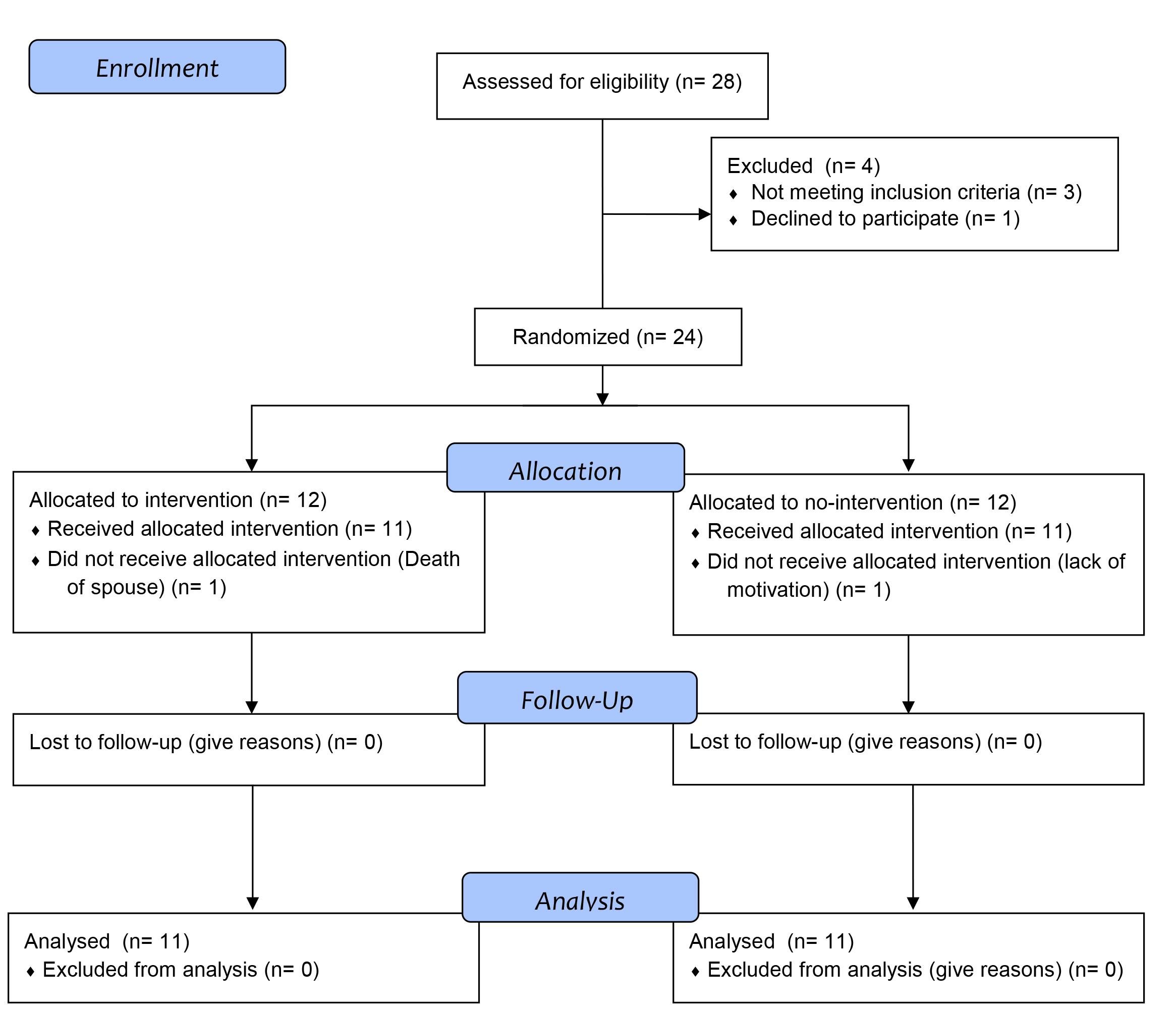
Figure 1 CONSORT 2010 flow diagram.
DOI: https://doi.org/10.4414/smw.2020.20346
Exercise intolerance is a substantial limiting factor in the life of patients with acquired or congenital structural heart disease. Cardiac causes, including ventricular and valvular dysfunction, chronotropic incompetence and factors related to previous cardiac surgery, contribute to an impaired exercise tolerance. However, there are many other extracardiac factors related to exercise intolerance, such as parenchymal and vascular lung disease, pulmonary arterial hypertension, anaemia and iron-deficiency [1–3]. Patients with structural heard disease and chronic heart failure often have generalised myopathy involving the peripheral skeletal muscles as well as the respiratory muscles, which influences exercise capacity [4–6]. Moreover, inspiratory muscle strength expressed as maximum inspiratory pressure (MIP) is an independent predictor of prognosis [7] and is closely related to the sensation of dyspnoea [8]. In patients with complex congenital heart disease, respiratory muscle weakness is common and similar in extent to that in elderly patients with chronic heart failure from acquired heart disease. It is also associated with reduced exercise capacity [9]. Therefore, it appears obvious that in training the skeletal muscles, a particular attention should also be paid to the respiratory muscles and should be a therapy target to improve exercise capacity in patients with structural heart disease and heart failure. Structural physical training has shown beneficial effects on exercise capacity and respiratory muscle strength as well [10–13]. However, many patients with structural heart disease and heart failure do not participate in regular physical exercise for several reasons, such as physical disability, frailty and lack of motivation. Specific respiratory muscle training could be an addition or an alternative to the standardised cardiac rehabilitation to enhance exercise capacity without strenuous physical exertion [14]. The aim of this study was to investigate if regular singing in combination with structural breathing exercises would improve respiratory muscle strength, exercise capacity and quality of life in patients with acquired or congenital structural heart disease.
