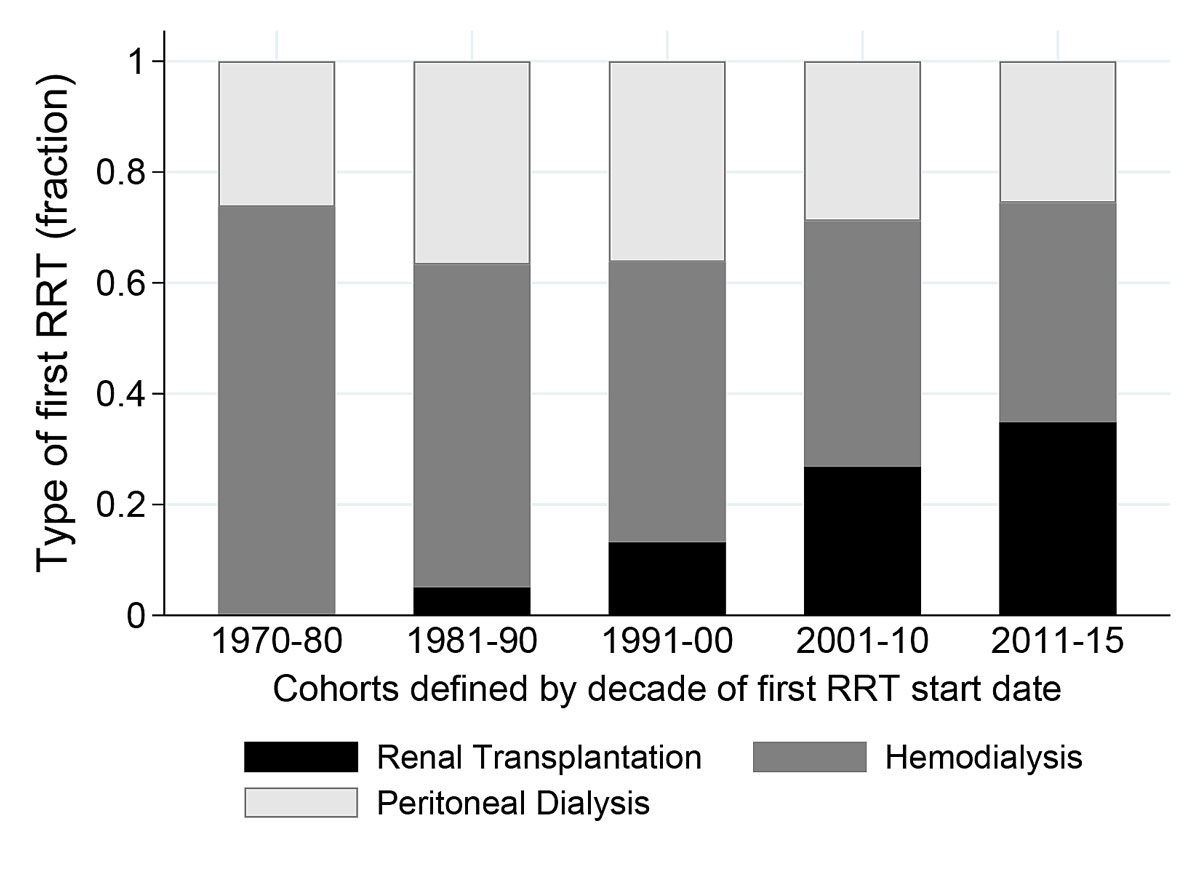
Figure 1 First renal replacement therapy (RRT) in different decades.
DOI: https://doi.org/10.4414/smw.2020.20300
congenital anomalies of the kidney and urinary tract
confidence interval
European Society for Paediatric Nephrology
end-stage renal disease
glomerular filtration rate
interquartile range
per million person-years
Swiss Association for Paediatric Nephrology
Swiss Paediatric Renal Registry
Chronic kidney disease is a rare but severe condition in childhood and adolescence, leading to end-stage renal disease (ESRD). Treatment – renal replacement therapy – includes haemodialysis, peritoneal dialysis and renal transplantation from deceased or living donors. ESRD is associated with high morbidity and mortality, including cardiovascular complications, growth retardation, psychosocial problems and impaired quality of life [1–6]. Renal transplantation improves long-term outcome and quality of life [7, 8], but the necessary immunosuppressive treatment leads to side effects, in particular infections and malignancies [9, 10].
National and international registries developed during recent years describe the incidence, prevalence, underlying primary disease, treatment modalities and complications in children with ESRD [11–15]. This allows long-term outcomes to be examined and comparisons between countries to be made [13, 16–20]. However, registries use different inclusion criteria and outcome parameters, and many started only recently. Longitudinal data describing time trends in primary diseases, treatment modalities and survival are rare [7, 21, 22].
The Swiss Paediatric Renal Registry (SPRR) was established as a prospective cohort study in 1970, together with the launch of paediatric renal replacement therapy in Switzerland. In 1970, haemodialysis and renal transplantation were introduced in Switzerland, followed by continuous ambulatory peritoneal dialysis. In 1995, renal replacement therapy was introduced for children with syndromes and variable cognitive impairment. Since 2009, data have been regularly delivered to the European Society of Paediatric Nephrology (ESPN) Registry. Previous SPRR publications have described the outcomes of 84 children treated from 1970 to 1994 [21], of 48 patients transplanted from 1992 to 1999 [23] and of 51 children treated from 2001 to 2014, describing the effect of the Swiss Organ Allocation System prioritising children and adolescents, introduced by law in Switzerland in 2007 [24].
This publication gives a comprehensive overview of the clinical epidemiology of ESRD in Switzerland during the past 45 years (1970 – 2015). It describes (1) the incidence of ESRD in children and adolescents, (2) the spectrum of primary renal disease, (3) trends in different treatment modalities (haemodialysis, peritoneal dialysis, renal transplantation) and 4) long-term outcomes.
The SPRR was built as a prospective cohort of all children who develop ESRD before adulthood. Children and adolescents newly diagnosed at one of the five Swiss paediatric nephrology units (University Children's Hospitals of Basel, Berne, Geneva, Lausanne and Zurich) were reported to the registry. Inclusion criteria were dialysis for more than three months and/or renal transplantation. Baseline data at the start of renal replacement therapy were complemented with annual follow-up data on treatment changes, graft failure and cause of death. Appendix 1 gives an overview of the data collected from 1970 to 2015. Ethical approval was granted to the SPRR through the Ethics Committee of the Canton of Bern (KEK-BE: 140/2015).
Incidence was calculated for the period 1981 to 2015 and for the age group 0 to 14 years. For this group, completeness of the incidence data was estimated at 95.5%. Incomplete patient inclusion was reported in the years before 1980 and in the age group 15 to 19 years, leading to the decision to exclude this data from the incidence calculation. The age-standardised annual incidence per million persons was calculated using the official end-year Swiss resident population as the denominator [25]. Patients living abroad were excluded because they were not represented in the reference population. The increase in incidence over time was tested using a linear regression model. Differences in incidence between boys and girls were examined using student’s t-tests.
