Mortality prediction in acute heart failure: scores or biomarkers?
DOI: https://doi.org/10.4414/smw.2020.20320
Desiree
Wusslerab, Eleni
Michoua, Maria
Belkina, Nikola
Kozhuharovac, Matthias
Dieboldac, Danielle M.
Gualandroad, Tobias
Breidthardtab, Christian
Muellera
aCardiovascular Research Institute Basel (CRIB) and Department of Cardiology, University Hospital Basel,
bDepartment of Internal Medicine, University Hospital Basel,
c
dHeart Institute (InCor),
Mortality prediction in acute heart failure: scores or biomarkers?
Summary
Acute heart failure (AHF) is a complex and heterogeneous syndrome not only associated with a concerning rise in incidence, but also with still unacceptably high rates of mortality and morbidity. As this dismal outcome is at least in part due to a mismatch between the severity of AHF and the intensity of its management, both in-hospital and immediately after discharge, early and accurate risk prediction could contribute to more effective, risk-adjusted management.
Biomarkers are noninvasive and highly reproducible quantitative tools that have improved the understanding of AHF pathophysiology. They can help guide the intensity of AHF management. In addition, using a statistical model to estimate risk from a combination of several predictor variables such as vital signs or demographics has gained more and more attention over recent years. In this context, the aim of a statistical model, which gives a so-called risk score, is to help clinicians to make more standardised decisions.
This review highlights recent advances and remaining uncertainties regarding risk stratification in AHF by characterising and comparing the potential of biomarkers and risk scores.
Abbreviations:
- ADHERE
-
Acute Decompensated Heart Failure National Registry
- AHF
-
acute heart failure
- AHFI
-
Acute Heart Failure Index
- ANP
-
atrial natriuretic peptide
- BNP
-
B-type natriuretic peptide
- CI
-
confidence interval
- cTn
-
cardiac troponin
- EHMRG
-
Emergency Heart Failure Mortality Risk Grade
- ELAN
-
European coLlaboration on Acute decompeNsated heart failure
- GDF-15
-
growth differentiation factor 15
- GWTG-HF
-
Get With the Guidelines Heart Failure
- HR
-
hazard ratio
- MEESSI-AHF
-
Multiple Estimation of risk based on the emergency department Spanish Score In patients with AHF
- MR-proADM
-
mid-regional pro-adrenomedullin
- MR-proANP
-
mid-regional pro-atrial natriuretic peptide
- NT-proBNP
-
N-terminal pro-B-type natriuretic peptide
- OHFRS
-
Ottawa Heart Failure Risk Scale
- OR
-
odds ratio
- sST2
-
soluble suppression of tumourigenicity 2
Introduction
Acute heart failure (AHF) is a heterogeneous syndrome with a concerning rise in incidence [1, 2]. Equally, AHF is a growing social and economic burden in terms of its unacceptably high mortality rates and healthcare costs [3]. The dismal outcome of patients with AHF is at least in part due to a mismatch between risk assessment and the intensity of AHF management, both in-hospital and immediately after discharge [4–6]. Early and accurate risk prediction using clinical risk scores or biomarkers could contribute to more precise, risk-adjusted management [7, 8] (fig. 1).
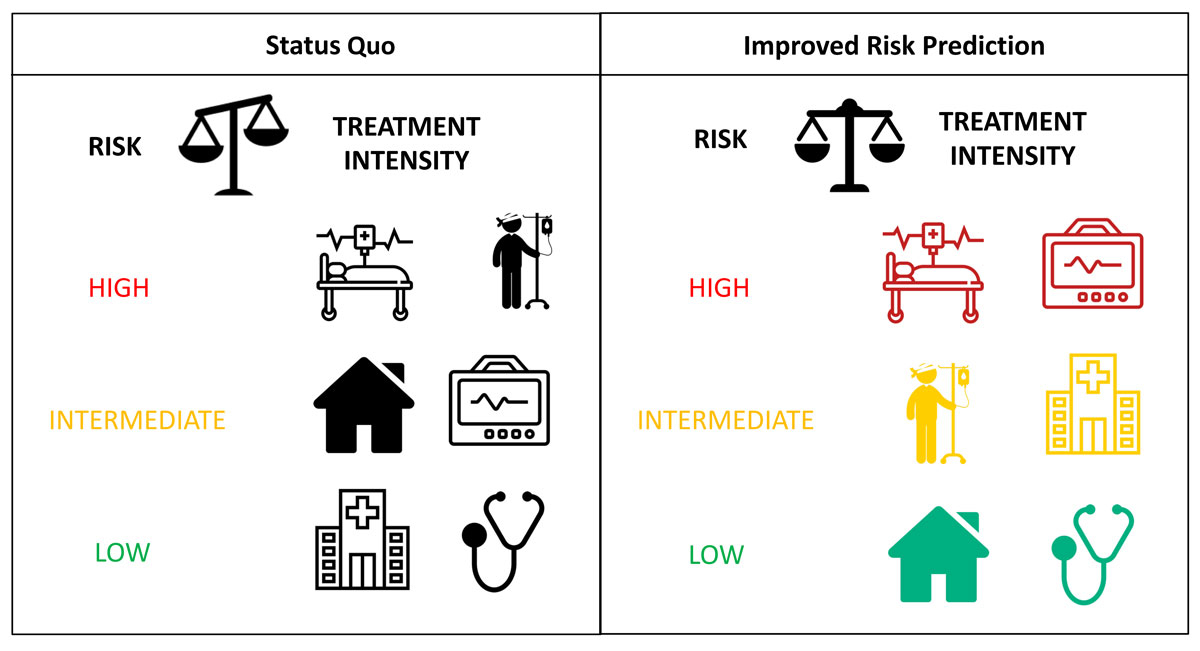
Figure 1
Status quo shows the current mismatch between risk categories and the intensity of management both in-hospital and immediately after discharge. For example, a recent study showed that the majority of patients classified as low-risk using a heart failure risk score were hospitalised despite the fact that adjusted ambulatory follow-up might have been the more accurate approach [8]. Improved risk prediction using clinical risk scores or biomarkers could contribute to more effective, risk-adjusted management. Therefore, high-risk patients could be transferred to an intensive care unit with continuous monitoring (marked in red), intermediate-risk patients could be hospitalised or temporarily treated in an intermediate care unit (marked in yellow), whereas low-risk patients could be safely discharged home with an adjusted ambulatory follow-up (marked in green).
Biomarkers quantify the pathophysiological processes central to a disease/syndrome and thereby provide a diagnostic and prognostic window [9–11]. Among the multitude of biomarkers evaluated in patients with AHF, natriuretic peptides (B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP)), used as quantitative markers of haemodynamic stress and heart failure, are by far the most important. Their use has been extensively validated in large diagnostic and prognostic studies and is consistently given a class I recommendation for AHF diagnosis in current guidelines [12, 13]. In addition, BNP and NT-proBNP concentrations measured at hospital discharge help to predict mortality and AHF rehospitalisation [14–16].
However, a single biomarker approach mostly quantifies one pathophysiologic pathway, and therefore risk stratification and adverse outcome prediction in AHF, a heterogeneous syndrome with various phenotypes, is often limited [17, 18]. Accordingly, the estimation of risk from a combination of several predictors using a statistical model could improve accuracy [19–21]. Therefore, several prognostic models, so-called risk scores, incorporating clinical parameters such as vital signs, demographics, and often biomarkers, have been developed [7, 22–24].
This review highlights recent advances and remaining uncertainties regarding risk stratification in AHF by characterising and comparing the potential of biomarkers and risk scores.
Mortality prediction in AHF: biomarkers
What makes a biomarker useful?
Given the vast number of emerging biomarkers reported to be associated with mortality in AHF, five criteria may help put data on biomarkers into clinical perspective and aid assessment of their potential to ultimately improve patient management [18, 25–27]:
- Pre-analytical and analytical aspects
- Often, pilot studies use dedicated blood sample preparation protocols that cannot be easily implemented into routine clinical practice, e.g. putting samples on ice immediately after blood draw or sample processing within minutes after blood draw [28]. This may be necessary for very unstable analytes, but would clearly reduce their utility in routine clinical practice. In addition, reasonable analytical precision needs to be demonstrated to ensure reproducibility of results. Turn-around time and the cost of measurement should also be reasonable.
- Accuracy
- Biomarker concentration should provide high accuracy for prognosis. Some biomarkers have only modest accuracy as single variables but may provide some additional value in incomplete multivariable models [29]. As these models only rarely reflect integrated clinical judgment, physicians should remain critical whenever the new biomarker itself lacks high accuracy.
- Incremental value
- In order to justify its possible application in routine clinical practice, a novel biomarker should add new and clinically useful information rather than restating information already available at the bedside or via the use of inexpensive, routinely available biomarkers. For instance, none of the thoroughly investigated renal biomarkers were able to convincingly demonstrate incremental value on top of old-fashioned creatinine for the early detection or prediction of acute kidney injury [30–32].
- Understanding pathophysiology
- The organ and/or cells contributing predominately to systemic concentrations of many biomarkers are incompletely understood, e.g., for sST2 or GDF-15. This uncertainty makes it much less likely that a specific therapeutic intervention can be identified. It also raises the possibility that extra-cardiac production is dominant even in AHF [33, 34].
- Guiding therapy (theragnostics)
- In order to plan clinical management, it is important to identify patients at very high risk. Ultimately, this information can only be used to alter outcomes if the very high risk is amendable by a change in management, e.g. more intense non-invasive monitoring while in hospital, more intense follow-up, or a specific modification of therapy. For instance, cardiac troponin concentrations are closely associated with mortality in AHF, but only when substantially elevated do they trigger a change in management, such as early coronary angiography when AMI is suspected as the AHF trigger.
Biomarkers involved in heart failure pathophysiology
Most biomarkers in current clinical evaluation can be assigned to one of the pathophysiologic pathways involved in the development and progression of heart failure [35] (fig. 2). The involvement of several pathophysiological pathways makes heart failure a complex, multiorgan disease with possible multiorgan failure due to proinflammatory activation, oxidative stress, ongoing ischaemia or unresolved congestion [36].