This single-centre, randomised and open-label interventional study was conducted in patients with known cardiomyopathy from acquired heart disease (ischaemic, valvular or dilated) or in patients with complex congenital heart disease (cyanotic congenital heart disease, Fontan palliation, subaortic right ventricle or repaired tetralogy of Fallot). This study was intended to be a pilot study. Patients were recruited at the outpatient Heart Failure Clinic and Congenital Heart Disease Clinic at the University Hospital Basel by the attending cardiologists. Entry criteria for the study were age ≥18 years and a previous history of symptomatic heart failure or complex congenital heart disease as described above. Exclusion criteria were acute coronary syndrome, cardiac surgery or heart failure hospitalisation within the previous 6 months; chronic metabolic, orthopaedic, or infectious disease; treatment with steroids, hormones, or cancer chemotherapy; severe exercise-induced asthma, being a professional singer or professionally playing a wind instrument, and enrolment in a cardiac rehabilitation programme. The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees (EKNZ 2017-02008). Written informed consent was obtained from all patients. The authors designed the study, gathered and analysed the data according to the CONSORT statement for reporting randomised controlled trials [15] (fig. 1 and supplementary table S1 in appendix 1), vouched for the data and analysis, wrote the paper and decided to submit it for publication.

Figure 1 CONSORT 2010 flow diagram.
Participants were randomised in a 1:1 ratio to the intervention or standard therapy group using electronically concealed randomisation lists. The randomisation was carried out block-wise by the Clinical Trial Unit of the Department of Clinical Research in Basel. Beside the standard treatment, patients in the interventional group participated in singing and breathing exercises conducted by a professional choir instructor for 12 weeks. The intervention consisted of weekly singing lessons for 90 minutes in a choir and additional instructions for daily breathing exercises at home (description of breathing exercises: videos “strawbreathing” and “pranayamabreathing”), which they should perform for 20 minutes daily. The control group received standard treatment alone without any specific physical exercise. Respiratory muscle strength (expressed as maximum inspiratory pressure [MIP] and maximum expiratory pressure [MEP]), exercise capacity (expressed as maximal oxygen uptake during exercise [MVO2]) and quality of life (quantified by the Minnesota living with heart failure questionnaire [MLHFQ] score) were assessed at baseline and after 12 weeks in all patients. The compliance with the breathing exercises at home was self-reported (numbers of days with breathing exercises of at least 20 minutes), whereas the participation in the choir rehearsals was recorded by the choir instructor.
Baseline characteristics including cardiac history, ejection fraction of the systemic ventricle measured by the modified Simpson method, presence of pulmonary hypertension defined as end-systolic pulmonary arterial pressure ≥40 mm Hg assessed by echocardiography and New York Heart Association (NYHA) functional class were collected from the available medical records. N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and kidney function were measured, and pulmonary function was assessed in all participants at baseline.
The primary endpoint was the difference in change of MIP (% of predicted) between both groups (∆MIP%predicted). Secondary endpoints were the difference in change in MEP (% predicted), MVO2 (% predicted) and MLHFQ score between both groups (∆MEP%predicted, ∆MVO2%predicted and ∆MLHFQ)
MIP and MEP were measured by experienced respiratory technicians at the Department of Pneumology, University Hospital Basel. The technicians were blinded to the randomisation. According to the clinical standard and recommended by the literature [16], MIP was measured after maximum expiration and MEP after maximum inspiration. A flanged mouthpiece was used and participants were asked to seal the mouthpiece with their hands during the manoeuvre to avoid air leak. Out of three valid measurements with a <10% variability, the highest measurement was used for analysis.
The study was substantially supported by a professional soprano singer, herself a cardiac patient with complex congenital heart disease regularly seen in our outpatient clinic. She played a major role in designing the intervention plan, the information material to support the intervention and the burden of the intervention from the patient’s perspective. She established the training plan for the breathing exercises, instructed study participants and lead the choir rehearsals. As a culmination of the choir rehearsals, a benefit concert was given with the support of 60 volunteers recruited from various lay choirs in the region of Basel, Switzerland.
