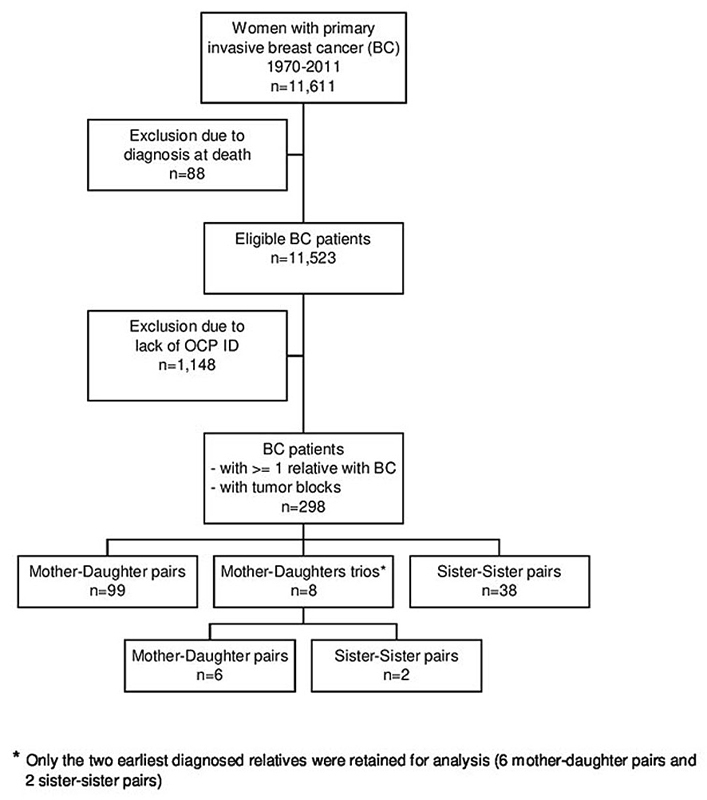
Figure 1 Flowchart of the study population.
DOI: https://doi.org/10.4414/smw.2020.20327
A family history of breast cancer is a well-documented risk factor: women with an affected mother or sister are at double the risk of the general population to develop a breast cancer [1]. However, the prognostic value of family history has not been clearly established. Several studies reported better survival for women with a positive family history of breast cancer as compared to those without [2, 3], whereas others reported no difference or worse survival [4–6]. A recent meta-analysis performed to clarify this issue demonstrated that having a first-degree relative with breast and/or ovarian cancer was associated with better overall survival and breast cancer-specific survival [7]. No clear conclusion could be drawn from subgroup analyses due to the limited number of studies available. Likewise, studies of tumour characteristics and prognosis in the context of a positive family history yielded mixed results and were mainly conducted in selected populations, particularly in high-risk families [8]. In two population-based studies, affected women with a positive family history of breast cancer had improved survival that could be attributable to different tumour characteristics rather than to differences in screening, detection method, or treatment [8, 9]. Finally, studies of intra-familial tumour characteristics and prognosis in population-based settings are very rare. In a previous study conducted among 160 mother-daughter and sister-sister pairs affected by breast cancer in Geneva, Switzerland, we found that breast cancer prognosis clusters within families and that the hereditary component is independent of patient and tumour characteristics and type of treatment [10]. Similar results were observed in a Swedish population-based cohort of 834 sister-sister affected pairs [11]. Both studies, however, lacked information on human epidermal growth factor receptor 2 (HER2) expression, which is a very important prognostic tumour characteristic [12]. Overexpression of HER2 is associated with aggressive tumour behaviour, resistance to therapies, and poor prognosis, especially prior to the introduction of a targeted immunotherapy.
The aim of the present study was to conduct a more comprehensive analysis of histopathological characteristics (histology, molecular subtype, tumour type and grade, tumour size, lymph node status, oestrogen receptor (ER) and progesterone receptor (PR) status, HER2 and Ki-67 status) through a centralised review of mother-daughter and sister-sister affected pairs using archived tumour blocks in Geneva. We evaluated intra-familial concordance of cancer pathology features and tested whether the clustering of breast cancer survival among family members is linked to patient and tumour characteristics.
Formal ethical approval and patient consent for this study was not required. The Geneva Cancer Registry has a general authorisation (Autorisation de la commission d'experts du secret professionnel en matière de recherche médicale: https://www.unige.ch/medecine/rgt/files/7914/6462/0509/Article321bis_1994_Texte_Commission_Experts.pdf; accessed on 18 December 2018) to collect nominative data, and to analyse the anonymised data.
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
We used data from the population-based Geneva Cancer Registry (GCR) that has been described in detail elsewhere [13]. Briefly, the database contains information on all cancers diagnosed in the resident population of the canton of Geneva (450,000 inhabitants) since 1970. The Cancer Registry extracts information from various sources, including pathology laboratories, public and private hospitals, private-sector physicians, and mortality data, and it is considered an accurate resource [14]. Recorded data include sociodemographic information, tumour characteristics using the World Health Organization (WHO) criteria [15], hormone receptor status, stage of disease at diagnosis, treatment during the first 6 months after diagnosis, occurrence of other primary cancers, and survival status. Cause of death is extracted from medical records.
