When a T cell engages a B cell: novel insights in multiple sclerosis
DOI: https://doi.org/10.4414/smw.2020.20330
Ivan
Jelcic, Mireia
Sospedra, Roland
Martin
Neuroimmunology and MS Research Section (nims), Neurology Clinic, University of Zurich,
When a T cell engages a B cell: novel insights in multiple sclerosis
Summary
Multiple sclerosis is an autoimmune disease of the central nervous system (CNS) in which autoreactive T cells are considered to be the major effector cells in orchestrating and promoting CNS injuries. However, B cells emerged as additional important cellular player in multiple sclerosis immunopathogenesis since B cell depletion therapy has been found to be very effective in reducing new relapses. This short review summarises important new insights into the interaction between these two cell populations and outlines recent observations regarding how memory B cells activate brain-homing autoreactive T cells in multiple sclerosis.
Multiple sclerosis is a chronic inflammatory autoimmune disease of the central nervous system (CNS) and is one of the leading causes of neurological disability among young individuals, particularly women. Although major advances have been achieved regarding our understanding of multiple sclerosis pathogenesis and in developing new treatments, the causes of this disease as well as the cellular and molecular mechanisms are not fully understood. Most evidence in patients and in animal models of multiple sclerosis has been pointing to a T helper cell-mediated autoimmune disease, where T cells react against CNS, mainly myelin, antigens. The importance of CD4+ T cells is further supported by genome-wide association studies in multiple sclerosis, which have identified some of the known major risk factors for multiple sclerosis, the HLA-DR15 haplotype, as well as a host of other immune-related risk genes that are involved in T cell activation and homeostasis (e.g., interleukin [IL]-7, IL-7R, IL-2RA, CD40, CD58) [1].
Although B cells also cross the blood brain barrier and oligoclonal antibody bands are present in the cerebrospinal fluid (CSF) of multiple sclerosis patients, there has not been any robust evidence for their pathogenic role in multiple sclerosis. This point of view changed drastically after B cell depletion therapy with the anti-CD20 antibody rituximab showed high efficacy in suppressing disease activity in multiple sclerosis patients, indicating that B cells contribute to immune-mediated inflammation in multiple sclerosis. However, the exact mechanism was unknown at that point [2]. Apart from their ability to produce pathogenic antibodies, B cells carry out several additional functions such as providing an important source of pro-inflammatory cytokines and acting as antigen-presenting cells. The fact that plasma cells are not targeted due to their low or absent expression of CD20 also suggests that antibody-independent functions are a plausible mechanism of anti-CD20 therapy. Interestingly, in depth analysis of several of the approved multiple sclerosis treatments such as interferon-β, fingolimod, alemtuzumab, dimethyl fumarate, and cladribine, has shown that they target not only T cells but also circulating B cells, in particular by reducing memory B cells. Atacicept on the other hand, which limits the survival of naïve B cells and plasma cells, but spares memory B cells, exacerbated multiple sclerosis disease activity [3].
A potential linkage between B and T cells was then demonstrated by the presence of tertiary lymphoid structures in the meninges of multiple sclerosis patients. These cell clusters, which are rich in B and T cells, can be found in close proximity to cortical brain lesions and play an important role in inducing inflammatory processes that lead to cortical neurodegeneration [4]. B cells express high levels of HLA class II and are very efficient antigen-presenting cells, especially if they capture their cognate antigen via the B cell receptor. An elegant animal study using transgenic mice that express a B cell receptor specific for a myelin protein as transgene provided good evidence that MHC class II-dependent B cell antigen presentation may be crucial in inducing neuroinflammation [5].
