
Figure 1 Visual field and optical coherence tomography findings in multiple sclerosis patients with homonymous visual field defects.
DOI: https://doi.org/10.4414/smw.2020.20319
Multiple sclerosis is an autoimmune inflammatory disorder characterised by demyelinating lesions of the central nervous system. The visual pathways are frequently involved. Whereas the antechiasmatic pathways are most commonly affected, resulting in either retrobulbar optic neuritis or papillitis, demyelination can affect any part of the visual pathway [1]. Approximately 30% of patients with multiple sclerosis present with optic neuritis/papillitis as the inaugural symptom of their disease, and 80% of patients will develop an optic neuritis along the course of their disease [2]. Although chiasmal syndrome has been reported in multiple sclerosis patients, it is rare [3]. Symptomatic retrochiasmal lesions of the visual pathways, resulting in homonymous visual field defects (HVFD), have been reported since 1890 [4]. However, there are only three published case series, which report five, eight and 18 cases of HVFD in multiple sclerosis respectively [5–7]. All other publications deal with single case reports. The frequency of HVFD in multiple sclerosis has been estimated at between 1.3% and 3.5% [5, 8–11]. Paradoxically, despite the low frequency of HVFD in multiple sclerosis, MRI and autopsy studies have shown that the retrochiasmal pathways are frequently affected by demyelinating lesions in multiple sclerosis (30-90%), with the periventricular area being the most common location of demyelinating lesions [8, 10]. According to Plant et al., the size of the demyelinating lesions is a predictor of HVFD, with only the largest lesions resulting in symptomatic HVFD [7]. The discrepancy between clinical data (low frequency of HVFD) and anatomical data (high incidence of retrochiasmal demyelinating lesions found by either autopsy or magnetic resonance imaging [MRI]) suggests that either most retrochiasmal lesions are asymptomatic, or that HVFD may be underdiagnosed in patients with multiple sclerosis [10, 12].
As well as MRI, nowadays there is another method to detect evidence of previous demyelinating retrochiasmal lesions: optical coherence tomography (OCT). Spectral-domain OCT is a relatively novel, noninvasive technique which allows the retinal structures to be assessed with a high resolution of 5-10µm. Furthermore, retinal segmentation permits the isolation and analysis of separate layers of the retina, namely the retinal ganglion cell layer. Any lesion along the afferent visual pathway can result in RGCL thinning. All antegeniculate lesions, i.e. optic nerve, optic chiasm, optic tract and lateral geniculate body, will result in retrograde axonal degeneration, resulting in a thinning of the RGCL [13, 14]. Experimental and clinical studies have also shown that transsynaptic retrograde degeneration can occur after lesions of the postgeniculate visual pathway [15–20]. However, retrograde transsynaptic degeneration is not present in all patients, is not associated with any particular pathology, and its exact mechanism is not yet elucidated.
In order to better characterise both the clinical profile and the evolution of HVFD in patients with multiple sclerosis, we performed a retrospective study of such patients. We also compared our results with those compiled from a search of the worldwide literature on the subject.
The study was ethically approved by the “Commission cantonale d’éthique de la recherche sur l’être humain” (CER‐VD).
We analysed the charts of all multiple sclerosis patients who presented HVFD and were examined in the Neuro-Ophthalmology Unit of the Hôpital Ophtalmique Jules-Gonin. Patients were retrieved from the neuro-ophthalmology database and anonymised data were extracted from their clinical charts. The period of recruitment ranged from 1994 to 2016. From 2013, all patients were investigated with spectral-domain OCT (Cirrus 5000, Carl Zeiss AG, Oberkochen, Germany). Segmentation of the macular OCT allowed measurements of the retinal ganglion cell layer (RGCL). All patients were examined by one of the study authors (FXB). Inclusion criteria were a diagnosis of multiple sclerosis and at least one episode of HVFD, documented by static automated perimetry (Octopus 300, program G1 or M2, Haag-Streit AG, Köniz, Switzerland). The diagnosis of multiple sclerosis was independently established according to the results of a clinical examination by a neurologist and the results of both MRI and lumbar puncture. The exclusion criterion was the presence of another pathology which could have contributed to the HVFD.
We extracted information on general demographics (age and gender), visual function at the time of the episode of HVFD (visual acuity; colour vision; visual field) and specific information on multiple sclerosis (date of diagnosis; type of multiple sclerosis, i.e. relapsing-remitting, primary progressive, secondary progressive) from the medical charts. Previous episodes of optic neuritis were recorded. Snellen visual acuity (VA) was converted into logMar scores for statistical purposes [21]. Normal visual acuity was defined as ≤ 0.0 logMar (≥10/10). Colour vision was tested using Ishihara pseudo-isochromatic plates and normal colour vision was defined by a score ≥ 11/13. Automated static perimetry data was analysed both qualitative and quantitative, at initial examination and also at follow-up visits.
We categorised HVFD into three types: homonymous hemianopsia, homonymous quadrantanopsia and homonymous scotoma. These homonymous defects could be either complete or partial. Recovery from the HVFD, on follow-up visual field examination, was either complete (no HVFD), partial (improvement of HVFD) or absent (no improvement of HVFD). Recurrences of HVFD and the results of cerebral MRI were also recorded when available.
