Outcomes after spinal stenosis surgery by type of surgery in adults aged 60 years and older
DOI: https://doi.org/10.4414/smw.2020.20325
Thomas
Degenabc*, Karina
Fischerab*, Robert
Theilerab, Stefan
Schärend, Otto W.
Meyerabc, Guido
Wannere, Patricia
Chocano-Bedoyaab, Hans-Peter
Simmencf, Urs D.
Schmidg, Johann
Steurerh, Hannes B.
Stähelini, Noemi
Mantegazzaab, Heike A.
Bischoff-Ferrariabc
aDepartment of Geriatrics and Aging Research,
bCentre on Aging and Mobility,
cSenior Traumatology Centre,
dDepartment of Spinal Surgery,
eDepartment of Trauma Surgery and Orthopaedic Surgery, Schwarzwald-Baar Teaching Hospital,
fDepartment of Traumatology,
gDepartment of Neurosurgery,
hHorten Centre for Clinical Research,
iDepartment of Geriatrics,
Outcomes after spinal stenosis surgery by type of surgery in adults aged 60 years and older
Summary
AIMS OF THE STUDY
Mobility disability due to spinal stenosis is common in the senior population and often surgery is warranted for patients with severe symptoms and neurological dysfunction. However, although current clinical guidelines recommend stabilisation surgery in addition to decompression in patients with spinal stenosis and instability due to degenerative spondylolisthesis, the relationship between outcomes and the specific type of surgery have not been well studied. We therefore assessed the postoperative recovery timeline for 12 months and compared patient-reported outcomes dependent on the extent of decompression and additional stabilisation among seniors undergoing spinal stenosis surgery.
METHODS
We investigated 457 patients (mean age 76.0 ± 10.7 years, 58% women) from a consecutive cohort prior to spinal stenosis surgery. Follow-up was at 3 or 6months and at 12 months postoperatively. At each visit, pain, neurological dysfunction and disability were assessed using the North American Spine Society questionnaire. Repeated-measures analysis compared outcomes by type of surgery adjusting for baseline symptoms, gender, age, number of comorbidities, centre and year of surgery.
RESULTS
Most improvement occurred within the first 3 to 6 months with little or no further improvement at 12 months. Over 12 months and in adjusted models, patients receiving one-segment versus multi-segment decompression experienced significantly greater reduction of pain (−49.2% vs −41.9%, p = 0.013) and neurological dysfunction (−37.1% vs −25.9%, p <0.0001), but only borderline greater reduction of disability (−32.7% vs −28.2%, p = 0.051). Moreover, reduction in pain and neurological function did not differ with or without additional stabilisation and extend of decompression. However, patients who received one-segment (−28.9%) or multi-segment (−28.3%) stabilisation experienced significantly less reduction in disability after surgery compared with those who were not stabilised (−34.1%, p <0.043).
CONCLUSIONS
Among senior patients undergoing spinal stenosis surgery, recovery was largely complete by 3 to 6 months after surgery and differed little by type of surgery independently of symptoms prior to surgery and other covariates. However we could document a trend toward more improvement in particularly neurological dysfunction and disability with less invasive surgery.
Introduction
Mobility disability due to spinal stenosis is common in the senior population, and is accompanied by a wide range of symptoms such as pain in the lower back and buttocks, thighs, and sometime calves provoked by walking or longer standing. Prevalence of symptomatic spinal stenosis among seniors aged 60 to 69 was found to be 19.4% in the Framingham Study, increasing further with age [1]. Similar data were presented from a population-based study in Japan [2–4]. Lumbar spinal stenosis is caused by progressive degeneration of intervertebral discs, facet joints and ligaments resulting in decreased vertebral height, which causes a narrowing the neural foramina and the spinal canal. This is further enhanced by age-related secondary osteoarthritis of the intervertebral joints. Degenerative instability with the development of spondylolisthesis and chronic degenerative slippage is a frequent sign of progressive disease [4, 5].
Symptoms of spinal stenosis include lower back pain, as well as pain and weakness in the legs, reduced ability to walk longer distances and increased frequency of falls [1, 5]. Whereas for mild symptoms, conservative treatment is recommended (pain medication, physiotherapy and steroid injection) [6–8], for patients with severe symptoms and neurological dysfunction surgery is warranted [9]. Notably, in patients aged 65 and older lumbar spinal stenosis is the most common indication for spinal surgery; it is similar to the findings of patient-reported outcome research into total joint replacement due to osteoarthritis of the hip [10]. It has been suggested that the prevalence of comorbid diseases affects outcomes more adversely than age [11].
Another source of outcome variability in seniors undergoing spinal stenosis surgery may be the extent of decompression and the need for additional stabilisation. Current guidelines recommend stabilisation surgery in patients with spinal stenosis and additional instability due to degenerative spondylolisthesis [12], but outcomes depending on type of surgery have not been well studied. Notably, as senior patients often present with a progressed stage of spinal stenosis, degenerative spondylolisthesis is highly prevalent [12]. Furthermore, as stabilisation in addition to decompression increases surgery time and therefore may carry extra risks in senior patients (i.e., infections, bleeding, delirium) [13], the outcome variation due to surgery technique might be of great clinical importance [14].