Starting from the observations that myelin-specific T cell clones can be activated by dendritic cells in the absence of exogenous antigens and also that peripheral blood mononuclear cells of multiple sclerosis patients reveal an increased HLA-dependent “spontaneous” T cell proliferation, also called autoproliferation, we reasoned that peripheral T cells from multiple sclerosis patients bear a higher propensity to react to self-peptides [6, 7]. We further demonstrated that the multiple sclerosis-associated HLA-DR15 haplotype and DR15-presented self-peptides contribute to this process, but which cellular interactions lead to increased autoproliferation and whether autoproliferating T cells have pathogenic potential remained elusive [7]. We therefore developed the in vitro system further by using the cell tracking dye CFSE to monitor autoproliferation of peripheral blood mononuclear cells and to dissect the cell types that are involved in this phenomenon [8]. Using this method, we showed an increased autoproliferation of classical and nonclassical CD4+ Th1 cells along with abundant levels of interferon-γ in multiple sclerosis patients, particularly in individuals with inactive disease and carrying the major genetic risk factor HLA-DR15. The activation of autoproliferating Th1 cells was triggered by HLA-TCR engagement, suggesting that self-peptides are involved in this process. Surprisingly, among the autoproliferating cells, we also identified a substantial fraction of memory B cells, which were capable of inducing a strong T cell activation in an HLA-DR dependent manner. Elimination of B cells in multiple sclerosis patients via anti-CD20 depletion therapy very effectively inhibited T cell activation, proliferation and pro-inflammatory cytokine production in vitro and in vivo, and thus supported the idea that the crosstalk between B and T cells are a key aspect to the increased autoproliferation in multiple sclerosis patients (fig. 1). Among the essential molecules that control B cell activation and hence also T cell proliferation, we identified the kinase, Bruton tyrosine kinase (BTK), which is mainly involved in B cell receptor, toll-like receptor and chemokine receptor signaling in B cells and is currently in clinical testing for multiple sclerosis [8]. By which signaling route Bruton tyrosine kinase exactly contributes and whether other factors such as Epstein-Barr virus infection of B cells and low vitamin D, which are known environmental risk factors in multiple sclerosis and also potentiate the risk of HLA-DR15 [9], influence this phenomenon needs to be further investigated.
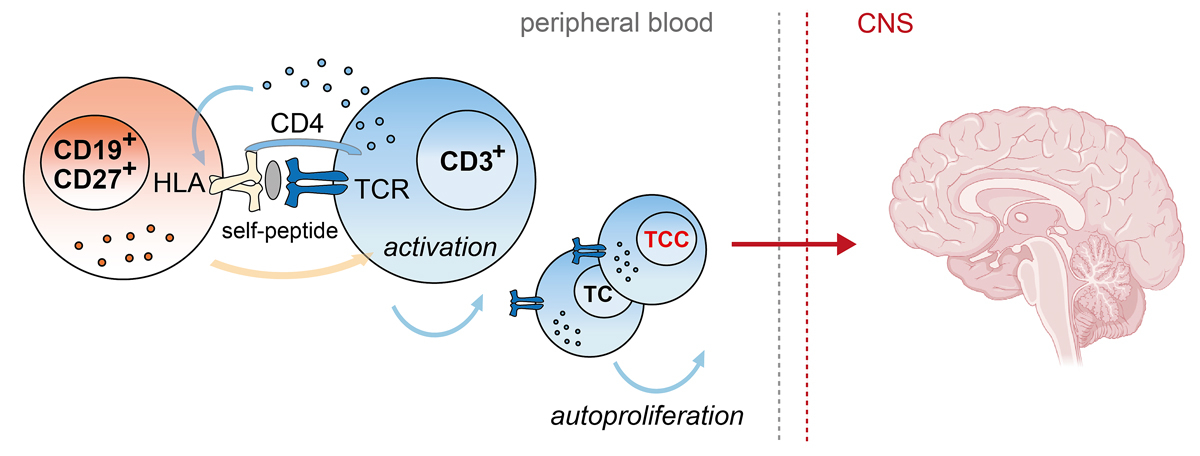
Figure 1 Physical crosstalk in which activation of Th1 cells (blue) by memory B cells (orange) leads to an increased spontaneous cell division, i.e., autoproliferation, in patients with multiple sclerosis. These autoproliferating T cells are enriched for brain-homing T cell clones (TCCs) that are specific for a protein, RASGRP2, which is expressed in B cells and in the brain. The brain image was created with BioRender.com.
Another important observation was that natalizumab, an anti-VLA4 antibody that prevents migration of leukocytes through the blood brain barrier and increases the number of circulating memory B cells, strongly increased autoproliferation and pro-inflammatory responses in multiple sclerosis patients. This observation is consistent with our findings that autoproliferation is particularly increased in clinically inactive multiple sclerosis patients, i.e., when autoreactive cells are likely to be present in the peripheral blood, and accords with the fact that discontinuation of natalizumab often leads to strong rebound activity. T cell receptor deep sequencing of active brain lesions and autoproliferating T cells from peripheral blood of two multiple sclerosis patients then proved that the latter are highly enriched for brain-homing T cells [8] (fig. 1).