We performed a literature review of HVFD in multiple sclerosis. We searched relevant papers on Pubmed and Google Scholar using the following keywords “multiple sclerosis, homonymous hemianopsia, homonymous quadrantanopsia, homonymous scotoma, visual homonymous defect, homonymous visual field defect, homonymous defect, visual pathway lesion, optic tract lesion, lateral geniculate body lesion, optic radiation lesion, retrochiasmal lesion”. Whenever possible, we retrieved from the article the following data: age and gender of the patients; type of multiple sclerosis; whether the HVFD was the first manifestation of multiple sclerosis; type of HVFD, its severity and evolution; location of the associated lesions.
In the neuro-ophthalmology database, 547 patients were coded as “multiple sclerosis” and 44/547 were also coded as “HVFD”. Amongst the 44 selected patients, we excluded 24 patients due to either another cause of the HVFD found on MRI, absence of follow-up, or lack of computerised visual field examination. Twenty patients were included in this study (table 1). There were 11 women (55%) and 9 men, and their median age was 35 years (range 16–52). Relapsing-remitting multiple sclerosis was the most common type (18/20; 90%), whereas primary progressive multiple sclerosis (1/20; 5%) and secondary progressive multiple sclerosis (1/20; 5%) were less frequent. In seven patients (35%), the HVFD was the inaugural symptom of multiple sclerosis. For the remaining thirteen patients, the HVFD occurred at a median time of 5.5 years (range 1 month to 16 years) after the diagnosis of multiple sclerosis. An HVFD was found fortuitously in five patients without any visual symptoms.
Table 1 Demographics of multiple sclerosis (MS) patients with homonymous visual field defects (HVFD).
| Pt | Age | Sex | MS | HVFD as presenting symptom | Previous optic neuritis | VA (/10) | Ishihara (/13) | HVFD | Recovery | Recurrence | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | Type | Within | ||||||||
| 1 | 44 | F | RR | – | R+L | 7 | 7 | 8 | 1 | PHH R | C | 10 weeks | – |
| 2 | 25 | F | RR | + | R+L | 5 | 2 | 7 | 1 | PHQ R | C | 12 weeks | – |
| 3 | 41 | F | RR | – | – | 10 | 10 | 11 | 11 | PHH L | C | 3 weeks | – |
| 4 | 22 | F | RR | – | – | 9 | 9 | 12 | 11 | PHQ R | X | 10 weeks | – |
| 5 | 16 | F | RR | – | – | 10 | 10 | 13 | 13 | PHQ L | C | 2 weeks | I |
| 6 | 35 | M | RR | – | – | 10 | 10 | 13 | 12 | CHH R | P | 58 weeks | – |
| 7 | 37 | M | RR | + | – | 15 | 12.5 | 12 | 12 | PHQ R | C | 12 weeks | – |
| 8 | 49 | F | RR | – | – | 12 | 12 | 13 | 13 | PHQ L | P | 16 weeks | – |
| 9 | 35 | M | PP | – | L | 12 | 1 | 11 | 0 | PHH R | P | 28 weeks | – |
| 10 | 28 | F | RR | + | – | 10 | 10 | 13 | 13 | PHQ L | C | 12 weeks | Cl |
| 11 | 28 | M | RR | – | R | 10 | 10 | 13 | 13 | CHQ R | C | 13 weeks | – |
| 12 | 51 | F | RR | – | R | 6 | 8 | 10 | 13 | PHQ L | C | 4 weeks | – |
| 13 | 52 | F | RR | + | – | 10 | 12 | 13 | 13 | CHH L | P | 34 weeks | Cl |
| 14 | 41 | M | RR | – | L | 12 | 12 | 13 | 13 | HS R | C | 12 weeks | – |
| 15 | 24 | M | RR | + | – | 10 | 10 | 13 | 13 | PHH L | C | 10 weeks | – |
| 16 | 33 | M | RR | + | – | 10 | 10 | 13 | 13 | HS R | C | 3 weeks | – |
| 17 | 47 | M | SP | – | – | 10 | 8 | 12 | 11 | PHQ R+L | X | 92 months | – |
| 18 | 26 | M | RR | + | – | 10 | 10 | 13 | 13 | PHH R | C | NA | – |
| 19 | 47 | F | RR | – | L | 12 | 10 | 12 | 1 | PHQ R | X | 16 weeks | – |
| 20 | 28 | F | RR | – | – | 10 | 10 | 11 | 11 | PHQ L | P | 28 weeks | – |
C = complete recovery; CHH = complete homonymous hemianopsia; CHQ = complete homonymous quadrantanopsia; Cl = contralateral; F = female; HS = homonymous scotoma; I = ipsilateral; L = left; LE = left eye; M = male; NA = not available; PHH = partial homonymous hemianopsia; P = partial recovery; PHQ = partial homonymous quadrantanopsia; PP = primary progressive; presenting symptom = HVFD as presenting symptom of MS; Pt = patient; R = right; RE = right eye; RR = relapsing-remitting; SP = secondary progressive; VA = visual acuity; X = no recovery
The results of central visual function tests at initial examination are summarised in table 2. Visual function of eyes without a previous history of optic neuritis (30 eyes) was normal for both visual acuity and Ishihara, whereas visual acuity and color vision were abnormal for the group of 10 eyes with previous episodes of optic neuritis.
