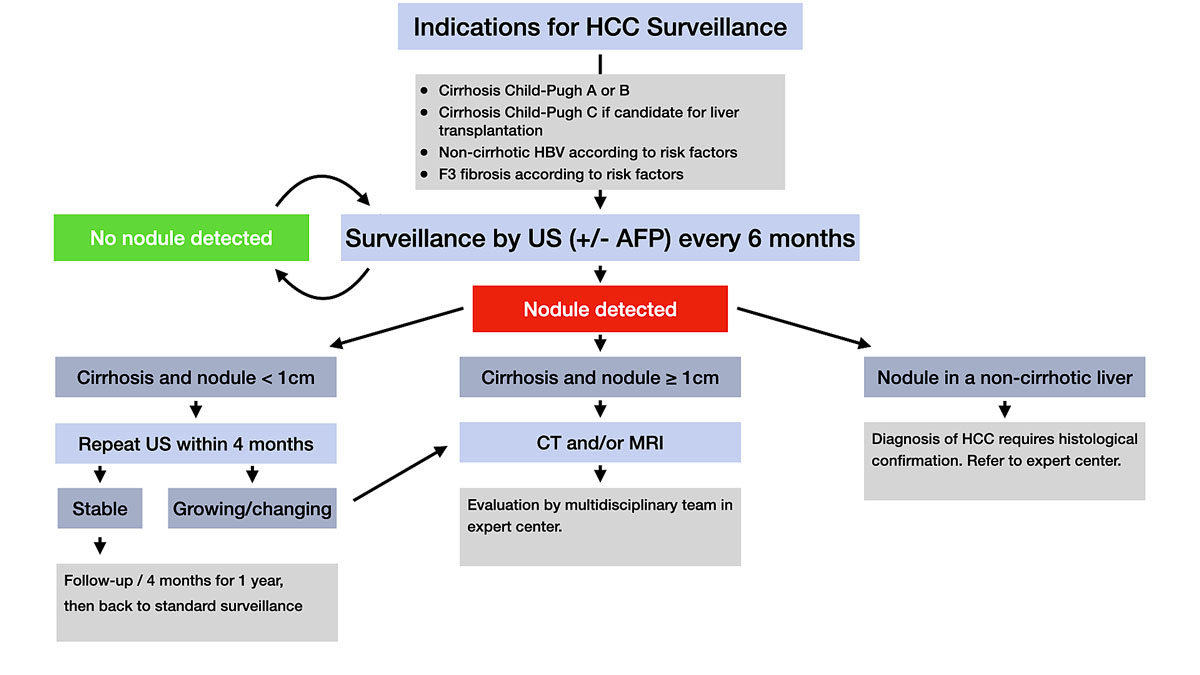
Figure 1 Overview of indications for HCC surveillance and recall policy in case of identification of a liver nodule.
AFP = alpha-fetoprotein; CT = computed tomography; HBV = hepatitis B virus; MRI = magnetic resonance imaging; US = ultrasound
DOI: https://doi.org/10.4414/smw.2020.20296
This document is an expert opinion statement of the Swiss Association for the Study of the Liver (SASL). Recommendations are based on guidelines on hepatocellular carcinoma (HCC) by the European Association for the Study of the Liver (EASL), the American Association for the Study of Liver Diseases (AASLD) and the European Society for Medical Oncology, as well as on the literature cited below.
HCC is the most common primary malignancy of the liver, comprising about 90% of primary liver cancers. Worldwide, liver cancer is the fifth most common cancer although there is significant geographical heterogeneity, with the highest incidence rates seen in East Asia and sub-Saharan Africa [1]. In Switzerland, the incidence of liver cancer was only the 11th of all cancers in men and the 20th in women in the period of 2011–15, but the impact on cancer mortality was disproportionally higher in the same period, as liver cancer was the fifth cause of cancer death in men and eighth in women [2].
Over 90% of HCCs develop in the setting of an underlying liver disease, especially chronic hepatitis C virus (HCV) or hepatitis B virus (HBV) infection, alcoholic liver disease and non-alcoholic fatty liver disease. In the Western world, HCV and alcoholic liver disease are the principal causes of HCC, whereas non-alcoholic fatty liver disease and non-alcoholic steatohepatitis are rapidly emerging causes of HCC, already becoming the fastest growing cause of HCC in liver transplant candidates in the USA [3, 4]. Prevention of HCC can therefore be achieved by preventing or treating the underlying cause of liver disease, such as vaccination against HBV, antiviral therapy for HBV and HCV, reducing alcohol intake and preventing obesity or encouraging coffee consumption [1, 5].
Cirrhosis is a strong risk factor for the development of HCC, especially when occurring in the context of viral hepatitis. Overall, one third of patients with cirrhosis will develop HCC during their lifetime, with a rate of approximately 1–8% per year depending on aetiology of liver disease, age, sex, stage of liver disease, presence of metabolic syndrome and diabetes, and additional factors. Several studies have shown that HCC can also occur in non-cirrhotic patients, in particular in patients with HBV infection and possibly non-alcoholic fatty liver disease. Stratification of HCC risk is key to rational implementation of HCC surveillance programmes in patients with chronic liver disease.
