PedAMINES: a disruptive mHealth app to tackle paediatric medication errors
DOI: https://doi.org/10.4414/smw.2020.20335
Frédéric
Ehrlera, Johan N.
Siebertb
aDivision of Medical Information Sciences, Department of Radiology and Medical Informatics,
bDepartment of Paediatric Emergency Medicine, Geneva Children’s Hospital,
PedAMINES: a disruptive mHealth app to tackle paediatric medication errors
Summary
Medication errors are among the most common medical adverse events and an important cause of patient morbidity and mortality, affecting millions of people worldwide each year. This problem is especially acute in paediatric settings, where most drugs given intravenously to children are provided in vials prepared for the adult population. This leads to the need for a specific, individual, weight-based drug-dose calculation and preparation for each child, which varies widely across age groups. This error-prone process places children at a high risk for life-threatening medication errors, particularly in stressful and critical situations, such as cardiopulmonary resuscitation. To limit and mitigate the likelihood of their occurrence, hospitals are increasingly adopting eHealth interventions aimed at supporting and securing each individual stage along the whole medication process, but there is mixed evidence regarding their positive contribution. These technologies are helpful as long as they are used within the scope of their application and users are aware of their limitations, as their introduction has sometimes led to new, often unforeseen, types of errors.
The aim of the present work is to provide an overview of some of the main eHealth interventions used across the various stages of the medication process and to highlight areas that require attention in order to implement successful digital technologies. More specifically, the contribution of eHealth technologies in paediatrics is discussed, including the out-of-hospital setting, as well as barriers to their implementation in low- and middle-income countries. Finally, we describe our own work in this field with regards to the development and use of an innovative, evidence-based mobile device application (PedAMINES) to address the unmet need of reducing paediatric medication errors, especially during cardiopulmonary resuscitation. The PedAMINES app has also the potential to make a very effective contribution to the goals of the Third World Health Organization Global Patient Safety Challenge to reduce severe, avoidable medication-associated harm by 50% in all countries over the next 5 years, including low- and middle-income countries.
Introduction
Unsafe medication practices and errors are a leading cause of injury and avoidable harm in healthcare systems worldwide. The global economic burden of medication errors has been estimated at US$ 42 billion annually [1]. In 2017, the World Health Organization (WHO) Third Global Patient Safety Challenge called for the reduction of serious and avoidable medication-associated harm by 50% in all countries over the following 5 years [1]. Providing medication at the point of care implies a five-stage process traditionally subdivided into: (1) prescribing/ordering; (2) transcribing; (3) dispensing; (4) administering; and (5) monitoring and reporting of medication effects on patients.
In a prospective cohort study in adults, Leape et al. observed that without the support of eHealth systems, most errors occurred during the physician prescription (39%) and nurse administration (38%) stages. The remaining errors were almost equally divided between transcription (12%) and dispensing (11%) [2]. To limit and mitigate the likelihood of medication errors, several eHealth interventions have been developed in recent decades to target and support each individual medication stage from prescribing to treatment monitoring [3]. Placed end-to-end, these interventions form a closed-loop digital medication management system that has largely become an international gold standard for hospitals striving to achieve the highest level of medication safety [4] (fig. 1). However, the introduction of these technologies has sometimes led to new, often unforeseen, types of errors and their implementation as a whole is not always feasible in all settings.
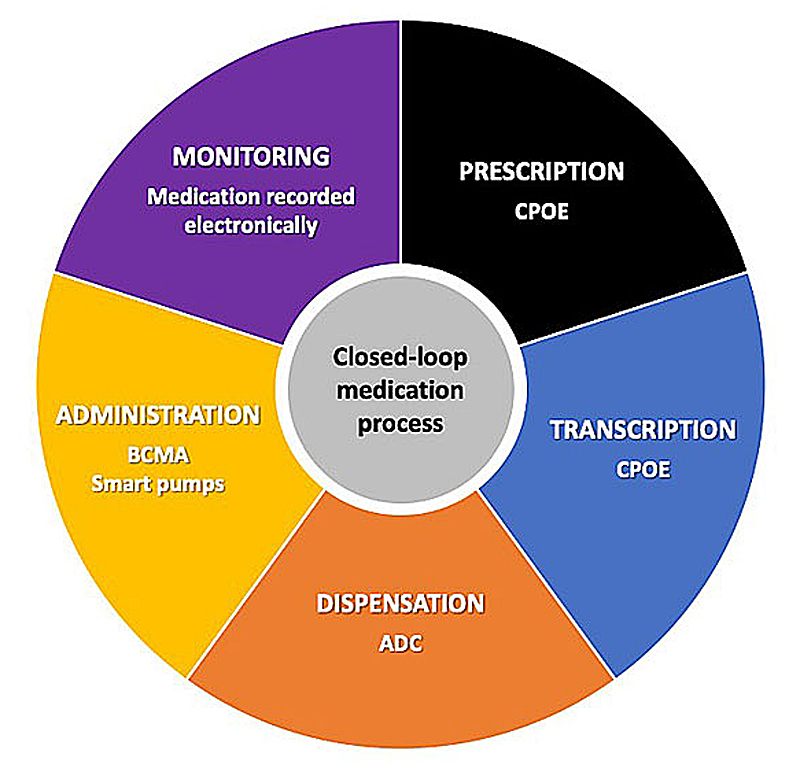
Figure 1
Closed-loop medication management process. This process completely encloses, supports and secures the medication process using digital eHealth technologies. Adapted from [5]. ADC = automated dispensing cabinet; BCMA = bar-coded medication administration; CPOE = computerised provider/physician order entry
In this narrative review, we first provide a non-exhaustive summary of some of the main eHealth interventions used across the various stages of the medication process and areas that could benefit from their application, with a special focus on the paediatric emergency field. We then describe our own work in this field with the development and use of an innovative mobile device application – the paediatric accurate medication in emergency situations (PedAMINES) app – designed as a step-by-step guide for the preparation to delivery of intravenous drugs to address the unmet need of reducing paediatric medication errors.
eHealth technologies and the medication management process
Prescription and transcription stages
Innovative interventions aimed at preventing in-hospital medication errors at the prescribing stage mainly focus on the transition from error-prone, handwritten, paper-based ordering to electronic prescribing (ePrescribing) through computerised provider/physician order entry (CPOE) systems. CPOE requires users to place medication orders into an electronic system, which then transmits the order directly to the recipient responsible for carrying out the order. These systems alleviate the problem of poor handwriting or incorrect transcription and, at a minimum, they ensure standardised, legible and complete orders, including drug name, dose and route [6]. They also often include functionalities such as drug dosage support, alerts about harmful interactions and built-in clinical decision support, which may further reduce errors [7]. Numerous systematic reviews have reported the overall efficiency of CPOE systems in decreasing prescription errors [8–12], some in specific areas of clinical care. For instance, a systematic review and meta-analysis observed that the transition from paper-based ordering to CPOE systems in adult and paediatric intensive care units was associated with an 85% reduction in prescribing error rates [13]. In four studies, the implementation of CPOE in paediatric emergency departments and intensive care units significantly reduced medication prescribing errors [14–17].
However, the optimistic adoption and widespread use of CPOE in paediatrics should be somewhat tempered because of its unintended consequences, including the potential for other errors and impediments to effective performance [18, 19]. Indeed, a growing body of evidence shows that CPOE (with or without clinical decision support) has the potential to introduce or facilitate new types of prescription errors, such as failure to follow established protocols, inexperience or lack of training in using the CPOE system, or ignoring/overriding alerts [20–23]. A systematic review observed that the prevalence of prescription errors related to the use of CPOE systems relative to all prescription medication errors varied widely across studies (6.1–77.7%; median 26.1%) [21]. Moreover, as stated by Kahn et al., the necessity in some hospitals to adapt CPOE designed for adults to meet the unique prescribing needs of children, as well as the lack of uniform design among studies involving CPOE in paediatrics, preclude wide generalisability of results on the true impact of CPOE in reducing prescription and transcription errors among paediatric patients [24].