Table 2 Visual function of patients with multiple sclerosis and homonymous visual field defects.
|
All eyes
(n = 40) |
Eyes without ON
(n = 31) |
Eyes with previous ON (n = 9) | ||||
|---|---|---|---|---|---|---|
| Right eye | Left eye | Right eye | Left eye | Right eye | Left eye | |
| Average VA (LogMAR) | +0.01 ± 0.10 | +0.10 ± 0.30 | 0.00 ± 0.00 | 0.00 ± 0.00 | +0.17 ± 0.11 | +0.39 ± 0.48 |
| Median VA (LogMAR) | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.14 |
| Min–max VA (LogMAR) | +0.30 to −0.18 | +1.18 to −0.09 | +0.05 to −0.18 | +0.10 to −0.09 | +0.30 to 0.00 | +1.18 to −0.08 |
| Average Ishihara (/13) | 12 ± 2 | 10 ± 5 | 12 ± 1 | 12 ± 1 | 10 ± 2 | 3 ± 5 |
| Median Ishihara (/13) | 12.5 | 12.5 | 13 | 13 | 9 | 1 |
| Min–max Ishihara (/13) | 7–13 | 0–13 | 11–13 | 11–13 | 7–13 | 0–13 |
ON = optic neuritis; VA = visual acuity
Eleven patients (55%) presented with homonymous quadrantanopsia, seven patients (35%) exhibited homonymous hemianopsia and two patients (10%) showed homonymous scotoma (fig. 1). An example of each type of homonymous defect is shown in figure 2. The majority of patients (17/20; 85%) presented with a partial HVFD. One patient exhibited a bilateral partial HVFD.

Figure 1 Visual field and optical coherence tomography findings in multiple sclerosis patients with homonymous visual field defects.
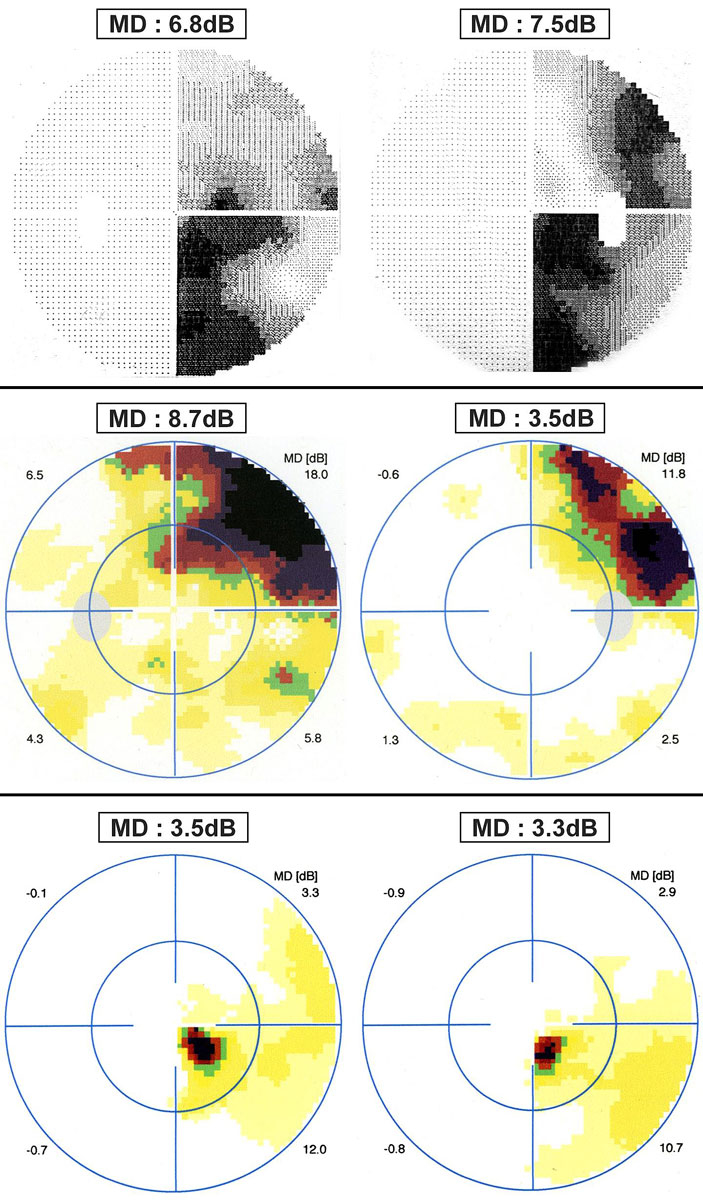
Figure 2 Examples of homonymous visual field defects in patients with multiple sclerosis. Top – right partial homonymous hemianopsia (30° visual field, program G1). Middle – partial right homonymous quadrantanopsia (30° visual field, program G1). Bottom – right paracentral inferior homonymous scotoma (central 10° visual field, program M2). MD = mean defect expressed in decibels (dB).
Results of MRI at the time of the HVFD were available for only 11 patients. Seven of them exhibited a lesion explaining the visual field defect. Lesions were present within the optic radiations (5/7; 71.4%), the optic tract (1/7; 14.3%) and the lateral geniculate nucleus (1/7; 14.3%).
Macular spectral-domain OCT was obtained for 10 patients (all examined after 2013). Analysis of RGCL thickness revealed three patients with homonymous thinning of the RGCL, six patients with diffuse unilateral or bilateral thinning of the RGCL resulting from previous episodes of optic neuritis, and one patient with normal RGCL thickness.
All patients benefited from follow-up automated static perimetry examinations. Follow-up duration varied from two weeks to 11 years, with a median time of 12 weeks. The recovery was complete in 12 patients (60%), and the median time to complete recovery was 10 weeks (2–13 weeks). One patient recovered completely but the time to full recovery could not be determined as the patient was examined only four years after the initial episode of HVFD. Incomplete recovery was documented in five subjects (25%) after a median follow-up time of 28 weeks (16–58 weeks). No recovery was found in three subjects (15%), despite a long median follow-up time of 17 weeks (10 weeks to 7.6 years). Worsening of the HVFD was documented in one of these three patients, who had a significant increase in mean defect during the follow-up period. Examples of VF recovery are depicted in figure 3. Overall, evolution of the HVFD was favourable in 85% of our patients.