Cancer surveillance is the periodic application of a diagnostic test to individuals at risk of developing a given cancer with an aim to reduce disease-related mortality. In line with a recent EASL clinical practice guideline, all patients with cirrhosis should be considered for HCC surveillance (fig. 1) [1]. Even in the absence of cirrhosis, some categories of patients with liver disease still exceed the incidence thresholds at which HCC surveillance is judged cost effective [1]. In Caucasian patients with HBV infection, a PAGE-B score (including age, gender and platelet count as predictive variables) of 10 or more is indicative of an intermediate to high risk of HCC, justifying surveillance, although this score requires further validation [1, 6]. For non-Caucasian HBV-infected individuals, there are no clear-cut guidelines, but individual risk assessment with known risk factors of HCC in this population (male sex, African or Asian descent, age [>40 years for men, >50 years for women], family history of HCC) should guide decisions about HCC surveillance. In addition, the EASL clinical practice guideline recommends that HCC surveillance be considered in patients with advanced Metavir fibrosis stage 3 (F3) based on individual risk assessment.

Figure 1 Overview of indications for HCC surveillance and recall policy in case of identification of a liver nodule.
AFP = alpha-fetoprotein; CT = computed tomography; HBV = hepatitis B virus; MRI = magnetic resonance imaging; US = ultrasound
HCC surveillance should be performed by means of liver ultrasound every 6 months [1]. The use of alpha-fetoprotein (AFP) for HCC surveillance is not recommended by the EASL clinical practice guideline. Clearly, there is no place for HCC surveillance by AFP alone. However, some data suggest that the addition of AFP to ultrasound may improve sensitivity of HCC surveillance [7]. For patients in whom ultrasound is not feasible or not conclusive, computed tomography (CT) and magnetic resonance imaging (MRI) can be considered.
Peculiar vascular derangements occurring during hepatic carcinogenesis are associated with distinct imaging characteristics in multiphase contrast-enhanced imaging techniques (CT, MRI, contrast enhanced ultrasound (CEUS)) that allow the diagnosis of HCC without a tumour biopsy [8]. We agree with the EASL clinical practice guideline that in patients with cirrhosis, diagnosis of HCC can be made by contrast-enhanced CT, MRI or CEUS, if a nodule is >1cm in size and shows hyperenhancement in the late arterial phase and washout in the venous and/or delayed phase [1]. Although CEUS can accurately characterise lesions in cirrhosis, in most patients, CT and MRI are preferred over CEUS because of their higher sensitivity, the analysis of the whole liver and the possibility to share images to be reviewed in other centres. In comparison with contrast-enhanced CT, MRI (in particular gadoxetic acid-enhanced MRI) has shown improved sensitivity, especially for small lesions [9]. Use of the “liver imaging reporting and data system” (CEUS/CT/MRI LI-RADS v2018) may increase specificity (at a cost of reduced sensitivity) of HCC diagnosis by imaging but has not yet been fully endorsed by EASL [10, 11].
Despite the availability of noninvasive diagnostic criteria for HCC, pathological diagnosis remains the gold standard. In cirrhotic patients, this may allow identification of non-HCC liver tumours, assessment of tumour differentiation and identification of premalignant lesions. In non-cirrhotic livers, the diagnosis of HCC requires histopathological confirmation. Risks of liver biopsy include bleeding and needle track seeding, although these risks remain low (<5%), especially in expert centres [1, 12].
When a hepatic nodule is detected in a patient with cirrhosis, diagnostic assessment will depend on the size of the nodule (fig. 1). Because of the lower probability of malignancy in smaller nodules, it is recommended to follow up nodule(s) less than 1 cm in diameter detected by ultrasound at ≤4-month intervals in the first year. In cirrhotic patients, the diagnosis of HCC for nodules of ≥1 cm in diameter in general can often be achieved with noninvasive methods. If imaging is inconclusive, biopsy of the lesion is recommended, with repeat biopsy indicated if inconclusive or discordant results are obtained. Because of the complexity of their management, cirrhotic patients with a new hepatic nodule should be evaluated in a referral centre with multidisciplinary teams including hepatologists, hepatobiliary surgeons, diagnostic and interventional radiologists, expert pathologists and oncologists, and regular hepatobiliary multidisciplinary meetings, which may be associated with improved outcomes in these patients [13].
