Introduction
In Switzerland, one woman in two and one man in five will sustain a fragility fracture after the age of 50. Hence the number of incident fragility fractures is at least 75,000 per year; 140,000 subjects have prevalent hip or vertebral fractures and 450,000 have osteoporosis (in 2010) [1]. In turn, the acute complications of osteoporosis are a major cause of hospitalisations and the chronic complications, such as the loss of mobility and independence in daily living activities, increase the needs for secondary care and nursing homes. Yet primary and secondary prevention of fragility fractures remains insufficient, as it is estimated that less than 10% of subjects with osteoporosis and only 20% of subjects with low-trauma fractures receive anti-osteoporotic medications [2]. Hence identification and treatment of subjects at high risk of fragility fractures remains critical.
Current guidelines from the Swiss Association against Osteoporosis (SVGO) dating from 2015 recommend therapy for men and women at increased fracture risk, specifically those with a vertebral or hip fracture; those with bone mineral density (BMD) T-score <−2.5 at spine or hip; and those with a high 10-year probability of major osteoporotic fractures calculated by using the fracture risk assessment tool FRAX . By high risk we mean at least equivalent to the 10-year risk of an individual of the same age and sex with a prevalent vertebral fracture, which in Switzerland corresponds to about 10% by age 50 years and 30% by age 72 years (fig. 1). In addition, patients receiving chronic glucocorticoid therapy, anti-aromatase therapy or androgen suppression therapy are also considered at high risk and recommended for osteoporosis treatment. More recently, SVGO also made recommendations regarding the duration and the sequence of osteoporosis therapy [3]. In particular, it was recommended that high risk patients, including those older than 65 yrs and fallers remain treated until reaching a bone mineral density (BMD) at hip better than −2.0 T-score.

Figure 1 Intervention thresholds for osteoporosis therapy based on the 10-year risk of a major osteoporotic fracture in Switzerland.
There are multiple classes of drugs available for the treatment of osteoporosis and fracture prevention, including: antiresorptives, namely, selective oestrogen receptor modulators (SERMs: raloxifene, basedoxifene), bisphosphonates (alendronate, risedronate, ibandronate, zoledronate) and monocloncal antibodies (denosumab); bone forming agents (teriparatide); and, very recently approved in Switzerland, a new monoclonal antibody, romosozumab, with dual antiresorptive and anabolic effects. As stated in the 2015 SVGO guidelines: “In general an anti-resorptive (AR) is recommended as first line (without specifying whether and when it should be SERMs, bisphosphonates or denosumab), whereas teriparatide (TPT) could be recommended as first line in case of severe osteoporosis (i.e., BMD <−2.5 + vertebral fracture).”
However, no specific treatment recommendations have been made so far in our country to guide therapy according to the baseline level of risk. Here we provide new guidance to categorise patients at various levels of risk based on their probability of a major osteoporotic fracture (rather than hip fracture only, which accounts for only a fraction of the former) and to choose the most appropriate first-line therapy for each risk category.
New evidence regarding patients at very high and/or imminent risk
Recent publications have provided new evidence regarding the identification of very high risk patients and the efficacy of certain treatments in this population. First, patients with a recent major osteoporotic fracture (spine, hip, pelvis, humerus or radius) are increasingly recognised as being at “imminent risk”, since the relative risk of a second fracture is increased several fold within the first 2 years after the index fracture but declines thereafter, and 40% to 60% of all recurrent fractures will occur within those 2 years [4]. According to some very recent Medicare data from the US, after 65 years of age, 25% of patients with a vertebral fracture and 15% or more of those with a hip or pelvis fracture will refracture within 2 years, whereas 10% or more of those with humerus or distal radius fractures will refracture within 2 years [5].
An important consequence of identifying subjects with recent fractures is the positive impact it has on the cost-benefits of therapy. Indeed, by taking into account the imminent risk of refracturing in these subjects, the number of secondary fractures potentially prevented by treatment would be doubled compared with subjects with similar fractures but in whom the recentness of the fracture has not been taken into account [6]. Moreover, it has been estimated that in these imminent-risk patients, parenteral therapy with denosumab, teriparatide or romosozumab would prevent more fractures than using an oral bisphosphonate [6].