The GCR regularly assesses survival in reference to the date of confirmed diagnosis or the date of hospitalisation (if it preceded the diagnosis and was related to the disease). In addition to passive follow-up through routine examination of death certificates and hospital records, an active follow-up is performed yearly using data from the Cantonal Population Office (OCP) regarding the registration of the resident population.
For this study we considered all women resident in the canton of Geneva diagnosed with a first primary invasive breast cancer between 1970 and 2011. Primary invasive breast cancer is a malignancy originating in the breast(s) with epithelial cancer cells from inside the milk ducts or lobules that have spread to the surrounding breast tissues. Breast cancer cases diagnosed at death (n = 88) were excluded. The final cohort consisted of 11,523 women with breast cancer.
Each resident in the Canton of Geneva is identified by a unique identification number. This number is registered in both OCP and GCR databases and was available for 10,375 patients of the cohort. Using the identification number as a key, a record linkage between these two databases was performed to identify the mother, daughter(s) and sister(s) of each breast cancer patient, and to retrieve their information about invasive breast cancer. Formal ethical approval and patient consent for this study was not required. The GCR has a general authorisation [16] to collect nominative data, and to analyse the anonymised data.
Within the 10,375 patients of the cohort, 298 had one or more relatives diagnosed with invasive breast cancer in the canton of Geneva, between 1970 and 2011, corresponding to 99 mother–daughter pairs, 8 mother-daughters trios and 38 sister-sister pairs. For the 8 trios, only the two earliest diagnosed relatives were retained for analysis (6 mother-daughter pairs and 2 sister-sister pairs). For the 105 mother-daughter pairs and the 40 sister-sister pairs, the patient with the earliest diagnosis of breast cancer was defined as the index case. Figure 1 describes the breast cancer patients selected for the study.

Figure 1 Flowchart of the study population.
To determine the uptake of genetic counselling and testing among the 290 patients of the cohort we performed a linkage with the database of the Oncogenetics and Cancer Prevention Unit at the Geneva University Hospitals. This unit has managed more than 4000 families since 1994 and has had the monopoly on genetic counselling and testing in the Geneva area until the end of 2017.
Primary tumour tissue specimens fixed into paraffin blocks of the 145 pairs of breast cancer patients and relatives were sought in the three laboratories of pathology existing in Geneva. Blocks were retrieved for 206 affected patients (69 mother-daughter pairs and 34 sister-sister pairs) and centralised at the Service of Clinical Pathology of the University Hospitals of Geneva for processing.
Slides from these patients were reviewed in a consensus meeting with four pathologists from the different laboratories (JCT, ES, DW, HB), who were unaware of the original diagnosis and clinical features. Tumour type and grade were determined according to the WHO classification [17].
Whole 4 μm sections were deparaffinised, rehydrated, and then submitted to immunohistochemistry with antigen retrieval on the Benchmark XT automated stainer (Ventana, Mannheim, Germany). Antibodies against ER (Ventana, Clone SP1, Cat num: 790-4324); PR (Ventana, Clone 1E2, Cat num: 790-2223); Ki-67 (Mib1 clone, DAKO, cat num M7240); HER2 (Ventana, Clone 4B5, cat num 790-2991) were used; HER2 chromogenic in situ hybridisation (Ventana, Dual ISH DNA Probe Cocktail Assay, cat num 780-4422) was performed. One slide per antibody was used.
We used the Allred score to quantify ER and PR expression by evaluating the percentage of stained cells (proportion score from 0 to 5) and the intensity of nuclear staining (intensity score from 0 to 3) [18]. If a patient's tumour expresses ER and/or PR, we can predict that this patient will positively benefit from endocrine therapy such as tamoxifen or aromatase inhibitors in postmenopausal women.
A semi-quantitative estimation was performed on the entire tumour for Ki-67 status. It was determined as the mean percentage of positive nuclei incorporating hot spots, according to the guideline [19].
HER2 expression was determined by in situ hybridisation as the first-line indication. If the technique failed, then we used immunohistochemistry (IHC) and completed by chromogenic in situ hybridisation (CISH) for scores 2+. HER2 status was considered positive when the ratio HER2/centromere enumeration probe for chromosome 17 (CEP17) was ≥ 2.0 or HER2 copies/cells > 6.0 and if an IHC score was 3+ according to the guidelines [20].
Two experts of breast pathology (JCT and ES) interpreted the staining
Intrinsic molecular subtypes based on St Gallen International Expert Consensus (2013) have classified breast carcinoma into luminal A, luminal B, HER2+, and triple-negative, depending on the expression of ER, PR, HER2, and Ki-67. Primary breast tumours were classified into four subgroups: luminal A (ER Allred >3, PR Allred ≥4, Ki-67 ≤20%, and HER2−), luminal B (ER Allred >3, PR Allred ≤3 or Ki-67 >20% and HER2−), triple negative (if ER Allred = 0, PR Allred = 0 and HER2-), and HER2 (if HER2+).
We compared index cases and relatives among mother-daughter pairs and sister-sister pairs for demographic, tumour, and treatment characteristics using chi-square tests.