The results of the study have been communicated to all study participants personally. Further impact of the study results and publication will be equally communicated with all participants, volunteers and other interested persons such as colleagues from different rehabilitation programmes in Switzerland and abroad.
All patients underwent symptom-limited cardiopulmonary exercise testing on a cycle ergometer (Schiller Reomed BP 200 Plus) in accordance with published guidelines [17]. Testing was done by three different standardised protocols according to the patients’ estimated capacity. The chosen protocol for an individual patient was the same for the entry test and the end of study testing. Participants were advised to interrupt the test at their individual maximum physical capacity. Expired gas was analysed breath by breath (Power Cube Electronic Gas analyser). In addition to heart rate and blood pressure, the following parameters were recorded at rest, at ventilator threshold (derived by the slope method) and at peak exercise by averaging five out of seven cycles: oxygen uptake (VO2), carbon dioxide output (VCO2) and minute ventilation (VE). The respiratory exchange ratio (VCO2 divided by VO2) at peak exercise was calculated in all participants and a value of >1.05 was a marker for maximum physical effort. Measurements were normalised for age, gender and height, and expressed as percentage of the predicted value. All participants underwent spirometry before exercise testing: forced vital capacity (FVC) and forced expiratory volume during the first second of expiration (FEV1) were measured.
Quality of life was assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) [18, 19]. This is a validated and widely used tool to assess quality of life in patients with chronic heart failure. It is a multidimensional questionnaire assessing the impact of heart failure on overall quality of life by the mean of 21 questions with a six-point rating scale, with 0 = none/not applicable to 5 = very much applicable, focusing on physical, socioeconomic and psychological/emotional aspects of life with heart failure. A score of <24 points is estimated to represent a good quality of life, a score of 24–45 points a moderate quality of life and a score of >45 points a poor quality of life [18, 20].
Data were analysed using SPSS® for Windows (version 23, SPSS, Chicago) and tested for normality with the Kolmogorov-Smirnov test. Descriptive data for continuous variables were presented as means with standard deviations (SDs) or as medians with interquartile ranges (IQRs) as appropriate.
For comparison between groups, continuous variables were evaluated using the independent Student t-test and proportions were evaluated using chi-square or Fischer-exact tests as appropriate. Linear univariate regression was used to determine relationships in the intervention group between ∆MIP%predicted and the baseline measurements of MIP % predicted and MVO2 % predicted. The Pearson correlation coefficient was used to determine the relationship between ∆MIP%predicted in the intervention group and the number of days of fulfilled breathing exercises at home. The sample size calculation was based on the primary endpoint – change of MIP in % predicted between patients with and without the intervention. Based on previous data [14], we estimated that a sample size of 11 individuals in each group would have a power of 80% to detect a 10% difference in MIP % predicted for a two-sided alpha set at 0.05.
Between January and March 2018, 24 patients were included in the study and randomised equally in two groups after their eligibility was confirmed. In each group one patient dropped out during the intervention period (fig. 1). Twenty-two patients were included in the analysis. Age and gender were well balanced. Baseline characteristics are described in table 1.
Table 1 Baseline characteristics.
|
All
(n = 22) |
Intervention
(n = 11) |
Control
(n = 11) |
|
|---|---|---|---|
| Age, years (SD) | 65 (19) | 64 (19) | 65 (19) |
| Male gender, n (%) | 10 (45) | 5 (45) | 5 (45) |
| BMI, kg/m2 (SD) | 25 (4) | 25 (4) | 26 (4) |
| Left ventricular ejection fraction in %, (SD) | 48 (13) | 47 (12) | 49 (14) |
| Cardiomyopathy, n (%) | |||
| – Coronary cardiomyopathy | 5 (23) | 2 (18) | 3 (27) |
| – Dilated cardiomyopathy | 4 (18) | 0 | 4 (36) |
| – Valvular cardiomyopathy | 5 (23) | 4 (36) | 1 (9) |
| – Congenital heart disease | 5 (23) | 3 (27) | 2 (18) |
| - Other | 3 (14) | 2 (18) | 1 (9) |
| Severe valvular disease, n (%) | 3 (14) | 2 (18) | 1 (9) |
| Pulmonary hypertension, n (% | 2 (9) | 2 (18) | 0 |
| Medication, n (%) | |||
| – ACE-inhibitors/ARBs | 14 (64) | 7 (64) | 7 (64) |
| – Diuretics | 9 (41) | 4 (36) | 5 (45) |
| – Beta-blockers | 14 (64) | 6 (55) | 8 (73) |
| – Aldactone | 8 (36) | 6 (55) | 2 (18) |
| Sinus rhythm, n (%) | 17 (75) | 9 (82) | 8 (73) |
| NT-proBNP, ng/l (IQR) | 560 (278-1272) | 443 (299–1011) | 572 (206–1824) |
| FEV1, % predicted, (SD) | 85 (20) | 85 (22) | 86 (19) |
| FVC, % predicted, (SD) | 82 (17) | 81 (20) | 82 (14) |
| FEV1/FVC in %, (SD) | 77 (18) | 75 (23) | 80 (10) |
ACE = angiotensin-converting-enzyme; ARB = angiotensin receptor blocker; BMI = body mass index; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IQR = interquartile range; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; SD = standard deviation
Of the five patients with coronary heart disease, two had prior surgical revascularisation and four had prior myocardial infarction. Of the five patients with valvular heart disease, three had prior aortic valve surgery and two had prior mitral valve surgery. Patients with congenital heart diseases included two patients with unoperated cyanotic heart disease (tricuspid atresia and double inlet left ventricle), one patient with a Fontan palliation and two patients with repaired tetralogy of Fallot and residual pulmonary regurgitation. The remaining two patients were diagnosed with hypertrophic obstructive cardiomyopathy, repaired atrial myxoma and hypertensive cardiopathy with reduced ejection fraction. All patients were in stable functional class NYHA I or II. None of the patients had evidence of obstructive lung disease.