Two recent major head-to-head trials have demonstrated the greater anti-fracture efficacy of teriparatide and romosozumab compared with oral bisphosphonates (risedronate and alendronate) in high-risk subjects defined by severe and/or multiple vertebral fractures (VERO [7] and ARCH [8], respectively). Notably, romosozumab also substantially increased BMD at spine and hip within 1 year and significantly more so than bisphosphonates or teriparatide [8, 9]. However, its benefits and risks should be evaluated carefully in patients at higher cardiovascular risk, since romosozumab was associated with more cardiovascular serious adverse events than alendronate in one study [8].
Accordingly, the International Osteoporosis Foundation (IOF) jointly with the European Society for the Clinical and Economic Aspects of Osteoporosis (ESCEO) have recently proposed an algorithm for the management of patients at low, high and very high risk of osteoporotic fractures, in which they still recommend a potent anti-resorptive (bisphosphonate or denosumab) in high-risk patients, but consideration of an anabolic agent (teriparatide, romosozumab) first in very high-risk patients [10]. In women with a recent major osteoporotic fracture, use of an anabolic drug for a short period (12–18 months) followed by an anti-resorptive over a total duration of therapy of 10 years would result in more fractures saved than use of an anti-resorptive followed by an anabolic drug over the same period [10].
Based on these new developments, we now delineate the various risk categories and recommended treatments for each level of risk in order to facilitate the management of osteoporosis in Switzerland, notwithstanding the current limitations for reimbursement of some drugs in our country.
Risk stratification
- Risk stratification should take into consideration previous fragility fractures, BMD, age and other clinical risk factors as encompassed in FRAX (table 1). Moreover, the risk of falls should be taken into consideration, as subjects with risk factors for falls (such as >1 fall in past year, Parkinson’s disease, urinary incontinence, etc.) may have a fracture risk that is nearly double that of individuals with similar bone risk profiles but without falls [11].
- In patients at risk, BMD should be evaluated at spine and hip using dual energy X-ray absorbtiometry (DXA). When possible, a vertebral fracture assessment and trabecular bone score by DXA could also be performed to further refine the risk evaluation.
- Bone turnover markers, particularly carboxy terminal crosslinked telopeptides of type 1 collagen (CTx) and procollagen type 1 N-terminal propeptide (P1NP), are not recommended for risk stratification. However they may be useful to guide treatment decisions in some risk categories (below) and to monitor treatment efficacy in all categories.
- Subjects should be considered at
- Subjects should be considered at
- For instance, a 55-year-old woman with a previous wrist fracture, body mass index (BMI) 20 kg/m
- Subjects with previous major osteoporotic fractures (>2 years) and/or FRAX probabilities above the intervention threshold but less than 20% above that limit (fig. 1) should be considered
- Subjects receiving chronic glucocorticoid, aromatase inhibitor or androgen suppression therapy should also be considered
- Subjects with BMD <−2.5 T-score but no previous fracture and a FRAX probability below the intervention threshold should be considered at
- Subjects with osteopenia (T-score >−2.5 and <−1.0) and no other risk factors should be considered at
Treatment by risk category
Despite numerous studies comparing the effects of various treatments on BMD changes, only a few trials, including the VERO and ARCH trials mentioned above, directly compared the anti-fracture efficacy of osteoporosis drugs in high-risk patients [7, 8, 12], and none has compared anabolics with the most potent anti-resorptives (zoledronate and denosumab). Moreover, a single trial specifically recruited patients with a recent hip fracture, hence at imminent risk, and demonstrated that a yearly infusion of zoledronate initiated within 3 months after the fracture reduced secondary clinical fractures by 32% and mortality by 25% compared with placebo (median follow-up 1.9 years) [13]. Post-hoc analyses of the FREEDOM trial also reported that denosumab significantly reduced vertebral and hip fractures in high risk subjects (>75 yrs, hip T-score <−3.0, previous fracture) [14]. Denosumab has also been shown to reduce fracture risk in other high-risk categories, namely in postmenopausal women with early breast cancer receiving an aromatase inhibitor [15], as well as in men with non-metastatic prostate cancer receiving androgen suppression therapy [16].
Treatment recommendations for the various risk categories are shown in figure 2 and explained below.
