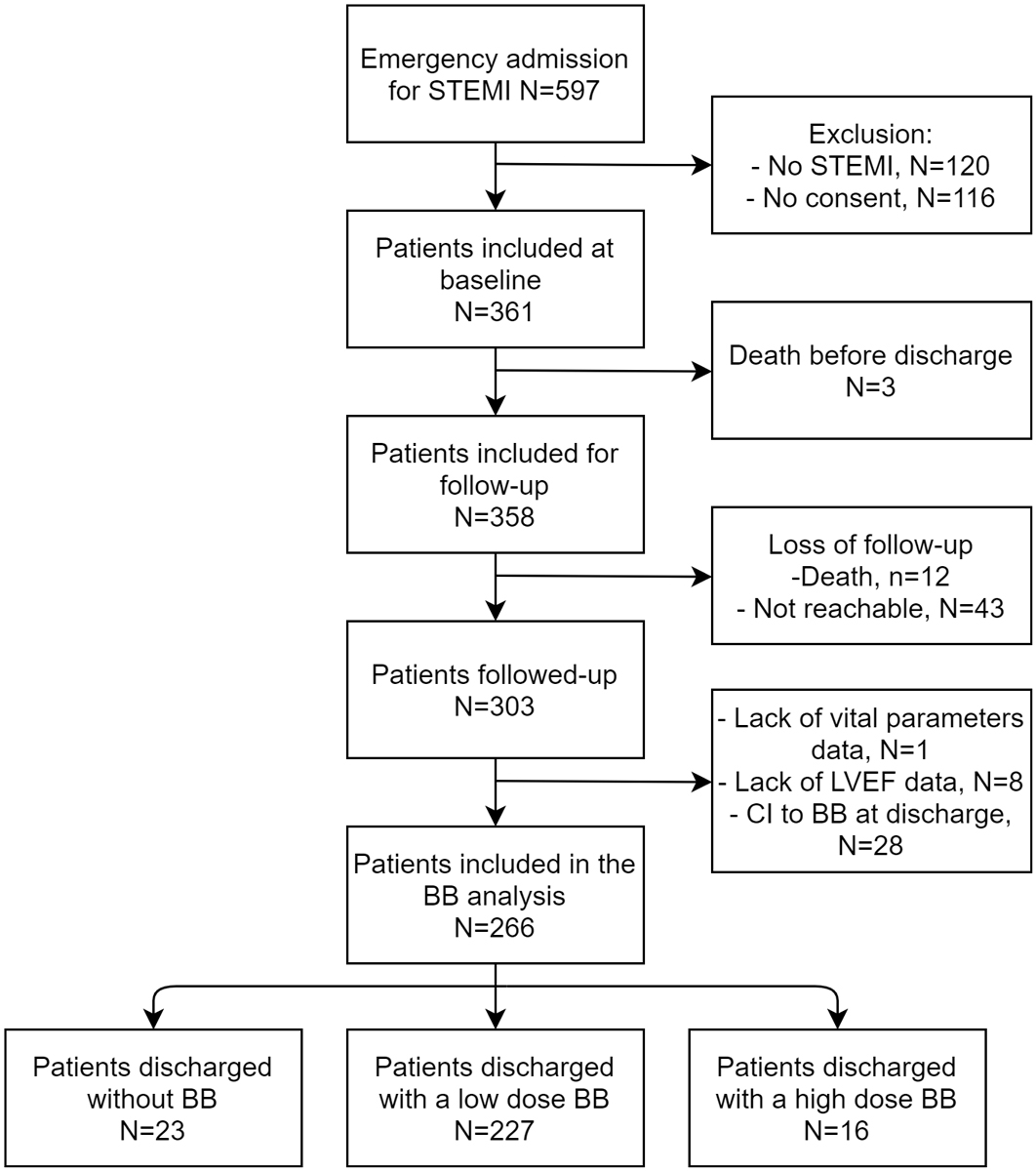
Figure 1 Flow chart of patient inclusion (15 April 2014 to 15 April 2016). STEMI = ST-segment elevation myocardial infarction; LVEF = left ventricular ejection fraction; CI = contraindication; BB = beta blocker
DOI: https://doi.org/10.4414/smw.2020.20321
For decades, beta-blockers have been known to improve survival after ST-segment elevation myocardial infarction (STEMI) [1–4]. The current guidelines of the European Society of Cardiology (ESC) and the American College of Cardiology Foundation/American Heart Association advocate beta-blocker use for secondary prevention in all patients who have had STEMIs, unless contraindicated [5, 6]. However, all studies of the efficacy of beta-blockers to date were conducted in the 1980s, before the introduction of reperfusion with percutaneous coronary intervention (PCI). With the use of this reperfusion strategy, beta-blocker use has been called into question. Recent studies have yielded stronger evidence for the beneficial effects of beta-blockers in high-risk patients, such as those with reduced left ventricular ejection fractions (LVEFs), anterior wall infarction or heart failure, than for low-risk patients [7–11]. Consequently, the current ESC guidelines provide distinct classes of recommendations according to LVEF function: class IIa level B for patients with LVEFs ≥40% and class I level A for patients with LVEFs <40% [5].