Most HCCs develop on the background of chronic necro-inflammatory liver diseases, and up to 80% arise in cirrhotic livers. Prognosis therefore depends not only on the grade and stage of the HCC, but also on the stage of the underlying liver disease. Whenever appropriate and feasible, the underlying liver disease should be treated. Hepatic resection is the treatment of choice for HCC in non-cirrhotic patients, regardless of tumour size. Management of HCC patients with concomitant liver cirrhosis is fundamentally different. Treatment allocation is based on staging systems that incorporate tumour burden, liver function and portal hypertension. We recommend use of the Barcelona Clinic Liver Cancer (BCLC) staging system with modifications (fig. 2). Modifications compared with the EASL clinical practice guidelines of 2018 [1] are: (1) the Eastern cooperative Oncology Group (ECOG) performance status is omitted, (2) preserved liver function includes patients with Child-Pugh stage B up to seven points, in the absence of clinically significant ascites, and (3) selective internal radiotherapy (SIRT) and stereotactic body radiotherapy (SBRT) are included. These modifications are not based on scientific evidence, but reflect the clinical practice in Swiss centres experienced in the management of patients with HCC. We strongly recommend that all HCC patients are referred to a centre with a specialised tumour board. All relevant management and treatment decisions should be discussed in multidisciplinary tumour boards composed of liver surgeons, gastroenterologists/hepatologists, medical oncologists, interventional radiologists, radiation therapists, radiologists and pathologists.
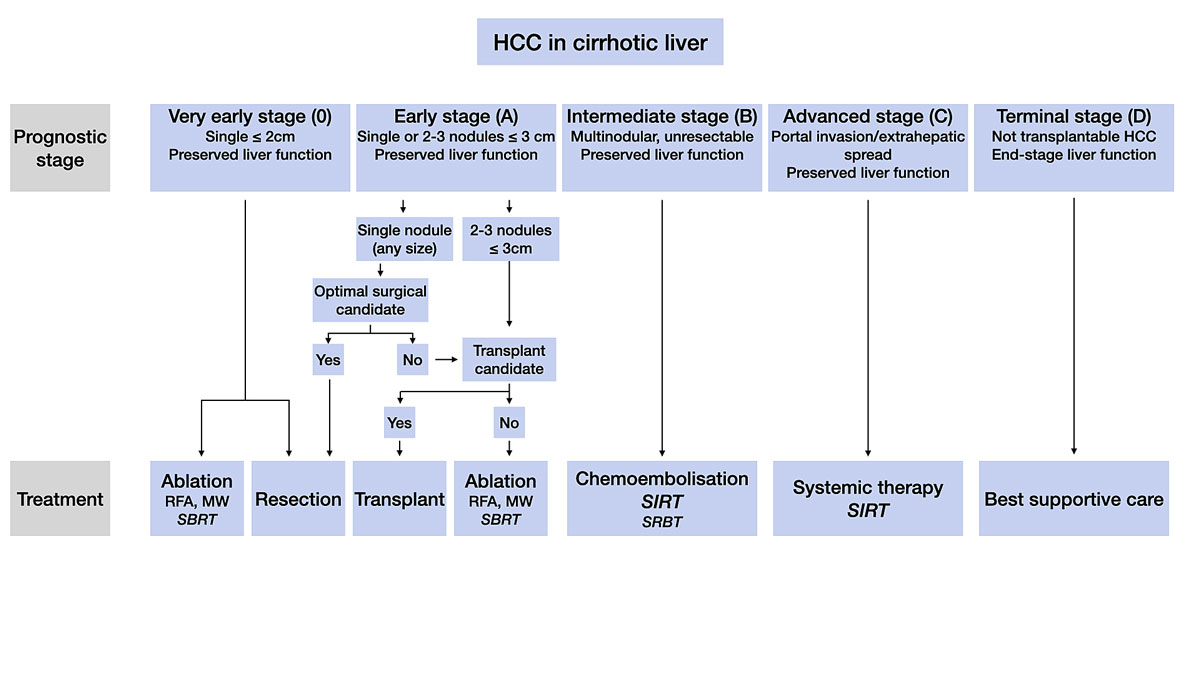
Figure 2 Modified Barcelona Clinic Liver Cancer (BCLC) staging system. Preserved liver function includes patients with Child-Pugh stage B up to seven points and no ascites. End-stage liver function are patients with >7 Child-Pugh points. Criteria for selection of patients for resection are discussed in the text. For details regarding treatments see text. Treatment modalities shown in italics are currently not supported by high-level scientific evidence.
MW = microwave ablation; RFA = radiofrequency ablation; SBRT = stereotactic body radiotherapy; SIRT = selective internal radiotherapy.
Patients with preserved liver function and a single ≤2 cm HCC (very early stage) should be managed by percutaneous ablation (radiofrequency or microwave ablation) or surgical resection. Both strategies offer similar outcomes, with expected five-year survival of 80–90% [14]. The location of the lesion is a key element in deciding between the two strategies. An HCC located deep in the liver should be primarily ablated, whereas more superficial lesions should be resected using a minimally invasive approach (laparoscopic or robot-assisted surgery). The advantage of the resection is linked to its associated pathological assessment; an ab initio transplantation should be discussed in patients at higher risk of recurrence (microvascular invasion, poor differentiation, or presence of satellite nodules seen on the resection specimen) [15]. When both ablation and resection are technically impossible (for example for HCCs located at the base of the hepatic veins), stereotactic body radiotherapy (SBRT) should be considered [16].