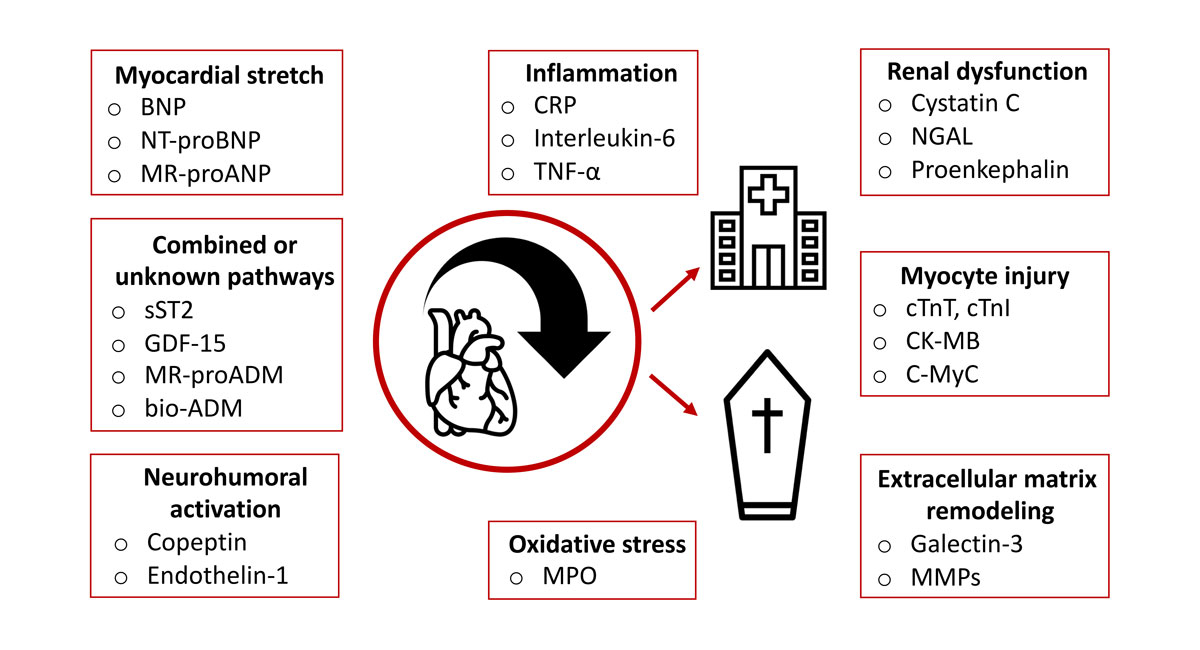
Figure 2 Overview of the pathophysiological pathways involved in heart failure and the corresponding biomarkers with prognostic relevance.
bio-ADM = bioactive adrenomedullin; BNP = B-type natriuretic peptide; CK-MB = creatine kinase myocardial band; C-MyC = cardiac myosin-binding protein C; CRP = C-reactive protein; cTnI = cardiac troponin I; cTnT = cardiac troponin T; GDF-15 = growth differentiation factor 15; MMP = matrix metalloproteinase; MR-proADM = mid-regional pro-adrenomedullin; MPO = myeloperoxidase; MR-proANP = mid-regional pro-atrial natriuretic peptide; NGAL = neutrophil gelatinase-associated lipocalin; NT-proBNP = N-terminal pro-B-type natriuretic peptide; sST2 = soluble suppression of tumourigenicity 2; TNF-α = tumour necrosis factor alpha.
Haemodynamic stress and myocardial stretch
Natriuretic peptides
In response to volume or pressure overload and the resulting myocardial wall stress, the biologically inert NT-proBNP and biologically active BNP are enzymatically cleaved from their precursor proBNP [37]. Since their clinical introduction as markers of myocardial wall stress and HF, the rapid detection of AHF among patients presenting with acute dyspnoea has improved substantially [38–42]. Accordingly, the diagnostic use of natriuretic peptides has received a class I recommendation in both the European and American practice guidelines [12, 43].
Besides their diagnostic value, BNP and NT-proBNP levels at hospital presentation are predictors of short- and long-term outcomes [14–16, 44–48]. For instance, in 48,629 AHF patients, BNP quartile was predictive of in-hospital mortality even after adjustment for common covariates [14]. However, the overall prognostic accuracy of BNP and NT-proBNP concentrations measured at presentation with AHF is only modest. When directly comparing pre-discharge values or the percentage change during hospitalisation with the admission values of natriuretic peptides, several studies have shown higher prognostic accuracy for measurements obtained during the course of hospitalisation [15, 49–53].
Both BNP and NT-proBNP concentrations are responsive to therapy during heart failure hospitalisation [15, 49–53]. Furthermore, the extent of decrease in plasma level is a strong predictor of outcome. For instance, in 171 AHF patients, treatment reduced BNP and NT-proBNP levels by more than 50% in survivors (p <0.001), while in non-survivors treatment did not significantly impact natriuretic peptide levels [53]. However, since the introduction of sacubitril/valsartan, this concept must be reconsidered. By inhibiting neprilysin, sacubitril inhibits the degradation of BNP, thereby increasing its concentration in blood and to some extent uncoupling its decrease from successful therapy [54, 55]. In contrast, NT-proBNP concentration is still closely correlated with subjective and objective measures of clinical improvement in patients on sacubitril/valsartan [54, 56].
Natriuretic peptides other than BNP or NT-proBNP
Like the B-type natriuretic peptides, atrial natriuretic peptide (ANP) is released into circulation as a consequence of increased myocardial wall stress. However, due to its very short half-life of 2–5 minutes, only the measurement of its more stable precursor protein proANP makes it available for AHF diagnosis and prognosis in clinical practice [57]. In three large diagnostic studies, mid-regional pro-atrial natriuretic peptide (MR-proANP) showed comparable diagnostic and prognostic accuracy to NT-proBNP [58–60]. Although ANP, and therefore MR-proANP, are considered to be released predominately by the atria, which is hypothesised to provide an additional window to the heart to BNP and NT-proBNP, which are considered to be secreted mainly by the ventricles [61], combined use of MR-proANP with BNP or NT-proBNP has not yet been found to provide clinically relevant incremental diagnostic or prognostic value [58–60]. A reason might be that left atrial stress and right ventricular/right atrial stress may also play a role in stimulating BNP and NT-proBNP release. This was suggested in a recent study in patients with aortic stenosis, as the correlation between BNP and pulmonary capillary wedge pressure was stronger than that between BNP and left ventricular end-diastolic pressure [62].
Combined/unknown pathways
Soluble suppression of tumourgenicity 2 (sST2)
sST2 is one isoform of ST2, a protein which is part of the interleukin-1 receptor family and is released under conditions of myocardial and vascular strain [63]. sST2 concentrations have moderate prognostic accuracy in both acute and chronic heart failure and seem to provide incremental value in some multivariable models [29, 64–68]. Early changes in sST2 may also provide prognostic value. For instance, one study showed that non-survivors had higher sST2 admission levels (median 108 vs 69 ng/ml, p <0.01) and also a lower decrease in sST2 (25% vs 42%, p = 0.01) during in-hospital treatment compared to survivors [66]. Additionally, sST2 concentrations appear to be responsive to therapy during HF hospitalisation [69, 70]. One of the main caveats regarding the use of sST2 is that its systemic concentrations seem to be the result of extra-cardiac production, with the predominant organ and/or cells yet to be identified [34]. This is in contrast to natriuretic peptides, whose cardiac production has been proven by studies measuring their trans-cardiac gradient [71, 72].
Growth differentiation factor 15 (GDF-15)
Similar pros and cons apply to GDF-15. The physiological expression of GDF-15 is mainly limited to the placenta and the prostate. However, the expression of GDF-15 in cardiomyocytes can be rapidly induced by inflammation or triggers of oxidative stress, including pressure overload in AHF [73]. Like sST2, GDF-15 concentrations are mainly the result of extra-cardiac production, with the predominant organ and/or cells yet to be identified [33]. Nevertheless, GDF-15 concentrations are strongly associated with death in multiple conditions, including AHF [74–76]. GDF-15 concentration was not modified by sacubitril/valsartan, whereas serelaxin administration was associated with greater decreases in GDF-15 compared to a placebo [75, 76].
Mid-regional pro-adrenomedullin (MR-proADM) and bioactive adrenomedullin (bio-ADM)
Adrenomedullin, a potent vasodilator with inotropic and natriuretic properties, is expressed in several tissues, such as the adrenal medulla, lungs and kidneys. Several studies have also shown a cardiac expression stimulated by both increased intracardiac filling pressure and volume overload [77, 78]. Adrenomedullin plasma levels are elevated in patients with chronic HF and correlate with disease severity [28, 77]. However, the clinical applicability of this biomarker is limited by the instability of the adrenomedullin plasma measurement. Therefore, MR-proADM, a more stable fragment from the same precursor as adrenomedullin, has emerged as a novel prognostic biomarker in AHF [58]. In addition, an assay exclusively measuring the biologically active amidated adrenomedullin (bio-ADM) is currently undergoing clinical evaluation [79].
Myocyte Injury
Cardiac troponin (cTn)
Most patients with AHF have cTn concentrations above the normal upper limit with a rise and/or fall even if AHF is not triggered by myocardial infarction. In the absence of evidence suggesting type 1 myocardial infarction, myocardial injury in patients with AHF [80] may have several different underlying pathophysiological processes, including cardiomyocyte apoptosis due to wall stress or toxicity related to inflammation [81]. The higher the cTn concentration in AHF, the higher the in-hospital and the long-term mortality, and the longer the length of stay [82–85]. Serial cTn measurements may allow monitoring of the response to therapy, and thereby provide additive information [85]. For instance, sacubitril/valsartan led to a 16% greater reduction in high-sensitivity cTnT concentration compared to enalapril after four weeks (p <0.001) [70].