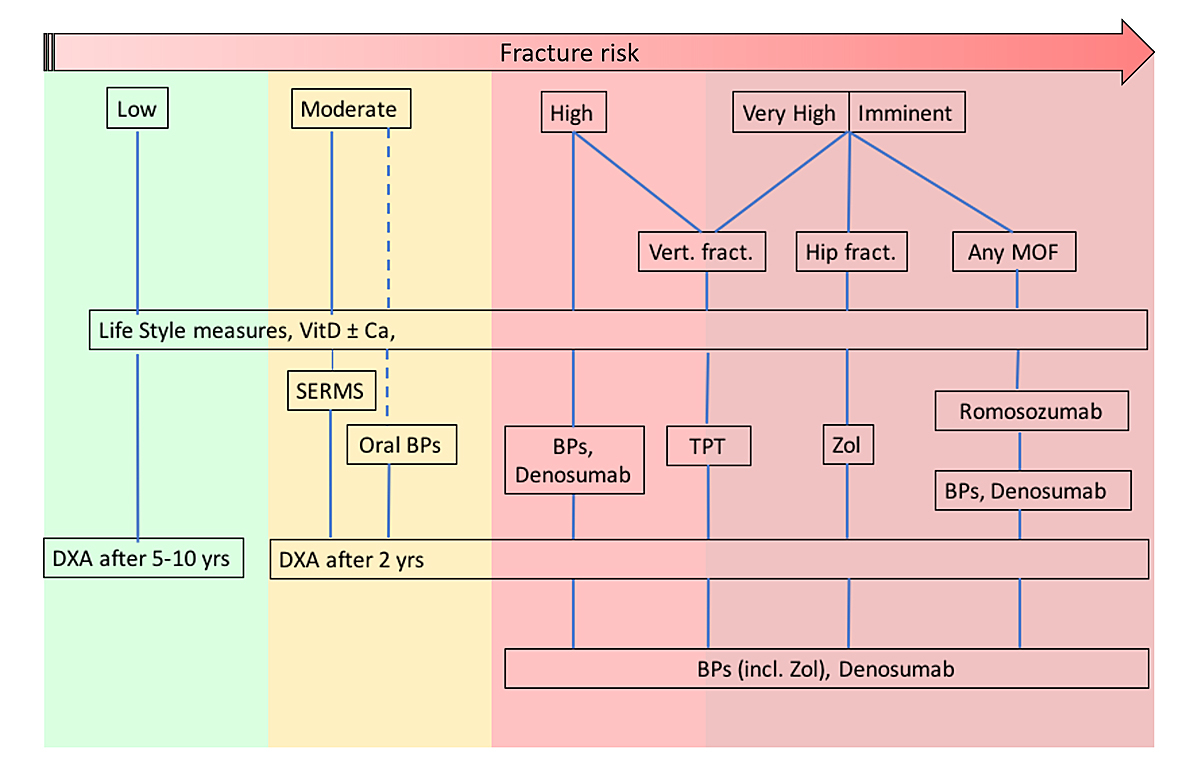
Figure 2 Treatment recommendations by level of risk. SERMS = selective oestrogen receptor modulators; BPs = bisphosphonates; Zol = zoledronate; TPT = teriparatide. Dashed lines indicate alternative treatments (see text for details).
- In patients at
- In patients at
- -– In all patients at
- In
- In
- In
- In
Discussion
These new recommendations on osteoporosis treatment differ from the previous SVGO guidelines (2015) by further defining four risk categories, including a new one – imminent and very high risk. Furthermore, among the armamentarium of osteoporosis drugs available, newly enriched by a potent anabolic agent with dual anti-resorptive properties, romosozumab, we now delineate which drugs should be used to inititate therapy according to the fracture risk category. In defined patients at imminent / very high risk in particular, a more aggressive approach with bone forming agents or anabolics is now recommended as first-line therapy, although some reimbursement issues may still be present. By recommending specific classes of drugs according to the level of fracture risk, we hope to facilitate the decision making of all doctors involved in the management of osteoporosis and thereby to narrow the treatment gap for this common disease. Nevertheless, we acknowledge that the threshold for “very high risk” as defined here is somewhat arbitrary. We intentionally chose a threshold for very high risk that is quite elevated in order to keep the size of the targeted population relatively limited, in consideration of the potential risks and/or higher costs of anabolic agents. The recent development of teriparatide biosimilars may actually favour its use among imminent / very high risk subjects. To note that, using a lower “very high risk” threshold in the UK population, it has been estimated that only about 15% of post-menopausal women will fall into that category at any age [10]. We therefore expect that only 10% or less of post-menopausal women in Switzerland will fall into that category. Further studies are needed in Switzerland and elsewhere to evaluate whether such threshold captures a sufficient proportion of subjects effectively at very high risk, or on the contrary is too restrictive, which would call for adapting our guidelines in the future.