European STEMI guidelines provide no beta-blocker dosage recommendations [5]. American STEMI guidelines advocate up-titration of the dose to a target of 200 mg metoprolol once daily or 25 mg carvedilol twice daily [6]. High beta-blocker doses (i.e., 200 mg metoprolol [2, 12], 50 mg carvedilol [7] or 180–240 mg propranolol [1]) have been used in all studies demonstrating the efficacy of these drugs to date. However, the patients included in these studies were younger and less polymorbid than those in the real-life population, and the studies were conducted in the pre-PCI era. Observational studies have been conducted to assess whether low beta-blocker doses are equivalent to high doses in terms of morbidity and mortality reduction after acute coronary syndrome (ACS) in the modern era [13–15]. None of these studies has shown that high beta-blocker doses are superior to lower doses, but they did not group patients according to LVEF, an important factor for long-term risk stratification. Thus, the use of high beta-blocker doses after myocardial infarction should still be assumed to be required to achieve effectiveness, particularly in patients with reduced LVEFs. When LVEF dysfunction is diagnosed, common practice is usually based on the guidelines for heart failure. These guidelines advocate the use of bisoprolol, metoprolol, carvedilol or nebivolol with evidence-based dosing and specific target doses [16].
In clinical practice, beta-blockers are introduced mainly at reference centers and at low doses due to tolerance issues such as hypotension, bradycardia and fatigue. Gradual up-titration requires time, but many patients admitted for STEMI are discharged home within a few days or transferred to district hospitals very shortly after the procedure. We believe that this situation leads to poor post-PCI up-titration, with many patients continuing to receive their initially prescribed doses.
Accordingly, we evaluated long-term beta-blocker prescription patterns in a cohort of patients with STEMI admitted to a university hospital in Switzerland. We determined the proportions of target-dose beta-blocker prescriptions at the time of discharge and one year post-PCI. We also assessed the proportion of up-titration among patients initially discharged with below-target doses and explored whether short hospital stays or being transferred to a district hospital increased the risk of not receiving beta-blockers at one year post-PCI.
This observational study was conducted with data from a local database containing all the data on ACS patients admitted to the University Hospital of Lausanne and who agreed to share their data. Only patients admitted to the University Hospital of Lausanne with suspicion of STEMI were considered for inclusion in this study. The local ethics committee of the canton of Vaud approved the local database in 2005 as part of the AMIS Plus project (n° 44/05).
We consecutively included all patients aged ≥18 years admitted to our hospital for STEMI between 15 April 2014 and 15 April 2016 who had confirmed STEMI diagnoses and the capacity for discernment and communication in French, and who provided written informed consent. The diagnosis of STEMI required clinical signs of characteristic retrosternal pain for <12 h and electrocardiographic evidence of ST-segment elevation in more than two contiguous derivations, a new bundle branch block, or ST-segment depression ≥0.5 mm in the V1–V3 leads. Patients anticipated to be unreachable after one year (e.g., because of homelessness or residing outside of Switzerland) and those with beta-blocker contraindications were excluded from the analysis. Recognised contraindications were hypotension (systolic blood pressure <100 mm Hg) and bradycardia (heart rate <60 bpm) on the day of discharge, acute heart failure, atrioventricular block, and beta-blocker intolerance or refusal (as noted in discharge letters and medical records). Patients for whom data (e.g., on LVEF or vital signs) were incomplete were also excluded.
All data were collected prospectively and entered into the local database. Baseline data were collected from the hospital’s electronic patient records and included characteristics of interest like age, sex, cardiovascular risk factors, co-morbidities, medical history, vital signs during hospitalisation, type of coronary artery disease, therapeutic strategy employed for the management of STEMI, and LVEF after the acute care. In cases of transfer, data on prescriptions at discharge were collected through agreements with the district hospitals. At the end of the inclusion period, we forwarded the list of transferred patients and their transfer dates to a responsible person in each of the district hospitals. The prescription list for each patient was then sent to us by post. One year after PCI, a pharmacist with training in study data collection contacted the participants by telephone and used a structured questionnaire to obtain information about their medical care, including all drug prescriptions, doses, and reasons for withdrawal or change; rehospitalisation and reinfarction; and cardiac rehabilitation programme participation. To enhance the accuracy of the information collected during these telephone interviews, participants were asked to read aloud the drug names and doses written on their medication packages. When a patient was unable to provide complete or clear information, the prescriber or pharmacy was contacted to obtain accurate and complete data.
The primary outcomes of interest were the prescribed beta-blocker dose category (at discharge and at one year) and the evolution of beta-blocker doses (beta-blocker introduction, withdrawal, up-titration, reduction, no change) during the year. We categorised beta-blocker dose as none, low (<50% target) or high ≥50% target). The target beta-blocker doses were those defined in the official drug information or in clinical trials: metoprolol 200 mg, carvedilol 50 mg, bisoprolol 10 mg, atenolol 100 mg and propranolol 180 mg (see table S1 in appendix 1).
Secondary outcomes were the proportions of patients in each of the beta-blocker dose categories (none, low and high) at discharge, assessed according to hospital type (university or district). We also descriptively analysed the effects of the length of university hospital stay, transfer to a district hospital and cardiac rehabilitation programme participation on beta-blocker prescription at one year.
Binary and categorical variables were expressed as frequencies with percentages and compared with chi-square tests. Continuous variables were expressed as median values with interquartile ranges (IQRs) and compared with Student’s t-tests. We used the chi-square test to assess differences in the proportions of patients with beta-blocker introduction and up-titration within the one-year study period, differences in beta-blocker prescription according to discharge hospital type, and the frequency of beta-blocker prescription at one year according to length of stay in the university hospital during the STEMI event (≤2 and >2 days). All analyses were stratified according to LVEF (≥40% and <40%). All tests were two tailed, with a significance level of p <0.05. The statistical analyses were performed using STATA software (version 14; Stata Corporation, College Station, TX, USA).