Dispensing stage
Computerised drug storage devices, such as automated dispensing cabinets (ADCs), have become widely used as decentralised medication-distribution systems at the point of care in emergency departments. ADCs are locking cabinets that electronically control access one at a time to compartmentalised medication via passwords or a fingerprint scan and provide an inventory control on their use. Although there is evidence that they enhance efficiency of medication distribution [25, 26], studies on their use have so far shown inconsistent results regarding their capacity to reduce non-negligible, medication administration errors [26, 27]. Moreover, ADCs themselves may give rise to medication errors. For example, errors that stem from overfilled drawers and the ensuing possibility of wrongly choosing too many vials of the prescribed drug, or choosing the wrong medication with look-alike drug names from an alphabetical pick list on the ADC screen (e.g., dopamine and dobutamine) [28]. Additionally, since refilling of the cabinet depends on human intervention, an upstream filling error by the pharmacy or a drug returned to the wrong space in the cabinet could be a future cause of medication error. To date, the usefulness of ADCs in paediatric emergency medicine, especially during cardiopulmonary resuscitation (CPR) and life-threatening events, has not been investigated.
Administration stage
The administration stage is the last moment to intercept any prior error that has occurred along the medication process before it reaches the patient. It is also an important source of errors. Barker et al. reported that, without any eHealth system support, every fifth medication dose administered was associated with an error, including the wrong time of administration (43%), omission of a dose (30%), wrong dose (17%) and other errors (10%) [29]. Non-technological solutions such as the use of prefilled color-coded syringes have been shown in a simulated model to decrease paediatric dosing errors at the administration stage [30]. However, these results were limited to direct intravenous pushes requiring no prior complex preparation. And the implementation and adoption of this solution in clinical practice remains to be assessed.
On the technological front, bar-coded medication administration (BCMA) technology has been identified as a way of reducing medication errors at the administration stage by ensuring the six “rights” of medication administration: the right patient, drug, dose, route, time and documentation. By means of a simple scan, this system reconciles the patient identity in the form of a bar-coded label or wristband and the bar-coded drug to be administered at the bedside. In the case of non-congruity, the nurse is notified prior to drug delivery. In early studies performed on in-house developed systems, BCMA technology was shown to reduce administration errors by up to 54%. However, later studies performed on commercial bar-coded systems demonstrated an inconsistent decrease in errors [31]. Furthermore, errors specifically caused by BCMA technology have been reported, such as mislabelling of medication with an incorrect bar code, lack of bar code, inability to scan the bar code, overriding of error warning, bar code not scanned, workarounds, wrong patient and system failure [32]. Unfortunately, there has been no study until now to assess whether BCMA might reduce medication errors during paediatric resuscitation at the administration stage.
Drug administration through smart infusion pumps is another eHealth intervention to prevent medication administration errors. These pumps allow users to input the required patient information and then select the desired drug from predefined built-in drug libraries. The pump then checks the nurse’s programmed medication administration against pre-established institutional limits [33]. When a programmed infusion outside the limits, the smart pump issues alerts before the infusion is started. Soft alerts prompt the user to reconsider administering a given dose, whereas hard alerts theoretically prevent the user from administering an infusion outside the recommended parameters. However, these alerts can be overridden to force the infusion start. Although increasingly used in some countries, with 88.1% of hospitals in the USA reporting their use in 2017 [34], there is mixed evidence that smart infusion pumps prevent medication error and adverse drug events [35, 36]. Issues that have been raised in the literature include workarounds, such as the use of basic infusions that do not employ the drug library dosing limits, improper patient or medication identification during pump programming and high override rates for soft alerts, all of which negate the safety features of smart pumps [37]. In addition, little is known about the kind of errors that smart pumps may actually introduce themselves with their use. It should also be kept in mind that when used during paediatric resuscitation, standardising drug concentrations of premixed drugs and varying infusion rates with smart pumps imply dealing with poor dose and rate precision in already unstable and critically-ill children. Furthermore, their price and the lack of a specialised pharmacy facility in many smaller hospitals limit their use, particularly in low- and middle-income countries. Thus, like other eHealth technologies, smart infusion pumps may be of great assistance in reducing medication errors, provided that their drawbacks are clearly borne in mind and risks associated with their use are never disregarded in order to achieve patient safety goals.
Areas for medication safety improvement through eHealth technology
eHealth in paediatric emergency medicine
Children represent a vulnerable population with specific medical needs compared to adults. Owing to paediatric-specific constitutional and physiological considerations, notably age-related variations in pharmacokinetics in terms of absorption, distribution, metabolism and elimination, they are particularly vulnerable to medication errors [38]. The latter occur in 5–27% of all paediatric prescriptions and cause significant mortality and morbidity, including 7000 patient deaths each year in the USA [39]. Errors can occur at any step during the medication process, from drug prescription to delivery. In paediatric emergency departments, one in every 32 prescriptions contains a 10-fold error on the recommended dose [40]. This becomes particularly concerning in paediatric critical situations, such as septic shock, cardiogenic shock, CPR and return of spontaneous circulation following CPR for cardiac arrest, where the accurate and safe preparation and administration of intravenous drugs is mandatory [30, 40, 41]. Notably, most drugs given intravenously to children are provided in vials originally prepared for the adult population. This leads to the need for a specific, individual, weight-based dose calculation and drug preparation for each child that varies widely across age groups [42]. This error-prone process and the lower dosing error tolerance of children [43, 44] place them at a high risk for life-threatening medication errors [40, 42, 45, 46].
Despite well-equipped and staffed environments with numerous available safeguards, direct intravenous medication errors have been reported in up to 41% of cases during simulated in-hospital paediatric resuscitations, 65% of which were incorrect medication dosage, thus making it the most common error [47]. In neonatal and paediatric intensive care units, errors in handwritten continuous infusion orders may be as high as to 70% [48]. The rate of medication errors further increases in critical care environments requiring the administration of several drugs which each may have its own concentration, dose and volume [40]. This is especially true in the high-risk out-of-hospital setting, where health care for children is often provided by caregivers who have little exposure to critically ill children and lack specialised paediatric skills required for weight-based drug dosage, conversion, calculation and dilution. In addition, they are often unfamiliar with their diseases and medication unique needs.
eHealth in the out-of-hospital setting
Medication errors in the prehospital setting are reported as occurring in more than 30% of all paediatric drugs administered [49]. In this particular context, initial care has to be delivered quickly by emergency medical services (EMS) in challenging field environments where resources and providers are limited [50]. Relying on his/her sole expertise and knowledge to make decisions during care provision, a single paramedic is often in charge of determining the patient’s weight, choosing the most suitable drug, calculating the drug dose and appropriate volume to inject, and administering it to the patient. However, as paramedics have little exposure to critically ill children, they lack they experience to administer emergency medications at paediatric doses [51], with minimal opportunities to gain and maintain competence in this skill [52, 53].
Until now, the use of technology to support paramedic practices has mainly concentrated on interventions to capture and transmit patient data through the use of telehealth/telemedicine [54–56]. In particular, Almadani et al. proposed an e-Ambulance framework to provide real-time remote patient monitoring with bidirectional communication between the EMS and off-site emergency department physicians. This system aimed to ensure optimal patient care during transportation and to prepare the incoming admission, while limiting ambulance transport time [57]. This latter consideration is particularly important. The chain of survival in advanced life support critically relies on early out-of-hospital CPR by the EMS [58] and the on-site administration of emergency drugs without delay [59–61] before a rapid transfer to the paediatric emergency department and advanced care. Indeed, during the first 15 minutes of paediatric CPR, survival and a favourable neurological outcome decrease linearly by 2.1% and 1.2% per minute, respectively [62], and rely in part on drug preparation time in out-of-hospital settings [60]. Among non-shockable paediatric out-of-hospital cardiac arrests, each minute of delay to epinephrine delivery is associated with a 9% decrease in survival odds [59, 60].
Unfortunately, to our knowledge, no study has ever assessed the impact of prehospital telemedicine on medication error rates. Additionally, although numerous interventions involving information technologies have been developed to improve the in-hospital security of the medication process [24], there has been no reciprocal intervention to reduce paediatric prehospital medication errors so far. Data regarding EMS-related prehospital medication errors and error prevention strategies are scarce [49, 63, 64]. Prehospital dosing errors were reported to affect approximately 56,000 children treated by EMS each year in the USA [49], with drugs administered outside of the proper dose range described in up to 39.8% of more than 5500 children. These results were similar to previous reporting that paramedics commit dosing errors 49–63% of the time, with miscalculation as a primary cause [45]. Although substantial, these errors are likely to be underestimated as not all are reported [49]. EMS are greatly exposed to opportunities for out-of-hospital medication errors, thus severely compromising patient safety. In this setting, children are likely to require immediate care [50] and resources are limited. Emotional anxiety, as well as exogenous conditions such as challenging field environments, parental stress and time pressure to prepare the drugs on-site are other factors encountered during prehospital paediatric resuscitation that potentially add to the complexity of the process [65, 66]. Providing EMS with ready access to a weight-estimation device app and a drug-dosing guide, such as the Broselow-Luten tape, was shown to lack sufficient accuracy and information to function independently as a complete resuscitation aid for the prevention of a high rate of prehospital medication errors [67, 68], with epinephrine dosing errors exceeding 60–70% [45]. Underlying causes of errors include the incorrect estimation of weight, incorrect use of the Broselow-Luten tape, incorrect recall of doses, difficulty with calculations under stress, mg/kg to milligrams to millilitre conversion errors, inaccurate measurement of volumes, and failure to cross-check doses between providers [67].