The aim of this observational study was to investigate the timeline of patient-reported recovery in the first year after spinal stenosis surgery and outcome variation by the extent of decompression and the need for additional stabilisation among senior patients age 60 years and older. To compare outcomes in the best possible way, our study adjusted for multiple factors, including symptoms prior to surgery, number of comorbid conditions, age, and gender.
Materials and methods
Study design and participants
We enrolled 524 consecutive patients age 60 years and older (mean age 75.6 ± 11.2 years, 58% women) who were scheduled for surgery to treat spinal stenosis in two large hospital centres in Switzerland (Basel University Hospital and Triemli City Hospital, Zurich) in a two-centre prospective “ real life” cohort study between January 2002 and December 2009. Exclusion criteria were fractures, spinal infections and tumours associated with oncological diseases. All of the 524 patients enrolled underwent baseline assessments prior to surgery. Post-surgical assessments of pain, neurological dysfunction and disability were performed at two time points: the first was either at 3 months (n = 277) or 6 months (n = 189) after surgery, depending on the centre, and the second at 12 months after the surgery (n = 457). Sixty-seven patients had incomplete follow-up data; these patients were therefore excluded from the statistical analysis (see table 1 below).
The study was approved by the local Ethics Committee of the Triemli City Hospital in Zurich, Switzerland (approval number: EK 33/02). As in 2002 written consent was not obligatory for observational studies in Switzerland, all participants were informed about the purpose of the study and gave oral consent to participate in the study prior to any study procedure. They gave written consent each time they filled out the electronic questionnaires with the study nurse.
Type of spinal stenosis surgery
The type of surgery performed on each patient with spinal stenosis was recorded and, as pre-defined for the purposes of this study, categorised according to two parameters: segmental decompression (one vs multiple) and segmental stabilisation (none, one, and multiple). Type of surgery was chosen by experienced spinal surgeons based on radiology findings and the clinical examination of each patient. The surgeons of the Triemli City Hospital exclusively use open microsurgical techniques with unilateral interlaminar fenestration (if necessary with arthrectomy, if appropriate with contralateral decompression “over the top”), but they never use full laminectomy for decompression of the lumbar spinal canal for spinal stenosis and grade 1 or 2 spondylolisthesis. The surgeons of the Spinal Surgery Unit Basel use open interlaminar bilateral decompression. They also never use laminectomy.
Assessment of pain, neurological dysfunction and disability
At baseline (before surgery), at 3 or 6 months (depending on the centre) and at 12 months, all participants were interviewed by a study nurse, in a standardised way, about their levels of back pain, neurological dysfunction and disability based on three subscales of the North American Spine Society (NASS) Lumbar Spine Outcome Assessment questionnaire [15, 16]. The NASS questionnaire assesses these characteristics as a score (0–100) on a scale from 0 (total absence of pain, no neurological dysfunction or disability) to 100 (maximum level of pain, neurological dysfunction or disability). The data were collected by a trained study nurse using the QUALITOUCH EDC software version 3.2 [16, 17].
Assessment of covariates
At the baseline assessments, age, gender, year of the surgery, and the clinical centre where the surgery was performed were recorded. Baseline levels of pain, neurological dysfunction and disability were assessed using the NASS questionnaire, and the presence of comorbid conditions (score: 0–3, 4–7 and 8+) before surgery was assessed using the Sangha comorbidity index (score range 0–12) [18].
Statistical analysis
Baseline characteristics were compared by using a χ2 test for categorical variables and a Student’s t-test for continuous variables.
In the primary analysis, overall differences in the impact of different types of decompression or stabilisation strategies on the repeatedly assessed NASS outcomes (changes from baseline in values of level of pain, neurological dysfunction and disability) over the total period of 12 months after spinal stenosis surgery were analysed using multivariable repeated-measures linear mixed effects ANCOVA models. Models included time, the main surgery strategies (decompression and stabilisation) and their interaction. Covariates adjusted in the ANCOVA models were gender, age, comorbidities (Sangha score 0–3, 4–7 and 8+), centre, year of the surgery and the baseline value of the respective outcome variable (pain, neurological dysfunction or disability).
To investigate the timeline of recovery as well as for a sensitivity analysis to compare the impact of segment decompression and stabilisation on NASS outcomes at each time point separately, differences in the NASS outcomes (change values of level of pain, neurological dysfunction or disability compared to baseline) between different types of decompression or stabilisation strategies were analysed at each time point (3 or 6 months, and 12 months) after spinal stenosis surgery using multivariable linear ANCOVA models. Models included the main surgery strategies (decompression and stabilisation) and their interaction. The same covariates as listed above for the primary analyses were adjusted in these ANCOVA models,
Statistical significance was set at p <0.05. All analyses were performed with SAS version 9.4.
Results
Characteristics of the study population
The mean baseline age of the 524 patients included in the prospective analysis was 75.6 years (standard deviation 11.2), 56% were women and the Sangha comorbidity index was 5.9 (2.6) (table 1). The pre-surgery NASS scores were 80.2 (16.4) for pain, 56.2 (28.0) for neurological dysfunction and 57.3 (18.9) for disability. Of the included patients, 68% (n = 354) required decompression of multiple segments and 51% (n = 266) underwent stabilisation of at least one segment; 27% (n = 143) needed one segment stabilised and 23% (n = 123) underwent stabilisation of multiple segments. Except for the centre where the surgery was performed and year of the surgery, there were no significant differences in baseline characteristics between participants with complete follow-up data (n = 457, 87%) and the 67 excluded participants.