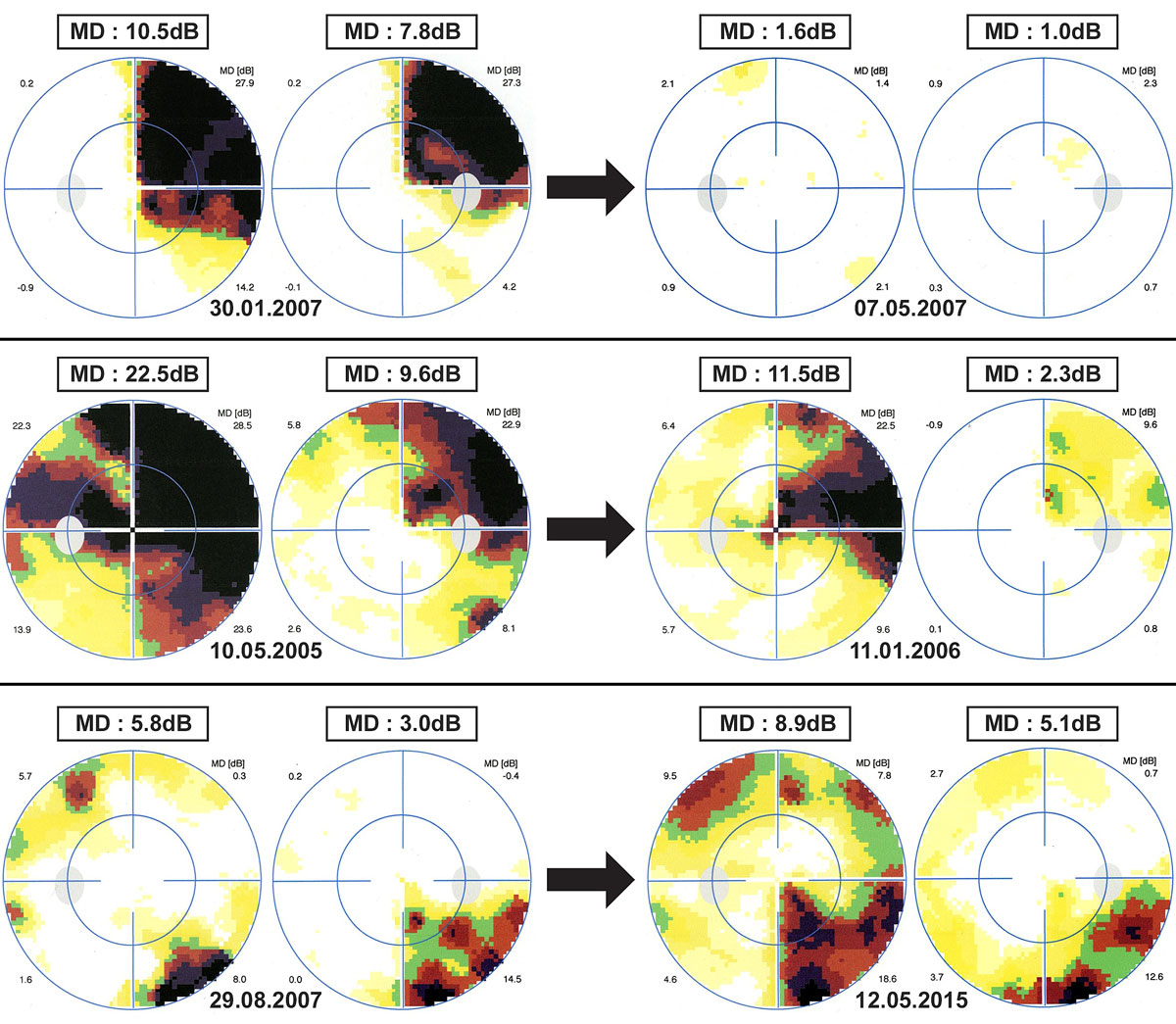
Figure 3 Examples of the types of evolution of homonymous visual field defects. Top – complete recovery of a right homonymous quadrantanopsia in 13 weeks (30° visual field, program G1). Middle – partial recovery of a partial right homonymous hemianopia after 28 weeks (30° visual field, program G1). Bottom – no recovery of a right inferior homonymous quadrantanopsia after more than 7 years (30° visual field, program G1). MD = mean defect expressed in decibels (dB).
A recurrent episode of HVFD occurred in three patients (15%). Each of these three patients had recovered from the initial episode when the second one occurred. All three patients eventually fully recovered.
A 28-year-old woman woke up with painless bilateral visual disturbance. Examination revealed a normal visual acuity and colour vision, but automated static perimetry revealed a left homonymous inferior quadrantanopsia. Cerebral MRI revealed a lesion on the right posterior thalamic region, also affecting the right optic radiations. A pseudotumoural form of multiple sclerosis was diagnosed. The visual field defect spontaneously resolved after three months (fig. 4). Two years later, she presented a recurrent episode of HVFD with a normal visual acuity and colour vision. An MRI showed two lesions in the white matter of the right hemisphere (periventricular region and optic radiations). Four months later, she had fully recovered. Four years after the inaugural HVFD, the patient was again symptomatic. She presented bilateral complete inferior visual field defect and MRI revealed bilateral lesions within the optic radiations. Recovery was again complete after four months. Thickness of the RGCL was normal in both eyes.