For calculating the agreement of the breast cancer histopathological characteristics among mother-daughter pairs and sister-sister pairs, we used the unweighted kappa statistics with 95% confidence intervals (95% CIs). The kappa coefficient is a standard tool for the analysis of chance-corrected agreement on a binary outcome between two observers or two independent events [21]. The agreement was calculated between index cases and relatives in a two-by-two table for each histopathological dichotomous variable (e.g., ductal breast cancer in index case yes/no versus ductal breast cancer in relative yes/no). The kappa statistic was interpreted as follows: <0 less to what would be expected by chance; 0.01–0.20 slight; 0.21–0.40 fair; 0.41–0.60 moderate; 0.61–0.80 substantial; >0.80 almost perfect agreement [22].
All women were followed for vital status up to 31 December 2016. Survival analyses were based on breast cancer-specific mortality. The person-time at risk started at the date of breast cancer diagnosis and continued until death, emigration or end of follow-up, whatever came first.
To classify relatives according to their index case’s survival, we modelled the 10-year survival rate of the index cases with Cox proportional hazards analysis, adjusting for age at diagnosis and year of diagnosis. We categorised all relatives based on the Martingale residuals of the Cox model of their index case into familial survival groups [23]. These residuals were calculated as observed minus expected mortality and values below, above and around zero correspond to better, worse or expected survival, respectively. We defined the good familial survival risk group as the first tertile of the Martingale residual distribution, the medium familial survival risk group as the second tertile and the poor familial survival risk group as the third tertile. We estimated breast cancer-specific standardised mortality ratios (SMRs) of relatives in the poor, medium and good familial survival risk groups. For this, we calculated the expected numbers of breast cancer deaths for each prognosis group by multiplying the person-years of observation of each group by the breast cancer mortality rates for the female population of Geneva (stratified for each 5-year age group and 5-year calendar period). SMRs for the three familial survival risk groups were then calculated by dividing the observed numbers of deaths by the expected numbers. Statistical significance and 95% CIs were estimated assuming a Poisson distribution.
We graphed breast cancer-specific survival according to survival risk groups using Kaplan-Meier survivor function [24].
We also assessed breast cancer-specific mortality in relatives in relation to the index cases’ survival, using a Cox regression analysis, adjusting for stage, ER status, PR status, and HER2 expression.
All statistical tests were two sided. A p‐value <0.05 was considered as sttistically significant. We used the STATA SE15.1 software (Stata Corporation, College Station, TX, USA) to carry out all analyses.
Patient and treatment characteristics of the 290 affected women are presented in table 1. As expected, the index cases were more often diagnosed with breast cancer in the earlier periods than their relatives (49% before 1993 versus 4.8%, respectively). They were on average older at breast cancer diagnosis (62.2 years versus 58.2 years, p = 0.011, respectively), more often treated with mastectomy (49.7% versus 24.8%, respectively), and less likely to receive hormonal therapy (50.3% versus 25.5%, respectively).
Table 1 Patient and treatment characteristics for mother-daughter, sister-sister and all pairs for the period 1970–2011, extracted from the Geneva Cancer Registry. Only pairs with known information for both index cases and relatives were considered.
| Mother-daughter pairs | Sister-sister pairs | All pairs | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Index cases
(n = 105) |
Relatives
(n = 105) |
p-value |
Index cases
(n = 40) |
Relatives
(n = 40) |
p-value |
Index cases
(n = 145) |
Relative
(n = 145) |
p-value | |
| Period of diagnosis, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| 1970–1984 | 26 (24.8) | 0 (0.0) | 7 (17.5) | 0 (0.0) | 33 (22.8) | 0 (0.0) | |||
| 1985–1993 | 30 (28.6) | 6 (5.7) | 8 (20.0) | 1 (2.5) | 38 (26.2) | 7 (4.8) | |||
| 1994–2002 | 30 (28.6) | 27 (25.7) | 18 (45.0) | 17 (42.5) | 48 (33.1) | 44 (30.3) | |||