Table 1 Baseline characteristics of the study population.
|
Characteristic
|
One-segment decompression
|
One-segment decompression and stabilisation
|
One-segment decompression, multi-segment stabilisation
|
Multi-segment decompression
|
Multi-segment decompression, one-segment stabilisation
|
Multi-segment decompression and stabilisation
|
p-value
|
Entire Population
(n = 524)
|
| n (%) |
66 (13%) |
84 (16%) |
20 (4%) |
192 (37%) |
59 (11%) |
103 (20%) |
|
524 (100%) |
| Gender, n (%) |
|
|
|
|
|
|
<0.0001 |
|
| ‒ Male |
40 (61%) |
27 (32%) |
4 (20%) |
108 (56%) |
18 (31%) |
32 (30%) |
|
228 (44%) |
| ‒ Female |
26 (39%) |
57 (68%) |
16 (80%) |
84 (44%) |
41 (69%) |
71 (70%) |
|
296 (56%) |
| Age*
|
70.6 (15.3) |
70.9 (11.9) |
77.6 (8.6) |
77.2 (10.3) |
77.7 (8.7) |
78.2 (8.4) |
|
75.6 (11.2) |
| Centre, n (%) |
|
|
|
|
|
|
<0.0001 |
|
| ‒ Basel |
41 (62%) |
34 (40%) |
5 (25%) |
117 (61%) |
47 (80%) |
23 (22%) |
|
267 (51%) |
| ‒ Zurich |
25 (38%) |
50 (60%) |
15 (75%) |
75 (39%) |
12 (20%) |
80 (78%) |
|
257 (49%) |
| Year of the surgery, n (%) |
|
|
|
|
|
|
<0.0001 |
|
| ‒ 2002/3 |
5 (8%) |
14 (17%) |
3 (15%) |
22 (12%) |
4 (7%) |
8 (8%) |
|
56 (11%) |
| ‒ 2004 |
14 (21%) |
13 (15%) |
9 (45%) |
39 (20%) |
9 (16%) |
27 (26%) |
|
111 (21%) |
| ‒ 2005 |
14 (21%) |
15 (18%) |
2 (10%) |
54 (28%) |
20 (34%) |
30 (29%) |
|
135 (26%) |
| ‒ 2006 |
12 (18%) |
17 (20%) |
0 (0%) |
24 (13%) |
6 (10%) |
18 (18%) |
|
77 (15%) |
| ‒ 2007–9 |
21 (32%) |
25 (30%) |
6 (30%) |
52 (27%) |
19 (33%) |
19 (19%) |
|
142 (27%) |
| Sangha comorbidity index (score 0–12)†
|
5.4 (2.3) |
5.9 (2.7) |
6.4 (2.3) |
6.1 (2.7) |
5.9 (2.9) |
6.1 (2.6) |
0.570 |
5.9 (2.6) |
| Pre-surgery pain (score 0-100)‡§
|
80.4 (15.4) |
80.7 (17.6) |
81.1 (16.1) |
79.4 (17.2) |
80.3 (16.1) |
80.83 (15.2) |
0.982 |
80.2 (16.4) |
| Pre-surgery neurological dysfunction (score 0–100)‡§
|
58.7 (26.8) |
55.6 (29.1) |
44.7 (28.2) |
57.5 (27.3) |
54.9 (29.4) |
55.5 (28.2) |
0.516 |
56.2 (28.0) |
| Pre-surgery disability (score 0–100)‡§
|
59.9 (18.6) |
57.6 (19.5) |
56.7 (15.8) |
56.1 (20.3) |
54.7 (17.5) |
59.3 (17.3) |
0.548 |
57.3 (18.9) |
Timeline of recovery after spinal stenosis surgery by type of surgery
Investigation of the timeline of recovery after spinal stenosis surgery by type of surgery (fig. 1, table 2) showed that for all outcomes and independent of symptoms prior to surgery, type of surgery, age, gender, comorbidity, year of surgery and centre, most improvement was achieved at 3 or 6 months with minimal or no further improvement at 12 months (table 2). Notably, the timeline of recovery for all three outcomes looked very similar regardless of surgery type (fig. 1).