The early stage includes patients with a single HCC >2 cm, or with 2–3 HCCs each ≤3 cm and preserved liver function. They have an expected five-year survival of 50–70%. Liver resection should be recommended for the patients with a single HCC (regardless of the size), preserved liver function and no clinically relevant portal hypertension (the hepatic venous pressure gradient should be <10 mm Hg). In fragile patients and/or in the presence of a more aggressive HCC (poorly differentiated, satellite nodules, high AFP), a loco-regional treatment (transarterial chemoembolisation [TACE] or SIRT) may be applied first. It helps to identify HCCs with the most favourable biology (no progression after loco-regional treatment) and can make surgery easier thanks to a decrease in the size of the lesion.
When surgery is not possible, and in patients with multiple HCCs, transplantation should be considered. In Switzerland, liver transplants are performed at the university centres of Bern, Geneva and Zurich. Candidate selection can be based on expanded criteria, which offer access to transplantation even for patients marginally beyond the Milan criteria (one HCC ≤5 cm, 2–3 HCCs each ≤3 cm). The most common criteria include the total tumour volume (TTV)/AFP score (TTV ≤115 cm3 and AFP ≤400 ng/ml), and the AFP score (based on HCC size, HCC number and AFP level) [17, 18]. Their use is based on the observation that patients with more advanced HCCs show good post-transplant outcomes as long as they have a low AFP level [19]. In addition, patients not meeting transplant criteria can also be considered for transplantation if they have been previously successfully downstaged using loco-regional means to transplant criteria (usually Milan or TTV/AFP). Overall, 80% five-year post-transplant survival can be expected. Of note, the average waiting time for a liver graft is over one year in Switzerland, and patients should undergo active and aggressive bridging treatment while on the list, using ablation and/or loco-regional treatments.
For patients who are not good candidates for resection or transplantation, percutaneous radiofrequency or microwave ablation are recommended. When ablation is technically impossible, SBRT should be considered [16].
Patients with unresectable and nontransplantable multinodular and/or large HCCs without vascular invasion or extrahepatic spread and with preserved liver function are classified in the intermediate stage (BCLC stage B). For these patients, TACE is the established first-line therapy [20]. The efficacy of TACE has been demonstrated in two randomised controlled trials [21, 22]. TACE consists of an intra-arterial infusion of a cytotoxic agent followed by embolisation of the tumour-feeding blood vessels. Super-selective chemoembolisation minimises the ischaemic insult to non-tumour tissue and is therefore recommended. Conventional lipiodol TACE and TACE with drug-eluting beads (TACE-DEB) seem to provide the same survival benefit [23]. Reduced portal vein blood flow (thrombus, hepatofugal blood flow) and extensive tumour burden (>50% of liver) increase the risk of hepatic decompensation after TACE [1, 24]. TACE is not recommended in patients with macrovascular tumour invasion into the portal venous system [1, 24]. TACE can be repeated in the case of incomplete tumour response or intrahepatic progression. TACE should not be repeated when no substantial reduction of tumour burden is achieved after two rounds. The combination of TACE with systemic therapies has not shown survival benefits compared with TACE alone [1, 24].
For patients in BCLC stage B who are not good candidates for TACE, potential alternative treatments are SIRT, external radiation therapy and systemic therapies.
Tumour invasion into the portal vein or extrahepatic spread define the advanced stage (BCLC stage C). For these patients, the standard of care is a systemic treatment. Conventional chemotherapies are ineffective and should not be used for the treatment of HCCs. There are now two first-line and several second-line therapies that are or will soon be available in Switzerland (fig. 3). SIRT can be the preferred treatment for selected stage C patients with portal vein invasion and no extrahepatic metastasis (see below).