Primary renal disease and all other analyses except incidence included all paediatric patients from the SPRR who gave informed consent or (if applicable) assent. Primary renal disease was categorised into congenital anomalies of the kidney and urinary tract (CAKUT), monogenetic hereditary diseases and acquired diseases. Primary renal diseases were described by number, frequency within the cohort, proportion of males diagnosed compared to females, age at onset of renal replacement therapy (described as mean and 95% confidence interval [CI]), and incidence (described as mean and 95% CI, using the incidence dataset).
First renal replacement therapy: For each decade (1970–80, 1981–90, 1991–00, 2001–10, 2011–15), the number of children treated with haemodialysis, peritoneal dialysis and pre-emptive renal transplantation was tabulated for the different age groups (0–4, 5–9, 10–14, 15–19 years). The percentages of children undergoing pre-emptive renal transplantation, haemodialysis and peritoneal dialysis were shown over the last 10 years (2006–2015) and over the whole study period by age group.
Duration of dialysis before first renal transplantation was calculated for each child. Children were grouped into cohorts depending on the decade in which renal replacement therapy was started. For each cohort, dialysis duration was displayed on a boxplot. The hypothesis that median waiting time on dialysis became shorter was tested using Mann-Whitney U-tests in which the most recent cohort (2011–2015) was compared to the earlier ones.
Graft donors in renal transplantation 1970–2015: Number of kidneys transplanted in Switzerland by type of graft donor was displayed graphically, summarised for five-year periods.
Graft and patient survival: Graft and patient survival were computed using Kaplan-Meier curves from four cohorts, i.e. children starting renal replacement therapy in 1970s, 1980s, 1990s and 2000s. Survival was compared between the four cohorts using log-rank tests. Overall patient survival rates after 5, 10 and 15 years were described. Graft and patient survival were censored at the end of the study or when the patient was lost to follow-up.
Causes of death were assessed from the hospital records.
STATA, version 15.1 was used to analyse the data (StataCorp. 2017. Stata Statistical Software: Release 15.1. College Station, TX: StataCorp LP).
Between 1981 and 2015, 227 children under 15 years of age started a chronic treatment for ESRD, the youngest being three weeks old. The mean incidence was 5.4 children per million children per year (per million person-years; pmpy) and had a tendency to increase over time (p = 0.085). The increase in incidence over time was most noticeable in children aged 0–4 years (in 1981–1984, incidence was 0 pmpy; in 1985–2015, mean incidence was 5.4, ranging from 0–16.5 pmpy). Incidence was higher in boys (6.3 pmpy) than in girls (4.5 pmpy, p = 0.009).
Out of all 367 children, 133 (36%) had CAKUT as the primary disease, 122 (33%) had a hereditary disease and 112 (31%) had an acquired disease (table 1). The most frequent diagnoses were obstructive uropathy with posterior urethral valves (incidence: 1.0 pmpy), medullary cystic disease including nephronophthisis (0.6 pmpy), congenital renal dysplasia with or without urinary tract malformation (0.6 pmpy) and congenital renal hypoplasia (0.4 pmpy). Mean age at onset of ESRD was 10.4 years across the whole study group. Onset of renal replacement therapy was later in patients with reflux-associated nephropathy (age 13.8 years) and Henoch-Schoenlein nephropathy (12.5 years) and earlier in children with congenital nephrotic syndrome (1.6 years) and atypical haemolytic uraemic syndrome (2.6 years, all p <0.0001). The remaining diagnoses were Alport’s syndrome, Eagle-Barrett syndrome, congenital nephrotic syndrome (Finnish type), Bardet-Biedl syndrome, Branchiootorenal syndrome, interstitial nephritis, IgA nephropathy, renal vein thrombosis, proliferative glomerulonephritis, systemic lupus erythematosus, Wilms tumour / Denys-Drash syndrome, granulomatosis with polyangiitis, diabetic nephropathy and anti-glomerular basement membrane disease, each of which affected fewer than five patients.
Table 1 Primary renal diseases.
| Primary renal disease | All paediatric patients | Males/ females | Age at start of RRT (years) |
Children
0–14 years* |
Incidence pmpy
(age 0–14 years)* |
|||
|---|---|---|---|---|---|---|---|---|
| n | % | Mean | 95% CI | n | Mean | 95% CI | ||
| All primary renal diseases | 367 | 100 | 1.4 | 10.4 | 9.9–11.0 | 227 | 5.4 | 4.7–6.1 |
| CAKUT | 133 | 36.2 | 2.4 | 10.6 | 9.6–11.5 | 78 | 1.9 | 1.5–2.2 |
| Renal hypoplasia | 33 | 9 | 1.8 | 10.7 | 8.9–12.4 | 18 | 0.4 | 0.2–0.6 |
| Congenital obstructive uropathy, posterior urethral valves† | 32 | 8.7 | n.a.† | 9.4 | 7.2–11.6 | 21 | 1.0† | 0.7–1.4 |
| Congenital renal dysplasia with or without urinary tract malformation | 36 | 9.8 | 2 | 9.5 | 7.4–11.5 | 25 | 0.6 | 0.4–0.8 |
| Reflux-associated nephropathy | 24 | 6.5 | 1.7 | 13.8 | 12.2–15.3 | 8 | 0.2 | 0.1–0.3 |
| Oligomeganephronia hypoplasia | 4 | 1.1 | 0.3 | 10.5 | 4.5–16.4 | 4 | 0.1 | 0.0–0.2 |
| Other forms of CAKUT | 4 | 1.1 | 0.3 | 11 | 0.0–20.0 | 2 | 0.0 | 0.0–0.1 |
| Monogenetic hereditary diseases | 122 | 33.2 | 1.3 | 10.5 | 9.5–11.5 | 85 | 2 | 1.6–2.5 |
| Medullary cystic disease, including nephronophthisis | 41 | 11.2 | 1 | 11.8 | 10.5–13.3 | 27 | 0.6 | 0.4–0.9 |
| Cystinosis | 17 | 4.6 | 1.1 | 11.3 | 9.8–12.9 | 13 | 0.3 | 0.1–0.5 |
| Nephrotic syndrome (unspecified) | 12 | 3.3 | 0.7 | 9 | 5.9–12.2 | 11 | 0.3 | 0.1–0.5 |
| Nephrotic syndrome (DMS) | 8 | 2.2 | 1 | 1.6 | 0.1–3.1 | 6 | 0.2 | 0.0–0.3 |
| Nephrotic syndrome (FSGS) | 6 | 1.6 | 2 | 10.4 | 3.9–17.0 | 5 | 0.1 | 0.0–0.2 |
| Polycystic kidneys (autosomal recessive) | 9 | 2.5 | 1.3 | 7.7 | 2.3–13.0 | 3 | 0.1 | 0.0–0.3 |
| Primary hyperoxaluria type 1 | 8 | 2.2 | 1.7 | 11.2 | 5.8–16.6 | 8 | 0.1 | 0.0–0.2 |
| Other monogenetic hereditary diseases | 21 | 5.7 | 3.2 | 12.4 | 10.0–14.9 | 12 | 0.3 | 0.1–0.5 |
| Acquired diseases | 112 | 30.6 | 0.8 | 10.1 | 9.2–11.1 | 64 | 1.5 | 1.2–1.9 |
| FSGS | 33 | 9 | 0.8 | 11.4 | 10.1–12.7 | 14 | 0.3 | 0.1–0.5 |
| Glomerulonephritis (unspecified) | 16 | 4.4 | 1 | 10.6 | 8.4–12.7 | 7 | 0.2 | 0.0–0.3 |
| Atypical haemolytic uraemic syndrome, (diarrhoea-negative) | 12 | 3.3 | 0.7 | 2.6 | 0.7–4.4 | 11 | 0.3 | 0.1–0.4 |
| Membranoproliferative glomerulonephritis | 12 | 3.3 | 0.7 | 11.4 | 8.8–14.0 | 7 | 0.2 | 0.0–0.3 |
| Cortical necrosis | 8 | 2.2 | 0.1 | 9.6 | 3.0–16.2 | 3 | 0.1 | 0.0–0.2 |
| Henoch-Schoenlein nephropathy | 5 | 1.4 | 0.3 | 12.5 | 10.9–14.2 | 4 | 0.1 | 0.0–0.2 |
| Other acquired diseases | 26 | 7.1 | 1 | 10.8 | 8.7–12.9 | 18 | 0.4 | 0.2–0.6 |
CI = confidence interval; CAKUT = congenital anomalies of the kidney and urinary tract; DMS = diffuse mesangial sclerosis; FSGS = primary focal segmental glomerulosclerosis; pmpy = per million population at risk per year; RRT = renal replacement therapy * Incidence was calculated using a subset of 227 children aged 0 to 14 years starting RRT in 1981–2015. † Congenital obstructive uropathy, posterior urethral valves concern only males. Male/female ratio is therefore not applicable (n.a.). Incidence was calculated using population at risk: boys aged 0 to 14 years.