Extracellular matrix remodelling
Galectin-3
Galectin‐3 belongs to a family of soluble lectins which play an important role in several pathophysiological processes such as inflammation and fibrosis. As these mechanisms are involved in the development of HF, a role of galectin-3 in HF pathophysiology has been suggested [86, 87]. In this context, recent studies have shown that galectin-3 is predictive of short-term mortality and rehospitalisation in patients presenting with AHF [88, 89]. However, two caveats apply to galactin-3. First, like sST2 and GDF-15, systemic galactin-3 concentrations in HF seem to depend mainly on extra-cardiac production [33]. Second, galactin-3 concentrations show a strong correlation with renal dysfunction. Therefore, at least some of their prognostic information may be purely a reflection of renal dysfunction [90].
Oxidative stress
Myeloperoxidase
Myeloperoxidase, a biomarker of inflammation and oxidative stress, is released from activated neutrophils, monocytes and from endothelial cells [91, 92]. Neutrophil activation and inflammation are associated with the development and progression of HF. Accordingly, myeloperoxidase levels are associated with increased mortality in AHF [93]. However, as oxidative stress is a rather unspecific element in AHF pathophysiology, the value of myeloperoxidase for AHF risk prediction appears comparably small.
Inflammation
C-reactive protein (CRP) and interleukin-6
Infectious diseases such as pneumonia are important and common precipitating factors in patients with AHF. However, even in the absence of an infection, mild increases in markers of systemic inflammation such as CRP or interleukin-6 are common in AHF [94]. In this context, it has been hypothesised that mesenteric arterial hypoperfusion combined with increased mesenteric venous pressure results in bacterial translocation from the bowel to the bloodstream. Therefore, heart failure severity correlates with the extent of inflammation [95, 96].
CRP is a member of the pentraxin family and is mainly produced in the liver in response to systemic inflammation processes. Several studies have documented increased mortality in AHF patients with continuously increasing CRP concentrations [97–102]. This association persists after adjusting for common covariates, including BNP [102]. Similar observations have been made for interleukin-6: this cytokine has independently predicted all-cause mortality in several studies [103–105]. In conclusion, markers of inflammation are rather unspecific and therefore seem to be less useful as single marker strategies in AHF risk prediction. However, their combination with established cardiac biomarkers such as natriuretic peptides may provide incremental value.
Mortality prediction in AHF: risk scores
What makes a risk score useful in HF?
Given the vast number of emerging AHF risk scores, eight criteria may help put data on AHF risk scores into clinical perspective and aid assessment of their potential to ultimately improve patient management [19–21]:
- Reliable clinical models should be developed using a large and high-quality dataset, possibly even one obtained from a multicentre study.
- The model should be based on a sound statistical analysis plan. In this context, it is important to avoid statistical overfitting, particularly when multiple candidate predictor variables are used in a relatively small dataset.
- A newly developed model must always be externally validated before its introduction to clinical practice can be considered. External validation using a dataset from a geographically different cohort is always preferred compared to a so-called time-dependent internal validation, which uses data from the same trial but obtained at a later time point as the derivation cohort.
- Ideally, a score should be developed in the setting in which it will be clinically applied. As most AHF patients present to the emergency department for initial treatment [106], the derivation cohort for an AHF risk score should be recruited from the emergency department in order to provide early and accurate risk stratification.
- Variables should be rapidly and routinely available and should assess disease severity as objectively as possible, rather than depend on the subjective judgment of a physician, to avoid interobserver variation.
- Direct comparison of a new score with an established score in the same dataset is recommended to document prognostic equivalence or even superiority.
- The accuracy of a risk score should be assessed by goodness of fit tests in the sense of near-optimal discrimination and calibration [107]. Furthermore, impact studies provide additional insights into a score´s impact on actual clinical decision-making and healthcare costs.
- Geographical differences in AHF outcomes and the potential prognostic impacts of novel therapeutic agents such as sacubitril/valsartan mean that the performance of a risk score may vary depending on geographical region, and possibly also change over time [54, 108, 109]. Therefore, it should be feasible to update existing models by adjusting the intercept or adding novel predictors such as biomarkers [110].
Table 1 gives an overview of selected risk scores and their characteristics. Those scores that were derived in emergency department populations are highlighted. The depicted scores were chosen in order to assess the development of AHF risk scores over time and to compare different characteristics such as data source, sample size and predictor variables. Figure 3 directly compares the predictor variables of selected risk scores regarding their availability in the emergency department.
Table 1 Summary of selected risk scores and their characteristics. Scores derived in ED populations are in bold type.
|
Risk score acronym
|
Data source and acquisition
|
Number of hospitals
(country)
|
Sample size
(derivation/ validation)
|
Model development
|
Data collection
|
No. variables included
|
Assessed risk
|
c-statistic
(derivation/ validation)
|
External validation
|
| Scores not including biomarkers as predictor variables |
| EFFECT (2003) [111] |
Administrative data
(retrospective) |
34 (derivation) 14 (validation)
(Canada) |
2624/1407 |
Logistic regression |
Hospitalised patients |
10/11†
|
30-day mortality
1-year mortality |
0.80/0.79 (30-day mortality)
0.77/0.76 (1-year mortality) |
No |
| ADHERE (2005) [112] |
Administrative data
(retrospective) |
263
(United States) |
33,046/32,229 |
Classification and regression tree |
Hospitalised patients |
3 |
In-hospital mortality |
0.69/0.67 |
No |
| AHFI (2005) [113] |
Administrative data
(retrospective) |
Pennsylvanian acute care centres
(United States) |
33,533/8384 |
Classification and regression tree |
Hospitalised patients |
21 |
In-hospital mortality and/or serious complications in survivors‡
|
Not given |
Prospective validation in 259 patients in another US-American State |
| GWTG-HF (2010) [114] |
Administrative data
(retrospective) |
198
(United States) |
27,850/11,933 |
Logistic regression |
Hospitalised patients |
7 |
In-hospital mortality |
0.75/0.75 |
No |
| Scores including biomarkers as variables |
|
EHMRG (2012) [
22
]
|
Administrative data
(retrospective)
|
86
(Canada)
|
7433/5158
|
Logistic regression
|
ED patients
|
10
|
7-day mortality
|
0.806/0.804
|
Administrative data from 6,708 patients in another Canadian state
Two prospective cohort studies (Spain n = 2137; Switzerland n = 849)§
|
|
OHFRS (2013) [
23
]
|
Observational cohort study
(prospective)
|
6
(Canada)
|
559/*
|
Logistic regression
|
ED patients
|
10
|
Combined 14-/30-day endpoint¶
|
0.77/0.77*
|
Clinical validation in 1,100 patients recruited in 6 Canadian EDs
|
| ADHF/NT-proBNP (2013) [115] |
Administrative data
(retrospective) |
1 (derivation)
2 (validation)
(Italy) |
453/371 |
Logistic regression |
Hospitalised patients |
8 |
1-year mortality |
0.839/0.768 |
No |
|
ELAN-HF (2014) [
116
]
|
Pooled data of 7 cohort studies
(prospective)
|
7
(Europe)
|
1,301/*
|
Cox regression
|
Hospitalised and ED patients
|
8
|
180-day mortality
|
0.76/–
|
Randomised trial of 325 patients in Italy
|
|
STRATIFY (2015) [
117
]
|
Observational cohort study
(prospective)
|
4
(United States)
|
1033/*
|
Logistic regression
|
ED patients
|
13
|
Combined 30-day endpoint‖
|
0.68/–
|
No
|
|
MEESSI-AHF (2017) [
7
]
|
Observational cohort study
(prospective)
|
34
(Spain)
|
4867/3229
|
Logistic regression
|
ED patients
|
13 or less
|
30-day mortality
|
0.836/0.828
|
Prospective cohort study including 1572 patients in Switzerland
|
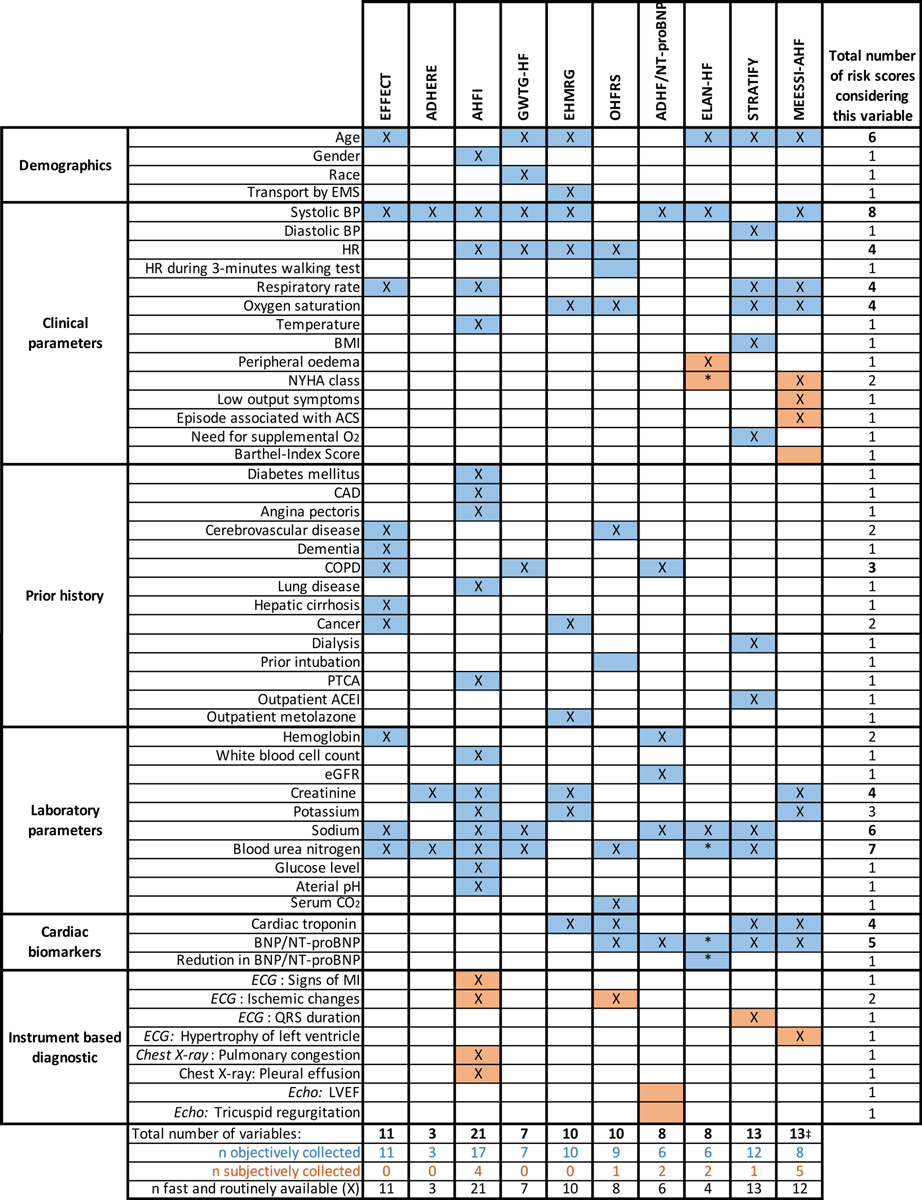
Figure 3 Overview of predictor variables included in selected risk scores.