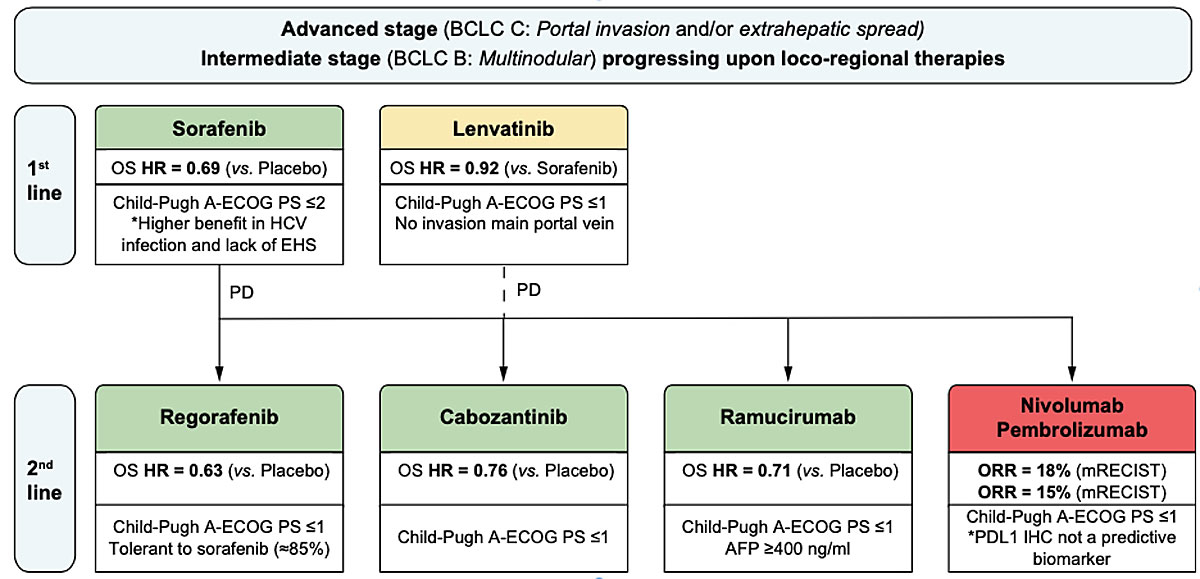
Figure 3 Treatment strategy for advanced hepatocellular carcinoma. Reproduced with permission from Llovet, Montal and Villanueva [25]. Key details of the patient populations are provided. Drugs in green have positive results from phase III trials with a superiority design, drugs in orange (lenvatinib) have positive results from phase III trials with a non-inferiority design. Drugs in red received accelerated approval by FDA based on results from phase II trials.
AFP = Alpha-fetoprotein; BCLC = Barcelona Clinic Liver Cancer (classification); ECOG PS = Eastern Cooperative Oncology Group performance status; EHS = extrahepatic spread; HCV = hepatitis C virus; HR = hazard ratio; mRECIST = modified Response Evaluation Criteria In Solid Tumors; ORR = objective response rate; OS = overall survival.
Sorafenib (Nexavar®) is the standard first-line systemic therapy for advanced HCC and the standard of care for earlier stage tumours not suitable for or progressing under loco-regional therapies [1, 24]. Sorafenib improved overall survival by 2–3 months in two pivotal phase III trials [26, 27]. Sorafenib is taken at a dose of 800 mg per day. The most frequent adverse effects are diarrhoea, hand-foot skin reactions, fatigue and hypertension. Patients should be seen at 2-week intervals for the first 2 months to proactively manage adverse effects [28]. Around 15% of patients are intolerant of sorafenib and another 35% of patients require dose reductions. There are currently no biomarkers to predict response or nonresponse in individual patients.
Lenvatinib (Lenvima®) was recently shown to be noninferior to sorafenib [29], and has been approved as first-line therapy in Switzerland. Its use is restricted to patients with advanced, unresectable HCCs with tumour burden <50% of the liver, no tumour invasion in the bile duct or main portal vein and preserved liver function (Child-Pugh A). Lenvatinib is given once daily at a dose of 8 mg or 12 mg for patients with body weight <60 kg or ≥60 kg, respectively. The overall frequency of adverse events during lenvatinib treatment is comparable to that with sorafenib. Hypertension and weight loss are more frequent with lenvatinib, whereas hand-foot skin reactions and alopecia are more frequent with sorafenib [29]. As with sorafenib, there are no biomarkers to predict response or nonresponse to lenvatinib in individual patients.
Patients with progressive disease on sorafenib who tolerate sorafenib well can be switched to second-line therapy with regorafenib (Stivarga®). Regorafenib was approved on the basis of a randomised phase III trial that showed improved overall survival (10.6 months median survival) compared with placebo (7.8 months median survival) in sorafenib nonresponders [30].
Cabozantinib (Cabometyx®) is not yet approved in Switzerland. In a recent phase III trial it demonstrated increased overall survival (10.2 months median survival) compared with placebo (8.0 months) [31]. Importantly, the trial included also patients who were intolerant to sorafenib. Cabozantinib is given once daily at a dose of 60 mg.
Ramucirumab (Cyramza®) is a human immunoglobulin monoclonal antibody that inhibits vascular endothelial growth factor receptor-2. It is given intravenously every 2 weeks at a dose of 8 mg/kg bodyweight. In a recent phase III trial that included patients who had previously received first-line sorafenib and had an AFP ≥400 ng/ml, ramucirumab was shown to improve overall survival compared with placebo (8.5 months vs 7.3 months) [32]. Cyramza® is not yet approved as second-line therapy for HCC in Switzerland.
Of note, the clinical trials that showed efficacy of regorafenib, cabozantinib and ramucirumab as second-line therapies after sorafenib did not have a treatment arm with continuous sorafenib treatment, but just a placebo arm. Given the rather small increases of median overall survival compared with placebo in all these trials, it is conceivable that continuous treatment with sorafenib after radiological progression would have been as effective as the active substances.