As first treatment, 194 (53%), 116 (32%) and 57 (15%) children underwent haemodialysis, peritoneal dialysis and pre-emptive renal transplantation respectively. Age at the beginning of the first renal replacement therapy was 0–4 years for 70 (19%), 5–9 years for 78 (21%), 10–14 years for 138 (38%) and 15–19 years for 81 (22%) patients. The choice of starting therapy differed between age groups (p <0.001). Children aged 0–4 years usually started renal replacement therapy with peritoneal dialysis, whereas older children started primarily with haemodialysis. Table 2 shows the time trends of first renal replacement therapy for different age groups. Pre-emptive renal transplantation became more frequent over time (fig. 1). Between 2001 and 2010, 27% (n = 25) of all first renal transplantation were pre-emptive, with 23 being living-related and only two (8%) from deceased donors. Between 2011 and 2015, 41% (n = 15) of all first renal transplantation were pre-emptive, with 11 (73%) originating from deceased donors.
Table 2 Time trends of first renal replacement therapy in 367 children of different ages.
| Age (years) |
0 to 4
n = 70 |
5 to 9
n = 78 |
10 to 14
n = 138 |
15 to 19
n = 81 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First RRT (n) | HD | PD | RTPL | HD | PD | RTPL | HD | PD | RTPL | HD | PD | RTPL |
| 1970–1980 | 1 | 2 | 0 | 11 | 6 | 0 | 23 | 5 | 0 | 7 | 2 | 0 |
| 1981–1990 | 1 | 9 | 0 | 4 | 8 | 3 | 20 | 11 | 1 | 21 | 1 | 0 |
| 1991–2000 | 2 | 19 | 2 | 10 | 7 | 3 | 21 | 4 | 2 | 13 | 3 | 4 |
| 2001–2010 | 1 | 18 | 2 | 9 | 2 | 8 | 21 | 6 | 8 | 13 | 2 | 8 |
| 2011–2015 | 1 | 11 | 1 | 6 | 0 | 1 | 6 | 0 | 10 | 4 | 0 | 3 |
| 1970–2015 | 6 | 59 | 5 | 40 | 23 | 15 | 91 | 26 | 21 | 58 | 8 | 15 |
| First RRT (%) | HD | PD | RTPL | HD | PD | RTPL | HD | PD | RTPL | HD | PD | RTPL |
| 1970–2015 | 8.6 | 84.3 | 7.1 | 51.3 | 29.5 | 19.2 | 65.9 | 18.8 | 15.2 | 71.6 | 9.9 | 18.5 |
| 2006*–2015 | 4.0 | 84.0 | 12.0 | 66.7 | 0.0 | 33.3 | 48.4 | 12.9 | 38.7 | 62.5 | 4.2 | 33.3 |
HD = haemodialysis; PD = peritoneal dialysis; RTPL = renal transplantation; RRT = renal replacement therapy * 2007: Introduction of the Swiss organ allocation system

Figure 1 First renal replacement therapy (RRT) in different decades.
Median waiting time on dialysis was shorter in the cohort of 2011–15 compared with the cohorts starting renal replacement therapy before 2001 (all p <0.01), but was comparable with the cohort of 2001–2010 (p = 0.38, fig. 2). For the five decades 1970–80, 81–90, 91–00, 2001–10 and 2011–15, median time on dialysis until transplantation changed from 0.87 years (interquartile range [IQR] 1.79–0.49) to 1.60 years (IQR 2.57–0.52), 0.95 years (IQR 2.33–0.51), 0.58 years (IQR 1.44–0.00) and 0.34 years (IQR 1.13–0.14), respectively.
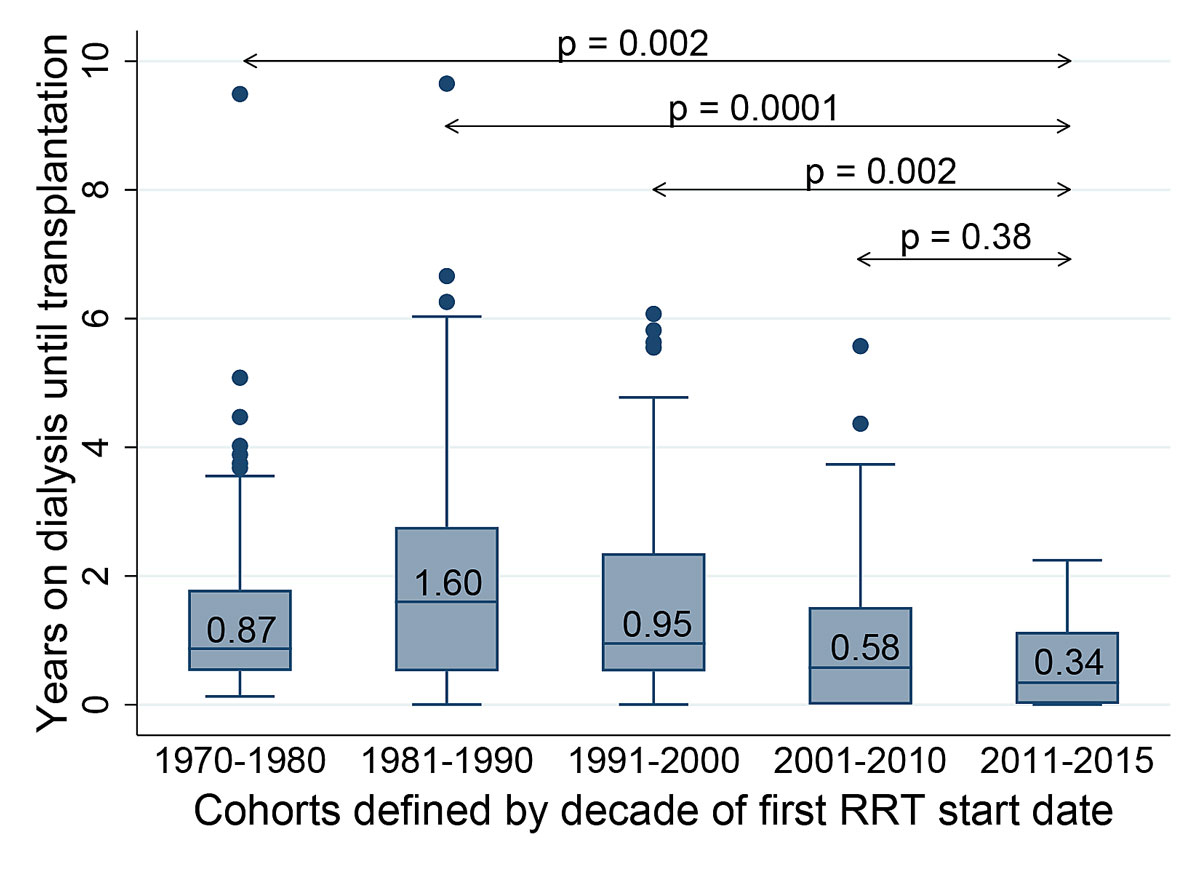
Figure 2 Duration of dialysis from start of first renal replacement therapy (RRT) until transplantation in different decades.