Patient compliance in the intervention group was as follows: on average, they took part in 13 of 14 weekly choir rehearsals and completed 86% (SD 14%) of the daily breathing exercises at home (72 [SD 12] of a total of 84 possible exercise days). None of the patients suffered any harm or unintended effects.
After 12 weeks, ∆MIP%predicted was significantly higher in the intervention group (∆MIP%predicted mean difference −27.5, 95% CI −46.9 to −8.14; p = 0.008; fig. 2a). ∆MIP in the intervention group was inversely correlated with baseline MIP (β = −0.611; p = 0.042), but did not correlate with baseline MVO2 (β = −0.428; p = 0.088). There was no significant correlation between ∆MIP %predicted in the intervention group and the number of days with fulfilled breathing exercise (Pearson correlation 0.42; p = 0.2). MEP % predicted did not change after 12 weeks (∆MEP%predicted mean difference −0.182, 95% CI −19.2 to 18.9; p = 1.0; fig. 2b). Detailed results of the respiratory muscle strength tests are described in table 2.

Figure 2 Primary and secondary endpoints. The figure shows the results at baseline (black bar) and after 12 weeks (grey bar) in the intervention (left bars) and in the control group (right bars). * marks the p-value of the mean change between both groups. Panel A (primary endpoint): maximum inspiratory pressure (MIP, %predicted). Panels B–D (secondary endpoints): maximum expiratory pressure (MEP, %predicted), maximum oxygen uptake during exercise (MVO2, %predicted), Minnesota living with heart failure questionnaire (MLFQ) scores.
Table 2 Indices of respiratory muscle strength, cardiopulmonary exercise testing and quality of life score
| Intervention (n = 11) | Control (n = 11) | Intervention versus control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BL | FU 12 | Change | p-value | BL | FU 12 | Change | p-value | Mean change | p-value | |
| MIP, kPa (SD) | 5.2 (2.0 | 6.2 (2.2) | 0.9 (2.3) | 0.02 | 6.2 (2.3) | 5.4 (2.6) | −0.9 (1.3) | 0.04 | 1.8 (0.5) | 0.002 |
| MIP, % predicted (SD) | 82 (33) | 96 (30) | 14 (21) | 0.05 | 98 (32) | 84 (33) | −14 (23) | 0.07 | 27.5 (9.3) | 0.008 |
| MEP, kPa (SD) | 7.6 (3.9) | 7.6 (4.0) | −0.06 (2.4) | 0.9 | 6.8 (2.7) | 6.6 (2.4) | −0.2 (1.2) | 0.6 | 0.3 (0.8 | 0.8 |
| MEP, % predicted (SD) | 81 (33) | 78 (24) | −3 (26) | 0.7 | 78 (33) | 75 (27) | 3 (16) | 0.5 | 0.2 (9.1) | |
| Respiratory exchange ratio (SD)* | 1.16 (0.05) | 1.14 (0.05) | 0.02 (0.04) | 0.12 | 1.16 (0.08) | 1.10 (0.08) | 0.06 (0.07) | 0.048 | 0.04 (0.03) | 0.15 |
| MVO2, ml/min/kg (SD)* | 15.6 (5.7) | 19.6 (6.9) | 4.1 (2.2) | <0.001 | 16.8 (7.7) | 19.4 (11.0) | 2.6 (4.1) | 0.1 | 1.5 (1.5) | 0.3 |
| MVO2, % predicted (SD) | 68 (18) | 86 (23) | 18 (12) | 0.001 | 78 (22) | 88 (28) | 10 (15) | 0.1 | 8 (6) | 0.2 |
| QoL Minnesota score (SD) | 14 (13) | 9 (9) | −5 (6) | 0.026 | 11 (10) | 14 (13) | 3 (5) | 0.1 | 8 (3) | 0.006 |
BL = baseline; FU12 = follow-up after 12 weeks; MIP = maximum inspiratory pressure; MEP = maximal expiratory pressure; MVO2= maximum oxygen uptake during exercise; QoL = quality of life; SD = standard deviation * 3 patients in the control group had to terminate prematurely and were excluded from the analysis.