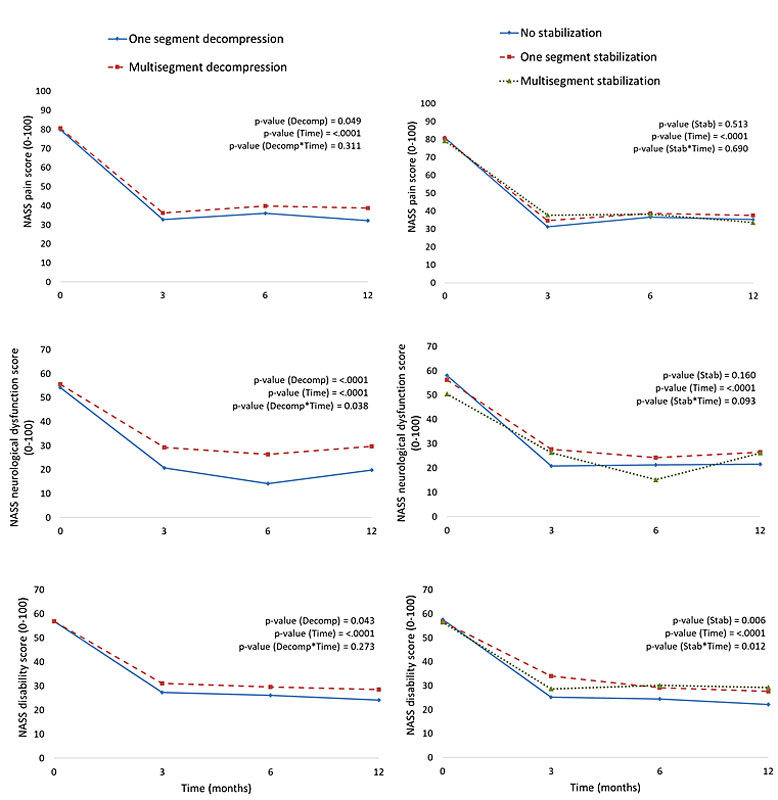
Figure 1 Timeline of recovery after spinal stenosis by decompression and stabilisation surgery types for the three outcomes North American Spine Society (NASS) pain scores, neurological dysfunction scores and disability scores over time. Data (n = 457) are means of absolute values of NASS outcomes prior to the surgery (month “0”) and at 3- 6-, and 12-month follow-up after spinal stenosis surgery based on repeated-measures linear mixed effects ANCOVA models.
Overall change in repeated NASS outcomes over 12 months after spinal stenosis surgery by type of surgery
With respect to the overall impact of type of surgery in the first year after spinal stenosis surgery (table 3), in adjusted analysis, patients who received one-segment decompression experienced a significantly greater reduction of pain (−49.2% vs −41.9%, p = 0.013) and neurological dysfunction (−37.1% vs −25.9%, p <0.0001), but only a borderline greater reduction of disability (−32.7% vs −28.2%, p = 0.051) during the first year compared with those who required decompression of multiple segments, independent of symptoms prior to surgery, extend of stabilisation and other covariates.
Table 2 Change in NASS outcomes in the first year after spinal stenosis surgery by time point.
|
Table 2: Change in NASS outcomes in the first year after spinal stenosis surgery by time point. |
|
Difference in NASS outcome scores (0-100) compared to baseline
*
|
Number of decompressed segments
|
p-value
|
Number of stabilised segments
|
p-value
|
|
One
|
Multiple
|
None
|
One
|
Multiple
|
|
Mean (SE)
|
Mean (SE)
|
Mean (SE)
|
Mean (SE)
|
Mean (SE)
|
|
Pain†
|
|
|
|
|
|
|
|
| Δ (3 months − BL) |
−47.1 (3.9) |
−40.7 (3.2) |
0.130 |
−49.5 (3.8) |
−43.2 (4.0) |
−39.1 (4.6) |
0.132 |
| Δ (6 months − BL) |
−43.0 (5.8) |
−39.3 (3.9) |
0.459 |
−38.4 (4.6) |
−39.0 (4.8) |
−46.0 (7.0) |
0.561 |
| Δ (12 months − BL) |
−49.2 (2.5) |
−41.9 (1.8) |
0.013
|
−46.1 (2.0) |
−44.8 (2.4) |
−45.6 (3.4) |
0.914 |
|
Neurological dysfunction†
|
|
|
|
|
|
|
|
| Δ (3 months − BL) |
−34.8 (3.9) |
−25.1 (3.2) |
0.020
|
−34.5 (3.8) |
−26.3 (4.0) |
−29.0 (4.6) |
0.246 |
| Δ (6 months − BL) |
−40.6 (6.2) |
−30.9 (4.2) |
0.075 |
−28.3 (5.0) |
−30.3 (5.1) |
−48.8 (7.4) |
0.027
|
| Δ (12 months − BL) |
−37.1 (2.4) |
−25.9 (1.7) |
<0.0001
|
−32.2 (1.9) |
−28.1 (2.3) |
−34.1 (3.2) |
0.194 |
|
Disability†
|
|
|
|
|
|
|
|
| Δ (3 months − BL) |
−30.5 (2.8) |
−24.5 (2.3) |
0.047
|
−33.3 (2.7) |
−22.4 (2.9) |
−26.7 (3.3) |
0.010
|
| Δ (6 months − B) |
−28.9 (4.6) |
−26.4 (3.1) |
0.522 |
−28.8 (3.7) |
−26.2 (3.9) |
−27.9 (5.5) |
0.740 |
| Δ (12 months − BL) |
−32.7 (2.0) |
−28.2 (1.4) |
0.051 |
−34.1 (1.6) |
−28.9 (1.9) |
−28.3 (2.6) |
0.043
|
Table 3 Overall change in repeated NASS outcomes over 12 months after spinal stenosis surgery by type of surgery and other covariates.