Figure 4 Recurrence of a homonymous visual field defect (HVFD), previously partially reported [22]. Top – inaugural left homonymous inferior quadrantanopsia, which disappeared completely after three months. Middle – 2 years later, recurrence of the left homonymous inferior quadrantanopsia, which this time resolved over four months. Bottom – 4 years after the initial episode, a second HVFD recurrence occurred with a bilateral inferior homonymous quadrantanopsia. Complete recovery of the HVFD was obvious after 3.5 months. MD = mean defect expressed in decibels (dB).
Our literature search found 29 publications, totalling 70 patients with multiple sclerosis and HVFD [4–7, 11, 22–46]. The gender of the patients was known in 57 cases. There were 35 women (61.4%) and 22 men. Their median age was 30 years (range 19-57). The type of multiple sclerosis was described in 56 cases: the relapsing-remitting type was the most common (98.2%) and only one case of primary progressive type was described. An HVFD was the inaugural symptom of multiple sclerosis in eight patients (11.4%).
Fifty-four patients had an HVFD detected by manual kinetic perimetry and 11 patients were examined with automated static perimetry. For the remaining five patients, no information was found regarding perimetric techniques. Forty-three patients (61.4%) showed homonymous hemianopsia, 13 patients (18.6%) exhibited homonymous quadrantanopsia, and homonymous scotoma was present in 14 patients (20%). The HVFD was partial in 31 patients (44.2%).
A definite lesion explaining the visual defect was reported in 44 patients, localised at either the optic radiations (30/44; 68.2%), the optic tract (12/44; 27.3%), or the lateral geniculate nucleus (2/44; 4.5%).
Regarding the evolution of the HVFD, only fifty-two patients were followed-up. The recovery was described as complete in 31 patients (59.6%), with a median time to recovery of 9 weeks (range 2 weeks to 12 months). An incomplete recovery was found in 16 subjects (30.8%). No recovery was found in five subjects (9.6%). No recurrent episodes of HVFD in multiple sclerosis patients were described in the literature.
Patients with multiple sclerosis who present an HVFD are mostly young patients with relapsing-remitting multiple sclerosis, which is consistent with the epidemiology of multiple sclerosis. Both men and women can be affected, with a slight female preponderance (three women, two men), and an HVFD can occur at any time during the course of the disease. One third of our patients (7/20) presented an HVFD as the inaugural symptom of multiple sclerosis, which is three times higher than the proportions previously reported in the literature. This could be related to the fact that nowadays, computerised automated static perimetry is mostly used to evaluate visual fields. As automated static perimetry is more sensitive than other perimetric techniques, it allows more subtle defects to be detected.
Regarding the type of HVFD, we found that partial HVFD were more frequent than complete HVFD. The size and location of a demyelinating lesion are two critical factors which determine whether a lesion will result in a partial or a complete HVFD. Within the optic tract, axons are compacted into a small area and lesions at this location are more likely to produce a complete HVFD. In contrast, within the optic radiations, axons are spread over a larger area and lesions at this location are more likely to a produce partial HVFD. In multiple sclerosis, most lesions associated with HVFD are small and located within the optic radiations. This can explain why most HVFD are partial. Furthermore, optic radiation lesions can be asymptomatic, as MRI studies have revealed that retrochiasmal demyelinating lesions are found more frequently than HVFD [8, 47].
After an episode of HVFD due to a demyelinating lesion, most patients experienced complete recovery of their visual fields (59% in our series and 60% in the literature) in a relatively short time. The median recovery time was ten weeks in our study, which is comparable with previous reports (median of nine weeks with only one patient taking more than 12 months to recover) [7]. A partial recovery was found in 23% of our patients, with a median follow-up of three years. This suggests that if a full visual field recovery is not achieved at three months, it will not occur.
Two patients in our study showed worsening of their HVFD without new clinical relapse. They were both diagnosed with a secondary progressive form of multiple sclerosis. Worsening of an HVFD without new relapses in a patient with relapsing-remitting multiple sclerosis could be a sign of the disease evolving towards a secondary progressive phase. Due to both the highest incidence of relapsing-remitting multiple sclerosis and the relatively long follow-up time in our study (average two years, maximum ten years), it is not surprising that we encountered relapses of HVFD (fig. 4). In our study 3/20 patients presented with recurrences of HVFD.
The sensitivity of OCT for detecting abnormalities can sometimes be higher than that of automated computerised static perimetry. For example, following full recovery after an episode of optic neuritis, all patients exhibited RGCL loss ranging from 5-27% [48]. A higher sensitivity of OCT (namely the measurement of RGCL thickness) compared to computerised visual field examinations was also suggested by our study. Four years after the onset of a completely resolved mild partial right homonymous hemianopsia, OCT revealed the persistence of homonymous thinning of the RGCL (fig. 5, Patient 18). In addition, OCT can sometimes reveal subclinical damage to the retrochiasmal visual pathways, as illustrated by a visually asymptomatic patient with multiple sclerosis (not from the current study) who exhibited a homonymous thinning of the RGCL on OCT, without any history of visual impairment in the past (fig. 6). Homonymous hemiatrophy of the ganglion cell layer was recently reported in a retrospective series of 47 patients with HVFD of various aetiologies [49]. The authors reported that such homonymous hemiatrophy could result from either direct retrograde axonal degeneration or transsynaptic retrograde axonal degeneration. They also reported a higher sensitivity of RGCL thickness measurement compared to peripapillary retinal nerve fibre layer measurement.