| 2003–2011 | 19 (18.1) | 72 (68.6) | 7 (17.5) | 22 (55.0) | 26 (17.9) | 94 (64.8) | |||
| Age at diagnosis, n (%) | <0.0001 | <0.005 | 0.062 | ||||||
| <50 years | 16 (15.2) | 39 (37.1) | 14 (35.0) | 4 (10.0) | 30 (20.7) | 43 (29.7) | |||
| 50–59 years | 19 (18.1) | 32 (30.5) | 14 (35.0) | 10 (25.0) | 33 (22.8) | 42 (29.0) | |||
| 60–69 years | 31 (29.5) | 20 (19.0) | 9 (22.5) | 13 (32.5) | 40 (27.6) | 33 (22.8) | |||
| ≥70 years | 39 (37.1) | 14 (13.3) | 3 (7.5) | 13 (32.5) | 42 (29.0) | 27 (18.6) | |||
| Socioeconomic status, n (%) | 0.056 | 0.158 | 0.262 | ||||||
| High | 19 (30.7) | 10 (16.14) | 5 (19.4) | 5 (17.9) | 24 (26.7) | 15 (16.7) | |||
| Medium | 31 (50.0) | 30 (48.0) | 13 (64.5) | 19 (67.9) | 44 (48.9) | 49 (54.4) | |||
| Low | 12 (19.4) | 22 (35.5) | 10 (16.1) | 4 (14.3) | 22 (24.4) | 26 (28.9) | |||
| Sector of care, n (%) | 0.334 | 0.502 | 0.240 | ||||||
| Private | 51 (48.6) | 58 (55.2) | 19 (47.5) | 22 (55.0) | 70 (48.3) | 80 (55.2) | |||
| Public | 54 (51.4) | 47 (44.8) | 21 (52.5) | 18 (45.0) | 75 (51.7) | 65 (44.8) | |||
| Surgery, n (%) | <0.001 | 0.202 | <0.0001 | ||||||
| Breast conservative | 43 (41.0) | 81 (77.1) | 24 (60.0) | 21 (52.5) | 67 (46.2) | 102 (70.3) | |||
| Mastectomy | 56 (53.3) | 20 (19.0) | 16 (40.0) | 16 (40.0) | 72 (49.7) | 36 (24.8) | |||
| None | 6 (5.7) | 4 (3.8) | 0 (0.0) | 3 (7.5) | 6 (4.1) | 7 (4.8) | |||
| Chemotherapy, n (%) | 0.234 | 0.061 | 1.00 | ||||||
| No | 76 (72.4) | 68 (64.8) | 22 (55.0) | 30 (75.0) | 98 (67.6) | 98 (67.6) | |||
| Yes | 29 (27.6) | 37 (35.2) | 18 (45.0) | 10 (25.0) | 47 (32.4) | 47 (32.4) | |||
| Radiotherapy, n (%) | 0.004 | 0.091 | 0.123 | ||||||
| No | 40 (38.1) | 21 (20.0) | 9 (22.5) | 16 (40.0) | 49 (33.8) | 37 (25.5) | |||
| Yes | 65 (61.9) | 84 (80.0) | 31 (77.5) | 24 (60.0) | 96 (66.2) | 108 (74.4) | |||
| Hormonal therapy, n (%) | <0.001 | 0.008 | <0.0001 | ||||||
| No | 55 (52.4) | 30 (28.6) | 18 (45.0) | 7 (17.5) | 73 (50.3) | 37 (25.5) | |||
| Yes | 50 (47.6) | 75 (71.4) | 22 (55.0) | 33 (82.5) | 72 (49.7) | 108 (74.4) | |||
| ‒ Antioestrogen | 17 | 42 | 8 | 19 | 25 | 61 | |||
| ‒ Antiaromatase | 6 | 22 | 1 | 7 | 7 | 29 | |||
| ‒ Others | 27 | 11 | 13 | 7 | 40 | 18 | |||
| Genetic consultation, n (%) | <0.001 | 0.274 | <0.001 | ||||||
| No | 95 (90.5) | 76 (72.4) | 34 (85.0) | 29 (72.5) | 129 (89.0) | 105 (72.4) | |||
| Yes | 10 (9.5) | 29 (27.6) | 6 (15.0) | 11 (27.5) | 16 (11.0) | 40 (27.6) | |||
| BRCA1/2 testing | |||||||||
| – BRCA1/2 not mutated | 6 | 12 | 4 | 5 | 10 | 17 | |||
| – BRCA1/2 mutated | 2 | 2 | 1 | 1 | 3 | 3 | |||
| BRCA1/2 not tested | 2 | 15 | 1 | 5 | 3 | 20 | |||
Considering the type of family relationship, index cases in mother-daughter pairs were significantly older at breast cancer diagnosis than their relatives (65.0 years versus 55.8 years, respectively, p <0.0001) and less often treated with breast-conservative surgery (41.0% versus 77.1%, respectively, p <0.001), radiotherapy (61.9% versus 80.0%, respectively, p = 0.004) and hormonotherapy (47.6% versus 71.4%, respectively, p <0.001). By contrast, the index cases in sister pairs were significantly younger at breast cancer diagnosis than relatives (54.7 years versus 64.6 years, respectively, p <0.0001), but they were also less often treated with hormonotherapy (55.0% versus 82.5%, respectively, p = 0.008). As expected, index cases were less likely to have an oncogenetic consultation than relatives in mother-daughter pairs (9.5% versus 27.6%, respectively, p <0.001) and sister-sister pairs (15.0% versus 27.5%, respectively, p = 0.274).
Based on the breast tumour pathology review, agreements of pathological characteristic features between index cases and relatives are presented for mother-daughter pairs (table 2) and sister pairs (table 3). No significant agreement was observed; in mother-daughter pairs, the highest kappa values were found for mixed histology tumours (kappa = 0.27, 95%CI −0.09 to 0.64), luminal A tumours (kappa = 0.24; 95% CI 0.01to 0.48), grade II and grade III tumours (kappa = 0.21; 95% CI −0.03 to 0.44 and kappa = 0.18, 95%CI −0.07 to 0.43, respectively) and HER2 status (kappa = 0.19, 95%CI −0.09 to 0.48). In sister-sister pairs, the highest kappa values were observed for T3–T4 tumours (kappa = 0.45, 95%CI 0.23 to 1), tumour stage (kappa = 0.44, 95%CI 0 to 0.88), lymph node status (kappa = 0.22, 95%CI −0.16 to 0.59) and triple-negative tumours (kappa = 0.21, 95%CI −0.29 to 0.7).