|
Parameter
|
Mean differences in NASS scores (0–100) compared with baseline*
|
|
Pain
|
p-value
|
Neurological dysfunction
|
p-value
|
Disability
|
p-value
|
| Decompression |
|
|
|
|
|
|
| ‒ One segment |
−49.2 (2.5) |
0.013
|
−37.1 (2.4) |
<0.0001
|
−32.7 (2.0) |
0.051 |
| ‒ Multi-segment |
−41.9 (1.9) |
|
−25.9 (1.7) |
|
−28.2 (1.4) |
|
| Stabilisation |
|
|
|
|
|
|
| ‒ None |
−46.1 (2.0) |
0.914 |
−32.2 (1.9) |
0.194 |
−34.1 (1.6) |
0.043
|
| ‒ One segment |
−44.8 (2.4) |
|
−28.1 (2.3) |
|
−28.9 (1.9) |
|
| ‒ Multi-segment |
−45.6 (3.4) |
|
−34.1 (3.2) |
|
−28.3 (2.6) |
|
| Gender |
|
|
|
|
|
|
| ‒ Men |
−46.6 (2.3) |
0.377 |
−31.4 (2.1) |
0.948 |
−31.0 (1.8) |
0.599 |
| ‒ Women |
−44.4 (1.8) |
|
−31.6 (1.7) |
|
−29.9 (1.4) |
|
| Age (5 years increase)†
|
−0.8 (0.6) |
0.195 |
−0.1 (0.6) |
0.857 |
−0.07 (0.5) |
0.884 |
| Sangha score (0–12) |
|
|
|
|
|
| ‒ 0–3 |
−46.4 (2.6) |
0.824 |
−31.9 (2.5) |
0.973 |
−29.6 (2.1) |
0.679 |
| ‒ 4–7 |
−45.8 (1.9) |
|
−31.4 (1.8) |
|
−31.5 (1.5) |
|
| ‒ 8+ |
−44.4 (2.6) |
|
−31.2 (2.4) |
|
−30.3 (2.0) |
|
| Baseline pain (10-unit increase)†
|
−7.2 (0.7) |
<0.0001
|
– |
|
– |
|
| Baseline neurological dysfunction (10-unit increase)†
|
– |
|
−7.7 (0.4) |
<0.0001
|
– |
|
| Baseline disability (10-unit increase)†
|
– |
|
– |
|
−5.6 (0.5) |
<0.0001
|
In adjusted analysis, reduction in pain and neurological dysfunction did not differ with or without additional segment stabilisation, independent of symptoms prior to surgery, extend of decompression and other covariates (table 3). However, patients who received one-segment (−28.9% reduction) or multi-segment (−28.3% reduction) stabilisation experienced significantly less reduction in disability than those who were not stabilised (−34.1% reduction, p = 0.043, table 3).
Pre-surgery levels of pain, neurological dysfunction and disability were important predictors of improvement after surgery, independent of type of surgery and other covariates (table 3). Notably, per 10% more severe symptoms prior to surgery, patients had on average a 7% additional reduction in pain, 8% greater reduction in neurological dysfunction, and 6% greater reduction in disability after spinal stenosis surgery (p <0.0001, table 3).
Sensitivity analysis
The results of the sensitivity analysis comparing the impact of type of surgery on pain, neurological dysfunction and disability at each time point separately were similar to those of the primary repeated-measures analysis (table 2). In adjusted analysis, patients who had decompression of only one segment experienced consistently greater improvement in all three outcomes compared with those who had multi-segment decompression, independent of symptoms prior to surgery, extent of stabilisation and other covariates. For pain reduction (p = 0.013), these differences were significant only at 12 months after surgery, and for disability (p = 0.047) only at 3 months after surgery. The differences in neurological dysfunction were significant at 3 months (p = 0.020) and 12 months (p <0.0001). Moreover, the estimated effect sizes were very similar to those obtained in the primary analysis. Additional segment stabilisation did not improve the results of decompression with respect to pain, independent of symptoms prior to surgery, extend of decompression and other covariates. For neurological dysfunction, differences were significant only at 6 months after surgery (p = 0.027). Patients who had segment stabilisation tended to have significantly worse results with respect to reduction in disability compared with those who did not (p = 0.043, table 2).
Discussion
In this prospective registry-based cohort study, we assessed 524 consecutive patients age 60 years and older prior to surgery for degenerative spinal stenosis, and were able to investigate 457 (87%) of them at 3- or 6- plus 12-month follow-up. Our results suggest that for pain, neurological dysfunction and disability, most improvements after spinal stenosis surgery were achieved within the first 3 or 6 months after surgery. Independent of symptoms prior to surgery and other covariates such as age, gender and comorbid conditions, we found little variation in outcome by type of surgery. However, our results support the conclusion that neurological dysfunction and disability may improve more with less invasive surgery. Also, we found no significant difference between the patients who underwent only decompression compared with patients who had both decompression and stabilisation surgery. In addition, benefits from surgery appeared to be most pronounced among patients with more severe symptoms prior to surgery, which is relevant to clinical care and outcome prediction after spinal stenosis surgery in senior patients.