Overall, 322 (88%) children undergoing renal replacement therapy were transplanted before the age of 20 years. A second graft was transplanted in 43 (13%) and a third in four patients (1%) during their time in paediatric care. Living-related renal transplantation was introduced in 1986. The cumulative number of renal transplantation per five years increased from 1970 to 1985, but then plateaued in 1986 before peaking from 2006–2010 (i.e., the time period after the change of the law favouring deceased donor organ allocation to children had been implemented in 2007) (fig. 3). Deceased donor organ donation increased steadily up to 1985, then declined up to 2005. Since 2006 it has increased again, whereas living-related donation has decreased markedly (fig. 3). Living donors were related to the transplant receivers in 111 donations; five were non-related altruistic donors.
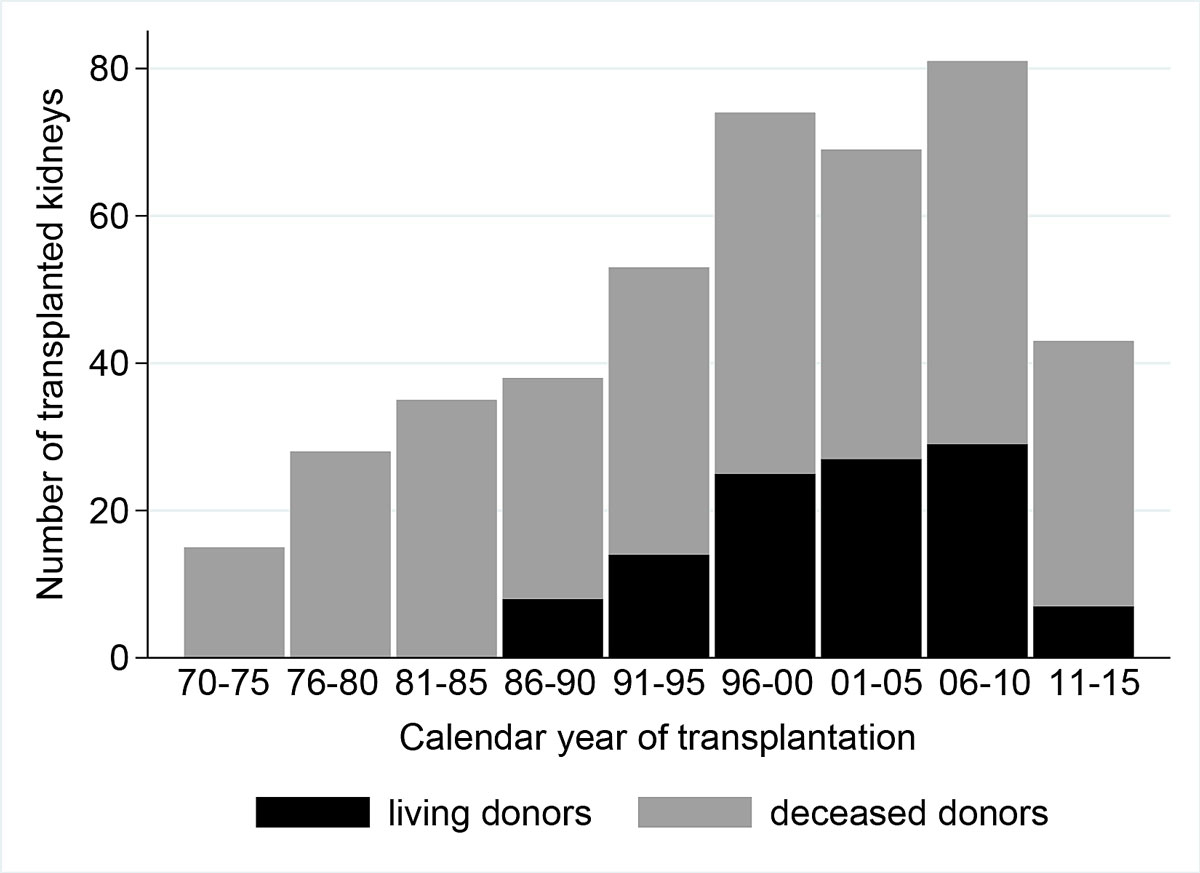
Figure 3 Number of transplanted grafts from deceased donors and living donors in paediatric patients in different periods.
Graft survival at five years is displayed by decade using Kaplan-Meier curves (fig. 4). Over the four decades (1970s, 1980s, 1990s and 2000s), the one-year graft survival rate changed from 0.76 (95% CI 0.60–0.86) to 0.80 (0.68–0.88), 0.89 (0.81–0.94) and 0.97 (0.90–0.99), respectively. The five-year graft survival rates in each decade were 0.44 (95% CI 0.28–0.58), 0.64 (0.54–0.79), 0.84 (0.73–0.90) and 0.89 (0.73–0.90), respectively. The pairwise comparison of graft survival between decades showed a gradual increase over time (70s versus 80s: p = 0.028; 80s versus 90s: p = 0.003; 90s versus 00s: p = 0.084).
