1173–84. doi:.https://doi.org/10.1093/eurjhf/hft134
8
Greene
SJ
,
Butler
J
,
Albert
NM
,
DeVore
AD
,
Sharma
PP
,
Duffy
CI
, et al.
Medical therapy for heart failure with reduced ejection fraction: The champ-hf registry. J Am Coll Cardiol. 2018;72(4):351–66. doi:.https://doi.org/10.1016/j.jacc.2018.04.070
9
Komajda
M
,
Anker
SD
,
Cowie
MR
,
Filippatos
GS
,
Mengelle
B
,
Ponikowski
P
, et al.; QUALIFY Investigators. Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016;18(5):514–22. doi:.https://doi.org/10.1002/ejhf.510
10
Mueller
C
,
Bally
K
,
Buser
M
,
Flammer
AJ
,
Gaspoz
JM
,
Mach
F
, et al.
Roadmap for the treatment of heart failure patients after hospital discharge: an interdisciplinary consensus paper. Swiss Med Wkly. 2020;150:w20159. doi:.https://doi.org/10.4414/smw.2020.20159
11
Packer
M
,
Poole-Wilson
PA
,
Armstrong
PW
,
Cleland
JG
,
Horowitz
JD
,
Massie
BM
, et al.; ATLAS Study Group. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation. 1999;100(23):2312–8. doi:.https://doi.org/10.1161/01.CIR.100.23.2312
12
Konstam
MA
,
Neaton
JD
,
Dickstein
K
,
Drexler
H
,
Komajda
M
,
Martinez
FA
, et al.; HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840–8. doi:.https://doi.org/10.1016/S0140-6736(09)61913-9
13
Ouwerkerk
W
,
Voors
AA
,
Anker
SD
,
Cleland
JG
,
Dickstein
K
,
Filippatos
G
, et al.
Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017;38(24):1883–90. doi:.https://doi.org/10.1093/eurheartj/ehx026
14
Mordi
IR
,
Ouwerkerk
W
,
Anker
SD
,
Cleland
JG
,
Dickstein
K
,
Metra
M
, et al.
Heart failure treatment up-titration and outcome and age: an analysis of BIOSTAT-CHF. Eur J Heart Fail. 2020. doi:.https://doi.org/10.1002/ejhf.1799
15
Rosano
GMC
,
Tamargo
J
,
Kjeldsen
KP
,
Lainscak
M
,
Agewall
S
,
Anker
SD
, et al.
Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018;4(3):180–8. doi:.https://doi.org/10.1093/ehjcvp/pvy015
16
McMurray
JJ
,
Packer
M
,
Desai
AS
,
Gong
J
,
Lefkowitz
MP
,
Rizkala
AR
, et al.; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:.https://doi.org/10.1056/NEJMoa1409077
17
Zannad
F
,
McMurray
JJ
,
Krum
H
,
van Veldhuisen
DJ
,
Swedberg
K
,
Shi
H
, et al.; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi:.https://doi.org/10.1056/NEJMoa1009492
18
Thomsen
RW
,
Nicolaisen
SK
,
Hasvold
P
,
Garcia-Sanchez
R
,
Pedersen
L
,
Adelborg
K
, et al.
Elevated potassium levels in patients with congestive heart failure: Occurrence, risk factors, and clinical outcomes: A Danish population-based cohort study. J Am Heart Assoc. 2018;7(11):7. doi:.https://doi.org/10.1161/JAHA.118.008912
19
Muzzarelli
S
,
Maeder
MT
,
Toggweiler
S
,
Rickli
H
,
Nietlispach
F
,
Julius
B
, et al.; TIME-CHF Investigators. Frequency and predictors of hyperkalemia in patients ≥60 years of age with heart failure undergoing intense medical therapy. Am J Cardiol. 2012;109(5):693–8. doi:.https://doi.org/10.1016/j.amjcard.2011.10.027
20
Vardeny
O
,
Claggett
B
,
Anand
I
,
Rossignol
P
,
Desai
AS
,
Zannad
F
, et al.; Randomized Aldactone Evaluation Study (RALES) Investigators. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7(4):573–9. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.114.001104
21
Rossignol
P
,
Dobre
D
,
McMurray
JJ
,
Swedberg
K
,
Krum
H
,
van Veldhuisen
DJ
, et al.
Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7(1):51–8. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.113.000792
22
Aldahl
M
,
Jensen
AC
,
Davidsen
L
,
Eriksen
MA
,
Møller Hansen
S
,
Nielsen
BJ
, et al.
Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38(38):2890–6. doi:.https://doi.org/10.1093/eurheartj/ehx460
23
Rossignol
P
,
Lainscak
M
,
Crespo-Leiro
MG
,
Laroche
C
,
Piepoli
MF
,
Filippatos
G
, et al.; Heart Failure Long-Term Registry Investigators Group. Unravelling the interplay between hyperkalaemia, renin-angiotensin-aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORP Heart Failure Long-Term Registry. Eur J Heart Fail. 2020;22(8):1378–89. doi:.https://doi.org/10.1002/ejhf.1793
24
Clase
CM
,
Carrero
JJ
,
Ellison
DH
,
Grams
ME
,
Hemmelgarn
BR
,
Jardine
MJ
, et al.; Conference Participants. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(1):42–61. doi:.https://doi.org/10.1016/j.kint.2019.09.018
25
Desai
AS
,
Vardeny
O
,
Claggett
B
,
McMurray
JJ
,
Packer
M
,
Swedberg
K
, et al.
Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril: A secondary analysis of the paradigm-hf trial. JAMA Cardiol. 2017;2(1):79–85. doi:.https://doi.org/10.1001/jamacardio.2016.4733
26
McMurray
JJV
,
Solomon
SD
,
Inzucchi
SE
,
Køber
L
,
Kosiborod
MN
,
Martinez
FA
, et al.; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi:.https://doi.org/10.1056/NEJMoa1911303
27
Elkinton
JR
,
Clark
JK
,
Squires
RD
,
Bluemle
LW, Jr
,
Crosley
AP, Jr
. Treatment of potassium retention in anuria with cation exchange resin; a preliminary report. Am J Med Sci. 1950;220(5):547–52. doi:.https://doi.org/10.1097/00000441-195022050-00010
28
Nasir
K
,
Ahmad
A
. Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad. 2014;26(4):455–8.
29
Lepage
L
,
Dufour
AC
,
Doiron
J
,
Handfield
K
,
Desforges
K
,
Bell
R
, et al.
Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in ckd. Clin J Am Soc Nephrol. 2015;10(12):2136–42. doi:.https://doi.org/10.2215/CJN.03640415
30
Harel
Z
,
Harel
S
,
Shah
PS
,
Wald
R
,
Perl
J
,
Bell
CM
. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e9–24. doi:.https://doi.org/10.1016/j.amjmed.2012.08.016
31
Http://www.Accessdata.Fda.Gov/drugsatfda_docs/label/2011/011287s023lbl.Pdf
2020
32
Hoy
SM
. Sodium zirconium cyclosilicate: A review in hyperkalaemia. Drugs. 2018;78(15):1605–13. doi:.https://doi.org/10.1007/s40265-018-0991-6
33
Pitt
B
,
Anker
SD
,
Bushinsky
DA
,
Kitzman
DW
,
Zannad
F
,
Huang
IZ
; PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–8. doi:.https://doi.org/10.1093/eurheartj/ehq502
34
Weir
MR
,
Bakris
GL
,
Bushinsky
DA
,
Mayo
MR
,
Garza
D
,
Stasiv
Y
, et al.; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21. doi:.https://doi.org/10.1056/NEJMoa1410853
35
Bakris
GL
,
Pitt
B
,
Weir
MR
,
Freeman
MW
,
Mayo
MR
,
Garza
D
, et al.; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The amethyst-dn randomized clinical trial. JAMA. 2015;314(2):151–61. doi:.https://doi.org/10.1001/jama.2015.7446
36
Agarwal
R
,
Rossignol
P
,
Romero
A
,
Garza
D
,
Mayo
MR
,
Warren
S
, et al.
Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10208):1540–50. doi:.https://doi.org/10.1016/S0140-6736(19)32135-X
37
Pitt
B
,
Bakris
GL
,
Weir
MR
,
Freeman
MW
,
Lainscak
M
,
Mayo
MR
, et al.
Long-term effects of patiromer for hyperkalaemia treatment in patients with mild heart failure and diabetic nephropathy on angiotensin-converting enzymes/angiotensin receptor blockers: results from AMETHYST-DN. ESC Heart Fail. 2018;5(4):592–602. doi:.https://doi.org/10.1002/ehf2.12292
38
Pitt
B
,
Bakris
GL
,
Bushinsky
DA
,
Garza
D
,
Mayo
MR
,
Stasiv
Y
, et al.
Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17(10):1057–65. doi:.https://doi.org/10.1002/ejhf.402
39
Rossignol
P
,
Williams
B
,
Mayo
MR
,
Warren
S
,
Arthur
S
,
Ackourey
G
, et al.
Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): results in the pre-specified subgroup with heart failure. Eur J Heart Fail. 2020;22(8):1462–71. doi:.https://doi.org/10.1002/ejhf.1860