During the study period, 597 patients were admitted to Lausanne University Hospital for suspected STEMI, and their data were entered into the local database. After applying the exclusion criteria, data from 358 patients were included at baseline, and 303 of these were followed for one year. After further exclusion of patients due to missing data (n = 9) or beta-blocker contraindication (hypotension, n = 14; bradycardia, n = 9; bradycardia and hypotension, n = 3; atrioventricular block, n = 1; acute heart failure, n = 1), data from 266 patients were included in the analysis (fig. 1).

Figure 1 Flow chart of patient inclusion (15 April 2014 to 15 April 2016). STEMI = ST-segment elevation myocardial infarction; LVEF = left ventricular ejection fraction; CI = contraindication; BB = beta blocker
The majority (77.1%) of patients were men, and the median age was 63.7 years (IQR 55.0–73.0). Cardiovascular risk factors were prevalent in the study population. Most patients were treated with PCI; 1.3% underwent coronary artery bypass grafting. About 15% of the cohort had LVEFs <40%. Patients without beta-blocker prescriptions at discharge were older than those with such prescriptions. All other characteristics were similar between the groups (table 1).
Table 1 Patient characteristics according to beta blocker (BB) prescription at discharge (n = 266).
| Characteristic |
No BB at discharge
(n = 23) |
BB at discharge
(n = 243) |
Low BB dose at discharge
(n = 227) |
High BB dose at discharge
(n = 16) |
|
|---|---|---|---|---|---|
| Age, median (IQR) | 67.6 (52.2–73.0) | 63.6 (55.3–73.0) | 62.9 (55.3–73.0) | 66.7 (54.9–71.5) | |
| Age group, years, n (%) | <55 | 8 (34.8) | 59 (24.3) | 55 (24.2) | 4 (25.0) |
| 55–64 | 1 (4.4) | 73 (30.0) | 70 (30.9) | 3 (18.8) | |
| 65–74 | 9 (39.1) | 63 (25.9) | 56 (24.7) | 7 (43.8) | |
| ≥75 | 5 (21.7) | 48 (19.8) | 46 (20.3) | 2 (12.5) | |
| Male, n (%) | 17 (73.9) | 188 (77.4) | 176 (77.5) | 12 (75.0) | |
| BMI, kg/m2, n (%) | <25 | 8 (34.8) | 91 (37.5) | 84 (37.0) | 7 (43.8) |
| 25–29.9 | 10 (43.5) | 101 (41.6) | 96 (42.3) | 5 (31.3) | |
| ≥30 | 5 (21.7) | 51 (21.0) | 47 (20.7) | 4 (25.0) | |
| CV risk factors, n (%) | |||||
| Smoking | Never | 5 (21.7) | 86 (35.4) | 78 (34.4) | 8 (50.0) |
| Former | 6 (26.1) | 66 (27.2) | 64 (28.2) | 2 (12.5) | |
| Current | 12 (52.2) | 91 (37.5) | 85 (37.4) | 6 (37.5) | |
| Family history of CAD* | 7 (30.4) | 67 (31.2) | 63 (31.2) | 4 (30.8) | |
| Hypertension | 10 (43.5) | 110 (45.3) | 99 (43.6) | 11 (68.8) | |
| Dyslipidaemia† | 14 (63.6) | 155 (64.9) | 146 (65.2) | 9 (60.0) | |
| Diabetes‡ | 4 (18.2) | 28 (11.6) | 26 (11.5) | 2 (12.5) | |
| Medical history, n (%) | |||||
| Comorbidities (any) | 8 (34.8) | 80 (32.9) | 75 (33.0) | 5 (31.3) | |
| History of ACS‡ | 2 (8.7) | 33 (13.7) | 32 (14.2) | 1 (6.3) | |
| History of CABG‡ | 0 (0.0) | 7 (2.9) | 7 (3.1) | 0 (0.0) | |
| History of PCI§ | 1 (4.4) | 34 (14.1) | 32 (14.2) | 2 (12.5) | |
| Vital signs,¶ median (IQR) | SBP (mm Hg) | 116 (104–130) | 116 (107–129) | 115 (107–129) | 121 (117–130) |
| DBP (mm Hg) | 71 (63–76) | 70 (62–79) | 69 (62–78) | 78.5 (71.5–83.5) | |
| HR (bpm) | 74 (64–81) | 68 (63–80) | 73 (64–81) | 80 (75–89) | |
| Laboratory values, median (IQR) | Total cholesterol (mmol/l)‖ | 5.0 (4.1–5.6) | 5 (4.2–5.8) | 5 (4.2–5.8) | 4.7 (4.1–5.2) |
| Creatinine (µmol/l)** | 80 (64–86) | 85 (74–98) | 86 (75–98) | 81 (65–99) | |
| Coronary disease type, n (%) | Monovessel | 8 (34.8) | 102 (42.0) | 96 (42.3) | 6 (37.5) |
| Multivessel | 15 (65.2) | 141 (58.0) | 131 (57.7) | 10 (62.3) | |
| LVEF, n (%) | <30% | 0 (0.0) | 9 (3.7) | 9 (4.0) | 0 (0.0) |
| 30–39% | 3 (13.0) | 37 (15.2) | 34 (15.0) | 3 (18.8) | |
| ≥40% | 20 (87.0) | 137 (81.1) | 184 (81.1) | 13 (81.3) | |
| Therapeutic strategy, n (%) | PCI | 23 (100.0) | 238 (97.9) | 222 (97.8) | 16 (100.0) |
| CABG | 0 (0.0) | 3 (1.2) | 3 (1.3) | 0 (0.0) | |
| Conservative treatment | 0 (0.0) | 2 (0.8) | 2 (0.9) | 0 (0.0) | |
| Co-prescriptions, n (%) | Aspirin | 23 (100.0) | 239 (98.4) | 223 (98.2) | 16 (100.0) |
| P2Y12 inhibitor | 23 (100.0) | 241 (99.2) | 225 (99.1) | 16 (100.0) | |
| Statin | 21 (91.3) | 234 (96.3) | 219 (96.5) | 15 (93.8) | |
| ACEI | 21 (91.3) | 230 (94.7) | 214 (94.3) | 16 (100.0) | |
ACEI = angiotensin converting-enzyme inhibitor ACS = acute coronary syndrome; BMI = body mass index; CABG = coronary artery bypass surgery; CAD = coronary artery disease; CV = cardiovascular; DBP = diastolic blood pressure; HR = heart rate; IQR = interquartile range; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; SBP = systolic blood pressure Data missing for *28, †5, ‡2, §1, ¶6, ‖40 and **15 cases.