All patients in the intervention group were able to complete the cardiopulmonary exercise test, whereas three patients in the control group had to terminate prematurely owing to back pain (two patients) or general discomfort (one patient). After 12 weeks, ∆MVO2 %predicted did not differ between both groups (∆MVO2%predicted 18 [SD 12%] vs 10 [SD 15%], p = 0.2, fig. 2c). Detailed results of the cardiopulmonary exercise tests are described in table 2 and supplementary table S2 in appendix 1.
After 12 weeks, quality of life score was significantly better in the intervention group than in the control group (∆MLHFQ score −5 [SD 6] vs 3 [SD 5], p = 0.006, fig. 2d and table 2).
This randomised clinical study in patients with acquired or congenital structural heart disease showed an increase in inspiratory muscle strength after a 12-week intervention with weekly choir singing rehearsals in combination with structured daily breathing exercises at home. We report seven major findings:
First, structured breathing exercises combined with regular singing rehearsals improved significantly the inspiratory muscle strength expressed as MIP. Exercise training is widely recognised as nonpharmacological intervention to improve exercise tolerance and quality of life in patients with chronic heart failure [13]. However, conventional physical exercise as instructed in many cardiac rehabilitation programmes does not specifically train the respiratory muscles. A previous randomised study in patients with chronic heart failure showed an even stronger increase of inspiratory muscle strength after a 12-week programme of inspiratory muscle training using an inspiratory muscle training device [14]. In another clinical trial comparing aerobic exercise alone and aerobic exercise plus inspiratory training showed a positive impact of inspiratory muscle training on physical capacity [21].
Second, there was no difference in the expiratory muscle strength expressed as MEP. In contrast to training with a handheld device, which strengthens the diaphragm muscles, breathing exercises such as in yoga-breathing strengthen the intercostal muscles. Therefore, breathing exercises such as those in our study focus less on the expiratory process, a fact that could be an explanation to why the MEP did not improve in our study.
Third, delta ΔMVO2 did not improve in our study because of the small sample size. Breathing exercises (yoga breathing) are a relaxation and meditation technique based on postures, exercises and breathing techniques, and has demonstrated beneficial effects in patients with chronic heart failure. In another trial, 19 patients were randomised to yoga breathing or standard medical therapy alone. After 8 weeks of training, MVO2 and quality of life significantly improved in the yoga group versus the control group [22]. The same research group demonstrated benefits of adding yoga to standard medical care in a small cohort of African American patients with heart failure by improving MVO, quality of life and inflammatory markers [23].
Fourth, baseline MIP showed a reverse correlation with the increase of MIP after 12 weeks of inspiratory muscle training. Therefore, baseline MIP might serve as a possible factor to predict the success of respiratory therapy.
Fifth, regular breathing exercise is an affordable intervention and can be performed by almost all patients, even in the frailest ones, the elderly and those with very advanced heart disease. These patients frequently are not capable of participating in regular physical exercise or cardiac rehabilitation programmes. Therefore, even minor changes in respiratory muscle strength and maximum oxygen uptake are difficult to obtain, and respiratory muscle training could be an available option to improve exercise capacity in these patients.
Sixth, there was a high compliance rate with the daily respiratory training due to the study concept, with additional weekly choir rehearsals. On average, patients fulfilled their daily breathing exercise programme on about six of seven days per week. Nevertheless, no correlation could be found between compliance with performing breathing exercises at home with an increase of inspiratory muscle strength in the intervention group. The weekly choir rehearsals became an entertaining and enjoyable moment of social interaction and they seem to enhance the motivation of performing daily yoga breathing exercises at home.
To our knowledge, group singing is a new therapy concept for patients with chronic heart failure and so far, no study has analysed beneficial effects of singing in a choir in this population. Seventh, we found an improved quality of life in the intervention group compared with patients who did not receive the intervention and did not participate in the choir. Singing is associated with a number of physiological changes. Studies have shown that singing can cause changes in neurotransmitters and hormones, including the upregulation of oxytocin, immunoglobulin A and endorphins, which improves immune function and increases happiness [24]. Additionally, in older patients, a group-singing programme with deep breathing training and song-learning can have a positive effect on memory, language, speech information processing, executive function and respiratory muscle strength [25]. The cardiorespiratory system is utilised during continuous singing training, resulting in improved respiratory muscles and an optimised breathing mode [24]. This improvement could also be demonstrated in patients with lung disease: a beneficial effect of singing during pulmonary rehabilitation was shown in patients suffering from chronic obstructive pulmonary disease (COPD) and other chronic respiratory disorders [26], as well as in children with cystic fibrosis [27].
Patients with generalised myopathy and reduced exercise capacity due to underlying cardiac disease and/or chronic heart failure benefit from physical activity and, as shown in our study, from respiratory therapy. However, it is unclear whether respiratory therapy has an additional effect in patients undergoing conventional cardiac rehabilitation. We are planning to investigate this question in a larger randomised multicentre trial.