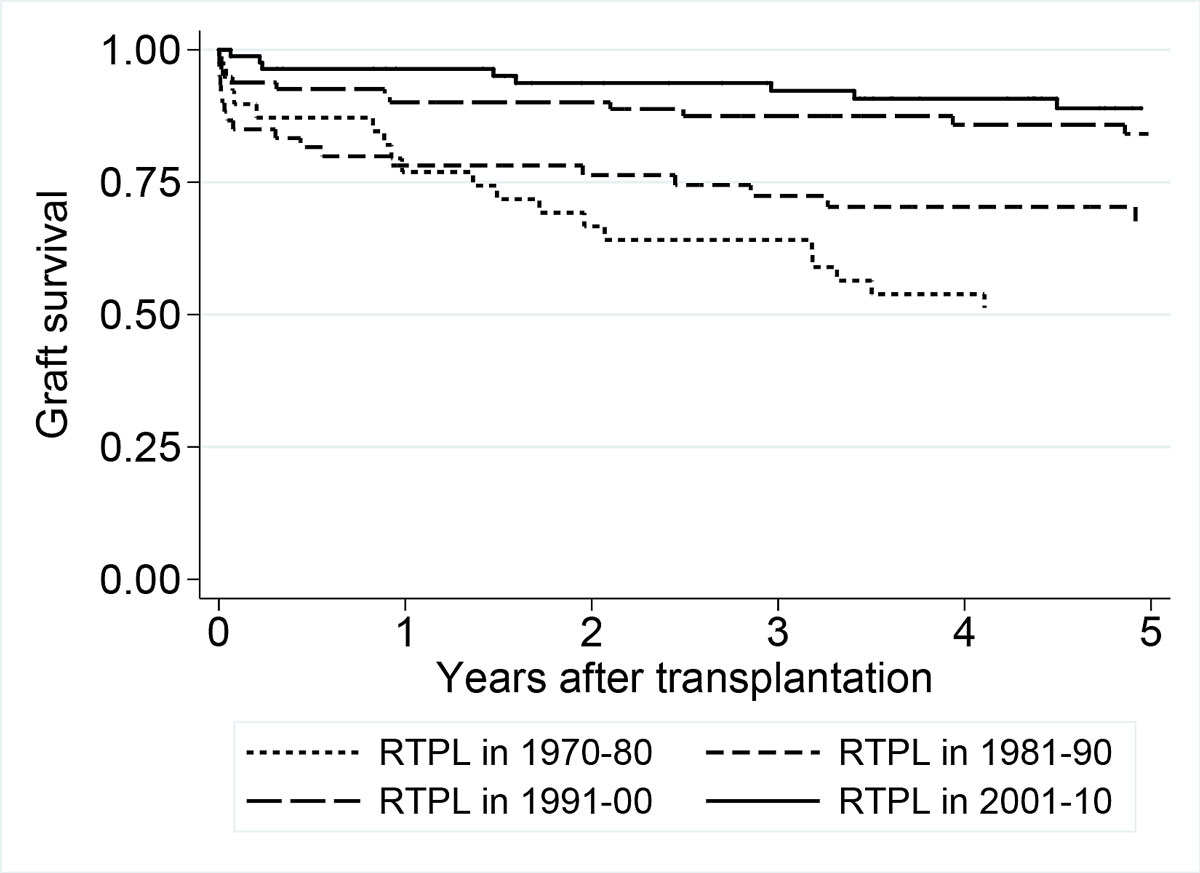
Figure 4 Renal transplantation (RTPL) graft survival displayed by decade of transplantation using Kaplan-Meier curves.
The patient survival rates for the entire cohort were 0.92 (n = 214; 95% CI 0.88–0.95), 0.87 (n = 143; 95% CI 0.81–0.91) and 0.79 (n = 81; Cl 0.70–0.86) at 5, 10 and 15 years, respectively (fig. 5). The five-year patient survival rates by ascending decade were 0.83 (95% CI 0.70–0.91), 0.99 (0.91–1.0), 0.93 (0.86–0.97) and 0.94 (0.86–0.97); the 10-year patient survival rates were 0.75 (95% CI 0.61–0.85), 0.96 (0.87–0.99), 0.88 (0.79–0.94) and 0.94 (0.86–0.97) (fig. 5). The highest patient survival rate was observed in the cohort starting renal replacement therapy in 1981–1990, in an era when preschool children and children with severe syndromic comorbidity had only limited access to chronic renal replacement therapy. Only 24 children in this cohort survived five years on renal replacement therapy, compared with 47 and 45 children in the 90s and 00s and with 15 children in the 70s. The pairwise comparison of patient survival between decades did not reveal significant differences between the curves (70s versus 80s: p = 0.20; 80s versus 90s: p = 0.79; 90s versus 00s: p = 0.74).
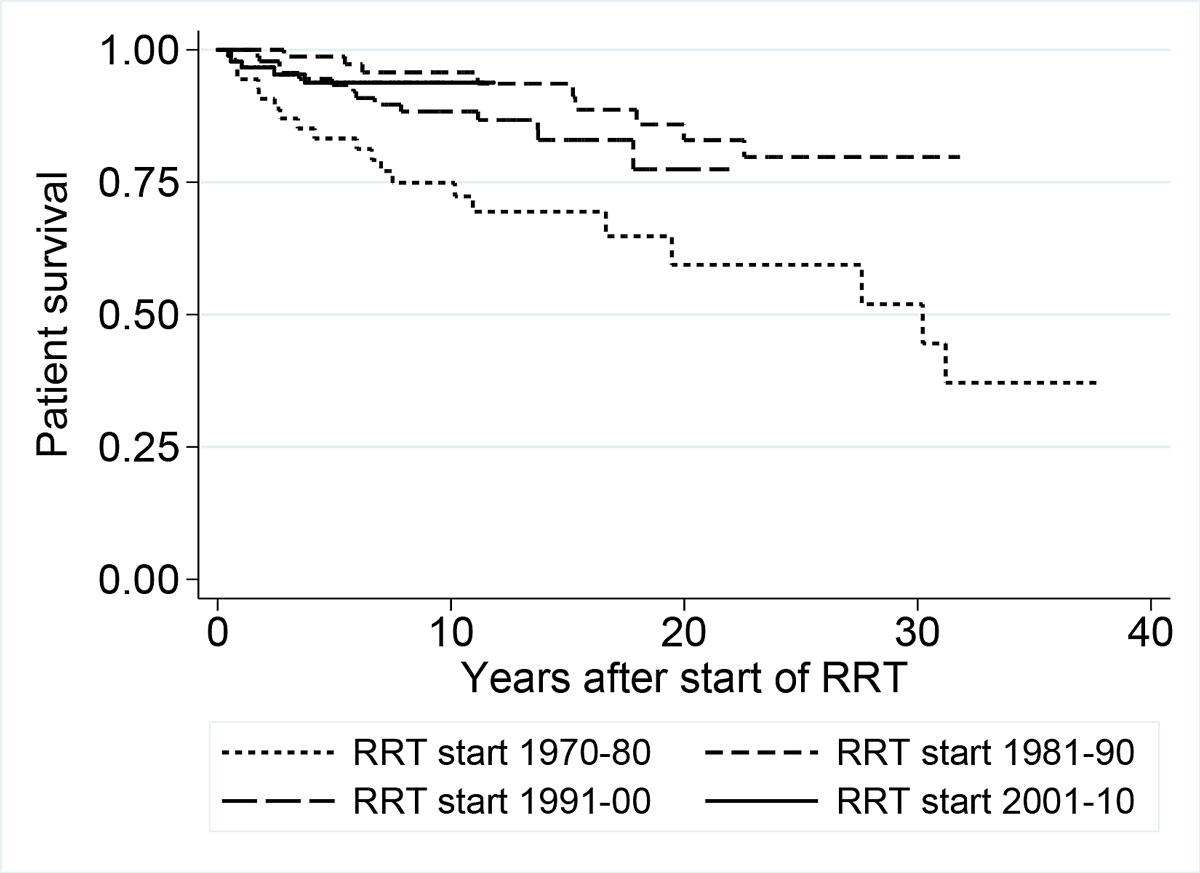
Figure 5 Renal replacement therapy (RRT) patient survival displayed by decade of start of treatment using Kaplan-Meier curves.