Table 2 Tumour characteristics of breast cancer in mother-daughter pairs (data from pathology review).
| Characteristics |
Index cases
(n = 69) |
Relatives
(n = 69) |
Concordant number | Kappa* | 95% CI |
|---|---|---|---|---|---|
| Histology, n (%) | |||||
| ‒ Ductal | 53 (76.8) | 56 (81.2) | 44 | 0.09 | −0.17 to 0.34 |
| ‒ Lobular | 8 (11.6) | 9 (13.0) | 2 | 0.13 | −0.17 to 0.43 |
| ‒ Mixed and others | 8 (11.6) | 4 (5.8) | 2 | 0.27 | −0.09 to 0.64 |
| Molecular subtype, n (%) | |||||
| ‒ Luminal A | 36 (52.2) | 38 (55.1) | 24 | 0.24 | 0.01 to 0.48 |
| ‒ Luminal B | 18 (26.1) | 16 (23.2) | 5 | 0.06 | −0.19 to 0.31 |
| ‒ HER2+ | 7 (10.1) | 13 (18.8) | 3 | 0.19 | −0.09 to 0.48 |
| ‒ Triple negative | 8 (11.6) | 2 (2.9) | 0 | −0.05 | −0.11 to 0.01 |
| Tumour size, n (%) | |||||
| ‒ T1 | 38 (55.1) | 48 (69.6) | 27 | 0.03 | −0.20 to 0.27 |
| ‒ T2 | 26 (37.7) | 15 (21.7) | 1 | −0.31 | −0.48 to 0.15 |
| ‒ T3–T4 | 5 (7.2) | 6 (8.7) | 0 | −0.09 | −0.14 to 0.03 |
| Lymph node status | −0.11 | −0.37 to 0.14 | |||
| ‒ Negative | 31 (49.2) | 41 (63.1) | 16 | ||
| ‒ Positive | 32 (50.8) | 24 (36.9) | 10 | ||
| Stage, n (%) | −0.02 | −0.25 to 0.21 | |||
| ‒ I–II | 55 (79.7) | 58 (84.1) | 46 | ||
| ‒ III–IV | 14 (20.3) | 11 (15.9) | 2 | ||
| Differentiation, n (%) | |||||
| ‒ Grade I | 11 (15.9) | 11 (15.9) | 3 | 0.13 | −0.15 to 0.42 |
| ‒ Grade II | 38 (55.1) | 39 (56.5) | 25 | 0.21 | −0.03 to 0.44 |
| ‒ Grade III | 20 (29.0) | 19 (27.5) | 8 | 0.18 | −0.07 to 0.43 |
| ER status, n (%) | 0.09 | −0.19 to -0.36 | |||
| ‒ Negative | 11 (15.9) | 8 (11.6) | 2 | ||
| ‒ Positive | 58 (84.1) | 61 (88.4) | 52 | ||
| PR status, n (%) | 0.03 | −0.22 to 0.27 | |||
| ‒ Negative | 20 (29.0) | 16 (23.2) | 5 | ||
| ‒ Positive | 49 (71.0) | 53 (76.8) | 38 | ||
| HER2 status, n (%) | 0.19 | −0.09 to 0.48 | |||
| ‒ Negative | 62 (89.9) | 56 (81.2) | 52 | ||
| ‒ Positive | 7 (10.1) | 13 (18.8) | 3 | ||
| Ki-67, n (%) | 0.05 | −0.2 to 0.3 | |||
| ‒ ≤20% | 45 (68.2) | 48 (69.6) | 32 | ||
| ‒ >20% | 21 (31.8) | 21 (30.4) | 7 |
* Unknown values not taken into account in kappa computation
Table 3 Tumour characteristics of breast cancer in sister pairs (data from pathology review).
| Characteristics |
Index cases
(n = 34) |
Relatives
(n = 34) |
Concordant number | Kappa* | 95% CI |
|---|---|---|---|---|---|
| Histology, n (%) | |||||
| ‒ Ductal | 28 (82.4) | 28 (82.4) | 23 | −0.01 | −0.36 to 0.34 |
| ‒ Lobular | 3 (8.8) | 1 (2.9) | 0 | −0.05 | −0.12 to 0.03 |
| ‒ Mixed and others | 3 (8.8) | 5 (14.7) | 1 | 0.16 | −0.29 to 0.6 |
| Molecular subtype, n (%) | |||||
| ‒ Luminal A | 20 (58.8) | 17 (50.0) | 9 | −0.12 | −0.46 to 0.23 |
| ‒ Luminal B | 9 (26.5) | 9 (26.5) | 3 | 0.09 | −0.28 to 0.46 |
| ‒ HER2+ | 1 (2.9) | 5 (14.7) | 0 | −0.05 | −0.14 to 0.04 |
| ‒ Triple negative | 4 (11.8) | 3 (8.8) | 1 | 0.21 | −0.29 to 0.7 |
| Tumour size, n (%) | |||||
| ‒ T1 | 17 (50.0) | 25 (73.5) | 14 | 0.11 | −0.2 to 0.42 |
| ‒ T2 | 13 (40.6) | 5 (15.6) | 1 | −0.11 | −0.38 to 0.17 |
| ‒ T3–T4 | 2 (6.3) | 2 (6.3) | 1 | 0.45 | −0.23 to 1 |
| Lymph node status | 0.22 | −0.16 to 0.59 | |||
| ‒ Negative | 17 (58.6) | 18 (62.1) | 12 | ||
| ‒ Positive | 12 (41.4) | 11 (37.9) | 6 | ||