Hence, the sole expertise of paramedics helped by conventional methods is not sufficient to ensure a fast and reliable conversion and preparation of paediatric emergency intravenous medication. In a US national survey among EMS, 72.6% of paramedics rated that an EMS-specific mobile device app would be helpful to very helpful in decreasing paediatric drug dosing errors [49]. To date, no mobile app has been shown to support the EMS medication process.
eHealth in low- and middle-income countries
Although low- and middle-income countries have the highest burden of disease [69], efforts to promote medication safety through technological innovations have mostly taken place in high-income countries. Indeed, the above-mentioned eHealth interventions are often costly and resource consuming, thus hindering their deployment in countries with limited financial resources for medical technology, low levels of education and poor infrastructures for delivering and maintaining technology. Furthermore, investing resources in new technologies for potential improvement should be put in perspective with the improvement linked to investment in more traditional healthcare activities [70]. In this context, the increasing coverage of around 40% of the population of low- and middle-income countries by mobile internet and the widespread use of smart mobile devices may play a pivotal role in the integration of mobile health (mHealth) solutions in limited-resource settings [71].
Mobile technologies allow cheap and easy-to-deliver interventions, but important barriers may limit their integration into existing healthcare systems and their use by frontline physicians and health workers. As recognised by Wallis et al., lack of adequate legislative and regulatory frameworks, such as laws to protect patient privacy, are likely to be the first barrier [72]. Technology-related issues, such as inadequate mobile and/or cellular infrastructure, prohibitive costs and unreliable technology, may represent a second barrier, whereas poorly designed devices/apps, difficulty changing clinical behaviour and poor technology literacy constitute a third barrier. Finally, poor access to the scientific literature and knowledge-sharing by researchers and colleagues in low- and middle-income countries must be addressed to improve the culture of medication errors as the overwhelming majority of publications originate in high-income countries and contribute to limit support for research and innovation in settings that need them most [73, 74]. Aware of these limitations, the integration of mHealth solutions into healthcare systems may be a way to reduce medication errors in low- and middle-income countries.
Role and contribution of mobile device apps to prevent medication errors
Several interventions involving information technologies have been developed to improve the security of the medication process. However, apart from CPOE systems and smart infusion pumps, few robust data are available to measure their real impact on patient safety [75]. The heterogeneity in current paediatric medication error intervention studies prevents the wide generalisability of results and yields unclear guidance to hospitals on which interventions are best to adopt [39]. In a systematic review and meta-analysis focused on the efficacy of interventions for reducing medication administration errors, Berdot et al. did not find evidence that technology-related interventions can effectively decrease medication errors [76]. Similar results were published by Rinke et al., who were unable to find bias-free, robust and rigorous evidence in the paediatric literature to recommend CPOE, bar coding technology and unit dose-dispensing systems to reduce medication errors [39]. In addition, there are usually challenges with regard to the costs involved, sustainability, staff acceptability and other implementation issues [75].
Some authors have advocated automated actions to reduce as much as possible tasks inducing the cognitive load during paediatric resuscitation, in order to optimise patient care and diminish medication errors [77]. In this, mobile device apps could be game changers. In the field of CPR, a recent study identified 34 available mobile apps on Google Play and Apple App stores [78]. However, many of these apps are marginally medically correct and have limited usability and poor user-friendliness [79]. It is also unknown whether they actually improve or impede clinical care, especially in paediatrics, since the effectiveness of most of these apps has not been validated in evidence-based studies [79]. In addition, the few reports published have been shown to be methodologically flawed [80, 81]. For instance, Baumann et al. recently conducted a randomised crossover trial among anaesthesiologists to assess the impact of a mobile app in reducing intravenous medication administration errors in adults and children [80]. They found that given drug doses were more frequently in the accurate dosing range in both age groups when administration was supported by their app. Unfortunately, as no carryover effect was investigated by the authors, the efficacy of the app could potentially have been different depending on the first method used in the crossover design (i.e., biased), and results should be interpreted with caution [82].
Although digitalisation has strongly influenced the way medications are provided in emergency care through the introduction of eHealth intervention [3], assistance for real-time medication delivery during CPR has been judged as almost impossible due to the specific constraints of the situation. As a consequence, in many critical situations, physicians and nurses are still dependent on paper-based support, empirical calculators or spreadsheets in order to ensure correct drug delivery. To address the above-mentioned flaws, we developed the PedAMINES app.
The PedAMINES mobile device app: development and characteristics
The PedAMINES project was launched at Geneva University Hospitals (Geneva, Switzerland). The app was developed with a user-centred and evidence-based approach, with emergency department caregivers, ergonomists and computer scientists from a research and development service. The key functionalities and processes to be implemented were identified following observations of paediatric resuscitation and focus groups [83]. The content, design, interface and usability of the app was assessed iteratively until the app was considered to work to the satisfaction of physicians and nurses.
The app lists all the available resuscitation drugs in alphabetical order with doses automatically adapted to the weight or age of the patient based on information entered when starting the app (age-based weight estimates are based on the Paediatric Advanced Life Support values set by the American Heart Association). Each of the listed drugs for direct intravenous injection or continuous infusion (highlighted in yellow) can be selected in a menu on the left of the app and are displayed on the right pane with a detailed preparation procedure according to a standardised and simplified pathway (fig.2). In the case of a direct intravenous injection, this pathway is composed of two steps: (1) drug selection; (2) conversion of the prescribed dose in mg/kg into a volume in ml; and, if necessary, an additional step for dilution of the initial drug concentration with compatible fluids (sodium chloride 0.9%, etc.). In the case of a continuous infusion, this pathway is composed of three steps: (1) drug selection; (2) dilution of the initial drug concentration; and (3) conversion of the prescribed dose rate in μg/kg per min into an infusion pump rate in ml/h. For each drug, the exact amount to prepare is clearly displayed and thus the necessity for calculations is avoided (fig. 2). This is based on the app’s ability to automatically calculate the optimal weight-based final volume to inject or infusion pump rate and to describe the preparation sequence required to achieve it, independent of the user’s competency in this domain.