At the time of discharge, 91% of the patients were prescribed beta-blocker, 83% of whom had low doses. At one year, the proportions with no, high or low beta-blocker doses had changed only slightly in all groups. No differences in beta-blocker introduction or up-titration to ≥50% of the target dose were observed between the LVEF groups. Overall, the rate of beta-blocker withdrawal was about 12%. Reasons for withdrawal were not provided in the study data. Among the patients discharged with high beta-blocker doses (n = 16), seven (43.8%) had their doses reduced and one (6.3%) had the beta-blocker withdrawn during the year (table 2).
Table 2 Beta-blocker (BB) prescription at discharge and at one year, stratified by left ventricular ejection fraction (LVEF), with evolution over this period (n = 266).
| Time of assessment | LVEF <40% (n = 49) | LVEF ≥40% (n = 217) | |||||
|---|---|---|---|---|---|---|---|
| No BB | Low BB dose | High BB dose | No BB | Low BB dose | High BB dose | ||
| Discharge, n (%) | 3 (6.1) | 43 (87.8) | 3 (6.1) | 20 (9.2) | 184 (84.8) | 13 (6.0) | |
| No change | 2 (66.7) | 37 (86.0) | 3 (100.0) | 15 (75.0) | 151 (82.1) | 5 (38.5) | |
| Introduction | 1 (33.3) | – | – | 5 (25.0) | – | – | |
| Withdrawal | – | 4 (9.3) | – | – | 24 (13.0) | 1 (7.7) | |
| Up-titration | – | 2 (4.7) | – | – | 9 (4.9) | – | |
| Dose reduction | – | – | – | – | – | 7 (53.8) | |
| 1 year, n (%) | 6 (12.2) | 38 (77.6) | 5 (10.2) | 40 (18.4) | 163 (75.1) | 14 (6.5) | |
Of the 243 patients discharged with beta-blocker prescriptions, 194 (86.6%) received metoprolol at a median daily dose of 25 mg (IQR, 12.5–37.5 mg). The remaining patients received bisoprolol (n = 14/243, 5.8%; median daily dose 2.5 mg, IQR 2.5–5 mg), carvedilol (n = 10/243, 4.1%; median daily dose 9.375 mg, IQR, 6.25–12 mg), nebivolol (n = 5/243, 2.1%; median daily dose 2.5 mg, IQR 2.5–5 mg) or propranolol (n = 1/224, 0.5%; daily dose 80 mg). All raw data on the beta-blocker prescriptions are provided in the table S2 in appendix 1.
More patients were discharged without beta-blocker prescriptions from district hospitals than from the university hospital. The proportion of patients with high beta-blocker doses at discharge was greater in the district hospital discharge group, regardless of LVEF. The differences between the proportions with no beta-blocker, low dose beta-blocker and high dose beta-blocker at discharge from the university hospital versus from district hospitals were significant in the LVEF <40% group (p = 0.03), but not in the LVEF ≥40% group (fig. 2).