The survival rates of children aged 0–2 years at 1, 2, 5 and 10 years were 0.97, 0.95, 0.92 and 0.82 (95% CI 0.83–1.0, 0.81–0.99, 0.77–0.97 and 0.64–0.91), respectively. The survival rates of children younger than two years with chronic renal replacement therapy in the cohorts starting renal replacement therapy in the 1990s and the 2000s are comparable (p = 0.81). The survival rate in children younger than two years with chronic renal replacement therapy in the 1970s and 1980s was 1.0 at five years. In total, a higher number of children aged 0–2 years survived five years with renal replacement therapy in the 1990s and 2000s (n = 20) than in the 1970s and 1980s (n = 8). In syndromic children, the survival rate at five years was 1.0 before and after 1990. A higher number of syndromic children survived five years with renal replacement therapy in the 1990s and 2000s (n = 19) than in the 1970s and 1980s (n = 6).
There were 48 deaths in 3546 years of follow-up, resulting in 13.5 deaths per 1000 patient years. Twenty-nine (8%) of the 367 patients recorded in the registry died before the age of 20 years. Of those, six died on haemodialysis (two with vasculitis, two with multiorgan failure, one with hypertensive cardiac failure and one with uraemia) and six died on peritoneal dialysis (two with septicaemia, two with multiorgan failure, one with hyperkalaemia and one with a malignant solid tumour). There were 17 deaths after renal transplantation (six with multi-organ failure, six with septicaemia, three with cardiac arrest and two with uraemia).
From the very start of paediatric kidney transplantation in Switzerland in 1970, paediatric nephrologists established a national registry. This allows us today to summarise long-term results from children undergoing renal replacement therapy and to compare our data with other registries. Time trends of incidence, primary renal disease, treatment modalities and outcomes in 367 children are available over a period of 45 years. Between 1981 and 2015, 227 children aged 0–14 years were diagnosed with ESRD, resulting in an incidence of 5.4 children pmpy. The UK reported a higher incidence of 7.9 pmpy for children <16 years [26]. Comparing incidence with other European countries is difficult because inclusion criteria vary between studies [13]. In Sweden, incidence was reported at 7.7 pmpy, with 118 children diagnosed with chronic renal disease, defined as a glomerular filtration rate (GFR) <30 ml/min per 1.73 m2 body surface in children aged 0.5 – 15 years [17]. The ItalKid project reported an incidence of 12.1 pmpy, with 1197 patients aged 0–19 years with moderate to severe chronic renal disease, defined as a GFR of <75 ml/min per 1.73 m2 body surface. This project reported a lower incidence of patients undergoing renal transplantation of 7.3/year/100 patients [18]. In other countries, incidence is higher than in this study, but these rates include not only patients undergoing renal transplantation, but also children with impaired GFR [16, 19, 20]. It is thus not surprising that the incidence we report for Switzerland is lower, as we included only children with ESRD and chronic renal replacement therapy. Similar to other reports [13], we found that boys were more often affected than girls. In the long observation period, we found a small increase in the incidence of renal replacement therapy of 0.6 pmpy over 10 years. This might be explained by improvements in the intensive care of children with acute kidney injury [27], resulting in higher numbers of children with more complex renal disorders and multi-organ involvement, including cognitive impairment in syndromic patients, on renal replacement therapy, especially at a younger age (even from the neonatal period) [22]. The calculation of incidence by primary renal disease was based on very small numbers. Some chance variation may explain differences from findings in other countries.
Across the 45-year study period, the primary renal disease leading to renal replacement therapy in Switzerland was CAKUT in 36%, hereditary disease in 33% and acquired disease in 31% of patients. Registries in the USA [28], Italy [18], Belgium [20] and the United Kingdom [26] have also found that CAKUT is the most common primary renal disease resulting in ESRD. Registries from the ESPN [29] and Australia [30] show incidences of CAKUT similar to our data. However, registries with a high proportion (48–59%) of CAKUT refer to patients <20 years with chronic kidney disease (GFR <75 ml/min/1.73 m2) and not to patients with ESRD [18, 20]. As the inclusion criteria of the SPRR are similar to those of the ESPN and the Australian registry, these results are comparable. Children and adolescents with CAKUT have renal impairment, but ESRD and renal replacement therapy happen later in life. The median age of patients undergoing renal replacement therapy is 31 years [15].
Observation of treatment modality over such a long time illustrates key developments in paediatric renal medicine and the progress that has been achieved. We found an age-related difference in the type of dialysis modality, especially during the first three decades. While haemodialysis was the first treatment of choice in patients >five years of age in the seventies, peritoneal dialysis became the preferred first-line treatment in under-fives later on, as the technique and dialysis solutions developed and were adapted to younger patients [31]. Peritoneal dialysis is widely considered to be the ideal first dialysis modality, especially in very young paediatric patients [32]. However, considering the whole period and including all age categories, 53% of all patients were initially treated with haemodialysis, 32% with peritoneal dialysis and only 15% got a pre-emptive renal transplant. These observations agree with European data which found that 17% of patients are transplanted pre-emptively [33]. Considering that the benefit in life expectancy of a pre-emptive renal transplantation compared with chronic dialysis is about 20 years [34] and that quality of life and long-term survival are superior in transplanted patients compared to those remaining on the waiting list [35], this number seems far too low. In terms of deceased donor organ donation, there is a wide disparity in European countries regarding organ allocation [33]. Based on a shortage of deceased donor organs in Switzerland, living donation became popular during the nineties, steadily increasing until 2006, but declining thereafter. This is because in 2007 an organ allocation system was introduced by law in Switzerland. This new system prioritises all patients <20 years, leading to shorter waiting times and the possibility of a pre-emptive deceased donor transplantation without prior dialysis [25]. The total number of children who were transplanted as first treatment grew consistently over time in all age groups.
We observed a consistent improvement in graft survival over time. This trend is explained by improved pre-transplant preparation, better donor selection, more potent and selective immunosuppression, optimised surgical techniques, better postoperative management and the possibility of adequate antiviral prophylaxis [36]. We observed an overall five-year graft survival of 72%. In other registries graft survival varied enormously, between 44% and 95% [37–42], depending on observation period, type of country and the availability of renal transplantation in the paediatric population. A Dutch study described a 10-year graft survival rate of 45% in 397 renal transplantation performed in 249 children [39]. For the same time period and a comparable patient cohort, the SPRR found a 10-year graft survival of 57% for Switzerland.
In terms of patient survival, in our study, 92%, 87% and 79% of the children survived the first 5, 10 and 15 years after diagnosis respectively. McDonald et al., in a long-term survival study including 1634 children from 1963 to 2002, also showed that patient survival steadily improved, but was still worse for the youngest patients aged < five years [7]. Twenty-nine of the 367 children in our cohort died. Deaths were due to a variety of reasons, but cardiovascular and infection-associated mortality were the most common [14, 43, 44]. In adults, causes of death changed over time, with cardiovascular mortality decreasing and infection-associated mortality increasing, the latter indicating side effects of more potent immunosuppression [45].
Notably, the economic status of a country remains the key determinant of long-term outcome in children undergoing renal replacement therapy [46]. In the majority of countries there is still a lack of financial and human resources, resulting in the inability to treat children with ESRD [47].