Long-term outcomes at 5 to 12 years after spinal stenosis surgery have been addressed in several studies [19–22] showing favourable results, but only limited data are available on the timeline of early recovery in the first year after surgery. The timeline of recovery for improvement in pain, neurological dysfunction and disability observed in our study suggests that most benefits to be expected in the first 12 months after surgery are achieved at the 3- to 6-month follow-up. We found little or no further improvement for all three symptoms thereafter. Thus, the timeline of recovery after spinal stenosis surgery is similar to that described for senior patients undergoing total hip replacement [23].
In a systematic review by Martin et al. [24] including 578 patients (253 enrolled in 4 randomised controlled trials and 325 included in 9 observational studies), a greater benefit for clinical outcome was reported for spinal stenosis surgery with additional stabilisation in comparison with decompression alone (relative risk 1.40, 95% confidence interval 1.04–1.89). Notably, however, the authors stated that the individual study quality was low, with sample sizes ranging from 19 to 102 patients. Alternatively, as suggested by our study among 451 senior patients followed up for 1 year with standardised assessments before and after surgery, additional stabilisation may not contribute to greater pain reduction and improvements in neurological function, independent of symptoms prior to surgery, extend of decompression and the other covariates.
In fact, our findings suggest that, in the first year after surgery, patients who received one-segment or multi-segment stabilisation experienced less improvement in disability after surgery compared with those who were treated with decompression alone. This finding is supported by data from the National Swedish Register for Spine Surgery (Swespine) among a total of 5390 patients [25]. At the 2-year follow-up, the authors found no significant difference in patient satisfaction between the decompression surgery for spinal stenosis with or without stabilisation for any of the outcomes and regardless of the presence of a pre-operative spondylolisthesis [25].
We found that, independent of symptoms prior to surgery, age, gender and additional stabilisation, the average improvement in neurological dysfunction was significantly better for one-segment decompression, with 37% improvement compared with 26% improvement for multi-segment decompression (p <0.0001). A similar pattern became apparent for reduction of pain and improvement in disability. A possible explanation may be that patients undergoing multi-segment decompression had more extensive degenerative changes over multiple segments than patients undergoing one-segment decompression, and therefore their outcome is expected to be worse. However, our analyses adjusted for symptoms prior to surgery (as well as age, gender and numbers of comorbidities), which could be considered a good measure of disease severity and risk profile prior to surgery. Moreover, our analyses suggest that worse symptoms prior to surgery are associated with significantly greater improvements after surgery for all three outcomes assessed in our study.
Notably, surgeons may choose more extensive surgical techniques in order to reduce the possibility of repeat surgery. This question could not be addressed in our study, but a study by Martin et al. [26] among 24,882 adults undergoing any spine surgery, provided mixed findings over a 11-year follow-up. Whereas patients with spondylolisthesis of any age had a lower incidence of reoperation after stabilisation than after decompression alone (17.1% vs 28.0%, p = 0.002), for all other diagnoses, the incidence of reoperation was higher with stabilisation than with decompression alone (21.5% vs 18.8%, p = 0.008). Supporting our findings that less invasive surgery may be more advantageous for clinical outcomes in senior patients with spinal stenosis, Deyo et al. documented that among 32,152 Medicare recipients undergoing spinal stenosis surgery, life-threatening complications occurred in 2.3% of patients with decompression alone compared with 5.6% among those with additional stabilisation [27]. This may in part be explained by the longer operating time associated with more extensive surgery.
Our study has several strengths. We followed up a large number of consecutive patients undergoing spinal stenosis surgery from two large hospital centres. Also the same outcome assessment (NASS) was applied in a standardised way prior to surgery, at 3 or 6 months, and 12 months after surgery.
However our study has also several limitations. The observational design, rather than a random allocation of type of surgery, limits our study. In addition, there was no predefined protocol between the surgeons upon the type of surgery. No data were collected on the prevalence of radiological spondylolisthesis or sagittal balance prior to surgery. However, we recognise that stabilisation is mainly used to avoid further slippage and that the comparison with or without stabilisation might be a too simple comparison.
The evaluation of clinical balance is not a standardised method in clinical practice. However, our assumption was that patients who received stabilisation in addition to decompression were most likely selected on the basis of prevalent instability in addition to spinal stenosis. Another limitation of our study may be the change of surgery techniques during the long observation period and the lack of long-term follow-up beyond one year, and thereby missing information on patients who required repeat surgery depending on type of surgery. In addition concomitant medication and postoperative physiotherapy were not recorded systematically. As this study was performed in a purely clinical setting it can support clinical signals which have been further analysed by a more sophisticated spinal stenosis study group (LSOS). These publications give additional information in this highly relevant research topic [28–31].
In summary, based on patient-reported outcomes prior to and after surgery, our findings suggest that the timeline for recovery after spinal stenosis surgery is largely complete by 3 to 6 months after surgery, and differs little by type of surgery, independent of baseline symptoms, gender, age and number of comorbidities. However, our data do provide a signal that for improvement in neurological dysfunction and disability less invasive surgery may be more advantageous in patients age 60 years and older, independent of symptoms prior to surgery.
Conclusion
Among senior patients undergoing spinal stenosis surgery, recovery may be largely complete by 3 to 6 months after surgery and differs little by the type of surgery. However, particularly neurological dysfunction and disability may improve more with less invasive surgery. Therefore, when surgery is considered, less invasive procedures may be warranted independent of pre-surgery symptoms.