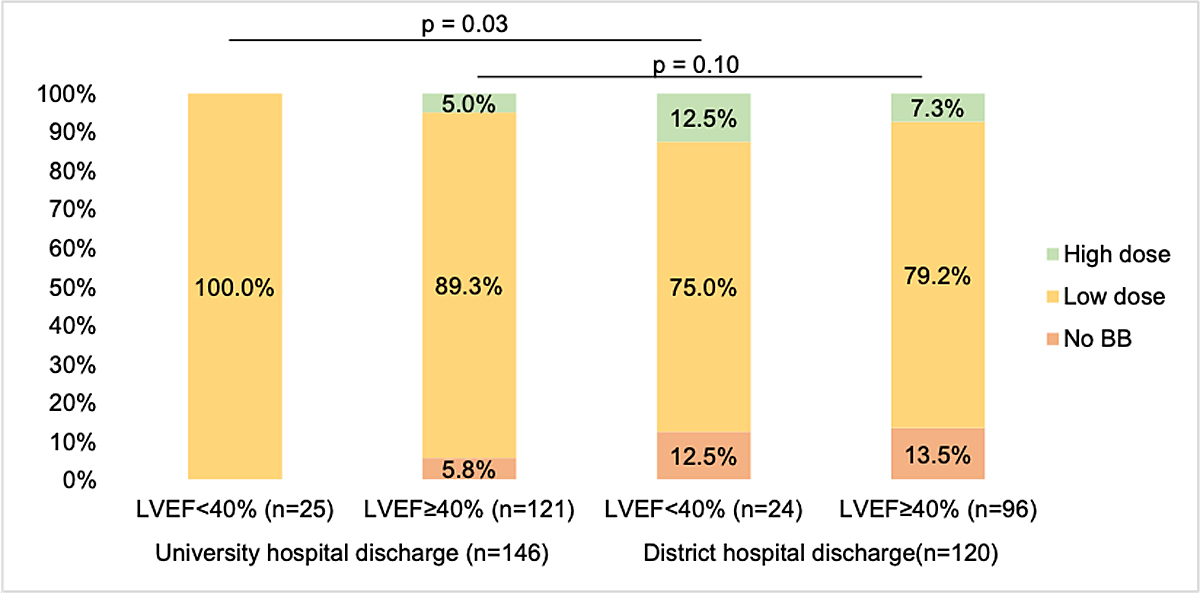
Figure 2 Beta-blocker prescription according to discharge hospital type (n = 266). BB = beta-blocker; LVEF = left ventricular ejection fraction
Table 3 shows that among patients with lengths of stay ≤2 days, the proportion of patients without beta-blocker prescriptions at one year was greater than the proportion of patients with such prescriptions (LVEF <40%: 4/6, 66.7% vs 9/43, 20.9%, p = 0.017; LVEF ≥40%: 24/40, 60.0% vs 56/177, 31.6%, p = 0.001). As university hospital stays ≤2 days correlated strongly with transfers to district hospitals (97.9% of such transfers occurred 1–2 days after STEMI), the same results were found for transfer to district hospitals. Rates of participation in cardiac rehabilitation programmes after discharge were similar between the groups, except among patients with no beta-blocker prescription and LVEFs ≥40%, of whom 40% did not participate in such programmes.
Table 3 Prescription of beta-blockers (BBs) at one year according to length of hospital stay, destination at discharge and participation in a cardiac rehabilitation (CR) programme and according to left ventricular ejection fraction (LVEF) (n = 266).
| LVEF <40% (n = 49) | LVEF ≥40% (n = 217) | |||||
|---|---|---|---|---|---|---|
|
No BB at 1 year
(n = 6) |
BB at 1 year
(n = 43) |
p-value |
No BB at 1 year
(n = 40) |
BB at 1 year
(n = 177) |
p-value | |
| Length of stay ≤2 days | 4 (66.7%) | 9 (20.9%) | 0.017 | 24 (60.0%) | 56 (31.6%) | 0.001 |
| Transfer to district hospital | 4 (66.7%) | 20 (46.5%) | 0.355 | 24 (60.0%) | 72 (40.7%) | 0.026 |
| No CR participation* | 1 (16.7%) | 7 (16.3%) | 0.980 | 16 (40.0%) | 17 (9.6%) | 0.000 |
* Data missing for six cases in the BB at one year group, two for LVEF < 40% and four for LVEF ≥ 40%.
We analysed the evolution of beta-blocker prescriptions among patients transferred to district hospitals (n = 120). Among patients discharged from the university hospital without beta-blocker, 69.0% (20/29) of those with LVEFs ≥40% and 83.3% (5/6) of those with LVEFs <40% subsequently had a beta-blocker prescribed at district hospital discharge (table 4).
Table 4 Changes in beta-blocker (BB) prescription from university to district hospital discharge in transferred patients (n = 120).
| Time of assessment | LVEF <40% (n = 24) | LVEF ≥40% (n = 96) | ||||||
|---|---|---|---|---|---|---|---|---|
| No BB | Low BB dose | High BB dose | No BB | Low BB dose | High BB dose | |||
| BB prescription at discharge from university hospital, n (%) | 6 (25.0) | 17 (70.8) | 1 (4.2) | 29 (30.2) | 65 (67.7) | 2 (2,1) | ||
| No change | 1 (16.7) | 12 (70.6) | 0 (0.0) | 9 (31.0) | 58 (89.2) | 1 (50.0) | ||
| Introduction | 5 (83.3) | – | – | 20 (69.0) | – | – | ||
| Withdrawal | – | 2 (11.8) | 0 (0.0) | – | 4 (6.2) | 0 (0.0) | ||
| Up-titration | – | 3 (17.7) | – | – | 3 (4.6) | – | ||
| Dose reduction | – | – | 1 (100.0) | – | – | 1 (50.0) | ||
| BB prescription at discharge from district hospital, n (%) | 3 (12.5) | 18 (75.0) | 3 (12.5) | 13 (13.5) | 76 (79.2) | 7 (7.3) | ||
LVEF = left ventricular ejection fraction
In this prospective cohort of patients hospitalised for STEMI, beta-blockers were prescribed at discharge in 91% of cases. At one year after discharge, this proportion remained high but had diminished slightly (82%). These beta-blocker prescription proportions are consistent with those found in other studies. For example, Auer et al. [17] reported a beta-blocker prescription rate of 93% for patients discharged after ACS from hospitals in Switzerland. However, our study adds important information about dosing and up-titration practices. We found that the doses prescribed at discharge were below those with established benefits: the majority of patients received doses <50% of the target doses. Moreover, up-titration was performed during the year in <5% of cases, with the consequence that only 7% of patients were receiving optimal doses at one year. These results were expected for patients with preserved LVEF, but are worrying for patients with reduced LVEFs, only 10% of whom had optimal doses at one year. The few studies from the US that have examined long-term beta-blocker dosing yield similar results [14, 18, 19]. These results reflect a change in contemporary practice, which is more focused on achieving a target heart rate (e.g., 60–70 bmp) than on up-titrating doses until the target dose chosen in initial clinical trials is reached. Moreover, more patients had their beta-blocker dose decreased or totally withdrawn than increased. This reflects a difference between real-life practice and that during clinical trials: real patients are older, have more comorbidities, suffer from side effects or do not want to take medicines. It is therefore more difficult to achieve the target dosing. However, many patients had no change at all in their beta-blocker prescription. Thus, we postulate that this is probably the consequence of short hospital stays and clinical inertia.