* Marked parameters of ELAN-HF were collected at hospital discharge.
‡ The MEESSI-AHF score is also calculable with fewer than 13 variables
Full names of risk scores: ADHERE = Acute Decompensated Heart Failure National Registry; ADHF/NT-proBNP = Acute Decompensated Heart Failure/N-terminal pro-B-type natriuretic peptide; AHFI = Acute Heart Failure Index; EFFECT = Enhanced Feedback For Effective Cardiac Treatment; EHMRG = Emergency Heart Failure Mortality Risk Grade; ELAN-HF = European coLlaboration on Acute decompeNsated Heart Failure; GWTG-HF = Get With the Guidelines Heart Failure; MEESSI-AHF = Multiple Estimation of risk based on the Emergency department Spanish Score In patients with AHF; OHFRS = Ottawa Heart Failure Risk Scale; STRATIFY = Improving Heart Failure Risk Stratification in the ED
ACEI = angiotensin converting-enzyme inhibitor; ACS = acute coronary syndrome; BMI = body mass index; BP = blood pressure; BNP = B-type natriuretic peptide; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; ECG = electrocardiogram. ED = emergency department; eGFR = estimated glomerular filtration rate; EMS = emergency medical service; HR = heart rate; LVEF = left ventricular ejection fraction = NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; PTCA = percutaneous transluminal coronary angioplasty
Risk scores without cardiac biomarkers as predictor variables
The Acute Decompensated Heart Failure National Registry (ADHERE)
The ADHERE decision tree consists of only three variables and was developed for predicting in-hospital mortality in AHF patients (table 1, fig. 3). Interestingly, the score incorporates serum creatinine and blood urea nitrogen as parameters, both of which reflect renal function. The underlying explanation could be that blood urea nitrogen is considered a marker of renal neurohumoral activation [118].
The c-statistic of the decision tree was 0.69 in the derivation cohort and 0.67 in the time-dependent internal validation cohort [112]. This discrimination is comparable with the performance of similar simple scores developed from smaller datasets [119]. Although the ADHERE decision tree cannot compete with other risk scores in terms of data source and acquisition, as it was derived from retrospectively analysed data from hospitalised AHF patients, the main advantage of this decision tool is its simple application, due to the use of only three objectively collectable and routinely available variables.
Acute Heart Failure Index (AHFI)
The AHFI was developed in a large, retrospective cohort study which constructed decision trees to identify patients at low risk of in-hospital mortality and serious medical complications [113]. The final prediction rule uses 21 variables, including demographics, clinical parameters, history variables and laboratory parameters. It identified 5758 (17.2%) patients as low-risk, of whom 1.4% experienced the combined primary outcome defined as in-hospital death or a predefined serious complication (table 1, fig. 3). A time-dependent internal validation and external validation in a single centre study showed slightly higher event rates in patients classified as low-risk: (1.7% and 2.5% vs 1.4%) [120, 121]. Like the ADHERE decision tool, the AHFI was derived retrospectively from administrative data, and furthermore, no measures of goodness of fit are given. However, its major disadvantage seems to be difficult handling due to a multitude of predictor variables.
Get With the Guidelines Heart Failure (GWTG-HF) risk score
The GWTG-HF risk score was derived from administrative data from hospitalised AHF patients for the prediction of in-hospital mortality [114]. Seven objective predictor variables routinely collectable at the time of admission were identified, and the constructed risk score showed an acceptable discrimination (c-statistic, 0.75) and calibration (Hosmer-Lemeshow = 0.242) when tested in the overall sample (table 1, fig. 3).
Even though the GWTGF-HF score, like the AHFI and the ADHERE decision tool, was derived from a large study population, data were again collected retrospectively from hospitalised patients. As an AHF risk score should be developed in the setting where it will be applied, data collection from the emergency department would be preferable.
Risk scores including cardiac biomarkers as predictor variables
Emergency Heart Failure Mortality Risk Grade (EHMRG)
The EHMRG was derived from administrative data obtained from patients presenting with AHF to Canadian emergency departments [22]. A logistic regression model for seven-day mortality was constructed to identify 10 predictor variables which are objectively collectable and readily available at emergency department presentation. The EHMRG consists of vital signs, clinical features and laboratory parameters, including cTn as a cardiac biomarker (table 1, fig. 3). Goodness of fit tests show an excellent discrimination of the EHMRG in both the derivation (c-statistic, 0.806) and internal validation cohorts (c-statistic, 0.804).
Recently the EHMRG was externally validated and refined using administrative data obtained in another Canadian state. External validation showed an acceptable discrimination for seven-day mortality of the EHMRG score (c-statistic, 0.732) and its variables (c-statistic, 0.747). However, calibration of the prediction rule in the external validation cohort was not reported [122]. Interestingly, the authors assessed several scenarios to improve the model´s discrimination by introducing new variables and removing existing ones. The addition of natriuretic peptides improved the performance of the original prediction rule, as indicated by a significantly higher discrimination (c-statistic, EHMRG variables 0.747 vs 0.760; p = 0.007) and a net reclassification index of 0.268 (p <0.0001) for predicting seven-day mortality. In addition, the removal of cTn resulted in an impaired performance compared to the original EHMRG variables (c-statistic decrease from 0.747 to 0.740, p = 0.025; net reclassification index −0.269, p <0.0001). Furthermore, the EHMRG showed an acceptable discrimination when externally validated for the prediction of 30-day mortality in Spain (c-statistic, 0.741) [7] and Switzerland (c-statistic, 0.765) [8]. Strengths of the EHMRG include development in emergency department patients, use of routinely available variables and an external validation. Unfortunately, palliative patients are excluded from the EHMRG and data were not prospectively recorded.
Ottawa Heart Failure Risk Scale (OHFRS)
The OHFRS was developed in a small, prospective multicentre study including AHF patients presenting to the emergency department [23]. A logistic regression analysis was used to predict a composite endpoint including 30-day all-cause mortality and other adverse events such as admission to a critical care unit within 14 days of the index emergency department presentation. The 10 predictor variables included in the final risk scale were history variables, vital signs on admission and biomarkers such as cTn and NT-proBNP (table 1, fig. 3). Goodness of fit tests showed an acceptable discrimination (c-statistic, 0.77) and calibration (Hosmer-Lemeshow p = 0.75). Clinical validation was performed to explore the potential impact of the OHFRS on clinical practice [123]. This analysis showed that compared to clinical practice, using an admission threshold of an OHFRS >1 would have increased sensitivity for adverse event prediction in AHF patients (71.8% vs 91.8%) but would also have increased admission rates (57.2% vs 77.6%).
Despite development in the emergency department, an acceptable discrimination and a clinical validation, the OHFRS is limited by some disadvantages. In comparison to other described AHF risk scores, a uniform application in the emergency department seems unlikely due to many exclusion criteria (e.g., oxygen saturation at admission <85%, exclusion of inhabitants of a nursing home) and use of a three-minute walking test as a predictor variable, even though only few patients are physically capable of completing this task during an episode of AHF.
European coLlaboration on Acute decompeNsated Heart Failure (ELAN) score
The ELAN-HF score was designed for 180-day discharge prognostication in patients admitted for AHF. It used data assembled from prospective European cohort studies [116]. Unlike the other HF risk scores, the ELAN score includes predictor variables collected either at presentation or discharge (table 1, fig. 3). Therefore, it is not intended to provide early risk stratification in the emergency department, but rather is to be used for post-discharge risk stratification. In addition, some of the included predictor variables, such as the patients´ NYHA class, depend on the subjective judgement of the attending physician. Natriuretic peptides are incorporated twice, as NT-proBNP discharge levels, as well as NT-proBNP percentage change during hospitalisation. The model was externally validated in a small, single centre European randomised trial [116]. However, as no measures of discrimination or calibration are given, further studies are required for a comprehensive evaluation of the ELAN-HF score.
Improving Heart Failure Risk Stratification in the emergency department (STRATIFY) decision tool
The STRATIFY scale was developed in a prospective, multicentre study to identify AHF patients at a low risk of adverse events such as death or myocardial infarction within 30 days of emergency department presentation [117]. Thirteen predictor variables readily available at emergency department presentation, including cTnI and NTproBNP, were identified, and a nomogram was developed to assist estimation of the 30-day risk of adverse events. The overall calibration of the STRATIFY decision tool was 0.68 and results were confirmed by internal validation using bootstrapping (table 1, fig. 3). Advantages of the STATIFY decision tool include development in a prospective, multicentre study and the use of variables readily available within a short time after emergency department presentation. However, in comparison to other risk scores, the discrimination is rather poor, the scale was derived from a small dataset, and no external validation was performed.