Acknowledgements
We thank the study nurses Maria Balsiger and Margarita Bünter for conducting the standardised assessments and interviews.
*
Shared first authorship
References
1
Kalichman
L
,
Cole
R
,
Kim
DH
,
Li
L
,
Suri
P
,
Guermazi
A
, et al.
Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9(7):545–50. doi:.https://doi.org/10.1016/j.spinee.2009.03.005
2
Ishimoto
Y
,
Yoshimura
N
,
Muraki
S
,
Yamada
H
,
Nagata
K
,
Hashizume
H
, et al.
Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthritis Cartilage. 2012;20(10):1103–8. doi:.https://doi.org/10.1016/j.joca.2012.06.018
3
Ishimoto
Y
,
Yoshimura
N
,
Muraki
S
,
Yamada
H
,
Nagata
K
,
Hashizume
H
, et al.
Association of Lumbar Spondylolisthesis With Low Back Pain and Symptomatic Lumbar Spinal Stenosis in a Population-based Cohort: The Wakayama Spine Study. Spine. 2017;42(11):E666–71. doi:.https://doi.org/10.1097/BRS.0000000000001960
4
Fraser
JF
,
Huang
RC
,
Girardi
FP
,
Cammisa
FP, Jr
. Pathogenesis, presentation, and treatment of lumbar spinal stenosis associated with coronal or sagittal spinal deformities. Neurosurg Focus. 2003;14(1):e6. doi:.https://doi.org/10.3171/foc.2003.14.1.7
5
Kim
HJ
,
Chun
HJ
,
Han
CD
,
Moon
SH
,
Kang
KT
,
Kim
HS
, et al.
The risk assessment of a fall in patients with lumbar spinal stenosis. Spine. 2011;36(9):E588–92. doi:.https://doi.org/10.1097/BRS.0b013e3181f92d8e
6
Lee
JH
,
Sung
E
. The effects of aquatic walking and jogging program on physical function and fall efficacy in patients with degenerative lumbar spinal stenosis. J Exerc Rehabil. 2015;11(5):272–5. doi:.https://doi.org/10.12965/jer.150231
7
Chou
R
,
Hashimoto
R
,
Friedly
J
,
Fu
R
,
Bougatsos
C
,
Dana
T
, et al.
Epidural Corticosteroid Injections for Radiculopathy and Spinal Stenosis: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(5):373–81. doi:.https://doi.org/10.7326/M15-0934
8
Friedly
JL
,
Comstock
BA
,
Turner
JA
,
Heagerty
PJ
,
Deyo
RA
,
Sullivan
SD
, et al.
A randomized trial of epidural glucocorticoid injections for spinal stenosis. N Engl J Med. 2014;371(1):11–21. doi:.https://doi.org/10.1056/NEJMoa1313265
9
Spinal stenosis. Severity determines treatment plan. Mayo Clin Health Lett. 2001;19(6):1–3.
10
Bischoff-Ferrari
HA
,
Lingard
EA
,
Losina
E
,
Baron
JA
,
Roos
EM
,
Phillips
CB
, et al.
Psychosocial and geriatric correlates of functional status after total hip replacement. Arthritis Rheum. 2004;51(5):829–35. doi:.https://doi.org/10.1002/art.20691
11
Sobottke
R
, et al.
Predictors of improvement in quality of life and pain relief in lumbar spinal stenosis relative to patient age: a study based on the Spine Tango registry. Eur Spine J. 2017;26(2):462–72. doi:.https://doi.org/10.1007/s00586-015-4078-8
12North American Spine Society. Diagnosis and treatment of degenerative spinal stenosis. 2011. Available from: https://www.spine.org/Documents/ResearchClinicalCare/Guidelines/LumbarStenosis.pdf [accessed 2015 Nov 19].
13
Birkelbach
O
,
Mörgeli
R
,
Spies
C
,
Olbert
M
,
Weiss
B
,
Brauner
M
, et al.
Routine frailty assessment predicts postoperative complications in elderly patients across surgical disciplines - a retrospective observational study. BMC Anesthesiol. 2019;19(1):204. doi:.https://doi.org/10.1186/s12871-019-0880-x
14
Vitaz
TW
,
Raque
GH
,
Shields
CB
,
Glassman
SD
. Surgical treatment of lumbar spinal stenosis in patients older than 75 years of age. J Neurosurg. 1999;91(2, Suppl):181–5.