We found that more patients were discharged without beta-blocker prescriptions from district hospitals (i.e., after transfer) than from the university hospital. Moreover, we demonstrated that a large proportion of patients with no beta-blocker prescription at one year had short university hospital stays for both LVEF groups. As a short length of stay was due mainly to rapid transfer to district hospitals after PCI, we analysed the evolution of beta-blocker prescription between discharge from the university hospital and discharge from the district hospital after transfer. We found that 25–30% of transferred patients did not have any beta-blocker prescriptions at the time of university hospital discharge. After transfer, beta-blocker were not introduced in the district hospitals for about 31% of patients with preserved LVEFs, and for 16.7% of those with reduced LVEFs. These findings imply that a rapid transfer to district hospitals, and thus a short length of stay in the university hospital, is probably a reason for poor beta-blocker prescription. As these patterns were investigated as secondary outcomes in this study, we did not statistically analyse them by accounting for cofounders. Thus, these findings should be taken with caution because many other factors (e.g., disease severity, contraindications not known during university hospital stay, patient intolerance) could have affected beta-blocker prescription during district hospital stays.
Several explanations can be offered for the prevalence of low beta-blocker doses at discharge, the low prevalence of beta-blocker up-titration and the relationship between short hospital stays and poor beta-blocker prescription. Beta-blocker prescription can be difficult due to known side effects such as dizziness and fatigue [20]. Additionally, in the early phase after STEMI, many patients develop bradycardia or hypotension, which contraindicate beta-blocker prescription. Hospital stays for STEMI have been shortened in recent years, with about half of patients transferred to district hospitals within 24–48 hours, leaving physicians with insufficient time for evidence-based medication prescription. The reasons for the lack of up-titration during university hospital stays are probably similar, as up-titration to a target dose is performed over a period of weeks. However, these issues do not explain the infrequency of up-titration or beta-blocker introduction after transfer from a university hospital. We hypothesise that prescribers at district hospitals and in ambulatory care settings have great confidence in the prescription decisions made by specialists at university hospitals, making them less likely to make changes. Additionally, as beta-blockers are not well tolerated in clinical practice, many patients probably report side effects such as fatigue, effort intolerance and sexual dysfunction during outpatient visits. As these side effects are dose related, general practitioners (GPs) may be reluctant to up-titrate. Moreover, as outpatients are not monitored, the fear of provoking bradycardia or atrioventricular block may lead prescribers to avoid up-titration. Finally, we believe that clinical inertia contributes to this trend. Dose up-titration is not the first therapeutic goal of GPs, who may not be familiar with target doses (especially given their absence from guidelines) and have other medical issues to address during patient visits. Our data did not indicate the type of physician (i.e., generalist vs cardiologist) in charge of prescription during outpatient care, or whether patients were followed via outpatient cardiology consultation at the university hospital. As Allen et al. [21] found that cardiologists were more likely than generalists to intensify medication therapy after myocardial infarction, such information would have enabled interesting comparisons that could have led to the proposal of strategies for improvement.
Overall, our results show that up-titration occurs infrequently in real-life practice, reflecting uncertainty about the optimal doses for patients with STEMI. These findings cannot be criticised for patients without reduced LVEF, because literature data do not provide sufficient evidence for this particular point. At the time that this article was written, patients were being recruited for two randomised controlled trials assessing the efficacy of beta-blockers in the modern era [22, 23]. These two studies will add important knowledge about the use of beta-blockers in patients with preserved LVEFs, and their findings will certainly help cardiologists decide whether they should prescribe beta-blockers to all patients after STEMI, and at which doses. In the meantime, we believe that beta-blockers should still be prescribed to all patients after STEMI if there is no contraindication. Up-titration should be employed specifically in patients with reduced LVEFs.
The poor beta-blocker prescription and up-titration practices revealed by this study demonstrate a need for improvement. The strong relationship between the type of healthcare provider and beta-blocker prescription implies that the quality of prescription could be optimised by improving the continuum of care. We found that many discharge letters did not contain high-quality information about the long-term management of patients with chronic illness and were not sufficient to ensure good communication between healthcare providers. Strategies such as improved care coordination have been shown to benefit the management of diverse chronic illnesses [24]. One study showed that clinical inertia could be reduced with interventions such as the provision of feedback and reminders (computerised or in face-to-face sessions with an endocrinologist) to the clinician at each visit of a patient with diabetes [25]. Regrettably, time and money are required for the development of such interventions. While these strategies could be developed over the long term, shorter-term strategies should be used in the meantime.