Since our study showed that choir singing in combination with breathing exercises improved quality of life in patients with heart disease, we will continue the “HeartChoir” independently of the study, and will include other patient groups with exercise intolerance (patients with pulmonary disease) and, thus, contribute to public health.
The study was powered to analyse the improvement of inspiratory muscle strength. Whether respiratory muscle training also leads to an improvement in exercise capacity in patients with structural heart disease could not be determined owing to the low number of patients in the study. We included patients with various types of cardiomyopathies and the patients’ characteristics were heterogeneous. The small sample size and the heterogeneity of the underlying cardiac disease limit the interpretation of the secondary endpoints. Larger studies are needed to analyse specific subgroups of cardiac diseases. A project with weekly rehearsals of choir singing limits the number of patients because it excludes patients from outer areas and patients who were not able to participate a result of mobility issues. Thus, a selection bias may be possible. The choir was led by only one instructor, so the effect of different instructors with different background and motivation is not shown. Lastly, given that our study was not blinded, the interpretation of the quality of life is limited.
Regular choir singing and respiratory muscle training improved respiratory muscle strength and quality of life in patients with chronic structural heart disease. Larger studies are needed to evaluate the effect of respiratory muscle training on exercise capacity and cardiac outcomes.
Table S1 CONSORT 2010 checklist of information to include when reporting a randomised trial.
| Section/topic | Item no. | Checklist item | Reported on page no.* |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 2 | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 4 |
| 2b | Specific objectives or hypotheses | 4 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 5 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | - | |
| Participants | 4a | Eligibility criteria for participants | 5 |
| 4b | Settings and locations where the data were collected | 5 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 5–6 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 6 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | – | |
| Sample size | 7a | How sample size was determined | 9 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | – | |
| Randomisation: | |||
| – Sequence generation | 8a | Method used to generate the random allocation sequence | 5–6 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 5–6 | |
| – Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 5–6 |
| – Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 5–6 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | – |
| 11b | If relevant, description of the similarity of interventions | – | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 9 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | – | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | Figure 1 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | Figure 1 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 10 |
| 14b | Why the trial ended or was stopped | – | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 9/table 1 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 10 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 10–11 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | – | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | – |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | 10 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 15 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 12–15 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 12–15 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 3 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | – |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 16 |
* Page numbers refer to the original manuscript