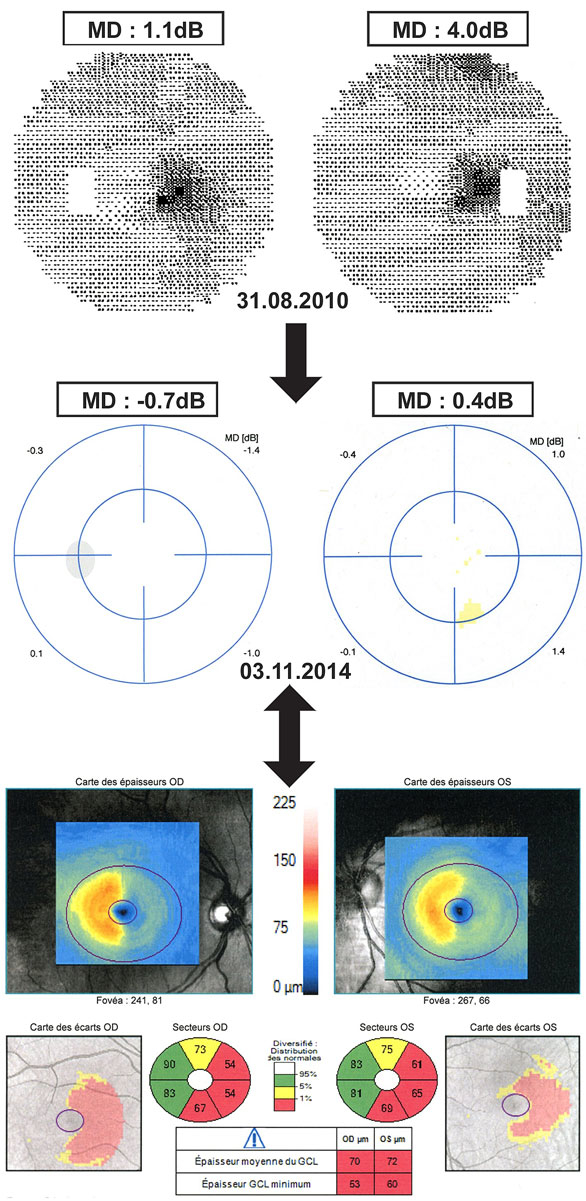
Figure 5 Persistence of homonymous retinal ganglion cell layer thinning after normalisation of the visual field. Top – initially (31 August 2010), the patient presented with a partial right homonymous scotoma and the mean defect (MD) was moderately elevated in both eyes. Middle – 4 years later (3 November 2014), the visual field was completely normal, with a normal MD in both eyes. Bottom – despite a full recovery of visual field, optical coherence tomography on 3 November 2014 showed a homonymous thinning of the retinal ganglion cell layer, visible on the thickness maps, the deviation maps and the sector maps. MD = mean defect expressed in decibels (dB).

Figure 6 Detection of asymptomatic retrochiasmal damage by optical coherence tomography (OCT). Top – normal visual field of a patient with multiple sclerosis who was never visually symptomatic. Bottom –OCT of the same patient, performed at the same date, demonstrated a homonymous left inferior thinning of the retinal ganglion cell layer, visible on the thickness maps, the deviation maps and the sector maps. MD = mean defect expressed in decibels (dB).
The incidence of HVFD in multiple sclerosis is probably underestimated. Some patients can exhibit only mild and partial HVFD, which could go unnoticed unless examined by static computerised perimetry. In our series, incidental partial HVFD were found in five visually asymptomatic patients at their regular ophthalmologic check-up. Systematic automated static perimetry in multiple sclerosis patients could potentially detect more cases of asymptomatic HVFD. Furthermore, it is possible that prospective OCT examinations could reveal additional cases of subclinical dysfunction of the retrochiasmal visual pathway, as illustrated in figure 6.
The weaknesses of our study are due to its retrospective nature. Not all patients benefited from a cerebral MRI at the time of HVFD diagnosis, and OCT was performed at the time of diagnosis in only 4/10 patients. A prospective study addressing these points would certainly allow to better determination of both the incidence and the clinical profile of HVFD in multiple sclerosis.
Homonymous visual field defects can occur in patients with multiple sclerosis. Their visual prognosis is good overall. The incidence of HVFD in multiple sclerosis might be higher than previously reported. Systematic examination with automated static perimetry and OCT might help to establish the true incidence of involvement of the retrochiasmal pathway in multiple sclerosis.
We would like to thank Mr Yann Leba for his assistance in making our figures.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Balcer LJ , Miller DH , Reingold SC , Cohen JA . Vision and vision-related outcome measures in multiple sclerosis. Brain. 2015;138(1):11–27. doi:.https://doi.org/10.1093/brain/awu335
2 Burton EV , Greenberg BM , Frohman EM . Optic neuritis: A mechanistic view. Pathophysiology. 2011;18(1):81–92. doi:.https://doi.org/10.1016/j.pathophys.2010.04.009
3 McDonald WI , Barnes D . The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J Neurol Neurosurg Psychiatry. 1992;55(9):747–52. doi:.https://doi.org/10.1136/jnnp.55.9.747
4 Bjerrum J . Et tilfaelde af hemianopsia partialis. Nord Ophtalm Tskr. 1890;3:71.
5 Boldt HA , Haerer AF , Tourtellotte WW , Henderson JW , Dejong RM . Retrochiasmal visual field defects from multiple sclerosis. Arch Neurol. 1963;8(5):565–75. doi:.https://doi.org/10.1001/archneur.1963.00460050115013
6 Beck RW , Schatz NJ , Savino J . Involvement of the optic chiasm, optic tract and geniculo-calcarine visual system in multiple sclerosis. Bull Soc Belge Ophtalmol. 1983;208(Pt 1):159–91.