Multiple Estimation of risk based on the Emergency department Spanish Score In patients with AHF (MEESSI-AHF)
The MEESSI-AHF risk score was derived to predict 30-day mortality in a large, prospective multicentre study of patients presenting with AHF to Spanish emergency departments [7]. The score consists of 13 variables which are partially subjectively collectable (symptoms of low output, NYHA class at admission) and partially objectively collectable (cTn, natriuretic peptide level at admission) (table 1, fig. 3). A time-dependent internal validation was performed, confirming excellent discrimination (c-statistic, 0.828) and calibration (Hosmer-Lemeshow p = 0.122). Compared to the EHMRG, the MEESSI score shows a significantly better discrimination for 30-day mortality (c-statistic, 0.83 vs 0.75, p <0.001). In addition, external validation in a different country confirmed after recalibration an excellent discrimination (c-statistic, 0.80) and calibration (Hosmer-Lemeshow p = 0.23) after it was recalibrated by a simple adjustment of the intercept [8]. Major advantages of the MEESSI score include development in the emergency department in a large, prospective multicentre study. Furthermore, several reduced MEESSI models, calculable with fewer than 13 variables and still exhibiting good discrimination (c-statistic, 0.784 to 0.829), are available. In addition, its calculation is simplified by an already established online calculator, and external validation in a different country has been performed. Further studies to investigate the actual impact of the MEESSI-AHF risk score on clinical decision-making in the emergency department and healthcare costs are warranted.
Outlook
Future studies should focus on three aspects. First, gaining a better understanding of the pathophysiology of those biomarkers strongly associated with mortality in AHF, such as cTn, adrenomedullin, sST2 and GDF-15. This is a prerequisite for the identification and evaluation of targeted interventions aiming to mitigate the detrimental causal factors. Second, combinations of the most promising and best-validated risk score, currently the MEESSI score, with some of the novel biomarkers should be tested in large studies for possible incremental value. Third, scores are often time-consuming and burdensome to calculate. IT-based solutions need to integrate the calculation and display of these scores and the quantified risk within electronic healthcare records.
Conclusions
Early risk prediction plays a key role in the subsequent management of patients presenting with AHF, a heterogeneous syndrome associated with still unacceptably high rates of mortality and morbidity. In order to optimise risk prediction and the intensity of management, clinicians should be aware of the following concepts.
- Biomarkers have improved the understanding of heart failure pathophysiology and can therefore contribute to adjusting the intensity of management in AHF.
- Among the large variety of biomarkers currently available, natriuretic peptides and cTn seem the most promising in this indication.
- However, as a heterogeneous syndrome with various phenotypes, a biomarker approach alone is insufficient for accurate risk prediction in AHF. Heart failure risk scores combining several predictor variables are more promising for helping clinicians to make decisions and customise the intensity of management.
- Among the described risk scores, those combining demographics and clinical parameters with biomarkers in a model with fast and routinely available variables seem the most promising tools for early and accurate risk stratification in the emergency department.
- For early risk stratification of patients with AHF in the emergency department, scores that were specifically derived and validated in emergency department cohorts should be used preferentially.
- Besides biomarkers, age, systolic blood pressure, respiratory rate, oxygen saturation, creatinine, electrolytes and blood urea nitrogen are the most commonly used predictor variables in the described risk scores (fig. 3).
- Among selected models, the MEESSI-AHF risk score currently seems to be the most promising tool for AHF risk prediction. This score was developed in the emergency department in a large derivation cohort, consists of fast and routinely available variables, exhibits highly accurate risk prediction and was externally validated in a different country from where it was developed.
References
1
Benjamin
EJ
,
Blaha
MJ
,
Chiuve
SE
,
Cushman
M
,
Das
SR
,
Deo
R
, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–603. doi:.https://doi.org/10.1161/CIR.0000000000000485
2
Shah
SJ
,
Katz
DH
,
Selvaraj
S
,
Burke
MA
,
Yancy
CW
,
Gheorghiade
M
, et al.
Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–79. doi:.https://doi.org/10.1161/CIRCULATIONAHA.114.010637
3
Jackson
SL
,
Tong
X
,
King
RJ
,
Loustalot
F
,
Hong
Y
,
Ritchey
MD
. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11(12):e004873. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.117.004873
4
Mueller
C
,
Christ
M
,
Cowie
M
,
Cullen
L
,
Maisel
AS
,
Masip
J
, et al.; Acute Heart Failure Study Group of the ESC Acute Cardiovascular Care Association. European Society of Cardiology-Acute Cardiovascular Care Association Position paper on acute heart failure: A call for interdisciplinary care. Eur Heart J Acute Cardiovasc Care. 2017;6(1):81–6. doi:.https://doi.org/10.1177/2048872615593279
5
Mebazaa
A
,
Yilmaz
MB
,
Levy
P
,
Ponikowski
P
,
Peacock
WF
,
Laribi
S
, et al.
Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine--short version. Eur Heart J. 2015;36(30):1958–66. doi:.https://doi.org/10.1093/eurheartj/ehv066
6
Mueller
C
,
Bally
K
,
Buser
M
,
Flammer
AJ
,
Gaspoz
J-M
,
Mach
F
, et al.
Roadmap for the treatment of heart failure patients after hospital discharge: an interdisciplinary consensus paper. Swiss Med Wkly. 2020;150:w20159. doi:.https://doi.org/10.4414/smw.2020.20159
7
Miró
Ò
,
Rossello
X
,
Gil
V
,
Martín-Sánchez
FJ
,
Llorens
P
,
Herrero-Puente
P
, et al.; ICA-SEMES Research Group. Predicting 30-Day Mortality for Patients With Acute Heart Failure in the Emergency Department: A Cohort Study. Ann Intern Med. 2017;167(10):698–705. doi:.https://doi.org/10.7326/M16-2726
8
Wussler
D
,
Kozhuharov
N
,
Sabti
Z
,
Walter
J
,
Strebel
I
,
Scholl
L
, et al.
External Validation of the MEESSI Acute Heart Failure Risk Score: A Cohort Study. Ann Intern Med. 2019;170(4):248–56. doi:.https://doi.org/10.7326/M18-1967
9
Braunwald
E
. Another step toward personalized care of patients with heart failure. Eur J Heart Fail. 2015;17(10):988–90. doi:.https://doi.org/10.1002/ejhf.348
10
Bishu
K
,
Deswal
A
,
Chen
HH
,
LeWinter
MM
,
Lewis
GD
,
Semigran
MJ
, et al.
Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. 2012;164(5):763–770.e3. doi:.https://doi.org/10.1016/j.ahj.2012.08.014
11
Tromp
J
,
Khan
MAF
,
Klip
IT
,
Meyer
S
,
de Boer
RA
,
Jaarsma
T
, et al.
Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J Am Heart Assoc. 2017;6(4):e003989. doi:.https://doi.org/10.1161/JAHA.116.003989
12
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JGF
,
Coats
AJS
, et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
13
Mueller
C
,
McDonald
K
,
de Boer
RA
,
Maisel
A
,
Cleland
JGF
,
Kozhuharov
N
, et al.; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–31. doi:.https://doi.org/10.1002/ejhf.1494
14
Fonarow
GC
,
Peacock
WF
,
Phillips
CO
,
Givertz
MM
,
Lopatin
M
; ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–50. doi:.https://doi.org/10.1016/j.jacc.2007.02.037
15
Cheng
V
,
Kazanagra
R
,
Garcia
A
,
Lenert
L
,
Krishnaswamy
P
,
Gardetto
N
, et al.
A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–91. doi:.https://doi.org/10.1016/S0735-1097(00)01157-8
16
Lassus
J
,
Gayat
E
,
Mueller
C
,
Peacock
WF
,
Spinar
J
,
Harjola
V-P
, et al.; GREAT-Network. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168(3):2186–94. doi:.https://doi.org/10.1016/j.ijcard.2013.01.228
17
Rahimi
K
,
Bennett
D
,
Conrad
N
,
Williams
TM
,
Basu
J
,
Dwight
J
, et al.
Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2(5):440–6. doi:.https://doi.org/10.1016/j.jchf.2014.04.008
18
Braunwald
E
. Heart failure. JACC Heart Fail. 2013;1(1):1–20. doi:.https://doi.org/10.1016/j.jchf.2012.10.002
19
Steyerberg
EW
,
Moons
KGM
,
van der Windt
DA
,
Hayden
JA
,
Perel
P
,
Schroter
S
, et al.; PROGRESS Group. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10(2):e1001381. doi:.https://doi.org/10.1371/journal.pmed.1001381
20
Moons
KGM
,
Kengne
AP
,
Woodward
M
,
Royston
P
,
Vergouwe
Y
,
Altman
DG
, et al.
Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98(9):683–90. doi:.https://doi.org/10.1136/heartjnl-2011-301246
21
Moons
KGM
,
Kengne
AP
,
Grobbee
DE
,
Royston
P
,
Vergouwe
Y
,
Altman
DG
, et al.
Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98(9):691–8. doi:.https://doi.org/10.1136/heartjnl-2011-301247
22
Lee
DS
,
Stitt
A
,
Austin
PC
,
Stukel
TA
,
Schull
MJ
,
Chong
A
, et al.
Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767–75, W-261, W-262. doi:.https://doi.org/10.7326/0003-4819-156-11-201206050-00003
23
Stiell
IG
,
Clement
CM
,
Brison
RJ
,
Rowe
BH
,
Borgundvaag
B
,
Aaron
SD
, et al.
A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20(1):17–26. doi:.https://doi.org/10.1111/acem.12056
24
Collins
SP
,
Jenkins
CA
,
Harrell
FE, Jr
,
Liu
D
,
Miller
KF
,
Lindsell
CJ
, et al.