In order to identify the nature of self-peptides that activate autoproliferating and brain-homing T helper cells, we isolated T cell clones from the autoproliferating compartment, confirmed their presence in active brain lesions and subsequently tested their reactivity with an unbiased epitope discovery approach which resulted in the identification of a RASGRP2 peptide, a previously unknown target antigen in multiple sclerosis. RASGRP2, also known as CalDAG-GEFI, is a cytoplasmic protein that was mainly explored in the context of platelet formation, but has been reported to play a role in antigen receptor, integrin and dopamine receptor signaling. Interestingly, we found high levels of RNA and protein expression of RASGRP2 in cortical neurons of the grey matter as well as B cells. Thus, our findings provide an important novel concept of how an autoantigen can be expressed by the inducing antigen-presenting cell population in the periphery, which may activate autoreactive T helper cells and, upon migration to the brain, target the identical antigen that leads to CNS inflammation [8]. RASGRP2 may represent only one among several potential autoantigens in multiple sclerosis patients. Another study from our lab recently identified several epitopes from GDP L-fucose synthase that were strongly recognised by brain-infiltrating T cell clones, derived from the same multiple sclerosis patient in which RASGRP2-reactive T cell clones were found, and by CSF-infiltrating T cells from a larger cohort of multiple sclerosis patients [10]. Thus, the breadth of autoantigens in multiple sclerosis may be broader and more complex than previously assumed. Further studies are required to test larger number of patients and controls with regard to the reactivity to those potential novel autoantigens and also to clarify the relation between expression and preferential location within multiple sclerosis lesions or brain areas.
In summary, our findings offer a plausible explanation for why treatment with B cell-depleting agents is so effective in multiple sclerosis and might therefore pave the way for more precise therapeutic approaches in the future. This important link between B and T cells also raises a number of further questions, on topics such as (i) the origin of this interaction, i.e., which cell type initiates this inflammatory interaction, (ii) the breadth of antigen specificity of autoproliferating T cells, (iii) the activation signatures of autoproliferating cells, and (iv) their heterogeneity at the single cell level. Most importantly, the particular association of this phenomenon with the major risk factor for multiple sclerosis, HLA-DR15, once more demonstrates that more effort is needed in order to dissect this important genetic linkage, which may not only be key for a better understanding of multiple sclerosis pathogenesis but is also highly relevant for many other autoimmune diseases that also have strong associations with certain HLA alleles.
References
1
The International Mutiple Sclerosis Gentics consortium, Wellcome Trust Case Control Consortium 2. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9. doi:.https://doi.org/10.1038/nature10251
2
Hauser
SL
,
Waubant
E
,
Arnold
DL
,
Vollmer
T
,
Antel
J
,
Fox
RJ
, et al.; HERMES Trial Group. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. doi:.https://doi.org/10.1056/NEJMoa0706383
3
Li
R
,
Patterson
KR
,
Bar-Or
A
. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19(7):696–707. doi:.https://doi.org/10.1038/s41590-018-0135-x
4
Magliozzi
R
,
Howell
O
,
Vora
A
,
Serafini
B
,
Nicholas
R
,
Puopolo
M
, et al.
Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(4):1089–104. doi:.https://doi.org/10.1093/brain/awm038
5
Molnarfi
N
,
Schulze-Topphoff
U
,
Weber
MS
,
Patarroyo
JC
,
Prod’homme
T
,
Varrin-Doyer
M
, et al.
MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210(13):2921–37. doi:.https://doi.org/10.1084/jem.20130699
6
Kondo
T
,
Cortese
I
,
Markovic-Plese
S
,
Wandinger
KP
,
Carter
C
,
Brown
M
, et al.
Dendritic cells signal T cells in the absence of exogenous antigen. Nat Immunol. 2001;2(10):932–8. doi:.https://doi.org/10.1038/ni711
7
Mohme
M
,
Hotz
C
,
Stevanovic
S
,
Binder
T
,
Lee
JH
,
Okoniewski
M
, et al.
HLA-DR15-derived self-peptides are involved in increased autologous T cell proliferation in multiple sclerosis. Brain. 2013;136(6):1783–98. doi:.https://doi.org/10.1093/brain/awt108
8
Jelcic
I
,
Al Nimer
F
,
Wang
J
,
Lentsch
V
,
Planas
R
,
Jelcic
I
, et al.
Memory B Cells Activate Brain-Homing, Autoreactive CD4+ T Cells in Multiple Sclerosis. Cell. 2018;175(1):85–100.e23. doi:.https://doi.org/10.1016/j.cell.2018.08.011
9
Olsson
T
,
Barcellos
LF
,
Alfredsson
L
. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25–36. doi:.https://doi.org/10.1038/nrneurol.2016.187
10
Planas
R
,
Santos
R
,
Tomas-Ojer
P
,
Cruciani
C
,
Lutterotti
A
,
Faigle
W
, et al.
GDP-l-fucose synthase is a CD4+ T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci Transl Med. 2018;10(462):eaat4301. doi:.https://doi.org/10.1126/scitranslmed.aat4301