7 Plant GT , Kermode AG , Turano G , Moseley IF , Miller DH , MacManus DG , et al. Symptomatic retrochiasmal lesions in multiple sclerosis: clinical features, visual evoked potentials, and magnetic resonance imaging. Neurology. 1992;42(1):68–76. doi:.https://doi.org/10.1212/WNL.42.1.68
8 Ormerod IEC , Miller DH , McDonald WI , du Boulay EPGH , Rudge P , Kendall BE , et al. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain. 1987;110(6):1579–616. doi:.https://doi.org/10.1093/brain/110.6.1579
9 Lehoczky T . Pathologic changes in the optic system in disseminated sclerosis. Acta Morphol Acad Sci Hung. 1954;4(3):395–408.
10 Castro SM , Damasceno A , Damasceno BP , Vasconcellos JP , Reis F , Iyeyasu JN , et al. Visual pathway abnormalities were found in most multiple sclerosis patients despite history of previous optic neuritis. Arq Neuropsiquiatr. 2013;71(7):437–41. doi:.https://doi.org/10.1590/0004-282X20130058
11 Hawkins K , Behrens MM . Homonymous hemianopia in multiple sclerosis. With report of bilateral case. Br J Ophthalmol. 1975;59(6):334–7. doi:.https://doi.org/10.1136/bjo.59.6.334
12 Sisto D , Trojano M , Vetrugno M , Trabucco T , Iliceto G , Sborgia C . Subclinical visual involvement in multiple sclerosis: a study by MRI, VEPs, frequency-doubling perimetry, standard perimetry, and contrast sensitivity. Invest Ophthalmol Vis Sci. 2005;46(4):1264–8. doi:.https://doi.org/10.1167/iovs.03-1213
13 Fisher JB , Jacobs DA , Markowitz CE , Galetta SL , Volpe NJ , Nano-Schiavi ML , et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324–32. doi:.https://doi.org/10.1016/j.ophtha.2005.10.040
14 Gabilondo I , Sepúlveda M , Ortiz-Perez S , Fraga-Pumar E , Martínez-Lapiscina EH , Llufriu S , et al. Retrograde retinal damage after acute optic tract lesion in MS. J Neurol Neurosurg Psychiatry. 2013;84(7):824–6. doi:.https://doi.org/10.1136/jnnp-2012-304854
15 Van Buren JM . Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry. 1963;26(5):402–9. doi:.https://doi.org/10.1136/jnnp.26.5.402
16 Reich DS , Smith SA , Gordon-Lipkin EM , Ozturk A , Caffo BS , Balcer LJ , et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol. 2009;66(8):998–1006. doi:.https://doi.org/10.1001/archneurol.2009.107
17 Rocca MA , Mesaros S , Preziosa P , Pagani E , Stosic-Opincal T , Dujmovic-Basuroski I , et al. Wallerian and trans-synaptic degeneration contribute to optic radiation damage in multiple sclerosis: a diffusion tensor MRI study. Mult Scler. 2013;19(12):1610–7. doi:.https://doi.org/10.1177/1352458513485146
18 Meier PG , Maeder P , Kardon RH , Borruat FX . Homonymous Ganglion Cell Layer Thinning After Isolated Occipital Lesion: Macular OCT Demonstrates Transsynaptic Retrograde Retinal Degeneration. J Neuroophthalmol. 2015;35(2):112–6.
19 Mitchell JR , Oliveira C , Tsiouris AJ , Dinkin MJ . Corresponding Ganglion Cell Atrophy in Patients With Postgeniculate Homonymous Visual Field Loss. J Neuroophthalmol. 2015;35(4):353–9. doi:.https://doi.org/10.1097/WNO.0000000000000268
20 Meier P , Maeder P , Borruat FX . Darstellung von transsynaptischer retrograder Degeneration bei homonymem RGCL-Verlust mittels OCT [Transsynaptic Retrograde Degeneration: Clinical Evidence with Homonymous RGCL Loss on OCT]. Klin Monatsbl Augenheilkd. 2016;233(4):396–8. doi:.https://doi.org/10.1055/s-0041-111535
21 Holladay JT . Proper method for calculating average visual acuity. J Refract Surg. 1997;13(4):388–91.
22 Kawasaki A , Borruat FX . Photophobia associated with a demyelinating lesion of the retrochiasmal visual pathway. Am J Ophthalmol. 2006;142(5):854–6. doi:.https://doi.org/10.1016/j.ajo.2006.05.026
23 Ronne H . Vorkommen enies hemianopischen zentralscotomsb. disseminierte sclerose. Klin Mbl Augenheilkd. 1912;14:446.
24 Bjerrum J . Nord ophtal. Tidjskr. 1912;3:71.
25Wilbrand H, Saenger A. Die Erkrankungen des Opticusstammes. In Die Neurologie des Auges. Wiesbaden: J. F. Bergmann; 1913;5.
26 Ronne H . Vorkommen enies hemianopischen zentralscotomsb, Disseminierte sclerose. Klin Mbl Augenheilkd. 1915;55:68.
27 Bielschowsky A . Klin Mbl Augenheilkd. 1933;90:542.
28Traquair HM. An introduction to clinical perimetry. 4th edition. London: Kimpton; 1942 pp. 259–60.