Notes
KL has received a research grant from Amgen and was principal investigator in several multicentre clinical trials of Amgen UCB and MSD. SF has received research and/or consulting grants from Amgen, UCB, Agnuvos, Alixiun, Gedeon Rühte and Galapájus.
References
1 Svedbom A , Ivergård M , Hernlund E , Rizzoli R , Kanis JA . Epidemiology and economic burden of osteoporosis in Switzerland. Arch Osteoporos. 2014;9(1):187. doi:.https://doi.org/10.1007/s11657-014-0187-y
2 Suhm N , Lamy O , Lippuner K ; OsteoCare study group. Management of fragility fractures in Switzerland: results of a nationwide survey. Swiss Med Wkly. 2008;138(45-46):674–83.
3 Meier C , Uebelhart B , Aubry-Rozier B , Birkhäuser M , Bischoff-Ferrari HA , Frey D , et al. Osteoporosis drug treatment: duration and management after discontinuation. A position statement from the SVGO/ASCO. Swiss Med Wkly. 2017;147:w14484.
4 Kanis JA , Johansson H , Odén A , Harvey NC , Gudnason V , Sanders KM , et al. Characteristics of recurrent fractures. Osteoporos Int. 2018;29(8):1747–57. doi:.https://doi.org/10.1007/s00198-018-4502-0
5 Balasubramanian A , Zhang J , Chen L , Wenkert D , Daigle SG , Grauer A , et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int. 2019;30(1):79–92. doi:.https://doi.org/10.1007/s00198-018-4732-1
6 Pinedo-Villanueva R , Charokopou M , Toth E , Donnelly K , Cooper C , Prieto-Alhambra D , et al. Imminent fracture risk assessments in the UK FLS setting: implications and challenges. Arch Osteoporos. 2019;14(1):12. doi:.https://doi.org/10.1007/s11657-019-0569-2
7 Kendler DL , Marin F , Zerbini CAF , Russo LA , Greenspan SL , Zikan V , et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40. doi:.https://doi.org/10.1016/S0140-6736(17)32137-2
8 Saag KG , Petersen J , Brandi ML , Karaplis AC , Lorentzon M , Thomas T , et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017;377(15):1417–27. doi:.https://doi.org/10.1056/NEJMoa1708322
9 McClung MR , Grauer A , Boonen S , Bolognese MA , Brown JP , Diez-Perez A , et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20. doi:.https://doi.org/10.1056/NEJMoa1305224
10 Kanis JA , Harvey NC , McCloskey E , Bruyère O , Veronese N , Lorentzon M , et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12. doi:.https://doi.org/10.1007/s00198-019-05176-3
11 Huntjens KM , van Geel TA , van Helden S , van den Bergh J , Willems P , Winkens B , et al. The role of the combination of bone and fall related risk factors on short-term subsequent fracture risk and mortality. BMC Musculoskelet Disord. 2013;14(1):121. doi:.https://doi.org/10.1186/1471-2474-14-121
12 Miller PD , Hattersley G , Riis BJ , Williams GC , Lau E , Russo LA , et al.; ACTIVE Study Investigators. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016;316(7):722–33. doi:.https://doi.org/10.1001/jama.2016.11136
13 Lyles KW , Colón-Emeric CS , Magaziner JS , Adachi JD , Pieper CF , Mautalen C , et al.; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–809. doi:.https://doi.org/10.1056/NEJMoa074941
14 Boonen S , Adachi JD , Man Z , Cummings SR , Lippuner K , Törring O , et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96(6):1727–36. doi:.https://doi.org/10.1210/jc.2010-2784
15 Gnant M , Pfeiler G , Dubsky PC , Hubalek M , Greil R , Jakesz R , et al.; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–43. doi:.https://doi.org/10.1016/S0140-6736(15)60995-3
16 Smith MR , Egerdie B , Hernández Toriz N , Feldman R , Tammela TL , Saad F , et al., Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–55. doi:.https://doi.org/10.1056/NEJMoa0809003
17 Meier C , Lamy O , Krieg MA , Mellinghoff HU , Felder M , Ferrari S , et al. The role of teriparatide in sequential and combination therapy of osteoporosis. Swiss Med Wkly. 2014;144:w13952. doi:.https://doi.org/10.4414/smw.2014.13952
Notes
KL has received a research grant from Amgen and was principal investigator in several multicentre clinical trials of Amgen UCB and MSD. SF has received research and/or consulting grants from Amgen, UCB, Agnuvos, Alixiun, Gedeon Rühte and Galapájus.