Table S2 Remaining indices of respiratory muscle strength and cardiopulmonary exercise testing.
| Intervention (n = 11) | Control (n = 8) | Intervention versus control | ||||||||
| BL | FU12 | Change | p-value | BL | FU12 | Change | p-value | Mean change | p-value | |
| T50MIP, seconds | 2.0 ± 1.6 | 2.4 ± 1.2 | 0.4 ± 0.6 | 0.07 | 2.2 ± 0.8 | 2.3 ± 0.8 | 0.06 ± 0.6 | 0.7 | 0.3 ± 0.2 | 0.3 |
| T50MEP, seconds | 1.6 ± 1.4 | 2.5 ± 1.2 | 0.9 ± 1.8 | 0.1 | 2.2 ± 1.3 | 2.4 ± 1.5 | 0.3 ±1.7 | 0.6 | 0.6 ± 0.7 | 0.4 |
| Watt, (SD) | 91 (32) | 95 (40) | 4 (11) | 0.3 | 116 (60) | 112 (65) | −4 (12) | 0.4 | 8 (5) | 0.2 |
| Watt, % predicted, (SD) | 70 (20) | 72 (24) | 2 (7) | 0.3 | 78 (26) | 75 (33) | −3 (10) | 0.4 | 5 (4) | 0.2 |
| METS, (SD) | 4.4 (1.6) | 5.6 (2.0) | 1.1 (0.7) | <0.001 | 4.8 (2.2) | 5.5 (3.1) | 0.8 (1.2) | 0.1 | 0.4 (0.4) | 0.4 |
| VO2 at AT, % of MVO2, (SD) | 52 (14) | 73 (26) | 21 (17) | 0.002 | 71 (11) | 83 (12) | 12 (7) | 0.003 | 9 (7) | 0.2 |
| O2 pulse, ml/beat | 8.9 (2.5) | 10.5 (3.7) | 1.6 (1.8) | 0.016 | 9.3 (4.0) | 12.1 (5.5) | 2.8 (2.6) | 0.17 | −1.2 (1.0) | 0.3 |
| O2 pulse, % predicted, (SD) | 86 (17) | 102 (29) | 16 (19) | 0.019 | 83 (26) | 108 (31) | 25 (18) | 0.006 | −9 (9) | 0.3 |
| VE/VCO2 slope, (SD) | 32.7 (8.1) | 31.9 (6.5) | −0.9 (4.3) | 0.5 | 33.7 (5.0) | 29.8 (4.7) | −3.9 (5.1) | 0.09 | −3.0 (2.2) | 0.2 |
| Heart rate rest, bpm, (SD) | 83 (15) | 82 (20) | 0 (14) | 1.0 | 84 (18) | 77 (16) | −7 (8) | 0.5 | −7 (6) | 0.2 |
| Heart rate max, bpm, (SD) | 126 (29) | 131 (25) | 6 (13) | 0.2 | 141 (18) | 122 (15) | −18 (15) | 0.01 | −24 (6) | 0.002 |
| Blood pressure rest, mm Hg, (SD) | 128 (33) | 120 (27) | −9 (16) | 0.1 | 140 (26) | 133 (16) | −7 (16) | 0.2 | 1 (7) | 0.9 |
| Blood pressure max, mm Hg, (SD) | 168 (39) | 165 (29) | -2 (17) | 0.7 | 189 (24) | 188 (19) | 1 (16) | 0.8 | −8 (16) | 0.9 |
| SpO2 rest in %, (SD) | 93 (6) | 93 (4) | 0.1 (2.3) | 0.9 | 93 (5) | 92 (5) | 0.5 (1.5) | 0.4 | 1 (3) | 0.6 |
| SpO2 max in %, (SD) | 90 (11) | 90 (11) | 0.1 (4.4) | 0.9 | 90 (14) | 90 (11) | 0.1 (3) | 0.9 | 0 (2) | 1.0 |
| VE max, % predicted, (SD) | 57 (17) | 76 (17) | 19 (19) | 0.007 | 69 (8) | 74 (22) | 4 (22) | 0.6 | 15 (10) | 0.1 |
| VT max, % predicted, (SD) | 76 (26) | 83 (26) | 8 (13) | 0.077 | 88 (18) | 84 (23) | −4 (17) | 0.5 | 12 (7) | 0.1 |
| RR max, % predicted, (SD) | 113 (15) | 121 (17) | 9 (13) | 0.061 | 119 (19) | 121 (19) | 2 (13) | 0.7 | 7 (20) | 0.3 |
AT = anaerobic threshold; BL = baseline; FU12 = follow-up after 12 weeks; MET = metabolic equivalent of task; MVO2 = maximum oxygen uptake during exercise; RR = respiratory rate; SD = standard deviation; SpO2 = oxygen saturation; VE max = maximum ventilation; VE/VCO2 = ventilatory equivalent for carbon dioxide; VT = tidal volume
We would like to thank Miss Jenny Hoegstroem, professional soprano singer, for her substantial contribution to the elaboration of the intervention plan, her professional involvement as a choir instructor and her indispensable lead during the final benefit concert. We equally would like to thank all study participants and the numerous choir participants who, thanks to their commitment, made this choir project and the benefit concert possible.
The study was supported by internal funds of the Cardiovascular Research Institute Basel, University Hospital Basel, University of Basel, Switzerland.
CM reports grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the University of Basel, University Hospital Basel, Abbott, Alere, BRAHMS, Pronota, Roche, Sphingotec, and Singulex during the conduct of the study and personal fees from Abbott, Alere, Amgen, AstraZeneca, BioMérieux, Boehringer Ingelheim, Bristol-Myers Squibb, BRAHMS, Cardiorentis, Novartis, Roche, Sanofi, Siemens, and Singulex outside the submitted work. OP reports personal fees from Vifor Pharma, Novartis Pharma, and MSD unrelated to the submitted work. DT reports grants from the Swiss National Science Foundation and personal fees from Actelion outside the submitted work. KA has received a research grant from the Swiss Academy of Medical Sciences and the Bangerter Foundation (YTCR 09/19). Authors not named here have disclosed no conflict of interest.