15
Daltroy
LH
,
Cats-Baril
WL
,
Katz
JN
,
Fossel
AH
,
Liang
MH
. The North American spine society lumbar spine outcome assessment Instrument: reliability and validity tests. Spine. 1996;21(6):741–8. doi:.https://doi.org/10.1097/00007632-199603150-00017
16
Schaeren
S
,
Bischoff-Ferrari
HA
,
Knupp
M
,
Dick
W
,
Huber
JF
,
Theiler
R
. A computer touch-screen version of the North American Spine Society outcome assessment instrument for the lumbar spine. J Bone Joint Surg Br. 2005;87-B(2):201–4. doi:.https://doi.org/10.1302/0301-620X.87B2.15548
17
Kirrstetter
AR
,
Brenig
C
,
Gengenbacher
M
,
Meier
B
,
Ott
A
,
Theiler
R
. Erfahrungen bei der Messung der Ergebnisqualität in der interventionellen Schmerztherapie [Experience in measuring the quality of treatment in interventional pain therapy : The Activity Index on a touchscreen PC]. Schmerz. 2017;31(2):131–8. Article in German. doi:.https://doi.org/10.1007/s00482-016-0173-y
18
Sangha
O
,
Stucki
G
,
Liang
MH
,
Fossel
AH
,
Katz
JN
. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63. doi:.https://doi.org/10.1002/art.10993
19
Micankova Adamova
B
,
Vohanka
S
,
Dusek
L
,
Jarkovsky
J
,
Bednarik
J
. Prediction of long-term clinical outcome in patients with lumbar spinal stenosis. Eur Spine J. 2012;21(12):2611–9. doi:.https://doi.org/10.1007/s00586-012-2424-7
20
Galiano
K
,
Obwegeser
AA
,
Gabl
MV
,
Bauer
R
,
Twerdy
K
. Long-term outcome of laminectomy for spinal stenosis in octogenarians. Spine. 2005;30(3):332–5. doi:.https://doi.org/10.1097/01.brs.0000152381.20719.50
21
Hurri
H
,
Slätis
P
,
Soini
J
,
Tallroth
K
,
Alaranta
H
,
Laine
T
, et al.
Lumbar spinal stenosis: assessment of long-term outcome 12 years after operative and conservative treatment. J Spinal Disord. 1998;11(2):110–5. doi:.https://doi.org/10.1097/00002517-199804000-00003
22
Shabat
S
,
Arinzon
Z
,
Folman
Y
,
Leitner
J
,
David
R
,
Pevzner
E
, et al.
Long-term outcome of decompressive surgery for lumbar spinal stenosis in octogenarians. Eur Spine J. 2008;17(2):193–8. doi:.https://doi.org/10.1007/s00586-007-0514-8
23
Bachmeier
CJ
,
March
LM
,
Cross
MJ
,
Lapsley
HM
,
Tribe
KL
,
Courtenay
BG
, et al.; Arthritis Cost and Outcome Project Group. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9(2):137–46. doi:.https://doi.org/10.1053/joca.2000.0369
24
Martin
CR
,
Gruszczynski
AT
,
Braunsfurth
HA
,
Fallatah
SM
,
O’Neil
J
,
Wai
EK
. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine. 2007;32(16):1791–8. doi:.https://doi.org/10.1097/BRS.0b013e3180bc219e
25
Försth
P
,
Michaëlsson
K
,
Sandén
B
. Does fusion improve the outcome after decompressive surgery for lumbar spinal stenosis?: A two-year follow-up study involving 5390 patients. Bone Joint J. 2013;95-B(7):960–5. doi:.https://doi.org/10.1302/0301-620X.95B7.30776
26
Martin
BI
,
Mirza
SK
,
Comstock
BA
,
Gray
DT
,
Kreuter
W
,
Deyo
RA
. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32(3):382–7. doi:.https://doi.org/10.1097/01.brs.0000254104.55716.46
27
Deyo
RA
,
Mirza
SK
,
Martin
BI
,
Kreuter
W
,
Goodman
DC
,
Jarvik
JG
. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–65. doi:.https://doi.org/10.1001/jama.2010.338
28
Burgstaller
JM
,
Wertli
MM
,
Ulrich
NH
,
Pichierri
G
,
Brunner
F
,
Farshad
M
, et al.; LSOS Study Group. Evaluating the Minimal Clinically Important Difference of EQ-5D-3L in Patients with Degenerative Lumbar Spinal Stenosis: A Swiss Prospective Multi-Center Cohort Study. Spine. 2020 March 19. Volume Publish Ahead of Print. doi:.https://doi.org/10.1097/BRS.0000000000003501
29
Betz
M
,
Burgstaller
JM
,
Held
U
,
Andreisek
G
,
Steurer
J
,
Porchet
F
, et al.; LSOS Study Group. Influence of Paravertebral Muscle Quality on Treatment Efficacy of Epidural Steroid Infiltration or Surgical Decompression in Lumbar Spinal Stenosis-Analysis of the Lumbar Spinal Outcome Study (LSOS) Data: A Swiss Prospective Multicenter Cohort Study. Spine. 2017;42(23):1792–8. doi:.https://doi.org/10.1097/BRS.0000000000002233
30
Ulrich
NH
,
Burgstaller
JM
,
Gravestock
I
,
Pichierri
G
,
Wertli
MM
,
Steurer
J
, et al.
Outcome of unilateral versus standard open midline approach for bilateral decompression in lumbar spinal stenosis: is “over the top” really better? A Swiss prospective multicenter cohort study. J Neurosurg Spine. 2019;31(2):1–10. doi:.https://doi.org/10.3171/2019.2.SPINE181309
31
Held
U
,
Steurer
J
,
Pichierri
G
,
Wertli
MM
,
Farshad
M
,
Brunner
F
, et al.
What is the treatment effect of surgery compared with nonoperative treatment in patients with lumbar spinal stenosis at 1-year follow-up?
J Neurosurg Spine. 2019;31(2):1–9. doi:.https://doi.org/10.3171/2019.1.SPINE181098