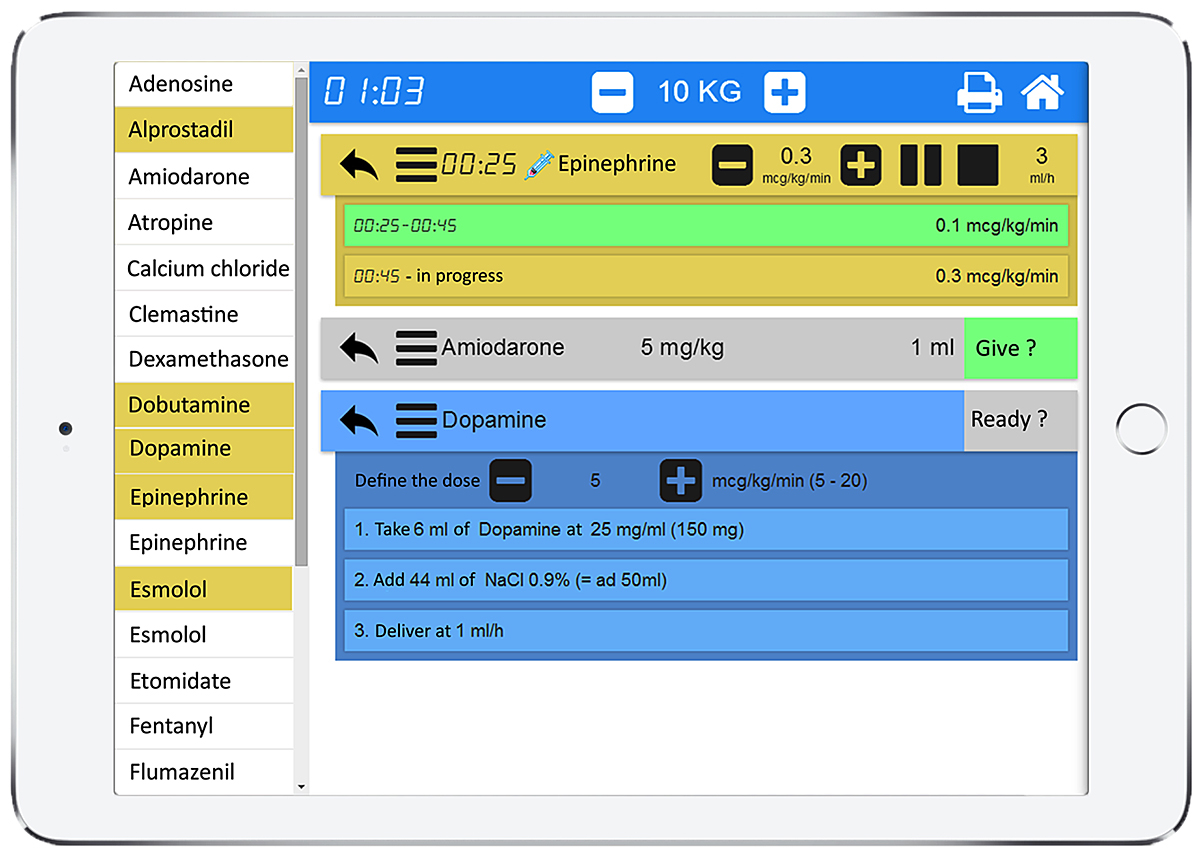
Figure 2
PedAMINES screenshot. List of bolus intravenous drugs (white boxes) and drugs for continuous infusion (yellow boxes) are selectable in the left margin of the application. The right window shows drugs selected by the nurse for a child weighing 10 kg. In this screenshot example, epinephrine is being delivered at 0.3 µg/kg/min. Amiodarone is selected and ready to be injected, waiting for the nurse’s confirmation. Dopamine preparation is displayed step-by-step. The printer logo in the upper right corner indicates that all actions performed by the nurses are sequentially saved in historic files that can be retrieved and printed at any time.
When administering continuous infusions, the user can start, pause, stop, increase or decrease the perfusion rate at any time. Multiple drugs can be prepared and run in parallel, including continuous infusions. Colour-coded boxes are used to display the stages of the current medication process: drugs selected and preparation steps (in blue), drugs ready for administration (in grey), running continuous infusions (in yellow), and drugs already delivered (in green) (table 1).
Table 1 Information displayed on the app when preparing drugs.
|
Stage
|
Drug type
|
Colour
|
Option
|
Displayed information
|
| Preparation |
Direct intravenous bolus, continuous infusion |
Blue |
Dose increments |
Drug name, dose, preparation steps |
| Ready to be administered |
Direct intravenous bolus, continuous infusion |
Grey |
|
Drug concentration and volume to be administered |
| Administration in process |
Continuous infusion |
Yellow |
Prescribed dose rate in μg/kg/min, dose rate increment, options to pause, start, stop the infusion |
Time since start of the infusion, drug name, dose rate in μg/kg/min, options, and infusion pump rate in mL/h |
| Administered |
Direct intravenous bolus, continuous infusion |
Green |
|
Amount/volume administered, time of administration |
For direct intravenous boluses, once administered, the state of the drug switches directly to the “administered” stage in green, whereas for continuous infusions the app displays the timed ongoing process. Once the drug is delivered to the patient, the user has to click on the stop button and the stage automatically switches to “administered” with a summary of the total amount of drug administered and time spent to achieve it. At the top of the screen, a header displays a timer indicating either the time since the start of the medication process (e.g., during CPR) or the current time. This information is provided to avoid the loss of sense of time that is frequent during stressful situations such as CPR and may preclude timely adherence to resuscitation guidelines or reporting of procedures in a timely manner. Finally, once the whole process is completed, all actions done by the user, as well as drug concentrations used, flow rates, etc., are sequentially saved locally on the device in historical files to preserve information that can be retrieved at any time for debriefing or medicolegal purposes. Historical files can also be erased or safely exported in pdf format to be saved in the patient’s electronic health records (fig. 3).
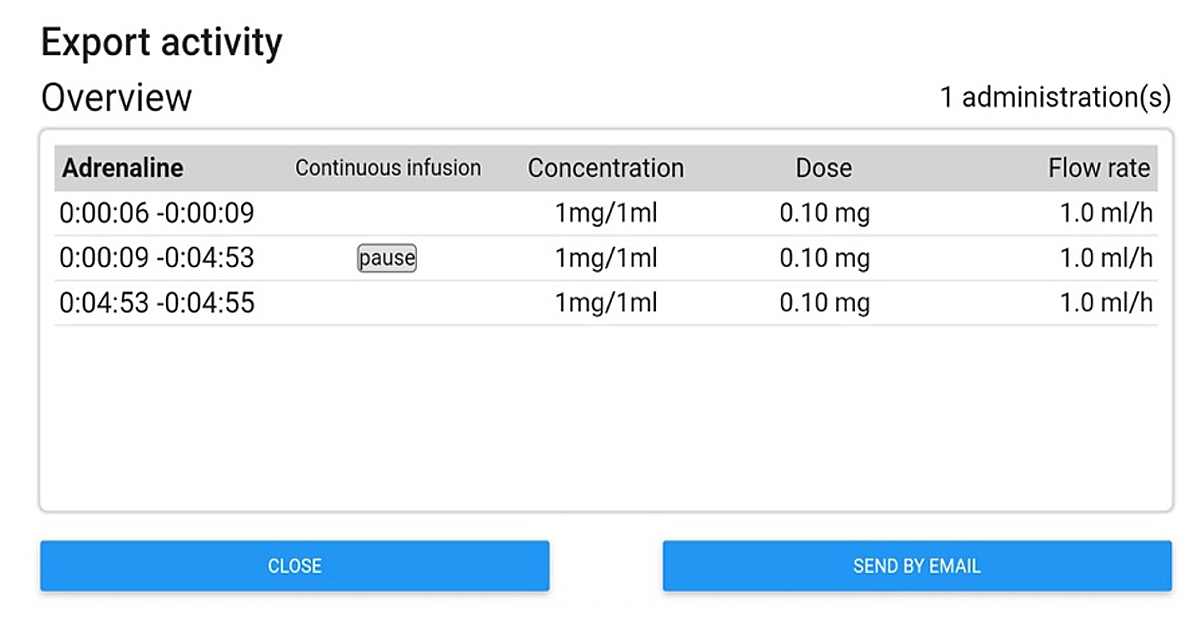
Figure 3 Screenshot of the export activity window.
Importantly, the app contains a drug editor that allows users to create exhaustive lists of drugs they use in their own settings and to edit them according to local formulations and habits (fig. 4). Sharing the lists between caregivers and institutions is possible through upload and download options via identity codes created by the list owner. Then, when requested by another user, the list owner can decide whether to share the list by providing the unique identity code for that list in order for the other user to retrieve the list on the server and to download it on his device. Once this is done, the user can either use it as is or modify the list on his or her device. But in no case will these modifications affect the original list saved on the server. This new list will have to be uploaded by the new user onto the server under a new name and with a new unique identity code. For that purpose, it was necessary to install a server on the secured infrastructure of Geneva University Hospitals and to link it to a database storing the existing lists. Once the list(s) is uploaded on a device, the app has full functionality without the requirement of cellular or WiFi connectivity and can be used anywhere, including in remote areas.