In Europe and in the US, immunotherapy with nivolumab and pembrolizumab can be considered in patients who are intolerant to or have progressed under sorafenib treatment. In Switzerland, nivolumab (Opdivo®) can be used as second-line after sorafenib in a compassionate use programme after approval by the health insurers. The accelerated approvals of nivolumab (2017) and pembrolizumab (2018) by the US Food and Drug Administration (FDA) were based on phase II trials.
The efficacy of nivolumab as a first-line therapy has been evaluated in a prospective randomised controlled phase III trial (CheckMate-459). Results of the study were presented at the EMSO 2019: nivolumab did not achieve the primary endpoint of the study, which was defined as improved overall survival compared with sorafenib.
The efficacy of pembrolizumab as a second-line therapy after sorafenib has been evaluated in randomised, placebo-controlled phase III study (Keynote-240). The study results were presented at the 2019 ASCO annual meeting. The study did not meet its co-primary endpoint of progression-free survival and overall survival.
The combination of atezolizumab (Tecentriq®) and bevacizumab (Avastin®) as a first-line therapy has been evaluated in phase III trial (IMbrave150). The study reached its co-primary endpoint of overall survival and progression-free survival compared with standard-of-care sorafenib. The hazard ratio for death with atezolizumab-bevacizumab as compared with sorafenib was 0.58 (95% confidence interval [CI] 0.42–0.79; p <0.001) [33]. Median progression-free survival was also significantly longer in the atezolizumab-bevacizumab group (hazard ratio for disease progression or death 0.59, 95% CI 0.47–0.76; p <0.001). It is expected that the combination of Tecentriq® and Avastin® will soon become available as first-line therapy for unresectable HCC.
The nonspecific blockade of inhibitory mechanisms by immune checkpoint inhibitor therapy can cause a discrete group of immune-related adverse events. Most of them are mild to moderated, but serious and even life-threatening immune-related adverse events occur. Effective management of immune-related adverse events depends on early recognition and prompt intervention with immune suppression and/or immunomodulatory strategies [34–36].
The role of immunotherapies as first- and second-line therapies for intermediate and advanced stage HCC will have to be evaluated in further clinical trials.
SIRT with yttrium-90 (90Y) loaded microspheres is an alternative treatment for patients with macrovascular tumour invasion in the portal venous system. Because 90Y microspheres have a weak embolic effect, SIRT can be performed in a lobar, sectorial or segmental approach, even in patients with portal vein thrombosis [37]. SIRT is a demanding procedure that requires a close collaboration between interventional radiologists, nuclear medicine specialists, radiopharmacists and physicists. Because of current reimbursement schemes, it is done in an ambulatory setting in Switzerland. Compared with sorafenib, SIRT provides better local tumour control and is better tolerated. However, overall survival is not improved [38, 39]. Therefore, SIRT is not generally recommended as first-line therapy for advanced HCC. However, it can be recommended in individual patients as first-line or as second-line treatment after multidisciplinary tumour board discussion.
HCC patients with end-stage liver disease (Child-Pugh C) and/or poor ECOG performance status who are not candidates for liver transplantation have a dismal prognosis with a life expectancy of 3–4 months. These patients do not profit from tumour-directed therapies. Instead, they should receive palliative support for nutrition, pain and psychological distress.
Response to therapy is monitored with dynamic CT or MRI using the “response evaluation criteria in solid tumours” (RECIST) or modified RECIST for HCC (mRECIST) criteria [40, 41] (fig. 4).
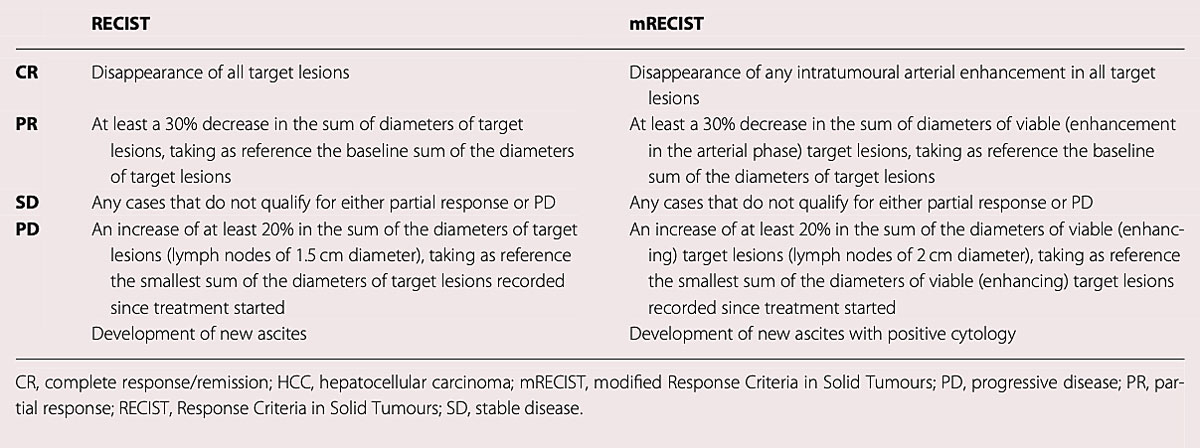
Figure 4 Response assessment by RECIST v1.1. and mRECIST for HCC. Reproduced with permission from the European Society for Medical Oncology (ESMO) clinical practice guidelines [24].