Identification of Emergency Department Patients With Acute Heart Failure at Low Risk for 30-Day Adverse Events: The STRATIFY Decision Tool. JACC Heart Fail. 2015;3(10):737–47. doi:.https://doi.org/10.1016/j.jchf.2015.05.007
25
Morrow
DA
,
de Lemos
JA
. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115(8):949–52. doi:.https://doi.org/10.1161/CIRCULATIONAHA.106.683110
26
Tang
WHW
,
Francis
GS
,
Morrow
DA
,
Newby
LK
,
Cannon
CP
,
Jesse
RL
, et al.; National Academy of Clinical Biochemistry Laboratory Medicine. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116(5):e99–109.
27
van Kimmenade
RRJ
,
Januzzi
JL, Jr
. Emerging biomarkers in heart failure. Clin Chem. 2012;58(1):127–38. doi:.https://doi.org/10.1373/clinchem.2011.165720
28
Nishikimi
T
,
Saito
Y
,
Kitamura
K
,
Ishimitsu
T
,
Eto
T
,
Kangawa
K
, et al.
Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26(6):1424–31. doi:.https://doi.org/10.1016/0735-1097(95)00338-X
29
Rehman
SU
,
Mueller
T
,
Januzzi
JL, Jr
. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52(18):1458–65. doi:.https://doi.org/10.1016/j.jacc.2008.07.042
30
Wettersten
N
,
Horiuchi
Y
,
van Veldhuisen
DJ
,
Mueller
C
,
Filippatos
G
,
Nowak
R
, et al.
Short-term prognostic implications of serum and urine neutrophil gelatinase-associated lipocalin in acute heart failure: findings from the AKINESIS study. Eur J Heart Fail. 2020;22(2):251–63. doi:.https://doi.org/10.1002/ejhf.1642
31
Murray
PT
,
Wettersten
N
,
van Veldhuisen
DJ
,
Mueller
C
,
Filippatos
G
,
Nowak
R
, et al.
Utility of Urine Neutrophil Gelatinase-Associated Lipocalin for Worsening Renal Function during Hospitalization for Acute Heart Failure: Primary Findings of the Urine N-gal Acute Kidney Injury N-gal Evaluation of Symptomatic Heart Failure Study (AKINESIS). J Card Fail. 2019;25(8):654–65. doi:.https://doi.org/10.1016/j.cardfail.2019.05.009
32
Maisel
AS
,
Wettersten
N
,
van Veldhuisen
DJ
,
Mueller
C
,
Filippatos
G
,
Nowak
R
, et al.
Neutrophil Gelatinase-Associated Lipocalin for Acute Kidney Injury During Acute Heart Failure Hospitalizations: The AKINESIS Study. J Am Coll Cardiol. 2016;68(13):1420–31. doi:.https://doi.org/10.1016/j.jacc.2016.06.055
33
Du
W
,
Piek
A
,
Schouten
EM
,
van de Kolk
CWA
,
Mueller
C
,
Mebazaa
A
, et al.
Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics. 2018;8(15):4155–69. doi:.https://doi.org/10.7150/thno.26055
34
Kaye
DM
,
Mariani
JA
,
van Empel
V
,
Maeder
MT
. Determinants and implications of elevated soluble ST2 levels in heart failure. Int J Cardiol. 2014;176(3):1242–3. doi:.https://doi.org/10.1016/j.ijcard.2014.07.206
35
Braunwald
E
. Biomarkers in heart failure. N Engl J Med. 2008;358(20):2148–59. doi:.https://doi.org/10.1056/NEJMra0800239
36
Zymliński
R
,
Sokolski
M
,
Biegus
J
,
Siwołowski
P
,
Nawrocka-Millward
S
,
Sokolska
JM
, et al.
Multi-organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur J Heart Fail. 2019;21(6):744–50. doi:.https://doi.org/10.1002/ejhf.1378
37
Kim
HN
,
Januzzi
JL, Jr
. Natriuretic peptide testing in heart failure. Circulation. 2011;123(18):2015–9. doi:.https://doi.org/10.1161/CIRCULATIONAHA.110.979500
38
Maisel
AS
,
Krishnaswamy
P
,
Nowak
RM
,
McCord
J
,
Hollander
JE
,
Duc
P
, et al.; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–7. doi:.https://doi.org/10.1056/NEJMoa020233
39
Maeda
K
,
Tsutamoto
T
,
Wada
A
,
Hisanaga
T
,
Kinoshita
M
. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5):825–32. doi:.https://doi.org/10.1016/S0002-8703(98)70041-9
40
Drexler
B
,
Heinisch
C
,
Balmelli
C
,
Lassus
J
,
Siirilä-Waris
K
,
Arenja
N
, et al.
Quantifying cardiac hemodynamic stress and cardiomyocyte damage in ischemic and nonischemic acute heart failure. Circ Heart Fail. 2012;5(1):17–24. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.111.961243
41
Mueller
C
,
Scholer
A
,
Laule-Kilian
K
,
Martina
B
,
Schindler
C
,
Buser
P
, et al.
Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647–54. doi:.https://doi.org/10.1056/NEJMoa031681
42
McCullough
PA
,
Nowak
RM
,
McCord
J
,
Hollander
JE
,
Herrmann
HC
,
Steg
PG
, et al.
B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416–22. doi:.https://doi.org/10.1161/01.CIR.0000025242.79963.4C
43
Yancy
CW
,
Jessup
M
,
Bozkurt
B
,
Butler
J
,
Casey
DE, Jr
,
Drazner
MH
, et al.
2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52. doi:.https://doi.org/10.1161/CIR.0b013e31829e8807
44
Harrison
A
,
Morrison
LK
,
Krishnaswamy
P
,
Kazanegra
R
,
Clopton
P
,
Dao
Q
, et al.
B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med. 2002;39(2):131–8. doi:.https://doi.org/10.1067/mem.2002.121483
45
Zairis
MN
,
Tsiaousis
GZ
,
Georgilas
AT
,
Makrygiannis
SS
,
Adamopoulou
EN
,
Handanis
SM
, et al.
Multimarker strategy for the prediction of 31 days cardiac death in patients with acutely decompensated chronic heart failure. Int J Cardiol. 2010;141(3):284–90. doi:.https://doi.org/10.1016/j.ijcard.2008.12.017
46
Januzzi
JL, Jr
,
Sakhuja
R
,
O’donoghue
M
,
Baggish
AL
,
Anwaruddin
S
,
Chae
CU
, et al.
Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166(3):315–20. doi:.https://doi.org/10.1001/archinte.166.3.315
47
van Kimmenade
RRJ
,
Pinto
YM
,
Bayes-Genis
A
,
Lainchbury
JG
,
Richards
AM
,
Januzzi
JL, Jr
. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386–90. doi:.https://doi.org/10.1016/j.amjcard.2006.02.043
48
Januzzi
JL
,
van Kimmenade
R
,
Lainchbury
J
,
Bayes-Genis
A
,
Ordonez-Llanos
J
,
Santalo-Bel
M
, et al.
NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330–7. doi:.https://doi.org/10.1093/eurheartj/ehi631
49
Dhaliwal
AS
,
Deswal
A
,
Pritchett
A
,
Aguilar
D
,
Kar
B
,
Souchek
J
, et al.
Reduction in BNP levels with treatment of decompensated heart failure and future clinical events. J Card Fail. 2009;15(4):293–9. doi:.https://doi.org/10.1016/j.cardfail.2008.11.007
50
Kociol
RD
,
Horton
JR
,
Fonarow
GC
,
Reyes
EM
,
Shaw
LK
,
O’Connor
CM
, et al.
Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4(5):628–36. doi:.https://doi.org/10.1161/CIRCHEARTFAILURE.111.962290
51
Bettencourt
P
,
Azevedo
A
,
Pimenta
J
,
Friões
F
,
Ferreira
S
,
Ferreira
A
. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–74. doi:.https://doi.org/10.1161/01.CIR.0000144310.04433.BE
52
O’Brien
RJ
,
Squire
IB
,
Demme
B
,
Davies
JE
,
Ng
LL
. Pre-discharge, but not admission, levels of NT-proBNP predict adverse prognosis following acute LVF. Eur J Heart Fail. 2003;5(4):499–506. doi:.https://doi.org/10.1016/S1388-9842(03)00098-9
53
Noveanu
M
,
Breidthardt
T
,
Potocki
M
,
Reichlin
T
,
Twerenbold
R
,
Uthoff
H
, et al.
Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and long-term outcome in acute decompensated heart failure. Crit Care. 2011;15(1):R1. doi:.https://doi.org/10.1186/cc9398
54
McMurray
JJV
,
Packer
M
,
Desai
AS
,
Gong
J
,
Lefkowitz
MP
,
Rizkala
AR
, et al.; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:.https://doi.org/10.1056/NEJMoa1409077
55
Solomon
SD
,
Zile
M
,
Pieske
B
,
Voors
A
,
Shah
A
,
Kraigher-Krainer
E
, et al.; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–95. doi:.https://doi.org/10.1016/S0140-6736(12)61227-6
56
Ibrahim
NE
,
McCarthy
CP
,
Shrestha
S
,
Gaggin
HK
,
Mukai
R
,
Szymonifka
J
, et al.
Effect of Neprilysin Inhibition on Various Natriuretic Peptide Assays. J Am Coll Cardiol. 2019;73(11):1273–84. doi:.https://doi.org/10.1016/j.jacc.2018.12.063
57
Yandle
TG
,
Richards
AM
,
Nicholls
MG
,
Cuneo
R
,
Espiner
EA
,
Livesey
JH
. Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. Life Sci. 1986;38(20):1827–33. doi:.https://doi.org/10.1016/0024-3205(86)90137-2
58
Maisel
A
,
Mueller
C
,
Nowak
R
,
Peacock
WF
,
Landsberg
JW
,
Ponikowski
P
, et al.
Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55(19):2062–76. doi:.https://doi.org/10.1016/j.jacc.2010.02.025
59
Shah
RV
,
Truong
QA
,
Gaggin
HK
,
Pfannkuche
J
,
Hartmann
O
,
Januzzi
JL, Jr
. Mid-regional pro-atrial natriuretic peptide and pro-adrenomedullin testing for the diagnostic and prognostic evaluation of patients with acute dyspnoea. Eur Heart J. 2012;33(17):2197–205. doi:.https://doi.org/10.1093/eurheartj/ehs136
60
Potocki
M
,
Breidthardt
T
,
Reichlin
T
,
Hartwiger
S
,
Morgenthaler
NG
,
Bergmann
A
, et al.
Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure. J Intern Med. 2010;267(1):119–29. doi:.https://doi.org/10.1111/j.1365-2796.2009.02135.x
61
Iwanaga
Y
,
Nishi
I
,
Furuichi
S
,
Noguchi
T
,
Sase
K
,
Kihara
Y
, et al.
B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47(4):742–8. doi:.https://doi.org/10.1016/j.jacc.2005.11.030
62
Maeder
MT
,
Weber
L
,
Ammann
P
,
Buser
M
,
Ehl
NF
,
Gerhard
M
, et al.
Relationship between B-type natriuretic peptide and invasive haemodynamics in patients with severe aortic valve stenosis. ESC Heart Fail. 2020;7(2):577–87. doi:.https://doi.org/10.1002/ehf2.12614
63
Weinberg
EO
,
Shimpo
M
,
Hurwitz
S
,
Tominaga
S
,
Rouleau
JL
,
Lee
RT
. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107(5):721–6. doi:.https://doi.org/10.1161/01.CIR.0000047274.66749.FE
64
Januzzi
JL, Jr
,
Peacock
WF
,
Maisel
AS
,
Chae
CU
,
Jesse
RL
,
Baggish
AL
, et al.
Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50(7):607–13. doi:.https://doi.org/10.1016/j.jacc.2007.05.014
65
Boisot
S
,
Beede
J
,
Isakson
S
,
Chiu
A
,
Clopton
P
,
Januzzi
J
, et al.
Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Card Fail. 2008;14(9):732–8. doi:.https://doi.org/10.1016/j.cardfail.2008.06.415
66
Breidthardt
T
,
Balmelli
C
,
Twerenbold
R
,
Mosimann
T
,
Espinola
J
,
Haaf
P
, et al.
Heart failure therapy-induced early ST2 changes may offer long-term therapy guidance. J Card Fail. 2013;19(12):821–8. doi:.https://doi.org/10.1016/j.cardfail.2013.11.003
67
Manzano-Fernández
S
,
Januzzi
JL
,
Pastor-Pérez
FJ
,
Bonaque-González
JC
,
Boronat-Garcia
M
,
Pascual-Figal
DA
, et al.
Serial monitoring of soluble interleukin family member ST2 in patients with acutely decompensated heart failure. Cardiology. 2012;122(3):158–66. doi:.https://doi.org/10.1159/000338800
68
Aimo
A
,
Vergaro
G
,
Ripoli
A
,
Bayes-Genis
A
,
Pascual Figal
DA
,
de Boer
RA
, et al.
Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail. 2017;5(4):287–96. doi:.https://doi.org/10.1016/j.jchf.2016.12.016
69
Maisel
A
,
Xue
Y
,
van Veldhuisen
DJ
,
Voors
AA
,
Jaarsma
T
,
Pang
PS
, et al.
Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study). Am J Cardiol. 2014;114(5):737–42. doi:.https://doi.org/10.1016/j.amjcard.2014.05.062
70
Morrow
DA
,
Velazquez
EJ
,
DeVore
AD
,
Prescott
MF
,
Duffy
CI
,
Gurmu
Y
, et al.
Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J. 2019;40(40):3345–52. doi:.https://doi.org/10.1093/eurheartj/ehz240
71
Tsutamoto
T
,
Sakai
H
,
Ishikawa
C
,
Fujii
M
,
Tanaka
T
,
Yamamoto
T
, et al.
Direct comparison of transcardiac difference between brain natriuretic peptide (BNP) and N-terminal pro-BNP in patients with chronic heart failure. Eur J Heart Fail. 2007;9(6-7):667–73. doi:.https://doi.org/10.1016/j.ejheart.2007.01.003
72
Maeder
MT
,
Mariani
JA
,
Kaye
DM
. Hemodynamic determinants of myocardial B-type natriuretic peptide release: relative contributions of systolic and diastolic wall stress. Hypertension. 2010;56(4):682–9. doi:.https://doi.org/10.1161/HYPERTENSIONAHA.110.156547
73
Ago
T
,
Sadoshima
J
. GDF15, a cardioprotective TGF-β superfamily protein. Circ Res. 2006;98(3):294–7. doi:.https://doi.org/10.1161/01.RES.0000207919.83894.9d
74
Chan
MMY
,
Santhanakrishnan
R
,
Chong
JPC
,
Chen
Z
,
Tai
BC
,
Liew
OW
, et al.
Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2016;18(1):81–8. doi:.https://doi.org/10.1002/ejhf.431
75
Cotter
G
,
Voors
AA
,
Prescott
MF
,
Felker
GM
,
Filippatos
G
,
Greenberg
BH
, et al.
Growth differentiation factor 15 (GDF-15) in patients admitted for acute heart failure: results from the RELAX-AHF study. Eur J Heart Fail. 2015;17(11):1133–43. doi:.https://doi.org/10.1002/ejhf.331
76
Bouabdallaoui
N
,
Claggett
B
,
Zile
MR
,
McMurray
JJV
,
O’Meara
E
,
Packer
M
, et al.; PARADIGM-HF Investigators and Committees. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2018;20(12):1701–9. doi:.https://doi.org/10.1002/ejhf.1301
77
Jougasaki
M
,
Wei
C-M
,
McKinley
LJ
,
Burnett
JC, Jr
. Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92(3):286–9. doi:.https://doi.org/10.1161/01.CIR.92.3.286
78
Nagaya
N
,
Satoh
T
,
Nishikimi
T
,
Uematsu
M
,
Furuichi
S
,
Sakamaki
F
, et al.
Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101(5):498–503. doi:.https://doi.org/10.1161/01.CIR.101.5.498
79
Self
WH
,
Storrow
AB
,
Hartmann
O
,
Barrett
TW
,
Fermann
GJ
,
Maisel
AS
, et al.
Plasma bioactive adrenomedullin as a prognostic biomarker in acute heart failure. Am J Emerg Med. 2016;34(2):257–62. doi:.https://doi.org/10.1016/j.ajem.2015.10.033
80
Thygesen
K
,
Alpert
JS
,
Jaffe
AS
,
Chaitman
BR
,
Bax
JJ
,
Morrow
DA
, et al.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64. doi:.https://doi.org/10.1016/j.jacc.2018.08.1038
81
Januzzi
JL, Jr
,
Filippatos
G
,
Nieminen
M
,
Gheorghiade
M
. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33(18):2265–71. doi:.https://doi.org/10.1093/eurheartj/ehs191
82
Del Carlo
CH
,
Pereira-Barretto
AC
,
Cassaro-Strunz
C
,
Latorre
MR
,
Ramires
JAF
. Serial measure of cardiac troponin T levels for prediction of clinical events in decompensated heart failure. J Card Fail. 2004;10(1):43–8. doi:.https://doi.org/10.1016/S1071-9164(03)00594-3
83
Peacock
WF, 4th
,
De Marco
T
,
Fonarow
GC
,
Diercks
D
,
Wynne
J
,
Apple
FS
, et al.; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358(20):2117–26. doi:.https://doi.org/10.1056/NEJMoa0706824
84
You
JJ
,
Austin
PC
,
Alter
DA
,
Ko
DT
,
Tu
JV
. Relation between cardiac troponin I and mortality in acute decompensated heart failure. Am Heart J. 2007;153(4):462–70. doi:.https://doi.org/10.1016/j.ahj.2007.01.027
85
Metra
M
,
Nodari
S
,
Parrinello
G
,
Specchia
C
,
Brentana
L
,
Rocca
P
, et al.
The role of plasma biomarkers in acute heart failure. Serial changes and independent prognostic value of NT-proBNP and cardiac troponin-T. Eur J Heart Fail. 2007;9(8):776–86. doi:.https://doi.org/10.1016/j.ejheart.2007.05.007
86
Sharma
UC
,
Pokharel
S
,
van Brakel
TJ
,
van Berlo
JH
,
Cleutjens
JPM
,
Schroen
B
, et al.
Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110(19):3121–8. doi:.https://doi.org/10.1161/01.CIR.0000147181.65298.4D
87
Dumic
J
,
Dabelic
S
,
Flögel
M
. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760(4):616–35. doi:.https://doi.org/10.1016/j.bbagen.2005.12.020
88
van Kimmenade
RR
,
Januzzi
JL, Jr
,
Ellinor
PT
,
Sharma
UC
,
Bakker
JA
,
Low
AF
, et al.
Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48(6):1217–24. doi:.https://doi.org/10.1016/j.jacc.2006.03.061
89
Miró
Ò
,
González de la Presa
B
,
Herrero-Puente
P
,
Fernández Bonifacio
R
,
Möckel
M
,
Mueller
C
, et al.
The GALA study: relationship between galectin-3 serum levels and short- and long-term outcomes of patients with acute heart failure. Biomarkers. 2017;22(8):731–9. doi:.https://doi.org/10.1080/1354750X.2017.1319421
90
Zamora
E
,
Lupón
J
,
de Antonio
M
,
Galán
A
,
Domingo
M
,
Urrutia
A
, et al.
Renal function largely influences Galectin-3 prognostic value in heart failure. Int J Cardiol. 2014;177(1):171–7. doi:.https://doi.org/10.1016/j.ijcard.2014.09.011
91
La Rocca
G
,
Di Stefano
A
,
Eleuteri
E
,
Anzalone
R
,
Magno
F
,
Corrao
S
, et al.