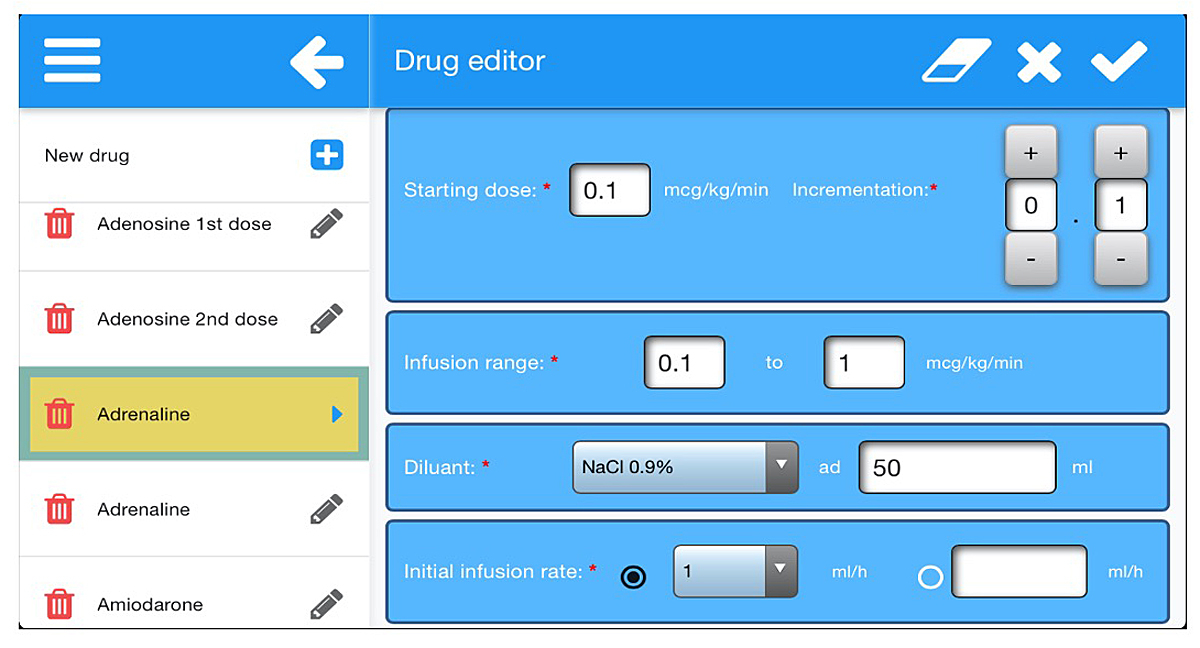
Figure 4
Screenshot of the drug editor. All drug-related information can be edited here.
As an attempt to improve one step further the safety of the medication process, we recently implemented cost-effective alternatives to ADC and BCMA on the app. This was achieved in the form of both a remote LED lighting solution, and an in-app matrix quick-response code recognition technology, respectively. The first system consists of LED strips placed at the level of the pharmacy drawers on which each LED can be turned on independently with assigned colours to indicate where to find each drug selected on the app. In this sense, the system is very similar to ADCs. However, our solution circumvents some flaws of traditional ADCs by offering a lightweight, affordable, flexible and scalable system to each region of the world, including low- and middle-income countries. The second system, based on quick-response codes, associates a unique matrix code to each type of drug at disposal. At the time of dispensing, this system allows users to scan the matrix code stick on the drug vial (or packaging) via the in-built camera of the device. The app drug editor on the other side allows association of any chosen quick-response codes to a specific drug. Matching codes validate the drug selection and authorise users to go further in the drug preparation and aims to prevent errors associated with selection of look-alike/sound-alike medications. In the case of non-matching codes, the app issues a warning and does not allow drug preparation. This system has the advantage of being totally free and resource-sparing as it only uses the camera features of the mobile device. It is also scalable to any location and settings since quick-response codes already in place on vials or packaging could be used without upstream manipulations or transformation, thus avoiding mislabelling of medication with an incorrect or missing bar code. However, workarounds are still possible without quick-response codes as the scanning option on the app can be deactivated. The impact of both systems on medication errors will be assessed in forthcoming studies. Finally, according to the six “rights” of medication administration, it is worth pointing out that the app, at the moment, does not have a dedicated solution to guarantee the choice of the “right patient” and “right indication”.
PedAMINES, an evidence-based app
In a previous multicentre, randomised, controlled, crossover trial, which was funded by the Swiss National Science Foundation (SNSF) and received the Pfizer Research Award in February 2020, we reported the ability of PedAMINES to significantly reduce the occurrence of in-hospital medication errors compared with an internationally used drug infusion rates table for the preparation of continuous drug infusion during simulation-based paediatric resuscitations [84] (video available at www.pedamines.com). One-hundred and twenty-eight nurses from paediatric emergency departments across six Swiss institutions were randomly assigned to participate in the trial. We observed that 96 of 128 drug administrations (75%) were associated with medication errors using the infusion rates table compared with only 9 of 128 (7%) delivered when guided by the app, thus representing a significant absolute reduction of 68% in medication errors. Additionally, the mean time to drug preparation and drug delivery were reduced by 45% and 40%, respectively. We detected no carryover effect and observed that hospital size and nurses’ experience did not modify the intervention effect.
The strengths of this trial were based on the methods used in accordance with guidelines for healthcare simulation research [85] and the previous single-centre pilot study conducted to validate the app [86]. There were some limitations associated with the trial. First, it was conducted during a resuscitation simulation-based scenario. Today, high-fidelity simulation is an essential investigative methodology to answer research questions that otherwise could not be answered during real CPR, as the diversity among patients and their diseases make CPR studies hard to standardise in critical situations [87]. Second, the 5-minute app training was just before the scenario. In real life, the interval between training and actual use would probably have been months. However, training with the app months before the study would have unblinded participants to its purpose and could have created a preparation bias.
In a second multicentre, randomised, controlled trial funded by the SNSF, we compared the PedAMINES app with conventional calculation methods for the preparation of direct intravenous administered emergency medications by 152 paramedics in several EMS located in different regions of Switzerland during simulation-based paediatric out-of-hospital cardiac arrest scenarios [88]. Enrolment was completed in January 2020 and publication is expected in a few months. Preliminary results are promising and we already anticipate a significant impact of the app on reducing prehospital paediatric medication errors. As most of the results obtained from simulation-based resuscitation studies agree with those obtained in real-life studies, we are confident that our results will be of great interest for a potential application in real-life situations. From perspective, using PedAMINES in low- and middle-income countries could be of particular interest, as it could overcome many of the above-mentioned limitations that generally hamper the implementation of new technologies in these countries.
Conclusion and future directions
Medication errors are a leading cause of global, potentially avoidable, morbidity and mortality and a serious challenge for our healthcare systems and societies. This might be partly mitigated by implementing adequate eHealth interventions. Although a growing body of evidence calls for their widespread implementation, important barriers may limit their use, such as their high costs or restricted scalability across countries. However, efforts should be made to ensure that vulnerable populations, particularly children, or those living in remote areas, benefit from these interventions. Similar to others, we believe that mHealth technologies can play a major role in this process. To the best of our knowledge, PedAMINES is the only evidence-based mobile app to assist medical drug preparation for in-hospital and out-of-hospital paediatric resuscitation, with the ability to reduce medication errors as well as time to drug delivery. We consider that this mobile app has the potential to change critical care clinical practice when intravenous drugs have to be prepared and to improve quality of care in the paediatric vulnerable population. Its development also contributes to the goals of the WHO Third Global Patient Safety Challenge, which aims to reduce severe, avoidable medication-associated harm by 50% in all countries over the next 5 years [1]. It remains to be determined whether the use of PedAMINES in real-life situations may similarly reduce medication error rates, especially in the prehospital setting and in remote areas where paramedics and health workers are less exposed to paediatric resuscitation.
Acknowledgements
We thank Rosemary Sudan for editorial assistance.
Author contributions
FE and JNS drafted and equally revised the article. JNS did the review of evidence.
References
1
Donaldson
LJ
,
Kelley
ET
,
Dhingra-Kumar
N
,
Kieny
MP
,
Sheikh
A
. Medication without harm: WHO’s third global patient safety challenge. Lancet. 2017;389(10080):1680–1. doi:.https://doi.org/10.1016/S0140-6736(17)31047-4
2
Leape
LL
,
Bates
DW
,
Cullen
DJ
,
Cooper
J
,
Demonaco
HJ
,
Gallivan
T
, et al.; ADE Prevention Study Group. Systems analysis of adverse drug events. JAMA. 1995;274(1):35–43. doi:.https://doi.org/10.1001/jama.1995.03530010049034
3
Weant
KA
,
Bailey
AM
,
Baker
SN
. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med. 2014;6:45–55. doi:.https://doi.org/10.2147/OAEM.S64174
4
Austin
JA
,
Smith
IR
,
Tariq
A
. The impact of closed-loop electronic medication management on time to first dose: a comparative study between paper and digital hospital environments. Int J Pharm Pract. 2018;26(6):526–33. doi:.https://doi.org/10.1111/ijpp.12432
5
Bhatti
S
. Adoption of closed loop medicines administration into the NHS. Pharm J. 2019. Available at: https://www.pharmaceutical-journal.com/opinion/blogs/adoption-of-closed-loop-medicines-administration-into-the-nhs/20206864.blog?firstPass=false.