Follow-up abdominal CT or MRI for patients who underwent radical treatments (resection or radio frequency ablation) should be done every 3 months during the first 2 years and surveillance every 6 months thereafter. Patients with intermediate or advanced stages of HCC who are treated with TACE or systemic agents are evaluated for tumour progression by dynamic CT or MRI every 3 months to guide therapy decisions. Some centres also include chest CTs for regular follow-up imaging examinations. If AFP is elevated at baseline, it can be informative during follow-up as well.
The clinical approach to patients with HCC is based on systematic staging followed by stage-specific treatments. Staging should be done by up-to-date imaging protocols with CT or MRI. There are several ongoing controversies in regard to stage-specific treatment allocations. In very early (0) and early stage (A) HCCs, there is still very limited scientific evidence to direct the choice between resection, ablation and SBRT. There is also an ongoing controversy on the prioritisation of patients with HCC on liver transplant lists. For advanced stage (C) patients, the lack of predictive biomarkers of response to the different first- and second-line drugs precludes a rational choice in many patients. Further clinical studies are urgently needed. We strongly recommend that all HCC patients are referred to a centre with a specialised gastrointestinal-hepatobiliary tumour board.
No financial support and no other potential conflict of interest relevant to this article were reported.
1 Galle PR , Forner A , Llovet JM , Mazzaferro V , Piscaglia F , Raoul J-L , et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:.. Correction in: J Hepatol. 2019;70(4):817. doi:https://doi.org/10.1016/j.jhep.2018.03.019
2Cancer data extracted from the Swiss national dataset managed by the Foundation National Institute for Cancer Epidemiology and Registration (NICER). [Internet]. [cited 2018 Dec 17] Available from: http://www.nicer.org/en/statistics-atlas/cancer-incidence/.
3 Kulik L , El-Serag HB . Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi:.https://doi.org/10.1053/j.gastro.2018.08.065
4 Younossi Z , Stepanova M , Ong JP , Jacobson IM , Bugianesi E , Duseja A , et al.; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–755.e3. doi:.https://doi.org/10.1016/j.cgh.2018.05.057
5 Bravi F , Tavani A , Bosetti C , Boffetta P , La Vecchia C . Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017;26(5):368–77. doi:.https://doi.org/10.1097/CEJ.0000000000000252
6 Papatheodoridis G , Dalekos G , Sypsa V , Yurdaydin C , Buti M , Goulis J , et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64(4):800–6. doi:.https://doi.org/10.1016/j.jhep.2015.11.035
7 Tzartzeva K , Obi J , Rich NE , Parikh ND , Marrero JA , Yopp A , et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1. doi:.https://doi.org/10.1053/j.gastro.2018.01.064
8 Bruix J , Sherman M , Llovet JM , Beaugrand M , Lencioni R , Burroughs AK , et al.; EASL Panel of Experts on HCC; European Association for the Study of the Liver. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35(3):421–30. doi:.https://doi.org/10.1016/S0168-8278(01)00130-1
9 Lee YJ , Lee JM , Lee JS , Lee HY , Park BH , Kim YH , et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275(1):97–109. doi:.https://doi.org/10.1148/radiol.14140690
10 Mitchell DG , Bruix J , Sherman M , Sirlin CB . LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056–65. doi:.https://doi.org/10.1002/hep.27304
11CT/MRI LI-RADSVR v2018. [cited; Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?%20la=en
12 Silva MA , Hegab B , Hyde C , Guo B , Buckels JA , Mirza DF . Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57(11):1592–6. doi:.https://doi.org/10.1136/gut.2008.149062
13 Sinn DH , Choi GS , Park HC , Kim JM , Kim H , Song KD , et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019;14(1):e0210730. doi:.https://doi.org/10.1371/journal.pone.0210730
14 Cucchetti A , Piscaglia F , Cescon M , Colecchia A , Ercolani G , Bolondi L , et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–7. doi:.https://doi.org/10.1016/j.jhep.2013.04.009
15 Ferrer-Fàbrega J , Forner A , Liccioni A , Miquel R , Molina V , Navasa M , et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. 2016;63(3):839–49. doi:.https://doi.org/10.1002/hep.28339
16 Rajyaguru DJ , Borgert AJ , Smith AL , Thomes RM , Conway PD , Halfdanarson TR , et al. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol. 2018;36(6):600–8. doi:.https://doi.org/10.1200/JCO.2017.75.3228
17 Toso C , Asthana S , Bigam DL , Shapiro AM , Kneteman NM . Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49(3):832–8. doi:.https://doi.org/10.1002/hep.22693