1 Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JGF , Coats AJS , et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
2 Stout KK , Broberg CS , Book WM , Cecchin F , Chen JM , Dimopoulos K , et al.; American Heart Association Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Cardiovascular Radiology and Imaging. Chronic Heart Failure in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation. 2016;133(8):770–801. doi:.https://doi.org/10.1161/CIR.0000000000000352
3 Diller GP , Dimopoulos K , Okonko D , Li W , Babu-Narayan SV , Broberg CS , et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112(6):828–35. doi:.https://doi.org/10.1161/CIRCULATIONAHA.104.529800
4 Clark AL , Poole-Wilson PA , Coats AJ . Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28(5):1092–102. doi:.https://doi.org/10.1016/S0735-1097(96)00323-3
5 Mancini DM , Henson D , LaManca J , Levine S . Evidence of reduced respiratory muscle endurance in patients with heart failure. J Am Coll Cardiol. 1994;24(4):972–81. doi:.https://doi.org/10.1016/0735-1097(94)90858-3
6 Hammond MD , Bauer KA , Sharp JT , Rocha RD . Respiratory muscle strength in congestive heart failure. Chest. 1990;98(5):1091–4. doi:.https://doi.org/10.1378/chest.98.5.1091
7 Meyer FJ , Borst MM , Zugck C , Kirschke A , Schellberg D , Kübler W , et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. 2001;103(17):2153–8. doi:.https://doi.org/10.1161/01.CIR.103.17.2153
8 Mancini DM , Henson D , La Manca J , Donchez L , Levine S . Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation. 1995;91(2):320–9. doi:.https://doi.org/10.1161/01.CIR.91.2.320
9 Greutmann M , Le TL , Tobler D , Biaggi P , Oechslin EN , Silversides CK , et al. Generalised muscle weakness in young adults with congenital heart disease. Heart. 2011;97(14):1164–8. doi:.https://doi.org/10.1136/hrt.2010.213579
10 Lavie CJ , Milani RV , Littman AB . Benefits of cardiac rehabilitation and exercise training in secondary coronary prevention in the elderly. J Am Coll Cardiol. 1993;22(3):678–83. doi:.https://doi.org/10.1016/0735-1097(93)90176-2
11 Anderson L , Oldridge N , Thompson DR , Zwisler AD , Rees K , Martin N , et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67(1):1–12. doi:.https://doi.org/10.1016/j.jacc.2015.10.044
12 Slimani M , Ramirez-Campillo R , Paravlic A , Hayes LD , Bragazzi NL , Sellami M . The Effects of Physical Training on Quality of Life, Aerobic Capacity, and Cardiac Function in Older Patients With Heart Failure: A Meta-Analysis. Front Physiol. 2018;9:1564. doi:.https://doi.org/10.3389/fphys.2018.01564
13 Piepoli MF , Davos C , Francis DP , Coats AJ ; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328(7433):189. doi:.https://doi.org/10.1136/bmj.37938.645220.EE
14 Dall’Ago P , Chiappa GR , Guths H , Stein R , Ribeiro JP . Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol. 2006;47(4):757–63. doi:.https://doi.org/10.1016/j.jacc.2005.09.052
15 Schulz KF , Altman DG , Moher D ; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–32. doi:.https://doi.org/10.7326/0003-4819-152-11-201006010-00232
16 Evans JA , Whitelaw WA . The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54(10):1348–59.
17 Ross RM . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(10):1451 , author reply 1451. doi:.https://doi.org/10.1164/ajrccm.167.10.950
18 Rector TS , Cohn JN ; Pimobendan Multicenter Research Group. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Am Heart J. 1992;124(4):1017–25. doi:.https://doi.org/10.1016/0002-8703(92)90986-6
19 Morcillo C , Aguado O , Delás J , Rosell F . Utilidad del Minnesota Living With Heart Failure Questionnaire en la evaluación de la calidad de vida en enfermos con insuficiencia cardiac[Utility of the Minnesota Living With Heart Failure Questionnaire for assessing quality of life in heart failure patients]. Rev Esp Cardiol. Article in Spanish. 2007;60(10):1093–6. doi:.https://doi.org/10.1157/13111242
20 Behlouli H , Feldman DE , Ducharme A , Frenette M , Giannetti N , Grondin F , et al. Identifying relative cut-off scores with neural networks for interpretation of the Minnesota Living with Heart Failure questionnaire. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6242–6. doi:.https://doi.org/10.1109/IEMBS.2009.5334659
21 Winkelmann ER , Chiappa GR , Lima CO , Viecili PR , Stein R , Ribeiro JP . Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J. 2009;158(5):768.e1–7. doi:.https://doi.org/10.1016/j.ahj.2009.09.005
22 Pullen PR , Nagamia SH , Mehta PK , Thompson WR , Benardot D , Hammoud R , et al. Effects of yoga on inflammation and exercise capacity in patients with chronic heart failure. J Card Fail. 2008;14(5):407–13. doi:.https://doi.org/10.1016/j.cardfail.2007.12.007
23 Pullen PR , Thompson WR , Benardot D , Brandon LJ , Mehta PK , Rifai L , et al. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc. 2010;42(4):651–7. doi:.https://doi.org/10.1249/MSS.0b013e3181bf24c4
24 Kang J , Scholp A , Jiang JJ . A Review of the Physiological Effects and Mechanisms of Singing. J Voice. 2018;32(4):390–5. doi:.https://doi.org/10.1016/j.jvoice.2017.07.008
25 Fu MC , Belza B , Nguyen H , Logsdon R , Demorest S . Impact of group-singing on older adult health in senior living communities: A pilot study. Arch Gerontol Geriatr. 2018;76:138–46. doi:.https://doi.org/10.1016/j.archger.2018.02.012
26 Herer B . Éducation respiratoire par le chant au cours d’un programme de réhabilitation respiratoire [Outcomes of a pulmonary rehabilitation program including singing training]. Rev Mal Respir. 2013;30(3):194–202. Article in French. doi:.https://doi.org/10.1016/j.rmr.2012.10.602
27 Irons JY , Petocz P , Kenny DT , Chang AB . Singing as an adjunct therapy for children and adults with cystic fibrosis. Cochrane Database Syst Rev. 2016;9:CD008036. doi:.https://doi.org/10.1002/14651858.CD008036.pub4