6
Ranji
SR
,
Rennke
S
,
Wachter
RM
. Computerised provider order entry combined with clinical decision support systems to improve medication safety: a narrative review. BMJ Qual Saf. 2014;23(9):773–80. doi:.https://doi.org/10.1136/bmjqs-2013-002165
7
Radley
DC
,
Wasserman
MR
,
Olsho
LE
,
Shoemaker
SJ
,
Spranca
MD
,
Bradshaw
B
. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc. 2013;20(3):470–6. doi:.https://doi.org/10.1136/amiajnl-2012-001241
8
Kaushal
R
,
Shojania
KG
,
Bates
DW
. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163(12):1409–16. doi:.https://doi.org/10.1001/archinte.163.12.1409
9
Shamliyan
TA
,
Duval
S
,
Du
J
,
Kane
RL
. Just what the doctor ordered. Review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res. 2008;43(1 Pt 1):32–53. doi:.https://doi.org/10.1111/j.1475-6773.2007.00751.x
10
Eslami
S
,
de Keizer
NF
,
Abu-Hanna
A
. The impact of computerized physician medication order entry in hospitalized patients--a systematic review. Int J Med Inform. 2008;77(6):365–76. doi:.https://doi.org/10.1016/j.ijmedinf.2007.10.001
11
van Rosse
F
,
Maat
B
,
Rademaker
CM
,
van Vught
AJ
,
Egberts
AC
,
Bollen
CW
. The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. Pediatrics. 2009;123(4):1184–90. doi:.https://doi.org/10.1542/peds.2008-1494
12
Nuckols
TK
,
Smith-Spangler
C
,
Morton
SC
,
Asch
SM
,
Patel
VM
,
Anderson
LJ
, et al.
The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev. 2014;3(1):56. doi:.https://doi.org/10.1186/2046-4053-3-56
13
Prgomet
M
,
Li
L
,
Niazkhani
Z
,
Georgiou
A
,
Westbrook
JI
. Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. J Am Med Inform Assoc. 2017;24(2):413–22. doi:.https://doi.org/10.1093/jamia/ocw145
14
Sethuraman
U
,
Kannikeswaran
N
,
Murray
KP
,
Zidan
MA
,
Chamberlain
JM
. Prescription errors before and after introduction of electronic medication alert system in a pediatric emergency department. Acad Emerg Med. 2015;22(6):714–9. doi:.https://doi.org/10.1111/acem.12678
15
Potts
AL
,
Barr
FE
,
Gregory
DF
,
Wright
L
,
Patel
NR
. Computerized physician order entry and medication errors in a pediatric critical care unit. Pediatrics. 2004;113(1):59–63. doi:.https://doi.org/10.1542/peds.113.1.59
16
Sard
BE
,
Walsh
KE
,
Doros
G
,
Hannon
M
,
Moschetti
W
,
Bauchner
H
. Retrospective evaluation of a computerized physician order entry adaptation to prevent prescribing errors in a pediatric emergency department. Pediatrics. 2008;122(4):782–7. doi:.https://doi.org/10.1542/peds.2007-3064
17
Vardi
A
,
Efrati
O
,
Levin
I
,
Matok
I
,
Rubinstein
M
,
Paret
G
, et al.
Prevention of potential errors in resuscitation medications orders by means of a computerised physician order entry in paediatric critical care. Resuscitation. 2007;73(3):400–6. doi:.https://doi.org/10.1016/j.resuscitation.2006.10.016
18
Patel
VL
,
Kannampallil
TG
. Human factors and health information technology: current challenges and future directions. Yearb Med Inform. 2014;9:58–66. doi:.https://doi.org/10.15265/IY-2014-0005
19
Han
YY
,
Carcillo
JA
,
Venkataraman
ST
,
Clark
RS
,
Watson
RS
,
Nguyen
TC
, et al.
Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2005;116(6):1506–12. doi:.https://doi.org/10.1542/peds.2005-1287
20
Slight
SP
,
Eguale
T
,
Amato
MG
,
Seger
AC
,
Whitney
DL
,
Bates
DW
, et al.
The vulnerabilities of computerized physician order entry systems: a qualitative study. J Am Med Inform Assoc. 2016;23(2):311–6. doi:.https://doi.org/10.1093/jamia/ocv135
21
Korb-Savoldelli
V
,
Boussadi
A
,
Durieux
P
,
Sabatier
B
. Prevalence of computerized physician order entry systems-related medication prescription errors: A systematic review. Int J Med Inform. 2018;111:112–22. doi:.https://doi.org/10.1016/j.ijmedinf.2017.12.022
22
Carli
D
,
Fahrni
G
,
Bonnabry
P
,
Lovis
C
. Quality of decision support in computerized provider order entry: systematic literature review. JMIR Med Inform. 2018;6(1):e3. doi:.https://doi.org/10.2196/medinform.7170
23
Koppel
R
,
Metlay
JP
,
Cohen
A
,
Abaluck
B
,
Localio
AR
,
Kimmel
SE
, et al.
Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–203. doi:.https://doi.org/10.1001/jama.293.10.1197
24
Kahn
S
,
Abramson
EL
. What is new in paediatric medication safety?
Arch Dis Child. 2019;104(6):596–9. doi:.https://doi.org/10.1136/archdischild-2018-315175
25
Grissinger
M
. Safeguards for Using and designing automated dispensing cabinets. P&T. 2012;37(9):490–530.
26
Cottney
A
. Improving the safety and efficiency of nurse medication rounds through the introduction of an automated dispensing cabinet. BMJ Qual Improv Rep. 2014;3(1):u204237.w1843. doi:.https://doi.org/10.1136/bmjquality.u204237.w1843
27
Tsao
NW
,
Lo
C
,
Babich
M
,
Shah
K
,
Bansback
NJ
. Decentralized automated dispensing devices: systematic review of clinical and economic impacts in hospitals. Can J Hosp Pharm. 2014;67(2):138–48. doi:.https://doi.org/10.4212/cjhp.v67i2.1343
28
Paparella
S
. Automated medication dispensing systems: not error free. J Emerg Nurs. 2006;32(1):71–4. doi:.https://doi.org/10.1016/j.jen.2005.11.004
29
Barker
KN
,
Flynn
EA
,
Pepper
GA
,
Bates
DW
,
Mikeal
RL
. Medication errors observed in 36 health care facilities. Arch Intern Med. 2002;162(16):1897–903. doi:.https://doi.org/10.1001/archinte.162.16.1897
30
Moreira
ME
,
Hernandez
C
,
Stevens
AD
,
Jones
S
,
Sande
M
,
Blumen
JR
, et al.
Color-coded prefilled medication syringes decrease time to delivery and dosing error in simulated emergency department pediatric resuscitations. Ann Emerg Med. 2015;66(2):97–106.e3. doi:.https://doi.org/10.1016/j.annemergmed.2014.12.035
31
Truitt
E
,
Thompson
R
,
Blazey-Martin
D
,
NiSai
D
,
Salem
D
. NiSai D, Salem D. Effect of the implementation of barcode technology and an electronic medication administration record on adverse drug events. Hosp Pharm. 2016;51(6):474–83. doi:.https://doi.org/10.1310/hpj5106-474
32
Cochran
GL
,
Jones
KJ
,
Brockman
J
,
Skinner
A
,
Hicks
RW
. Errors prevented by and associated with bar-code medication administration systems. Jt Comm J Qual Patient Saf. 2007;33(5):293–301, 245. doi:.https://doi.org/10.1016/S1553-7250(07)33034-1
33
Giuliano
KK
. IV smart pumps: the impact of a simplified user interface on clinical use. Biomed Instrum Technol. 2015;49(s4):13–21. doi:.https://doi.org/10.2345/0899-8205-49.s4.13
34
Schneider
PJ
,
Pedersen
CA
,
Scheckelhoff
DJ
. ASHP national survey of pharmacy practice in hospital settings: Dispensing and administration-2017. Am J Health Syst Pharm. 2018;75(16):1203–26. doi:.https://doi.org/10.2146/ajhp180151
35
Franklin
BD
. ‘Smart’ intravenous pumps: how smart are they?