18 Duvoux C , Roudot-Thoraval F , Decaens T , Pessione F , Badran H , Piardi T , et al.; Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986–94.e3, quiz e14–5. doi:.https://doi.org/10.1053/j.gastro.2012.05.052
19 Toso C , Meeberg G , Hernandez-Alejandro R , Dufour JF , Marotta P , Majno P , et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. 2015;62(1):158–65. doi:.https://doi.org/10.1002/hep.27787
20 European Association For The Study Of The Liver, European Organisation For Research and Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. doi:.https://doi.org/10.1016/j.jhep.2011.12.001
21 Llovet JM , Real MI , Montaña X , Planas R , Coll S , Aponte J , et al.; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9. doi:.https://doi.org/10.1016/S0140-6736(02)08649-X
22 Lo CM , Ngan H , Tso WK , Liu CL , Lam CM , Poon RT , et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71. doi:.https://doi.org/10.1053/jhep.2002.33156
23 Golfieri R , Giampalma E , Renzulli M , Cioni R , Bargellini I , Bartolozzi C , et al.; PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–64. doi:.https://doi.org/10.1038/bjc.2014.199
24 Vogel A , Cervantes A , Chau I , Daniele B , Llovet J , Meyer T , et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–55. doi:.https://doi.org/10.1093/annonc/mdy308
25 Llovet JM , Montal R , Villanueva A . Randomized trials and endpoints in advanced HCC: Role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–77. doi:.https://doi.org/10.1016/j.jhep.2019.01.028
26 Cheng AL , Kang YK , Chen Z , Tsao CJ , Qin S , Kim JS , et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:.https://doi.org/10.1016/S1470-2045(08)70285-7
27 Llovet JM , Ricci S , Mazzaferro V , Hilgard P , Gane E , Blanc JF , et al.; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi:.https://doi.org/10.1056/NEJMoa0708857
28 Brose MS , Frenette CT , Keefe SM , Stein SM . Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41(Suppl 2):S1–16. doi:.https://doi.org/10.1053/j.seminoncol.2014.01.001
29 Kudo M , Finn RS , Qin S , Han KH , Ikeda K , Piscaglia F , et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. doi:.https://doi.org/10.1016/S0140-6736(18)30207-1
30 Bruix J , Qin S , Merle P , Granito A , Huang YH , Bodoky G , et al.; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:.https://doi.org/10.1016/S0140-6736(16)32453-9
31 Abou-Alfa GK , Meyer T , Cheng AL , El-Khoueiry AB , Rimassa L , Ryoo BY , et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379(1):54–63. doi:.https://doi.org/10.1056/NEJMoa1717002
32 Zhu AX , Kang YK , Yen CJ , Finn RS , Galle PR , Llovet JM , et al.; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. doi:.https://doi.org/10.1016/S1470-2045(18)30937-9
33 Finn RS , Qin S , Ikeda M , Galle PR , Ducreux M , Kim TY , et al.; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–905. doi:.https://doi.org/10.1056/NEJMoa1915745
34 Puzanov I , Diab A , Abdallah K , Bingham CO, 3rd , Brogdon C , Dadu R , et al.; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:.https://doi.org/10.1186/s40425-017-0300-z
35 Haanen J , Carbonnel F , Robert C , Kerr KM , Peters S , Larkin J , et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. doi:.https://doi.org/10.1093/annonc/mdx225
36 Sangro B , Chan SL , Meyer T , Reig M , El-Khoueiry A , Galle PR . Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72(2):320–41. doi:.https://doi.org/10.1016/j.jhep.2019.10.021
37 Kulik LM , Carr BI , Mulcahy MF , Lewandowski RJ , Atassi B , Ryu RK , et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47(1):71–81. doi:.https://doi.org/10.1002/hep.21980
38 Chow PKH , Gandhi M , Tan SB , Khin MW , Khasbazar A , Ong J , et al.; Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36(19):1913–21. doi:.https://doi.org/10.1200/JCO.2017.76.0892
39 Vilgrain V , Pereira H , Assenat E , Guiu B , Ilonca AD , Pageaux GP , et al.; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–36. doi:.https://doi.org/10.1016/S1470-2045(17)30683-6
40 Eisenhauer EA , Therasse P , Bogaerts J , Schwartz LH , Sargent D , Ford R , et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi:.https://doi.org/10.1016/j.ejca.2008.10.026
41 Lencioni R , Llovet JM . Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:.https://doi.org/10.1055/s-0030-1247132
Reviewed and approved by: Philip Bruggmann, Andreas Cerny, Valerie McLin, Joachim Mertens, Darius Moradpour, Laura Rubbia-Brandt and David Semela as council members of the Swiss Association for the Study of the Liver (SASL) and by Bruno Balsiger, Peter Bauerfeind, Jan Borovicka, Lukas Degen, Gian Dorta, Tobias Ehmann, Jean-Louis Frossard, Christoph Gubler, Beat Müllhaupt, Florian Riniker and Kaspar Truninger as council members of the Swiss Society of Gastroenterology (SGG-SSG)
No financial support and no other potential conflict of interest relevant to this article were reported.