| Stage, n (%) | 0.44 | 0 to 0.88 | |||
| ‒ I–II | 27 (81.8) | 28 (84.8) | 24 | ||
| ‒ III–IV | 6 (18.2) | 5 (15.2) | 3 | ||
| Differentiation, n (%) | |||||
| ‒ Grade I | 10 (29.4) | 5 (14.7) | 1 | −0.08 | −0.37 to 0.21 |
| ‒ Grade II | 15 (44.1) | 19 (55.9) | 7 | −0.16 | −0.5 to 0.18 |
| ‒ Grade III | 9 (26.5) | 10 (29.4) | 2 | −0.09 | −0.43 to 0.24 |
| ER status, n (%) | 0.15 | −0.3 to 0.6 | |||
| ‒ Negative | 4 (11.8) | 4 (11.8) | 1 | ||
| ‒ Positive | 30 (88.2) | 30 (88.2) | 27 | ||
| PR status, n (%) | −0.03 | −0.38 to 0.32 | |||
| ‒ Negative | 10 (29.4) | 11 (32.4) | 3 | ||
| ‒ Positive | 24 (70.6) | 23 (67.6) | 16 | ||
| HER2 status, n (%) | −0.05 | −0.14 to 0.04 | |||
| ‒ Negative | 33 (97.1) | 29 (85.3) | 28 | ||
| ‒ Positive | 1 (2.9) | 5 (14.7) | 0 | ||
| Ki-67, n (%) | 0.12 | −0.29 to 0.53 | |||
| ‒ ≤20% | 25 (78.2) | 28 (82.4) | 21 | ||
| ‒ >20% | 7 (21.8) | 6 (17.6) | 2 |
* Unknown values not taken into account in kappa computation
The 290 patients included in the study were followed on average for 10.7 years (mean time for mother-daughter pairs: 9.8 years; mean time for sister-sister pairs: 12.1 years). As expected, it was higher for index cases than for relatives (13.8 years and 9.3 years in mother-daughter pairs; 14.8 years and 9.7 years in sister-sister pairs). At the end of the follow‐up period, 88 of the 145 index cases were deceased (39 of breast cancer) and 42 of the 145 relatives (17 of breast cancer). After regrouping relatives into familial survival risk categories based on their index cases’ breast cancer-specific survival, those in the good familial survival risk category had a nine-fold increased risk of death from breast cancer when compared to the Geneva population (SMR 9.4, 95% CI 3.0–29.0), whereas those in the poor familial survival risk category had an almost 24-fold higher risk of death from breast cancer (SMR 23.6, 95% CI 11.8–47.1) (table 4). Breast cancer-specific survival among relatives was lower, although not significantly (p = 0.14), in the poor familial risk category than in medium and good familial risk categories (fig. 2). In multivariate Cox regression analysis, the 10-year risk of breast cancer-specific death estimate was not significantly increased for relatives in the poor familial survival compared to patients in the good familial survival risk group, after adjustment for tumour stage, ER and PR status, and HER2 expression (adjusted hazard ratio 1.78, 95% CI 0.26–12.23) (table 5).
Table 4 Standardised mortality ratios (SMRs) for breast cancer among relatives according to survival risk category.
| Survival category* | n | Person-years | Observed | Expected | SMR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Good | 48 | 510.32 | 3 | 0.32 | 9.35 | 3.02–29.00 | <0.005 |
| Medium | 48 | 384.44 | 6 | 0.31 | 19.31 | 8.68–42.98 | <0.001 |
| Poor | 49 | 468.03 | 8 | 0.34 | 23.57 | 11.79–47.13 | <0.001 |
* 10-year breast cancer-specific survival modelled by multivariate Cox analysis adjusted for age at diagnosis and calendar year
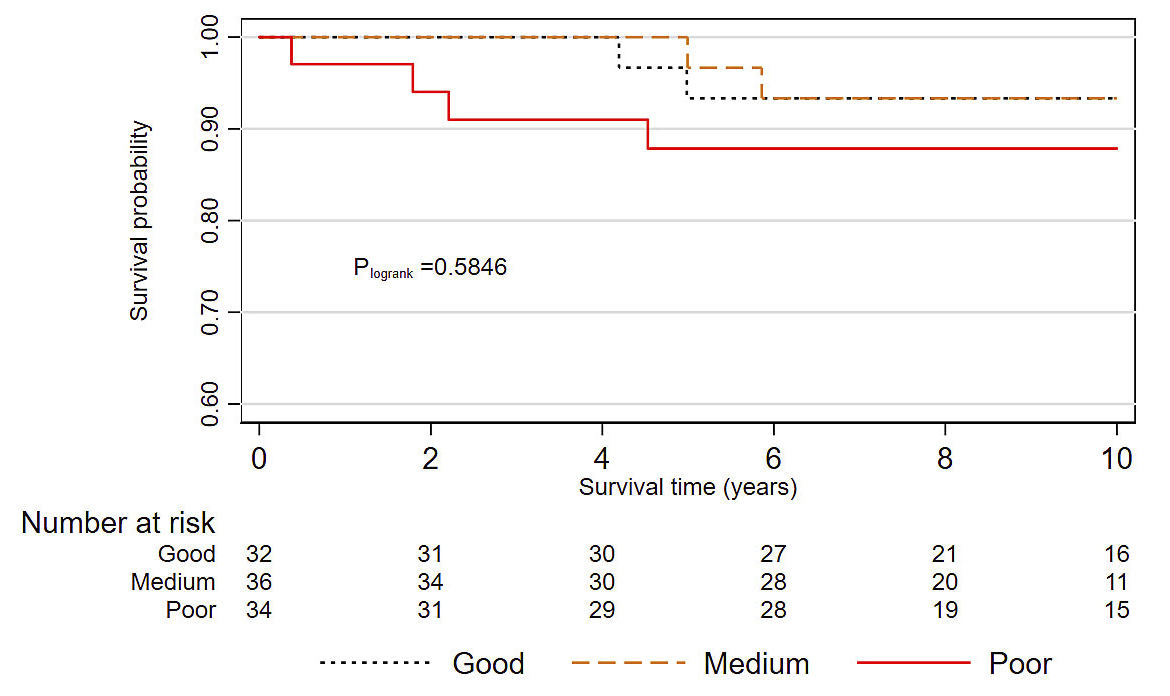
Figure 2 Breast cancer-specific survival in relatives according to familial survival categories (Kaplan-Meier method).