The strength of the SPRR is the high completeness of its data for all children aged 0 to 14 years treated with renal replacement therapy for chronic ESRD after 1980 and resident in Switzerland. Although the small size of the cohort does not allow short-term developments to be studied, the SPPR contributes to international collaborations, especially to the ESPN registry. Existing for nearly 50 years, the cohort is a rich resource for analysing long-term developments. A limitation is the lack of clinical follow-up data after transition. However, we plan to link the registry with the adult renal registry in our country. In summary, over time, a higher number of children on renal replacement therapy survived, graft survival improved, and dialysis duration before renal transplantation decreased.
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
The appendix is available as a separate file at https://smw.ch/article/doi/smw.2020.20300.
We thank the patients and their families, the members of the Swiss Paediatric Renal Registry (SPRR) and the Swiss Association for Paediatric Nephrology (SAPN): H. Chehade, R. Laux-End, P. Parvex, C. Rudin, S. Tschumi, G. Simonetti and E. Leumann. SwissPedRegistry, a member of SwissPedNet, supported this trial with its research infrastructure.
This study was funded by the University Children’s Hospital, University of Zurich (1970–2018); the Institute of Social and Preventive Medicine, University of Bern (2008–18); private donors; Swiss Ped Registry (2019); Bernischer Hilfsbund (2011–13) and Kids Kidney Care (2012).
The authors declare that they have no conflict of interest.
1 Rees L . Long-term outcome after renal transplantation in childhood. Pediatr Nephrol. 2009;24(3):475–84. doi:.https://doi.org/10.1007/s00467-007-0559-2
2 Falger J , Latal B , Landolt MA , Lehmann P , Neuhaus TJ , Laube GF . Outcome after renal transplantation. Part I: intellectual and motor performance. Pediatr Nephrol. 2008;23(8):1339–45. doi:.https://doi.org/10.1007/s00467-008-0795-0
3 Falger J , Landolt MA , Latal B , Rüth EM , Neuhaus TJ , Laube GF . Outcome after renal transplantation. Part II: quality of life and psychosocial adjustment. Pediatr Nephrol. 2008;23(8):1347–54. doi:.https://doi.org/10.1007/s00467-008-0798-x
4 Mekahli D , Shaw V , Ledermann SE , Rees L . Long-term outcome of infants with severe chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(1):10–7. doi:.https://doi.org/10.2215/CJN.05600809
5 Kari JA , Gonzalez C , Ledermann SE , Shaw V , Rees L . Outcome and growth of infants with severe chronic renal failure. Kidney Int. 2000;57(4):1681–7. doi:.https://doi.org/10.1046/j.1523-1755.2000.00013.x
6 Schaefer F , Doyon A , Azukaitis K , Bayazit A , Canpolat N , Duzova A , et al.; 4C Study Consortium. Cardiovascular Phenotypes in Children with CKD: The 4C Study. Clin J Am Soc Nephrol. 2017;12(1):19–28. doi:.https://doi.org/10.2215/CJN.01090216
7 McDonald SP , Craig JC ; Australian and New Zealand Paediatric Nephrology Association. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350(26):2654–62. doi:.https://doi.org/10.1056/NEJMoa031643
8 Smith JM , Martz K , Blydt-Hansen TD . Pediatric kidney transplant practice patterns and outcome benchmarks, 1987-2010: a report of the North American Pediatric Renal Trials and Collaborative Studies. Pediatr Transplant. 2013;17(2):149–57. doi:.https://doi.org/10.1111/petr.12034
9 Smith JM , Dharnidharka VR . Viral surveillance and subclinical viral infection in pediatric kidney transplantation. Pediatr Nephrol. 2015;30(5):741–8. doi:.https://doi.org/10.1007/s00467-014-2866-8
10 Mynarek M , Hussein K , Kreipe HH , Maecker-Kolhoff B . Malignancies after pediatric kidney transplantation: more than PTLD? Pediatr Nephrol. 2014;29(9):1517–28. doi:.https://doi.org/10.1007/s00467-013-2622-5
11 van der Heijden BJ , van Dijk PC , Verrier-Jones K , Jager KJ , Briggs JD . Renal replacement therapy in children: data from 12 registries in Europe. Pediatr Nephrol. 2004;19(2):213–21. doi:.https://doi.org/10.1007/s00467-003-1376-x
12 Tizard EJ , Verrina E , van Stralen KJ , Jager KJ . Progress with the European Society for Paediatric Nephrology (ESPN)/ERA-EDTA Registry for children with established renal failure (ERF). Nephrol Dial Transplant. 2009;24(9):2615–7. doi:.https://doi.org/10.1093/ndt/gfp275
13 Harambat J , van Stralen KJ , Kim JJ , Tizard EJ . Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27(3):363–73. doi:.https://doi.org/10.1007/s00467-011-1939-1
14 Chesnaye N , Bonthuis M , Schaefer F , Groothoff JW , Verrina E , Heaf JG , et al.; ESPN/ERA–EDTA registry. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr Nephrol. 2014;29(12):2403–10. doi:.https://doi.org/10.1007/s00467-014-2884-6
15 Harambat J , Bonthuis M , Groothoff JW , Schaefer F , Tizard EJ , Verrina E , et al. Lessons learned from the ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2016;31(11):2055–64. doi:.https://doi.org/10.1007/s00467-015-3238-8
16 Deleau J , Andre JL , Briancon S , Musse JP . Chronic renal failure in children: an epidemiological survey in Lorraine (France) 1975-1990. Pediatr Nephrol. 1994;8(4):472–6. doi:.https://doi.org/10.1007/BF00856534
17 Esbjörner E , Berg U , Hansson S ; Swedish Pediatric Nephrology Association. Epidemiology of chronic renal failure in children: a report from Sweden 1986-1994. Pediatr Nephrol. 1997;11(4):438–42. doi:.https://doi.org/10.1007/s004670050312
18 Ardissino G , Daccò V , Testa S , Bonaudo R , Claris-Appiani A , Taioli E , et al.; ItalKid Project. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111(4):e382–7. doi:.https://doi.org/10.1542/peds.111.4.e382
19 Bek K , Akman S , Bilge I , Topaloğlu R , Calişkan S , Peru H , et al. Chronic kidney disease in children in Turkey. Pediatr Nephrol. 2009;24(4):797–806. doi:.https://doi.org/10.1007/s00467-008-0998-4
20 Mong Hiep TT , Ismaili K , Collart F , Van Damme-Lombaerts R , Godefroid N , Ghuysen MS , et al. Clinical characteristics and outcomes of children with stage 3-5 chronic kidney disease. Pediatr Nephrol. 2010;25(5):935–40. doi:.https://doi.org/10.1007/s00467-009-1424-2
21 Gämperli A , Leumann E , Neuhaus TJ , Schlumpf R , Largiadèr F . [25 years of dialysis and kidney transplantation in children and adolescents]. Schweiz Med Wochenschr. 1996;126(3):77–85. Article in German.
22 Chesnaye NC , van Stralen KJ , Bonthuis M , Harambat J , Groothoff JW , Jager KJ . Survival in children requiring chronic renal replacement therapy. Pediatr Nephrol. 2018;33(4):585–94. doi:.https://doi.org/10.1007/s00467-017-3681-9
23 Leumann E , Goetschel P , Neuhaus TJ , Ambühl PM , Candinas D . [Pediatric kidney transplantation and living donors--invaluable by virtue of necessity]. Schweiz Med Wochenschr. 2000;130(43):1581–9. Article in German.