BMJ Qual Saf. 2017;26(2):93–4. doi:.https://doi.org/10.1136/bmjqs-2016-005302
36
Schnock
KO
,
Dykes
PC
,
Albert
J
,
Ariosto
D
,
Call
R
,
Cameron
C
, et al.
The frequency of intravenous medication administration errors related to smart infusion pumps: a multihospital observational study. BMJ Qual Saf. 2017;26(2):131–40. doi:.https://doi.org/10.1136/bmjqs-2015-004465
37
Melton
KR
,
Timmons
K
,
Walsh
KE
,
Meinzen-Derr
JK
,
Kirkendall
E
. Smart pumps improve medication safety but increase alert burden in neonatal care. BMC Med Inform Decis Mak. 2019;19(1):213. doi:.https://doi.org/10.1186/s12911-019-0945-2
38
American Academy of Pediatrics Committee on Drugs, American Academy of Pediatrics Committee on Hospital Care. Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112(2):431–6. doi:.https://doi.org/10.1542/peds.112.2.431
39
Rinke
ML
,
Bundy
DG
,
Velasquez
CA
,
Rao
S
,
Zerhouni
Y
,
Lobner
K
, et al.
Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134(2):338–60. doi:.https://doi.org/10.1542/peds.2013-3531
40
Kaufmann
J
,
Laschat
M
,
Wappler
F
. Medication errors in pediatric emergencies: a systematic analysis. Dtsch Arztebl Int. 2012;109(38):609–16. doi:.https://doi.org/10.3238/arztebl.2012.0609
41
Flannery
AH
,
Parli
SE
. Medication errors in cardiopulmonary arrest and code-related situations. Am J Crit Care. 2016;25(1):12–20. doi:.https://doi.org/10.4037/ajcc2016190
42
Gonzales
K
. Medication administration errors and the pediatric population: a systematic search of the literature. J Pediatr Nurs. 2010;25(6):555–65. doi:.https://doi.org/10.1016/j.pedn.2010.04.002
43
Kearns
GL
,
Abdel-Rahman
SM
,
Alander
SW
,
Blowey
DL
,
Leeder
JS
,
Kauffman
RE
. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67. doi:.https://doi.org/10.1056/NEJMra035092
44
Manrique-Rodríguez
S
,
Sánchez-Galindo
A
,
Fernández-Llamazares
CM
,
López-Herce
J
,
Rodríguez-Gómez
M
,
Echarri-Martínez
L
, et al.
Preparation of intravenous drug administration guidelines for a pediatric intensive care unit. J Infus Nurs. 2014;37(1):35–43. doi:.https://doi.org/10.1097/NAN.0000000000000019
45
Hoyle
JD, Jr
,
Davis
AT
,
Putman
KK
,
Trytko
JA
,
Fales
WD
. Medication dosing errors in pediatric patients treated by emergency medical services. Prehosp Emerg Care. 2012;16(1):59–66. doi:.https://doi.org/10.3109/10903127.2011.614043
46
Kämäräinen
A
. Out-of-hospital cardiac arrests in children. J Emerg Trauma Shock. 2010;3(3):273–6. doi:.https://doi.org/10.4103/0974-2700.66531
47
Porter
E
,
Barcega
B
,
Kim
TY
. Analysis of medication errors in simulated pediatric resuscitation by residents. West J Emerg Med. 2014;15(4):486–90. doi:.https://doi.org/10.5811/westjem.2014.2.17922
48
Lehmann
CU
,
Kim
GR
,
Gujral
R
,
Veltri
MA
,
Clark
JS
,
Miller
MR
. Decreasing errors in pediatric continuous intravenous infusions. Pediatr Crit Care Med. 2006;7(3):225–30. doi:.https://doi.org/10.1097/01.PCC.0000216415.12120.FF
49
Hoyle
JD, Jr
,
Crowe
RP
,
Bentley
MA
,
Beltran
G
,
Fales
W
. Pediatric prehospital medication dosing errors: a national survey of paramedics. Prehosp Emerg Care. 2017;21(2):185–91. doi:.https://doi.org/10.1080/10903127.2016.1227001
50
Cottrell
EK
,
O’Brien
K
,
Curry
M
,
Meckler
GD
,
Engle
PP
,
Jui
J
, et al.
Understanding safety in prehospital emergency medical services for children. Prehosp Emerg Care. 2014;18(3):350–8. doi:.https://doi.org/10.3109/10903127.2013.869640
51
Kaji
AH
,
Gausche-Hill
M
,
Conrad
H
,
Young
KD
,
Koenig
WJ
,
Dorsey
E
, et al.
Emergency medical services system changes reduce pediatric epinephrine dosing errors in the prehospital setting. Pediatrics. 2006;118(4):1493–500. doi:.https://doi.org/10.1542/peds.2006-0854
52
Su
E
,
Schmidt
TA
,
Mann
NC
,
Zechnich
AD
. A randomized controlled trial to assess decay in acquired knowledge among paramedics completing a pediatric resuscitation course. Acad Emerg Med. 2000;7(7):779–86. doi:.https://doi.org/10.1111/j.1553-2712.2000.tb02270.x
53
Shah
MN
,
Cushman
JT
,
Davis
CO
,
Bazarian
JJ
,
Auinger
P
,
Friedman
B
. The epidemiology of emergency medical services use by children: an analysis of the National Hospital Ambulatory Medical Care Survey. Prehosp Emerg Care. 2008;12(3):269–76. doi:.https://doi.org/10.1080/10903120802100167
54
Winburn
AS
,
Brixey
JJ
,
Langabeer
J, 2nd
,
Champagne-Langabeer
T
. A systematic review of prehospital telehealth utilization. J Telemed Telecare. 2018;24(7):473–81. doi:.https://doi.org/10.1177/1357633X17713140
55
Sibson
L
. The use of telemedicine technology to support in pre-hospital patient care. Journal of Paramedic Practice.
2014;6(7):344–53. doi:.https://doi.org/10.12968/jpar.2014.6.7.344
56
Kim
H
,
Kim
S-W
,
Park
E
,
Kim
JH
,
Chang
H
. The role of fifth-generation mobile technology in prehospital emergency care: An opportunity to support paramedics. Health Policy Technol. 2020;9(1):109–14. doi:.https://doi.org/10.1016/j.hlpt.2020.01.002
57Almadania B, Bin-Yahyaa M, Shakshukib EM. E-AMBULANCE: Real-time integration platform for heterogeneous medical telemetry system. The 5th International Conference on Current and Future Trends of Information and Communication Technologies in Healthcare (ICTH 2015) 2015.
58
Foltin
GL
,
Richmond
N
,
Treiber
M
,
Skomorowsky
A
,
Galea
S
,
Vlahov
D
, et al.
Pediatric prehospital evaluation of NYC cardiac arrest survival (PHENYCS). Pediatr Emerg Care. 2012;28(9):864–8. doi:.https://doi.org/10.1097/PEC.0b013e3182675e70
59
Fukuda
T
,
Kondo
Y
,
Hayashida
K
,
Sekiguchi
H
,
Kukita
I
. Time to epinephrine and survival after paediatric out-of-hospital cardiac arrest. Eur Heart J Cardiovasc Pharmacother. 2018;4(3):144–51. doi:.https://doi.org/10.1093/ehjcvp/pvx023
60
Hansen
M
,
Schmicker
RH
,
Newgard
CD
,
Grunau
B
,
Scheuermeyer
F
,
Cheskes
S
, et al.; Resuscitation Outcomes Consortium Investigators. Time to epinephrine administration and survival from nonshockable out-of-hospital cardiac arrest among children and adults. Circulation. 2018;137(19):2032–40. doi:.https://doi.org/10.1161/CIRCULATIONAHA.117.033067
61
Rittenberger
JC
,
Bost
JE
,
Menegazzi
JJ
. Time to give the first medication during resuscitation in out-of-hospital cardiac arrest. Resuscitation. 2006;70(2):201–6. doi:.https://doi.org/10.1016/j.resuscitation.2005.12.006
62
Matos
RI
,
Watson
RS
,
Nadkarni
VM
,
Huang
HH
,
Berg
RA
,
Meaney
PA
, et al.; American Heart Association’s Get With The Guidelines–Resuscitation (Formerly the National Registry of Cardiopulmonary Resuscitation) Investigators. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127(4):442–51. doi:.https://doi.org/10.1161/CIRCULATIONAHA.112.125625
63
Misasi
P
,
Keebler
JR
. Medication safety in emergency medical services: approaching an evidence-based method of verification to reduce errors. Ther Adv Drug Saf. 2019;10:2042098618821916. doi:.https://doi.org/10.1177/2042098618821916
64
Walker
D
,
Moloney
C
,
SueSee
B
,
Sharples
R
. Contributing factors that influence medication errors in the prehospital paramedic environment: a mixed-method systematic review protocol. BMJ Open. 2019;9(12):e034094. doi:.https://doi.org/10.1136/bmjopen-2019-034094
65
Cushman
JT
,
Fairbanks
RJ
,
O’Gara
KG
,
Crittenden
CN
,
Pennington
EC
,
Wilson
MA
, et al.