Table 5 Breast cancer-specific survival in relatives.
| Index cases | Relatives | ||
|---|---|---|---|
| 10-year risk of breast cancer-specific death | |||
| Hazard ratio (HR) | 95% CI | p-value | |
| Survival category* | |||
| Good | 1 (reference) | ||
| Medium | 1.37 | 0.17 –10.83 | 0.76 |
| Poor | 1.78 | 0.26 –12.23 | 0.56 |
| Stage | |||
| I | 1 (reference) | ||
| II | 6.98 | 0.67 –72.56 | 0.10 |
| III–IV | 14.8 | 1.15 –190.02 | 0.04 |
| Oestrogen receptor status | |||
| Negative | 1 (reference) | ||
| Positive | 0.66 | 0.04 –11.07 | 0.77 |
| Progesterone receptor status | |||
| Negative | 1 (reference) | ||
| Positive | 0.52 | 0.05 –5.42 | 0.58 |
| HER2 amplification status | |||
| No | 1 (reference) | ||
| Yes | 0.51 | 0.06 –4.11 | 0.53 |
* 10-year breast cancer-specific survival modelled by multivariate Cox analysis adjusted for age at diagnosis and calendar year
The aim of the present study was to conduct a comprehensive analysis of histopathological characteristics amongst mother-daughter and sister-sister affected pairs using archived tumour blocks in Geneva in order to evaluate intra-familial concordance of cancer pathology features and test whether the association of breast cancer survival among family members is linked to patient and tumour characteristics.
Family history is a well-established risk factor for breast cancer but its association with tumour characteristics at the time of diagnosis is unclear and existing literature is scarce. Smaller tumour size and/or less advanced stage for family history-positive cases has been reported in some but not other prior studies [8]. In the present population-based study, the results of kappa statistics showed that breast tumour characteristics in mother-daughter and sister-sister pairs did not have marked agreement. The analysis of the SMRs showed that relatives of breast cancer patients in any survival category experienced a much higher risk of breast cancer death than that expected in the general population of the same age and period. Our results do not confirm, however, that relatives in the poor survival category are at higher risk of breast cancer death than those in the good survival group.
The findings about the concordance of tumour features were generally in line with previous ones on Swedish sister pairs [11]. Our study is the first to report on tumour features in both family members based on a centralised pathology review and tumour analysis from archived blocks. In such a long follow-up study (from 1970 to 2011), some tumour characteristics in older time periods might not have been as accurate as in recent years, which can be the source of a potential bias toward underestimation of concordance [20]. Furthermore, agreement for HER2 amplification status could not be investigated as it was not routinely assessed prior to 2003.
Few population-based studies have considered family history in relation to invasive breast cancer survival. We previously reported concordance in good and poor survival between mother-daughter pairs and sister pairs [10]. Similar results were observed in Sweden using the nationwide Family-Cancer Database [11, 25]. These findings could likely be explained by genetic factors. Moreover, the lack of information on strong prognostic factors, in particular HER2 expression status, might potentially contribute to the observed results. It is also possible that the survival concordance in families is related to behavioural effects, such as active management of medical treatment in some families and avoidance in others, as well as treatment choices and lifestyle, the inheritance of host characteristics affecting for instance the ability to mount an effective anti-tumoural immune response or to respond to cancer therapy, and other unknown factors. In the present study, we did not find significant concordance in good and poor breast cancer-specific survival, likely because of the small number of family pairs and deaths from breast cancer. We also performed screening for germline BRCA1/2 pathogenic variants among 13 index cases and 20 relatives.
Large-scale studies with accurate data on strong prognosticators are still needed to confirm the hypothetical familial inheritance of breast cancer prognosis.
We thank Patrizia Bordignon for the immunohistochemistry study and in situ hybridisation work. We also thank Massimo Usel for helping in statistical analyses.
This work was supported by grants KLS 02544-02-2010 and KFS 2946-02-2012 from the Swiss Cancer League.
The authors declare that they have no conflict of interest.