Our findings lead us to emphasise the unequivocal importance of beta-blocker prescription and up-titration for patients with reduced LVEFs. One strategy to enhance communication between healthcare providers (i.e., district hospitals, outpatient cardiologists, GPs, cardiac rehabilitation centres and pharmacies), as well as with patients, would be to discharge these patients from the university hospital with treatment plans containing information about their long-term management. These plans could contain recommendations for beta-blocker dose up-titration within several weeks of discharge. Where beta-blocker have not been introduced during the university hospital stay, the reason for non-prescription and, if appropriate, recommendations for beta-blocker introduction and up-titration, should be provided in the plan. Such improvements in communication should be implemented urgently for high-risk patients.
Our study has several strengths. To the best of our knowledge, our study is the first in Switzerland to report beta-blocker prescriptions after STEMI over a period of one year, including the dosing and up-titration. Our results represent real-life conditions and raise the issue of clinical inertia. Although our results cannot be generalised to every hospital in the world, we are convinced that the problem of clinical inertia is known everywhere. Our results can therefore be used as a benchmark and as a reminder of the importance of up-titration after discharge.
The major limitation of our study is the small study population, due in part to considerable loss to follow-up. Moreover, we had to exclude several patients because some of their data were lacking, which is also a source of bias. Lost patients were significantly younger than those who completed follow-up and may have had more severe disease and lower beta-blocker tolerance. However, we were able to observe clear trends in this small sample, which should be confirmed in a larger cohort.
Another limitation is related to the lack of some relevant information, such as pre-admission prescription data. A patient taking beta-blockers before STEMI would probably have been more likely to be on target-dose beta-blockers during hospitalisation than beta-blocker naïve patients. In addition, we were not able to assess reasons for beta-blocker withdrawal or dose reduction during the one-year follow-up period. During telephone interviews, many patients could not describe their practitioners’ reasons for beta-blocker withdrawal or introduction. Withdrawal was likely performed for good reasons (i.e. intolerance) for many patients, but conditions such as bradycardia or hypotension may have subsequently normalised in some patients, permitting re-prescription. Furthermore, some patients might have provided inaccurate information. Finally, we did not have data on patients’ vital parameters and LVEF after one year. LVEF dysfunction can resolve during the period after STEMI, removing the need for beta-blocker use.
The results of the present study provide information on long-term beta-blocker prescription to patients admitted to a university hospital in Switzerland for STEMI. Although only a small proportion of these patients were discharged from the hospital with no beta-blocker prescription, we found that beta-blocker under-dosing is an issue, as only a very small proportion of patients were receiving target beta-blocker doses at one year after discharge. This underdosing is especially worrying for patients with reduced LVEFs. To optimise beta-blocker prescription at the time of discharge and in the long term, we suggest the provision of patient plans at the time of discharge to maximise the continuum of care through better communication between healthcare providers.
Table S1: Beta-blocker doses classified in two categories depending of the target dose (low dosing, <50% and high dosing, ≥50%).
Table S2: Beta-blockers prescribed at discharge and at one year for each participant (n = 266), classified by left ventricular ejection fraction (LVEF) category.
The appendix is available as a separate file at https://smw.ch/article/doi/smw.2020.20321.
No financial support was obtained for this study.
SF has received consulting fees from Bayer, Amgen and Cathworks and speaker fees from Bayer, Amgen and Biolvanile. The other authors have no conflict of interest. They received no grants from any funding agencies in the public, commercial, or not-for-profit sectors for this research.
1 A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247(12):1707–14. doi:.https://doi.org/10.1001/jama.1982.03320370021023
2 Hjalmarson A , Elmfeldt D , Herlitz J , Holmberg S , Málek I , Nyberg G , et al. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet. 1981;318(8251):823–7. doi:.https://doi.org/10.1016/S0140-6736(81)91101-6
3 Herlitz J , Elmfeldt D , Hjalmarson A , Holmberg S , Málek I , Nyberg G , et al. Effect of metoprolol on indirect signs of the size and severity of acute myocardial infarction. Am J Cardiol. 1983;51(8):1282–8. doi:.https://doi.org/10.1016/0002-9149(83)90299-0
4 Snow PJ . Effect of propranolol in myocardial infarction. Lancet. 1965;286(7412):551–3. doi:.https://doi.org/10.1016/S0140-6736(65)90863-9
5 Ibanez B , James S , Agewall S , Antunes MJ , Bucciarelli-Ducci C , Bueno H , et al.; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. doi:.https://doi.org/10.1093/eurheartj/ehx393