29 Malbran JL , Sitler R , Insousti T . Homonymous hemianopic paracentral scotoma. Arch Oftalmol B Aires. 1952;27:193.
30 Chamlin M , Davidoff LM . Homonymous hemianopia in multiple sclerosis. Neurology. 1954;4(6):429–37. doi:.https://doi.org/10.1212/WNL.4.6.429
31 François J , Verriest G . La névrite rétro-chiasmatique de la sclérose-enplaques[Retrochiasmatic neuritis in multiple sclerosis]. Ann Ocul (Paris). 1957;190(5):305–15.
32 Vedel-Jensen N . Optic tract neuritis in multiple sclerosis. Acta Ophthalmol (Copenh). 1959;37(5):537–45. doi:.https://doi.org/10.1111/j.1755-3768.1959.tb03466.x
33 Beck RW , Savino PJ , Schatz NJ , Smith CH , Sergott RC . Plaque causing homonymous hemianopsia in multiple sclerosis identified by computed tomography. Am J Ophthalmol. 1982;94(2):229–34. doi:.https://doi.org/10.1016/0002-9394(82)90080-0
34 Rosenblatt MA , Behrens MM , Zweifach PH , Forman S , Odel JG , Duncan CM , et al. Magnetic resonance imaging of optic tract involvement in multiple sclerosis. Am J Ophthalmol. 1987;104(1):74–9. doi:.https://doi.org/10.1016/0002-9394(87)90297-2
35 Slavin ML . Acute homonymous field loss: really a diagnostic dilemma. Surv Ophthalmol. 1990;34(5):399–407. doi:.https://doi.org/10.1016/0039-6257(90)90117-E
36 Vighetto A , Grochowicki M , Aimard G . Altitudinal Hemianopia in Multiple Sclerosis. Neuro-Ophthalmology. 1991;11(1):25–7. doi:.https://doi.org/10.3109/01658109109009638
37 Frederiksen JL , Larsson HB , Nordenbo AM , Seedorff HH . Plaques causing hemianopsia or quadrantanopsia in multiple sclerosis identified by MRI and VEP. Acta Ophthalmol (Copenh). 1991;69(2):169–77. doi:.https://doi.org/10.1111/j.1755-3768.1991.tb02707.x
38 Sanchez-Dalmau B , Goñi FJ , Guarro M , Roig C , Duch-Bordas F . Bilateral homonymous visual field defects as initial manifestation of multiple sclerosis. Br J Ophthalmol. 1991;75(3):185–7. doi:.https://doi.org/10.1136/bjo.75.3.185
39 Waldvogel D , Sturzenegger M , Ozdoba C , Schroth G . Hemiparesis and homonymous hemianopia as the presenting sign of multiple sclerosis. Neuro-Ophthalmology. 1991;11:25–7.
40 Borruat FX , Siatkowski RM , Schatz NJ , Glaser JS . Congruous quadrantanopia and optic radiation lesion. Neurology. 1993;43(7):1430–2. doi:.https://doi.org/10.1212/WNL.43.7.1430
41 Cesareo M , Pozzilli C , Ristori G , Roscioni AM , Missiroli A . Crossed quadrant homonymous hemianopsia in a case of multiple sclerosis. Clin Neurol Neurosurg. 1995;97(4):324–7. doi:.https://doi.org/10.1016/0303-8467(95)00053-M
42 Dogulu CF , Kansu T , Karabudak R . Alexia without agraphia in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;61(5):528. doi:.https://doi.org/10.1136/jnnp.61.5.528
43 Gündüz K , Cansu K , Bulduklar S , Saatçi I . Homonymous hemianopsia as the initial manifestation of multiple sclerosis. Ophthalmologica. 1998;212(3):215–20. doi:.https://doi.org/10.1159/000027283
44 Mao-Draayer Y , Panitch H . Alexia without agraphia in multiple sclerosis: case report with magnetic resonance imaging localization. Mult Scler. 2004;10(6):705–7. doi:.https://doi.org/10.1191/1352458504ms1075cr
45 Murai H , Kiyosawa M , Suzuki Y , Mizoguchi S , Ishii K , Ishikawa K , et al. A case of multiple sclerosis with homonymous hemianopia examined by positron emission tomography. Jpn J Ophthalmol. 2004;48(6):591–3. doi:.https://doi.org/10.1007/s10384-004-0128-1
46 Law SW , Lee AW , Chen CS . Multiple sclerosis presenting with homonymous hemianopia. Aust Fam Physician. 2009;38(10):795–6.
47 Hornabrook RS , Miller DH , Newton MR , MacManus DG , du Boulay GH , Halliday AM , et al. Frequent involvement of the optic radiation in patients with acute isolated optic neuritis. Neurology. 1992;42(1):77–9. doi:.https://doi.org/10.1212/WNL.42.1.77
48 Sherif M , Bergin C , Borruat FX . Wiederherstellung der normalen Sehkraft nach Neuritis nervi optici bei Patienten mit multipler Sklerose trotz signifikantem Verlust von retinalen Ganglienzellen [Normal visual recovery after optic neuritis despite significant loss of retinal ganglion cells in patients with multiple sclerosis]. Klin Monatsbl Augenheilkd. 2019;236(4):425–8. doi:.https://doi.org/10.1055/a-0853-1721
49 Mühlemann F , Grabe H , Fok A , Wagner F , Brügger D , Sheldon CA , et al. Homonymous hemiatrophy of ganglion cell layer from retrochiasmal lesions in the visual pathway. Neurology. 2020;94(3):e323–9. doi:.https://doi.org/10.1212/WNL.0000000000008738
F-XB. designed and directed the study. LS collected the data. F-XB and LS analysed the data and wrote the manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.