Oxidative stress induces myeloperoxidase expression in endocardial endothelial cells from patients with chronic heart failure. Basic Res Cardiol. 2009;104(3):307–20. doi:.https://doi.org/10.1007/s00395-008-0761-9
92
Nicholls
SJ
,
Hazen
SL
. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102–11. doi:.https://doi.org/10.1161/01.ATV.0000163262.83456.6d
93
Reichlin
T
,
Socrates
T
,
Egli
P
,
Potocki
M
,
Breidthardt
T
,
Arenja
N
, et al.
Use of myeloperoxidase for risk stratification in acute heart failure. Clin Chem. 2010;56(6):944–51. doi:.https://doi.org/10.1373/clinchem.2009.142257
94
Sato
Y
,
Takatsu
Y
,
Kataoka
K
,
Yamada
T
,
Taniguchi
R
,
Sasayama
S
, et al.
Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol. 1999;22(12):811–3. doi:.https://doi.org/10.1002/clc.4960221211
95
Peschel
T
,
Schönauer
M
,
Thiele
H
,
Anker
SD
,
Schuler
G
,
Niebauer
J
. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5(5):609–14. doi:.https://doi.org/10.1016/S1388-9842(03)00104-1
96
Krack
A
,
Sharma
R
,
Figulla
HR
,
Anker
SD
. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26(22):2368–74. doi:.https://doi.org/10.1093/eurheartj/ehi389
97
Alonso-Martínez
JL
,
Llorente-Diez
B
,
Echegaray-Agara
M
,
Olaz-Preciado
F
,
Urbieta-Echezarreta
M
,
González-Arencibia
C
. C-reactive protein as a predictor of improvement and readmission in heart failure. Eur J Heart Fail. 2002;4(3):331–6. doi:.https://doi.org/10.1016/S1388-9842(02)00021-1
98
Anand
IS
,
Latini
R
,
Florea
VG
,
Kuskowski
MA
,
Rector
T
,
Masson
S
, et al.; Val-HeFT Investigators. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112(10):1428–34. doi:.https://doi.org/10.1161/CIRCULATIONAHA.104.508465
99
Minami
Y
,
Kajimoto
K
,
Sato
N
,
Hagiwara
N
,
Takano
T
; ATTEND Study Investigators. C-reactive protein level on admission and time to and cause of death in patients hospitalized for acute heart failure. Eur Heart J Qual Care Clin Outcomes. 2017;3(2):148–56.
100
Siirilä-Waris
K
,
Lassus
J
,
Melin
J
,
Peuhkurinen
K
,
Nieminen
MS
,
Harjola
V-P
; FINN-AKVA Study Group. Characteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006;27(24):3011–7. doi:.https://doi.org/10.1093/eurheartj/ehl407
101
Mueller
C
,
Laule-Kilian
K
,
Christ
A
,
Brunner-La Rocca
HP
,
Perruchoud
AP
. Inflammation and long-term mortality in acute congestive heart failure. Am Heart J. 2006;151(4):845–50. doi:.https://doi.org/10.1016/j.ahj.2005.06.046
102
Park
JJ
,
Choi
D-J
,
Yoon
C-H
,
Oh
I-Y
,
Jeon
E-S
,
Kim
J-J
, et al.; KorHF Registry. Prognostic value of C-reactive protein as an inflammatory and N-terminal probrain natriuretic peptide as a neurohumoral marker in acute heart failure (from the Korean Heart Failure registry). Am J Cardiol. 2014;113(3):511–7. doi:.https://doi.org/10.1016/j.amjcard.2013.10.022
103
Maeda
K
,
Tsutamoto
T
,
Wada
A
,
Mabuchi
N
,
Hayashi
M
,
Tsutsui
T
, et al.
High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36(5):1587–93. doi:.https://doi.org/10.1016/S0735-1097(00)00912-8
104
Markousis-Mavrogenis
G
,
Tromp
J
,
Ouwerkerk
W
,
Devalaraja
M
,
Anker
SD
,
Cleland
JG
, et al.
The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21(8):965–73. doi:.https://doi.org/10.1002/ejhf.1482
105
Hamzic-Mehmedbasic
A
. Inflammatory Cytokines as Risk Factors for Mortality After Acute Cardiac Events. Med Arch. 2016;70(4):252–5. doi:.https://doi.org/10.5455/medarh.2016.70.252-255
106
Weintraub
NL
,
Collins
SP
,
Pang
PS
,
Levy
PD
,
Anderson
AS
,
Arslanian-Engoren
C
, et al.; American Heart Association Council on Clinical Cardiology and Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122(19):1975–96. doi:.https://doi.org/10.1161/CIR.0b013e3181f9a223
107
Steyerberg
EW
,
Vergouwe
Y
. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925–31. doi:.https://doi.org/10.1093/eurheartj/ehu207
108
Akiyama
E
,
Van Aelst
LNL
,
Arrigo
M
,
Lassus
J
,
Miró
Ò
,
Čelutkienė
J
, et al.; GREAT (Global Research on Acute Conditions Team) Network. East Asia may have a better 1-year survival following an acute heart failure episode compared with Europe: results from an international observational cohort. Eur J Heart Fail. 2018;20(6):1071–5. doi:.https://doi.org/10.1002/ejhf.1152
109
Motiejūnaitė
J
,
Akiyama
E
,
Cohen-Solal
A
,
Maggioni
AP
,
Mueller
C
,
Choi
D-J
, et al.
The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur Heart J. 2020;41(13):1357–64. doi:.https://doi.org/10.1093/eurheartj/ehaa071
110
Janssen
KJM
,
Moons
KGM
,
Kalkman
CJ
,
Grobbee
DE
,
Vergouwe
Y
. Updating methods improved the performance of a clinical prediction model in new patients. J Clin Epidemiol. 2008;61(1):76–86. doi:.https://doi.org/10.1016/j.jclinepi.2007.04.018
111
Lee
DS
,
Austin
PC
,
Rouleau
JL
,
Liu
PP
,
Naimark
D
,
Tu
JV
. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290(19):2581–7. doi:.https://doi.org/10.1001/jama.290.19.2581
112
Fonarow
GC
,
Adams
KF, Jr
,
Abraham
WT
,
Yancy
CW
,
Boscardin
WJ
; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572–80. doi:.https://doi.org/10.1001/jama.293.5.572
113
Auble
TE
,
Hsieh
M
,
Gardner
W
,
Cooper
GF
,
Stone
RA
,
McCausland
JB
, et al.
A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med. 2005;12(6):514–21. doi:.https://doi.org/10.1197/j.aem.2004.11.026
114
Peterson
PN
,
Rumsfeld
JS
,
Liang
L
,
Albert
NM
,
Hernandez
AF
,
Peterson
ED
, et al.; American Heart Association Get With the Guidelines-Heart Failure Program. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circ Cardiovasc Qual Outcomes. 2010;3(1):25–32. doi:.https://doi.org/10.1161/CIRCOUTCOMES.109.854877
115
Scrutinio
D
,
Ammirati
E
,
Guida
P
,
Passantino
A
,
Raimondo
R
,
Guida
V
, et al.
Clinical utility of N-terminal pro-B-type natriuretic peptide for risk stratification of patients with acute decompensated heart failure. Derivation and validation of the ADHF/NT-proBNP risk score. Int J Cardiol. 2013;168(3):2120–6. doi:.https://doi.org/10.1016/j.ijcard.2013.01.005
116
Salah
K
,
Kok
WE
,
Eurlings
LW
,
Bettencourt
P
,
Pimenta
JM
,
Metra
M
, et al.
A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart. 2014;100(2):115–25. doi:.https://doi.org/10.1136/heartjnl-2013-303632
117
Collins
SP
,
Jenkins
CA
,
Harrell
FE, Jr
,
Liu
D
,
Miller
KF
,
Lindsell
CJ
, et al.
Identification of Emergency Department Patients With Acute Heart Failure at Low Risk for 30-Day Adverse Events: The STRATIFY Decision Tool. JACC Heart Fail. 2015;3(10):737–47. doi:.https://doi.org/10.1016/j.jchf.2015.05.007
118
Testani
JM
,
Cappola
TP
,
Brensinger
CM
,
Shannon
RP
,
Kimmel
SE
. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58(4):375–82. doi:.https://doi.org/10.1016/j.jacc.2011.01.052
119
Arenja
N
,
Breidthardt
T
,
Socrates
T
,
Schindler
C
,
Heinisch
C
,
Tschung
C
, et al.
Risk stratification for 1-year mortality in acute heart failure: classification and regression tree analysis. Swiss Med Wkly. 2011;141:w13259. doi:.https://doi.org/10.4414/smw.2011.13259
120
Hsiao
J
,
Motta
M
,
Wyer
P
. Validating the acute heart failure index for patients presenting to the emergency department with decompensated heart failure. Emerg Med J. 2012;29(12):e5. doi:.https://doi.org/10.1136/emermed-2011-200610
121
Hsieh
M
,
Auble
TE
,
Yealy
DM
. Validation of the Acute Heart Failure Index. Ann Emerg Med. 2008;51(1):37–44. doi:.https://doi.org/10.1016/j.annemergmed.2007.07.026
122
Sepehrvand
N
,
Youngson
E
,
Bakal
JA
,
McAlister
FA
,
Rowe
BH
,
Ezekowitz
JA
. External Validation and Refinement of Emergency Heart Failure Mortality Risk Grade Risk Model in Patients With Heart Failure in the Emergency Department. CJC Open. 2019;1(3):123–30. doi:.https://doi.org/10.1016/j.cjco.2019.03.003
123
Stiell
IG
,
Perry
JJ
,
Clement
CM
,
Brison
RJ
,
Rowe
BH
,
Aaron
SD
, et al.
Prospective and Explicit Clinical Validation of the Ottawa Heart Failure Risk Scale, With and Without Use of Quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316–27. doi:.https://doi.org/10.1111/acem.13141