6 O’Gara PT , Kushner FG , Ascheim DD , Casey DE, Jr , Chung MK , de Lemos JA , et al., American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. doi:.https://doi.org/10.1161/CIR.0b013e3182742c84
7 Dargie HJ . Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–90. doi:.https://doi.org/10.1016/S0140-6736(00)04560-8
8 Huang BT , Huang FY , Zuo ZL , Liao YB , Heng Y , Wang PJ , et al. Meta-Analysis of Relation Between Oral β-Blocker Therapy and Outcomes in Patients With Acute Myocardial Infarction Who Underwent Percutaneous Coronary Intervention. Am J Cardiol. 2015;115(11):1529–38. doi:.https://doi.org/10.1016/j.amjcard.2015.02.057
9 De Luca G , de Boer MJ , Ottervanger JP , van ’t Hof AW , Hoorntje JC , Gosselink AT , et al. Impact of beta-blocker therapy at discharge on long-term mortality after primary angioplasty for ST-segment elevation myocardial infarction. Am J Cardiol. 2005;96(6):806–9. doi:.https://doi.org/10.1016/j.amjcard.2005.05.025
10 Kernis SJ , Harjai KJ , Stone GW , Grines LL , Boura JA , O’Neill WW , et al. Does beta-blocker therapy improve clinical outcomes of acute myocardial infarction after successful primary angioplasty? J Am Coll Cardiol. 2004;43(10):1773–9. doi:.https://doi.org/10.1016/j.jacc.2003.09.071
11 Nakatani D , Sakata Y , Suna S , Usami M , Matsumoto S , Shimizu M , et al.; Osaka Acute Coronary Insufficiency Study (OACIS) Investigators. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol. 2013;111(4):457–64. doi:.https://doi.org/10.1016/j.amjcard.2012.10.026
12 Rydén L , Ariniego R , Arnman K , Herlitz J , Hjalmarson A , Holmberg S , et al. A double-blind trial of metoprolol in acute myocardial infarction. Effects on ventricular tachyarrhythmias. N Engl J Med. 1983;308(11):614–8. doi:.https://doi.org/10.1056/NEJM198303173081102
13 Goldberger JJ , Bonow RO , Cuffe M , Liu L , Rosenberg Y , Shah PK , et al.; OBTAIN Investigators. Effect of Beta-Blocker Dose on Survival After Acute Myocardial Infarction. J Am Coll Cardiol. 2015;66(13):1431–41. doi:.https://doi.org/10.1016/j.jacc.2015.07.047
14 Allen JE , Knight S , McCubrey RO , Bair T , Muhlestein JB , Goldberger JJ , et al. β-blocker dosage and outcomes after acute coronary syndrome. Am Heart J. 2017;184:26–36. doi:.https://doi.org/10.1016/j.ahj.2016.10.012
15 Barron HV , Viskin S , Lundstrom RJ , Swain BE , Truman AF , Wong CC , et al. Beta-blocker dosages and mortality after myocardial infarction: data from a large health maintenance organization. Arch Intern Med. 1998;158(5):449–53. doi:.https://doi.org/10.1001/archinte.158.5.449
16 Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JGF , Coats AJS , et al.; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:.https://doi.org/10.1093/eurheartj/ehw128
17 Auer R , Gencer B , Räber L , Klingenberg R , Carballo S , Carballo D , et al. Quality of care after acute coronary syndromes in a prospective cohort with reasons for non-prescription of recommended medications. PLoS One. 2014;9(3):e93147. doi:.https://doi.org/10.1371/journal.pone.0093147
18 Goldberger JJ , Bonow RO , Cuffe M , Dyer A , Rosenberg Y , O’Rourke R , et al.; PACE-MI Investigators. beta-Blocker use following myocardial infarction: low prevalence of evidence-based dosing. Am Heart J. 2010;160(3):435–442.e1. doi:.https://doi.org/10.1016/j.ahj.2010.06.023
19 Gislason GH , Rasmussen JN , Abildstrøm SZ , Gadsbøll N , Buch P , Friberg J , et al. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27(10):1153–8. doi:.https://doi.org/10.1093/eurheartj/ehi705
20 Bhatt AS , DeVore AD , DeWald TA , Swedberg K , Mentz RJ . Achieving a Maximally Tolerated β-Blocker Dose in Heart Failure Patients: Is There Room for Improvement? J Am Coll Cardiol. 2017;69(20):2542–50. doi:.https://doi.org/10.1016/j.jacc.2017.03.563
21 Arnold SV , Spertus JA , Masoudi FA , Daugherty SL , Maddox TM , Li Y , et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62(19):1791–801. doi:.https://doi.org/10.1016/j.jacc.2013.04.102
22U.S. National Library of Medicine. Evaluation of decreased usage of Betablockers after myocardial infarction in the SWEDEHEART registry (REDUCE-SWEDEHEART). https://clinicaltrials.gov/ct2/show/NCT03278509?term=03278509&draw=2&rank=1. Accessed 2019 November 4.
23U.S. National Library of Medicine. Betablocker Treatment After Acute Myocardial Infarction in Patients Without Reduced Left Ventricular Systolic Function (BETAMI). https://clinicaltrials.gov/ct2/show/NCT03646357?term=03646357&draw=2&rank=1. Accessed 2019 November 4.
24 Peikes D , Chen A , Schore J , Brown R . Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–18. doi:.https://doi.org/10.1001/jama.2009.126
25 Ziemer DC , Doyle JP , Barnes CS , Branch WT, Jr , Cook CB , El-Kebbi IM , et al. An intervention to overcome clinical inertia and improve diabetes mellitus control in a primary care setting: Improving Primary Care of African Americans with Diabetes (IPCAAD) 8. Arch Intern Med. 2006;166(5):507–13. doi:.https://doi.org/10.1001/archinte.166.5.507
No financial support was obtained for this study.
SF has received consulting fees from Bayer, Amgen and Cathworks and speaker fees from Bayer, Amgen and Biolvanile. The other authors have no conflict of interest. They received no grants from any funding agencies in the public, commercial, or not-for-profit sectors for this research.