24 Weitz M , Sazpinar O , Schmidt M , Neuhaus TJ , Maurer E , Kuehni C , et al. Balancing competing needs in kidney transplantation: does an allocation system prioritizing children affect the renal transplant function? Transpl Int. 2017;30(1):68–75. doi:.https://doi.org/10.1111/tri.12874
25bfs.admin.ch. [Internet]. Neuchatel: Federal Statistical Office, Switzerland. Annual population statistics 1981 - 2010 (ESPOP), and Population and Households Statistics 2011 - 2015 (STATPOP). C2019 [cited 2019 Sep 27]. Available from: https://www.bfs.admin.ch/bfs/de/home/dienstleistungen/forschung/stat-tab-online-datenrecherche.html
26UK Renal Registry [Internet]. Bristol: UK Renal Registry 21st Annual Report – data to 31/12/2017; Chapter 7, Children un renal replacement therapy (RRT) for end-stage kidney disease (ESKD) in the UK in 2017; c2019 [cited 2020 Apr 19]. Available from: https://www.renalreg.org/wp-content/uploads/2019/05/21st_UKRR_Annual_Report_Ch7.pdf
27 Goldstein SL . Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial. 2011;24(2):187–91. doi:.https://doi.org/10.1111/j.1525-139X.2011.00834.x
28emmes.com. [Internet]. Rockville: Martz K, Stablein DM, Berlin S, Wright R, Fraser G, Thotapally K. North American Pediatric Renal Trials and Collaborative Studies, NAPRTCS, 2008 Annual Report; c2019 [cited 2019 Sep 27]. Available from: https://web.emmes.com/study/ped/annlrept/Annual%20Report%20-2008.pdf
29espn-reg.org. [Internet]. Amsterdam: van Stralen KJ, Verrina E, Schaefer F, Jager K. ESPN/ERA-EDTA registry annual report 2008; c2019 [cited 2019 Sep 27]. Available from: https://www.espn-reg.org/files/pd2008_espn__online.pdf.
30anzdata.org. [Internet]. Adelaide: McTaggart S MS, Henning P, Dent H. Paediatric Report. ANZDATA Registry Report 2009, Australia and New Zealand Dialysis and Transplant Registry; c2019 [cited 2019 Sep 27]. Available from: https://www.anzdata.org.au/wp-content/uploads/2016/12/Ch11-1.pdf.
31 Moncrief JW , Popovich RP , Nolph KD . The history and current status of continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1990;16(6):579–84. doi:.https://doi.org/10.1016/S0272-6386(12)81044-4
32 Feber J , Schärer K , Schaefer F , Míková M , Janda J . Residual renal function in children on haemodialysis and peritoneal dialysis therapy. Pediatr Nephrol. 1994;8(5):579–83. doi:.https://doi.org/10.1007/BF00858132
33 Harambat J , van Stralen KJ , Schaefer F , Grenda R , Jankauskiene A , Kostic M , et al. Disparities in policies, practices and rates of pediatric kidney transplantation in Europe. Am J Transplant. 2013;13(8):2066–74. doi:.https://doi.org/10.1111/ajt.12288
34 Kramer A , Stel VS , Tizard J , Verrina E , Rönnholm K , Pálsson R , et al. Characteristics and survival of young adults who started renal replacement therapy during childhood. Nephrol Dial Transplant. 2009;24(3):926–33. doi:.https://doi.org/10.1093/ndt/gfn542
35 Gillen DL , Stehman-Breen CO , Smith JM , McDonald RA , Warady BA , Brandt JR , et al. Survival advantage of pediatric recipients of a first kidney transplant among children awaiting kidney transplantation. Am J Transplant. 2008;8(12):2600–6. doi:.https://doi.org/10.1111/j.1600-6143.2008.02410.x
36 Verghese PS . Pediatric kidney transplantation: a historical review. Pediatr Res. 2017;81(1-2):259–64. doi:.https://doi.org/10.1038/pr.2016.207
37 Rees L , Shroff R , Hutchinson C , Fernando ON , Trompeter RS . Long-term outcome of paediatric renal transplantation: follow-up of 300 children from 1973 to 2000. Nephron Clin Pract. 2007;105(2):c68–76. doi:.https://doi.org/10.1159/000097601
38 Pitcher GJ , Beale PG , Bowley DM , Hahn D , Thomson PD . Pediatric renal transplantation in a South African teaching hospital: a 20-year perspective. Pediatr Transplant. 2006;10(4):441–8. doi:.https://doi.org/10.1111/j.1399-3046.2006.00489.x
39 Groothoff JW , Cransberg K , Offringa M , van de Kar NJ , Lilien MR , Davin JC , et al.; Dutch cohort study. Long-term follow-up of renal transplantation in children: a Dutch cohort study. Transplantation. 2004;78(3):453–60. doi:.https://doi.org/10.1097/01.TP.0000128616.02821.8B
40 Englund M , Berg U , Tydén G . A longitudinal study of children who received renal transplants 10-20 years ago. Transplantation. 2003;76(2):311–8. doi:.https://doi.org/10.1097/01.TP.0000076472.45979.65
41 Mehrabi A , Kashfi A , Tönshoff B , Feneberg R , Mehls O , Schemmer P , et al. Long-term results of paediatric kidney transplantation at the University of Heidelberg: a 35 year single-centre experience. Nephrol Dial Transplant. 2004;19(Suppl 4):iv69–74. doi:.https://doi.org/10.1093/ndt/gfh1046
42 Van Damme-Lombaerts R , Herman J , Coosemans W , Pirenne J . Pediatric renal transplantation: a single Belgian center experience over 20 years. Pediatr Transplant. 2001;5(6):447–51. doi:.https://doi.org/10.1034/j.1399-3046.2001.t01-1-00008.x
43 Parekh RS , Carroll CE , Wolfe RA , Port FK . Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141(2):191–7. doi:.https://doi.org/10.1067/mpd.2002.125910
44 Groothoff JW , Gruppen MP , Offringa M , Hutten J , Lilien MR , Van De Kar NJ , et al. Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int. 2002;61(2):621–9. doi:.https://doi.org/10.1046/j.1523-1755.2002.00156.x
45 Vogelzang JL , van Stralen KJ , Jager KJ , Groothoff JW . Trend from cardiovascular to non-cardiovascular late mortality in patients with renal replacement therapy since childhood. Nephrol Dial Transplant. 2013;28(8):2082–9. doi:.https://doi.org/10.1093/ndt/gft048
46 Mong Hiep TT , Janssen F , Ismaili K , Khai Minh D , Vuong Kiet D , Robert A . Etiology and outcome of chronic renal failure in hospitalized children in Ho Chi Minh City, Vietnam. Pediatr Nephrol. 2008;23(6):965–70. doi:.https://doi.org/10.1007/s00467-008-0752-y
47 Gulati S , Mittal S , Sharma RK , Gupta A . Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. 1999;13(7):594–6. doi:.https://doi.org/10.1007/s004670050750
This study was funded by the University Children’s Hospital, University of Zurich (1970–2018); the Institute of Social and Preventive Medicine, University of Bern (2008–18); private donors; Swiss Ped Registry (2019); Bernischer Hilfsbund (2011–13) and Kids Kidney Care (2012).
The authors declare that they have no conflict of interest.