1 Stratton MR , Rahman N . The emerging landscape of breast cancer susceptibility. Nat Genet. 2008;40(1):17–22. doi:.https://doi.org/10.1038/ng.2007.53
2 Malone KE , Daling JR , Weiss NS , McKnight B , White E , Voigt LF . Family history and survival of young women with invasive breast carcinoma. Cancer. 1996;78(7):1417–25. doi:.https://doi.org/10.1002/(SICI)1097-0142(19961001)78:7<1417::AID-CNCR7>3.0.CO;2-H
3 Jobsen JJ , van der Palen J , Brinkhuis M , Ong F , Struikmans H . Long-term effects of first degree family history of breast cancer in young women: Recurrences and bilateral breast cancer. Acta Oncol. 2016;55(4):449–54. doi:.https://doi.org/10.3109/0284186X.2015.1074281
4 Chang ET , Milne RL , Phillips KA , Figueiredo JC , Sangaramoorthy M , Keegan TH , et al. Family history of breast cancer and all-cause mortality after breast cancer diagnosis in the Breast Cancer Family Registry. Breast Cancer Res Treat. 2009;117(1):167–76. doi:.https://doi.org/10.1007/s10549-008-0255-3
5 Russo A , Herd-Smith A , Gestri D , Bianchi S , Vezzosi V , Rosselli Del Turco M , et al. Does family history influence survival in breast cancer cases? Int J Cancer. 2002;99(3):427–30. doi:.https://doi.org/10.1002/ijc.10342
6 Melvin JC , Wulaningsih W , Hana Z , Purushotham AD , Pinder SE , Fentiman I , et al. Family history of breast cancer and its association with disease severity and mortality. Cancer Med. 2016;5(5):942–9. doi:.https://doi.org/10.1002/cam4.648
7 Song JL , Chen C , Yuan JP , Sun SR . The association between prognosis of breast cancer and first-degree family history of breast or ovarian cancer: a systematic review and meta-analysis. Fam Cancer. 2017;16(3):339–49. doi:.https://doi.org/10.1007/s10689-017-9969-x
8 Malone KE , Daling JR , Doody DR , O’Brien C , Resler A , Ostrander EA , et al. Family history of breast cancer in relation to tumor characteristics and mortality in a population-based study of young women with invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2560–71. doi:.https://doi.org/10.1158/1055-9965.EPI-11-0781
9 Jannot AS , Usel M , Bouchardy C , Schubert H , Rapiti E . Breast cancer family history leads to early breast cancer detection and optimal management. Cancer Causes Control. 2017;28(9):921–8. doi:.https://doi.org/10.1007/s10552-017-0928-5
10 Verkooijen HM , Hartman M , Usel M , Benhamou S , Neyroud-Caspar I , Czene K , et al. Breast cancer prognosis is inherited independently of patient, tumor and treatment characteristics. Int J Cancer. 2012;130(9):2103–10. doi:.https://doi.org/10.1002/ijc.26206
11 Lindström LS , Li J , Lee M , Einbeigi Z , Hartman M , Hall P , et al. Prognostic information of a previously diagnosed sister is an independent prognosticator for a newly diagnosed sister with breast cancer. Ann Oncol. 2014;25(10):1966–72. doi:.https://doi.org/10.1093/annonc/mdu270
12 Ménard S , Fortis S , Castiglioni F , Agresti R , Balsari A . HER2 as a prognostic factor in breast cancer. Oncology. 2001;61(Suppl 2):67–72. doi:.https://doi.org/10.1159/000055404
13 Bouchardy C , Benhamou S , Schaffar R , Verkooijen HM , Fioretta G , Schubert H , et al. Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer. 2011;117(6):1288–95. doi:.https://doi.org/10.1002/cncr.25638
14International Agency for Research on Cancer. Cancer incidence in five continents, Vol IX. IARC Scientific Publications no 160. Lyon, France: IARC; 2008.
15Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al., eds. ICD‐O international classification of diseases for oncology. 3rd edn. Geneva: World Health Organization, 2000.
16Autorisation de la commission d'experts du secret professionnel en matière de recherche médicale: https://www.unige.ch/medecine/rgt/files/7914/6462/0509/Article321bis_1994_Texte_Commission_Experts.pdf. 2018.
17Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumors of the Breast. Lyon: IARC, 2012.
18 Allred DC , Harvey JM , Berardo M , Clark GM . Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68.
19 Dowsett M , Nielsen TO , A’Hern R , Bartlett J , Coombes RC , Cuzick J , et al.; International Ki-67 in Breast Cancer Working Group. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–64. doi:.https://doi.org/10.1093/jnci/djr393
20 Wolff AC , Hammond ME , Hicks DG , Dowsett M , McShane LM , Allison KH , et al.; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi:.https://doi.org/10.1200/JCO.2013.50.9984
21 Babaei M , Fallah M , Sundquist K , Hemminki K . Histological concordance in familial central nervous system tumors: Evidence from nationwide Swedish Family-Cancer Database. Cancer Epidemiol. 2015;39(3):334–9. doi:.https://doi.org/10.1016/j.canep.2015.03.004
22 Viera AJ , Garrett JM . Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3.
23Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. New York: Springer, 2003.
24 Kaplan EL , Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. doi:.https://doi.org/10.1080/01621459.1958.10501452
25 Hemminki K , Ji J , Försti A , Sundquist J , Lenner P . Survival in breast cancer is familial. Breast Cancer Res Treat. 2008;110(1):177–82. doi:.https://doi.org/10.1007/s10549-007-9692-7
Contributed equally
This work was supported by grants KLS 02544-02-2010 and KFS 2946-02-2012 from the Swiss Cancer League.
The authors declare that they have no conflict of interest.