Ambulance personnel perceptions of near misses and adverse events in pediatric patients. Prehosp Emerg Care. 2010;14(4):477–84. doi:.https://doi.org/10.3109/10903127.2010.497901
66
LeBlanc
VR
,
MacDonald
RD
,
McArthur
B
,
King
K
,
Lepine
T
. Paramedic performance in calculating drug dosages following stressful scenarios in a human patient simulator. Prehosp Emerg Care. 2005;9(4):439–44. doi:.https://doi.org/10.1080/10903120500255255
67
Lammers
R
,
Byrwa
M
,
Fales
W
. Root causes of errors in a simulated prehospital pediatric emergency. Acad Emerg Med. 2012;19(1):37–47. doi:.https://doi.org/10.1111/j.1553-2712.2011.01252.x
68
Wells
M
,
Goldstein
LN
,
Bentley
A
,
Basnett
S
,
Monteith
I
. The accuracy of the Broselow tape as a weight estimation tool and a drug-dosing guide - A systematic review and meta-analysis. Resuscitation. 2017;121:9–33. doi:.https://doi.org/10.1016/j.resuscitation.2017.09.026
69
Clifford
GD
. E-health in low to middle income countries. J Med Eng Technol. 2016;40(7-8):336–41. doi:.https://doi.org/10.1080/03091902.2016.1256081
70
Ochalek
J
,
Lomas
J
,
Claxton
K
. Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data. BMJ Glob Health. 2018;3(6):e000964. doi:.https://doi.org/10.1136/bmjgh-2018-000964
71World Telecommunication ITU. ITU Statistics. Individuals using the Internet, by level of development. 2020 [2020.04.04]. Available from: https://www.itu.int/en/ITU-D/Statistics/Pages/stat/default.aspx.
72
Wallis
L
,
Blessing
P
,
Dalwai
M
,
Shin
SD
. Integrating mHealth at point of care in low- and middle-income settings: the system perspective. Glob Health Action. 2017;10(sup3, suppl_3):1327686. doi:.https://doi.org/10.1080/16549716.2017.1327686
73
Bezuidenhout
L
,
Chakauya
E
. Hidden concerns of sharing research data by low/middle-income country scientists. Glob Bioet. 2018;29(1):39–54. doi:.https://doi.org/10.1080/11287462.2018.1441780
74
Langer
A
,
Díaz-Olavarrieta
C
,
Berdichevsky
K
,
Villar
J
. Why is research from developing countries underrepresented in international health literature, and what can be done about it?
Bull World Health Organ. 2004;82(10):802–3.
75
Acheampong
F
,
Anto
BP
,
Koffuor
GA
. Medication safety strategies in hospitals--a systematic review. Int J Risk Saf Med. 2014;26(3):117–31. doi:.https://doi.org/10.3233/JRS-140623
76
Berdot
S
,
Roudot
M
,
Schramm
C
,
Katsahian
S
,
Durieux
P
,
Sabatier
B
. Interventions to reduce nurses’ medication administration errors in inpatient settings: A systematic review and meta-analysis. Int J Nurs Stud. 2016;53:342–50. doi:.https://doi.org/10.1016/j.ijnurstu.2015.08.012
77
Luten
R
,
Wears
RL
,
Broselow
J
,
Croskerry
P
,
Joseph
MM
,
Frush
K
. Managing the unique size-related issues of pediatric resuscitation: reducing cognitive load with resuscitation aids. Acad Emerg Med. 2002;9(8):840–7. doi:.https://doi.org/10.1197/aemj.9.8.840
78
Metelmann
B
,
Metelmann
C
,
Schuffert
L
,
Hahnenkamp
K
,
Brinkrolf
P
. Medical correctness and user friendliness of available apps for cardiopulmonary resuscitation: systematic search combined with guideline adherence and usability evaluation. JMIR Mhealth Uhealth. 2018;6(11):e190. doi:.https://doi.org/10.2196/mhealth.9651
79
Lauridsen
KG
,
Nadkarni
VM
,
Berg
RA
. Man and machine: can apps resuscitate medical performance?
Lancet Child Adolesc Health. 2019;3(5):282–3. doi:.https://doi.org/10.1016/S2352-4642(19)30032-X
80
Baumann
D
,
Dibbern
N
,
Sehner
S
,
Zöllner
C
,
Reip
W
,
Kubitz
JC
. Validation of a mobile app for reducing errors of administration of medications in an emergency. J Clin Monit Comput. 2019;33(3):531–9. doi:.https://doi.org/10.1007/s10877-018-0187-3
81
Segal
JB
,
Arevalo
JB
,
Franke
MF
,
Palazuelos
D
. Reducing dosing errors and increasing clinical efficiency in Guatemala: first report of a novel mHealth medication dosing app in a developing country. BMJ Innov. 2015;1(3):111–6. doi:.https://doi.org/10.1136/bmjinnov-2015-000051
82
Cummings
P
. Carryover bias in crossover trials. Arch Pediatr Adolesc Med. 2010;164(8):703–5. doi:.https://doi.org/10.1001/archpediatrics.2010.126
83
Hagberg
H
,
Siebert
J
,
Gervaix
A
,
Daehne
P
,
Lovis
C
,
Manzano
S
, et al.
Improving drugs administration safety in pediatric resuscitation using mobile technology. Stud Health Technol Inform. 2016;225:656–7.
84
Siebert
JN
,
Ehrler
F
,
Combescure
C
,
Lovis
C
,
Haddad
K
,
Hugon
F
, et al.; PedAMINES Trial Group. A mobile device application to reduce medication errors and time to drug delivery during simulated paediatric cardiopulmonary resuscitation: a multicentre, randomised, controlled, crossover trial. Lancet Child Adolesc Health. 2019;3(5):303–11. doi:.https://doi.org/10.1016/S2352-4642(19)30003-3
85
Cheng
A
,
Kessler
D
,
Mackinnon
R
,
Chang
TP
,
Nadkarni
VM
,
Hunt
EA
, et al.; International Network for Simulation-based Pediatric Innovation, Research, and Education (INSPIRE) Reporting Guidelines Investigators. Reporting guidelines for health care simulation research: extensions to the CONSORT and STROBE statements. Simul Healthc. 2016;11(4):238–48. doi:.https://doi.org/10.1097/SIH.0000000000000150
86
Siebert
JN
,
Ehrler
F
,
Combescure
C
,
Lacroix
L
,
Haddad
K
,
Sanchez
O
, et al.
A mobile device app to reduce time to drug delivery and medication errors during simulated pediatric cardiopulmonary resuscitation: a randomized controlled trial. J Med Internet Res. 2017;19(2):e31. doi:.https://doi.org/10.2196/jmir.7005
87
Cheng
A
,
Auerbach
M
,
Hunt
EA
,
Chang
TP
,
Pusic
M
,
Nadkarni
V
, et al.
Designing and conducting simulation-based research. Pediatrics. 2014;133(6):1091–101. doi:.https://doi.org/10.1542/peds.2013-3267
88
Siebert
JN
,
Bloudeau
L
,
Ehrler
F
,
Combescure
C
,
Haddad
K
,
Hugon
F
, et al.
A mobile device app to reduce prehospital medication errors and time to drug preparation and delivery by emergency medical services during simulated pediatric cardiopulmonary resuscitation: study protocol of a multicenter, prospective, randomized controlled trial. Trials. 2019;20(1):634. doi:.https://doi.org/10.1186/s13063-019-3726-4



